
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 29 September 2023
Sec. Pediatric Critical Care
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1123405
This article is part of the Research TopicOptimizing Blood Pressure, Blood Flow, and Organ Perfusion in Neonates, Infants, and Children In the Perioperative and Critical Care SettingView all 7 articles
Cardiovascular instability and reduced oxygenation are regular perioperative critical events associated with anesthesia requiring intervention in neonates and young infants. This review article addresses the current modalities of assessing this population's adequate end-organ perfusion in the perioperative period. Assuring adequate tissue oxygenation in critically ill infants is based on parameters that measure acceptable macrocirculatory hemodynamic parameters such as vital signs (mean arterial blood pressure, heart rate, urinary output) and chemical parameters (lactic acidosis, mixed venous oxygen saturation, base deficit). Microcirculation assessment represents a promising candidate for assessing and improving hemodynamic management strategies in perioperative and critically ill populations. Evaluation of the functional state of the microcirculation can parallel improvement in tissue perfusion, a term coined as “hemodynamic coherence”. Less information is available to assess microcirculatory disturbances related to higher mortality risk in critically ill adults and pediatric patients with septic shock. Techniques for measuring microcirculation have substantially improved in the past decade and have evolved from methods that are limited in scope, such as velocity-based laser Doppler and near-infrared spectroscopy, to handheld vital microscopy (HVM), also referred to as videomicroscopy. Available technologies to assess microcirculation include sublingual incident dark field (IDF) and sublingual sidestream dark field (SDF) devices. This chapter addresses (1) the physiological basis of microcirculation and its relevance to the neonatal and pediatric populations, (2) the pathophysiology associated with altered microcirculation and endothelium, and (3) the current literature reviewing modalities to detect and quantify the presence of microcirculatory alterations.
Most clinical and blood pressure tools available to determine circulatory hemodynamic changes in the adult, pediatric, and neonatal populations evaluate macrocirculation as a surrogate of oxygen delivery and adequate end-organ perfusion pressure. The Surviving Sepsis Campaign Guidelines for managing septic shock and sepsis-associated organ dysfunction in children (1) recommend heart rate, capillary refill, and urinary output as clinical markers of cardiac output to assess fluid resuscitation. Gas exchange utilizes capnography, oxygen saturation probes, and blood gas analysis. Near-infrared spectroscopy (NIRS) noninvasively measures oxygen saturation in the vasculature, monitoring tissue perfusion and oxygen delivery (2). Addressing tissue perfusion meeting cellular metabolic demands requires the use of clinical biomarkers such as serum lactate (3, 4), mixed venous oxygen saturation (SmvO2) (5), and base deficit (6). Elevated lactate levels are recognized as an indirect marker for tissue hypoperfusion (7). Strategies to evaluate optimal organ resuscitation include serum lactate clearance and capillary refill (8). For the critically ill pediatric population, elevated lactate is consistently associated with mortality (9), including sepsis and septic shock (4). Targeting resuscitation to clear lactate decreases organ dysfunction (3). Although the macrocirculation assessment and monitoring of oxygen delivery is an accepted management strategy, a knowledge gap exists in addressing microcirculation as a treatment target in the resuscitation of the hemodynamically compromised critically ill pediatric patient.
Adequate tissue perfusion balances oxygen delivery (DO2) and oxygen consumption (VO2) along with the actual exchange of nutrients and waste products at the microcirculatory level. Anatomically, the microcirculation consists of blood vessels <20 µm in diameter (microvessels), including capillaries, arterioles, and venules (10). The capillaries are dynamic vascular vessels <10 µm between the arterioles containing smooth muscle cells and regulating blood flow and venules (Figure 1). The distribution and magnitude of blood flow is a coordinated interaction between arteriolar, capillary, and venular segments responding to metabolic demands (11). The capillaries within the microcirculation are important distribution centers delivering oxygen and nutrients, signaling molecules, and medication products to tissues and cells. They also support removing waste products and are essential in fluid movement and temperature control (12).
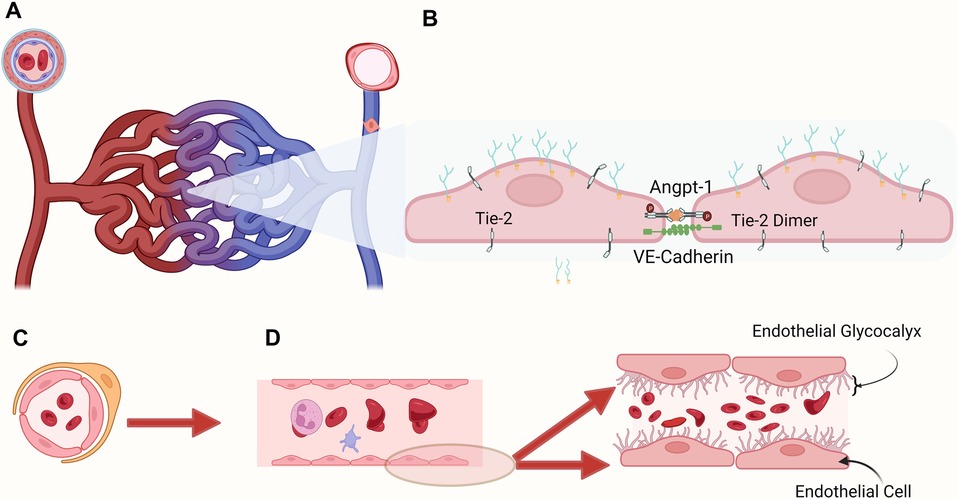
Figure 1. Microvasculature: (A) pericytes surround and support the capillaries, the most numerous and dynamic component of the microcirculation where gas and metabolite exchange occurs, and postcapillary venules, where endothelial cells lack tight junctions and are leakier than capillaries. (B) Representation of two endothelial cells joined transversally to maintain intact endothelium. (C) Cross sectional view of the microcirculation with red blood cells circulating in the inner section (D) Graphic representation of the glycocalyx covering the endothelial cells lining the blood vessel. The membrane bound main components include proteoglycans (syndecans, glypicans), glycoproteins (selectins, integrins). The endothelial surface layer includes hyaluronan, plasma proteins, and soluble proteoglycans. Shedding of endothelial glycocalyx (EG) components into the plasma is related to different critically ill conditions, potentially indicating the degree of endotheliopathy.
The parallel function of the macrocirculation with the microcirculation has been labeled hemodynamic coherence (13). However, optimal macrocirculation resuscitation does not necessarily reflect adequate microcirculatory perfusion. The dissociation that occurs between the macrocirculatory and microcirculatory circulations has been described in acute pathologic conditions, including shock (14, 15), hypoperfusion (16), cardiopulmonary bypass (17), and sepsis (18, 19) among the most commonly investigated.
Different techniques exist to assess the microcirculation (MC), detailed in Table 1 (10). Capillaroscopy (22) and laser scanning confocal imaging (23, 24) have limited use in the clinical setting. The introduction of handheld vital microscopy (HVM) using an illumination unit with green light (wavelength 548 nm for optimal oxyhemoglobin and deoxyhemoglobin light absorbance) and a light guide with a magnification lens (25) provided a potential venue to noninvasively obtain images useful for clinical assessment and treatment response at the bedside. The analysis and interpretation of HVM data involve two main components of oxygen-carrying capacity: red blood cell flow through the capillaries (oxygen delivery) and the density of the perfused capillaries (diffusive transport of oxygen) (10). Traditional microcirculatory parameters include total vessel density (TVD), functional capillary density (FCD), perfused vessel density (PVD), proportion of perfused vessels (PPV), microcirculatory flow index (MFI), and microcirculatory heterogeneity index (MHI) described in Table 2.
Three generations of HVMs have been developed (27). The first generation of HVM involved orthogonal polarization spectral (OPS) imaging (28) using light linearly polarized in one plane and collecting imaging through a second polarizer oriented in an orthogonal plane. A novel technique in HVM was developed in 2007 using sidestream dark field (SDF) imaging, improving quality imaging (29), currently used as a research tool assessing microcirculatory function in the clinical setting and animal models (16, 30). A newer HVM based on incident dark field illumination (IDF), considered a third-generation device, has been introduced in the clinical setting (31). This model combines high-density pixel-based imaging and short-pulsed illumination, providing high-resolution optics (27). Introducing an automatic algorithm software (MicroTools) eased data analysis collected by the HVM (32).
The inner section of the vascular system, the tunica intima, includes the endothelium, where endothelial cells (ECs) line the internal vascular system forming tight junctions, covered by the endothelial glycocalyx (EG), the luminal layer within the blood vessel and fundamental determinant of mechanotransduction and vascular permeability. The vascular endothelium is a highly specialized and physiologically important organ system regulating vascular permeability, vascular tone, cell adhesion, controls blood fluidity, allows macromolecular transfer between blood and tissue, and modulates immune cell recruitment and activation and platelet function (12). While ECs are first-line regulators of proinflammatory and immune responses, their role in neovascularization is also vital in tissue repair (33). The endothelial surface is lined by the endothelial glycocalyx (EG). It is composed of a glycan-rich layer consisting of highly sulfated, negatively charged glycosaminoglycans, including heparan sulfate and chondroitin sulfate attached to the endothelial surface-anchored proteoglycans: syndecans and glypicans (34). Damage to the endothelial glycocalyx can be assessed by screening for serum biomarkers. Recently, in addition to the assessment of the microcirculation, a non-invasive method to measure the size of the endothelial glycocalyx within the vascular microvessels became available, consisting of a handheld non-invasive camera collecting in vivo images of blood flow in the capillaries, coupled with the GlycoCheck™ software (35) measuring the thickness of the endothelial glycocalyx.
This review addresses (1) the physiological basis of microcirculation and its relevance to the neonatal and pediatric populations, (2) the pathophysiology associated with endothelial and endothelial glycocalyx dysfunction impacting the microcirculation function, and (3) the current literature reviewing modalities to detect and quantify the presence of microcirculatory alterations using HVM alone or coupled with endothelial glycocalyx assessment.
Search Strategy: In this review, the focus centers on the technology currently available to monitor microcirculation in the critically ill neonate and pediatric populations and its effectiveness during resuscitation. The template of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISM) (36) was used to collect and categorize the information. A systematic search was conducted in the following databases: PubMed®, MEDLINE OvidSP, and Google Scholar. Single and paired combinations of terms included “microcirculation”, “videomicroscopy”, and “children”. This review focuses on data published between 2010 and 2023. The data extraction was conducted by one investigator (GMA). The initial screening was completed independently from each other, and duplicates were deleted. The studies were merged for a second analysis. Eligibility and inclusion criteria encompassed prospective observational studies, cohort studies, systematic reviews, comprehensive reviews, and clinical trials. Screening criteria for inclusion included the following parameters: (1) Age group: the preterm, neonate, and children up to the age of 18 years, (2) the period reviewed covers the years 2010 and 2023 inclusively, (3) clinical condition labeled as critically ill or hemodynamically unstable, and (4) assessment of the microcirculation with handheld vital microscopy (HVM) ± assessment of the endothelial glycocalyx. We selected most of the reports with control cohorts in the prospective observation groups. We did not include studies describing the endothelium or endothelial glycocalyx assessment alone, only those combined with HVM assessments of the microcirculation. The results were qualitatively analyzed. The heterogeneity of patient populations, the small number of subjects in the reports, and the variety of techniques used for assessment challenged a quantitative analysis.
The number of pediatric and neonatal studies evaluating microcirculation using HVM in healthy and diseased children is minuscule compared to the available information published for the critically ill adult population. Entering the terms described in the methods section, we obtained 1,494 manuscripts from PubMed.gov, 1,430 references from Google Scholar, and 1,924 more from OVID Medline. The next step included the deletion of duplicate data (2,543 references). Abstracts from 2,305 studies were screened, and 153 full-text articles met the requirements for a full-text review. From this group, 32 manuscripts described the use of video microscopy in critically ill pediatric and neonate patients. This review describes the critically ill pediatric population of 16 pediatric (Table 3) and 11 neonatal (Table 4) reports. The working flowchart is presented in Figure 2. Most studies describe prospective observational reports involving a small number of patients. We also include five reviews evaluating microcirculation with and without endothelial biomarkers (Table 5). Maitoza et al. published the only systematic review using HVM in critically ill neonates and children and described 27 studies (64). In addition, two reviews of the literature describe the non-invasive measurements of the MC using HVM devices (OPS, SDF, and IDF) in neonates and pediatric populations (65) and the use of OPS and SDF in neonates and pediatrics (66). Top et al. reviewed nine studies using OPS and SDF in neonates and pediatric patients (67). Lastly, Puchwein-Schwepcke et al. published a mixed review of HVM combined with serum endothelial glycocalyx biomarkers (68).
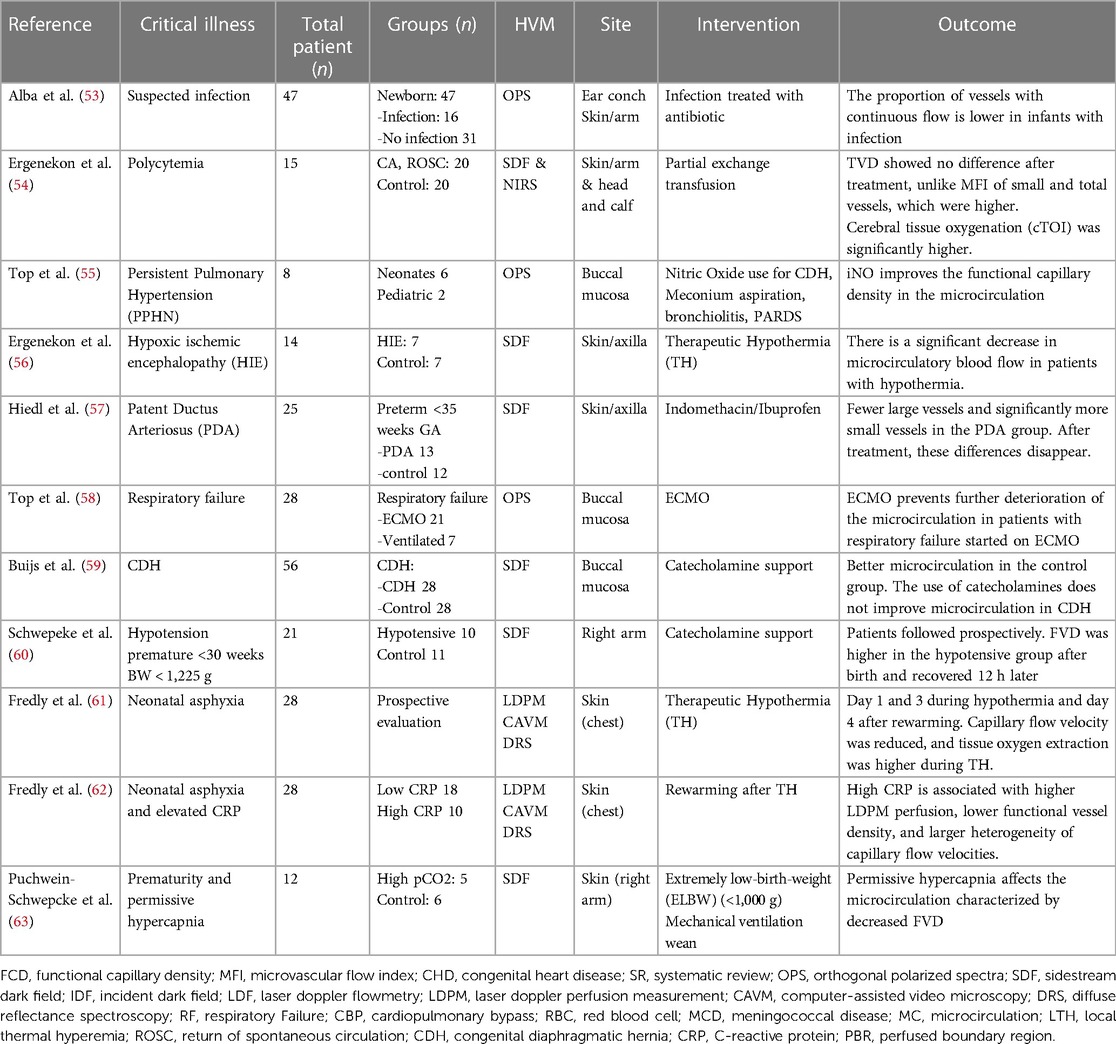
Table 4. Summary of critically ill neonatal and premature population studies using handheld vital microscopy (HVM).
A summary in Table 1 describes the multimodal techniques used for HVM. The methodology used by the investigators varied depending on the age group investigated and the HVM device used. The premature and neonatal populations showed the most anatomically diverse locations for microcirculation screens. Frequently reported regions for assessment reported in the premature and neonatal populations included the upper arm, nailbed, and ear conch. The buccal mucosa and the sublingual approaches were challenging to screen in the non-cooperative neonate and pediatric subjects, resulting in discomfort for the patient and poor image quality collection. Table 2 describes the measurement variables commonly reported using handheld devices adapted.
A summary of the details of the studies, findings, disease process, and conclusion is shown in a table format. Table 3 describes the pediatric group, and Table 4 describes the neonatal population findings. Table 5 includes a collection of recent reviews using HVM in the pediatric population, and one of the reports describes the use of HVM to examine microcirculation coupled with endothelial glycocalyx assessment.
Top et al. (37) used OPS to assess microcirculation characteristics 24 h after admission to the pediatric intensive care unit (PICU) for patients not surviving sepsis. The patients diagnosed with septic shock required fluid resuscitation, vasopressor/inotropes, and mechanical ventilation. On the first day, there were no differences in functional capillary density (FCD) between groups. Twenty-four hours later, the FCD significantly increased, and the microvascular flow index (MFI) improved in the survival group, suggesting that persistent microcirculatory abnormalities potentially lead to multiorgan system failure and death. Adult patients with sepsis demonstrate almost similar findings (69). A neonatal population study addressing infection showed a reduction in the proportion of vessels with continuous flow using the ear conch location with OPS (53). In meningococcal disease (MCD), Paize et al. compared 20 affected patients with 20 patients undergoing anesthesia for surgical procedures and 20 awake patients (38). This group used SDF in the sublingual location and measured microcirculation variables: microvascular flow index (MFI), capillary density (CD), proportion of perfused vessels (PPV), and perfused vessel density (PVD) in parallel to the endothelial biomarkers: intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin and P selectin. The patients with MCD were followed through their Pediatric Intensive Care Unit (PICU) stay. The authors found microcirculatory abnormalities correlating with biomarkers of endothelial dysfunction, and these abnormalities improved with clinical recovery. On a separate patient population, Fernandez-Sarmiento et al. published two cases with SARS-CoV-2-related multisystem inflammatory syndrome (MIS-C) investigating the endothelial glycocalyx using HVM with the Glycocheck™ software and plasma EG biomarkers revealing elevated plasma and imaging biomarkers of endothelial activation and endothelial glycocalyx degradation (48). Patients affected by severe malaria caused by Plasmodium falciparum were studied by Lyimo et al. (51). Using IDF of the buccal mucosa in patients with severe malaria compared to a control group, patients suffering from severe malaria demonstrated alterations in microcirculation and the endothelial glycocalyx.
Published work assessing medical interventions in shock management using HVM in combination with endothelial biomarkers and glycocalyx assessment has revealed that fluid administration and catecholamine use can be evaluated using HVM (26). A recent pediatric report demonstrated that fluid choice during resuscitation could have an unwanted impact on the endothelial system (49). This group compared a balanced solution for resuscitation with an unbalanced solution. The group used SDF with the GlycoCheck™ software and the endothelial serum biomarkers: angiopoietin-2 levels for vascular permeability and annexin A5 for apoptosis to determine microcirculation and endothelial responses. Unbalanced solutions increased endothelial degradation 6 h after fluid resuscitation compared to balanced solutions. These findings were also associated with a more significant elevation of biomarker levels and greater odds of metabolic acidosis and acute kidney injury returning to baseline levels 24 h later.
Several reports describe parameters obtained using HVM in neonates and children with congenital heart disease. Nussbaum and colleagues investigated the impact of cardiopulmonary bypass (CPB) on the microvasculature and endothelial glycocalyx using SDF and the GlycoCheck™ software. The microcirculation data demonstrated an acute reduction of the microvascular perfusion after cardiac surgery with CPB, particularly with aortic clamp and deep hypothermic cardiac arrest (showing decreased MFI and PVD). The perfused boundary region (PBR) increases as the glycocalyx is damaged. Immediately after surgery and CPB, PBR was increased, suggesting glycocalyx degradation. Nussbaum et al. found a relationship between changes in PBR and MFI and the need for mechanical ventilation and catecholamine support with longer CPB times (45). These results align with the study published by Bruegger et al. (70), where endothelial biomarkers Syndecan-1 and hyaluronan increased during, and after cardiac surgery. Similarly, in a more recent report, Erdem et al. (44) described the microcirculation in children with congenital heart disease before and during their cardiac surgical procedure and compared the microcirculation with a group of pediatric patients without heart disease undergoing surgery. Their findings showed decreased microcirculation perfusion and high vessel density in patients with congenital heart disease, with increased capillary recruitment. Specifically, both groups have similar perfused vessel densities and red blood cell velocities. However, children with CHD have less perfused vessels, lower perfusion quality, and higher small vessel densities.
More data addressing CHD has supported these findings. Scolletta et al. studied microcirculation in children with cyanotic and non-cyanotic heart disease. This group used HVM SDF sublingually in 24 children, 7 of them with cyanotic heart disease, and found that microcirculatory variables (PPV, TVD, and PVD) were different between patients with cyanosis and those without cyanosis (39). Gonzalez et al. included 14 patients with cyanosis and 16 without cyanosis and assessed the microcirculation after anesthesia induction using sublingual SDF (40). Their data demonstrated a higher TVD with an increase in small blood vessels in the sublingual microcirculation, possibly related to the chronic hypoxia in patients with cyanosis, potentially a mechanism of adaptation. Gonzalez Cortes et al, using SDF-HVM, found that children with CHD have higher small vessel density and higher density of perfused small vessels at baseline; and lower MFI and higher heterogeneity during surgery in 24 patients undergoing surgery under cardiopulmonary bypass surgery (42).
The data obtained from these different manuscripts show that patients with CHD have differences in microcirculation compared to patients with normal cardiac anatomy and physiology.
The concept of using HVM as a tool in the perioperative period was described by Hitly et al. These investigators used HVM data from 267 adult and pediatric patients undergoing surgery, diagnosed with sepsis and heart failure, and included healthy volunteers to validate a software algorithm to ease the HVM analysis (MicroTools) (50). The algorithm-based analysis of the sublingual microcirculation closely matched the manual analysis collected from the patient variety. The pediatric population included data from 120 perioperative patients undergoing elective cardiac surgery. The data was collected after anesthesia induction and included total vessel density (TVD) and functional capillary density (FCD). The authors did not specifically address the pediatric-specific data. However, in general, the authors found a strong correlation between the manual- vs. algorithm-based measurements in FCD, potentially suggesting that in the pediatric population, the algorithm-based measurements are reliable. Monitoring pediatric patients during high-risk surgeries depends on optimizing the macrocirculation. In a study completed by Wagner et al. (52), the authors addressed the feasibility of monitoring the microcirculation using handheld vital microscopy in a small group of pediatric patients. The microcirculation was assessed at four-time points during the perioperative period and was coupled with serum glycocalyx markers (syndecan-1 and hyaluronan). This effort was challenged by the number of personnel and logistics associated with its routine use in this clinical setting. In this small population, the findings showed microvascular changes and direct injury to the glycocalyx.
Schinagl et al. (47) explored the effects of blood transfusion on microcirculation. In a group of 19 patients with anemia, the authors found a lower TVD and higher RBC velocity than control patients. After blood transfusions, TVD increased with a concomitant decrease in RBC velocity in medium-sized vessels. Although these changes occurred in the patients with anemia after receiving RBCs, TVD and RBC velocities did not reach the control group values. From the 19 patients in this study, a subgroup analysis described nine patients with anemia and sepsis. These patients showed a lower TVD before transfusion, with a larger increase after transfusion than patients with anemia and without infection. The data was collected with HVM SDF assessing the buccal microcirculation. The control group included matched patients of age and sex receiving minor reconstructive surgery and without medical problems.
Buijs et al. studied the microcirculation after the return of spontaneous circulation (ROSC) in patients who suffered cardiac arrest (46). They used HVM SDF to investigate the microcirculation at the buccal mucosa during and after therapeutic hypothermia (TH). The prospective study covered four years, including 20 pediatric patients with cardiac arrest and age- and gender-matched control normothermic children without cardiorespiratory pathology. The goal for temperature in TH was set at 34.0°. During TH, all variables in the post-cardiac arrest group were lower than the control group and did not differ after returning to normothermia. Microcirculatory impairment was significantly present in the non-survivor group at the beginning of TH, and it is proposed that using non-invasive microcirculatory monitoring can be useful to the clinician for prognostication purposes. In a separate study including neonates, Ergenekon et al. used HVM SDF imaging from the axillary area for a group of 7 newborns who suffered hypoxic-ischemic encephalopathy (HIE) and underwent TH and head cooling (56). Microcirculatory blood flow significantly decreased during hypothermia, becoming similar to the control group after rewarming. Fredly et al. (61) used laser Doppler perfusion measurements (reduced during hypothermia) and diffuse reflectance spectroscopy (DRS) to assess microvascular oxygen extraction. The authors evaluated the capacity for oxygen delivery. They described a mean functional capillary density (FCD) higher during cooling and after rewarming in the group with HIE, along with a significant increase in oxygen extraction. Skin microcirculatory responses significantly differed after rewarming in a subgroup of patients with HIE and elevated CRP (62).
Top et al. investigated the effect of inhaled nitric oxide on neonates with respiratory failure using OPS in oral mucosa at two-time points: 1 h before and 1 h after the initiation of inhaled nitric oxide. Inhaled nitric oxide improved systemic microcirculation in patients with hypoxemic respiratory failure in the newborn population affected with pulmonary hypertension (55). Neonatal microcirculation was examined using HVM OPS imaging in patients with respiratory failure before extracorporeal membrane oxygenation (ECMO) and after initiating ECMO and compared with patients who remained on mechanical ventilation not supported with ECMO (58). These investigators noticed there was no change in microcirculation parameters with ECMO initiation. However, ECMO improved the microcirculation parameters compared to patients with respiratory failure and mechanical ventilation not receiving ECMO. In a more detailed investigation in patients treated with veno-venous (VV) and veno-arterial (VA) ECMO, Erdem et al. (43) aimed to determine the effects of ECMO in the sublingual microcirculation in pediatric and neonatal populations. Their data collection included the Pediatric Logistic Organ Dysfunction 2 (PELOD-2) score, the inotrope score (IS), and the vasoactive-inotrope score (VIS) for clinical data collection. They found no difference. They found that the microcirculatory parameters were not significantly different between VV and VA ECMO, and these parameters were no different in patients with CHD. Also, these parameters were not different between survivors and non-survivors. The authors discuss several possibilities for these findings, including starting ECMO in patients in extremis where the compensatory mechanisms for adequate microcirculation might not have been exhausted. Potentially, a possible explanation might include the heterogeneity of patients included in the study and not rendering adequate sample sizes for the diversity of pathologies included. The comparison in findings between these two manuscripts is challenged by the difference in patient populations included. The neonatal population tends to have more uniform pathologies than the pediatric population. Further studies with more homogeneous populations are needed to determine the effects of ECMO in the microcirculation.
HVM has been used to examine the microcirculation of premature and neonatal disease processes. Polycythemia requiring partial exchange transfusion showed no difference in TVD and higher MFI in total vessels with increased cerebral tissue oxygenation (54). The use of indomethacin in preterms with patent ductus arteriosus (PDA) was reported by Hiedl et al. (57) demonstrating fewer large vessels in the microcirculation of infants with PDA, with increment after medical treatment. Buijs et al. examined 28 newborns diagnosed with congenital diaphragmatic hernia (CDH). A subgroup was receiving vasopressor support and was compared to healthy newborns (59). Catecholamine support in patients with CDH improved the macrocirculatory parameters without improving the microcirculation. The severity of microcirculation dysfunction also predicted a poor clinical outcome and the need for extracorporeal membrane oxygenation (ECMO). VLBW patients with hypotension have higher functional vessel density when compared to a control group, a finding that resolves 12 h after birth (60). Puchwein-Schwepcke et al. studied the effects of hypercapnia in the extremely low birth weight (ELBW) premature population with weight <1,000 gms (63). The investigators noticed a significantly and progressively decreased functional vessel density (FVD), suggesting impaired peripheral microcirculation at the skin capillaries.
Several comprehensive reviews have summarized our current knowledge of the use of HVM in critically ill pediatric patients, detailed in Table 5. A recent report combined this approach with the assessment of the endothelial glycocalyx. The systematic review from Maitoza et al. includes microcirculation assessment information using HVM with literature published before 2020 (64). The authors observed several limitations in the manuscripts published, including study design, high subject dropout rate, and a need for standardized normal values for the investigated age populations. The authors highlight the need for future studies to define normal pediatric flow variables and more information describing treatment impact on the pediatric and neonatal microcirculation. Similar conclusions were echoed in the review completed by Erdem et al. (65). These authors highlight the advances in pediatric and neonatal microcirculation assessments, including the description of microcirculation differences between age groups, the presence of abnormal microcirculation in various disease processes despite maintaining normal macrocirculation values, and the persistent microcirculation abnormalities in patients with higher risk for mortality, emphasizing the need for age-related reference values. Kuiper et al. published a review highlighting the role of microcirculation monitoring in patients who later became hemodynamically unstable (66). Of interest to the pediatric critical care setting, Top et al. in 2011 (67) described a collection of reviews in neonates and pediatric patients, proposing microcirculation assessment as an essential hemodynamic variable to include during the evaluation and daily examination of the critically ill pediatric patient. In this review, the group emphasized the change in functional capillary density (δFCD) within the first two days in children affected by septic shock and compared those findings with the pediatric risk of mortality (PRISM). Their results described a better sensitivity and specificity for δFCD, previously reported by these authors as ΔFCD (37). More recently, Puchwein-Schwepcke et al. published a review where the endothelial glycocalyx (EG) was examined using HVM in combination with endothelial biomarkers (68). Their study describes the advances in microscopic technology to assess in vivo EG using a recently developed automated acquisition and analysis approach in combination with the determination of EG components as biomarkers: syndecan-1, chondroitin sulfate, hyaluronan, and heparan sulfate from serum and urine levels. In their review, the authors describe the tools to measure the EG and discuss the perfused boundary region (PBR), a variable used to measure the luminal part of the EG accessible to flowing erythrocytes, a concept validated in the adult population (35). This review summarizes the studies related to the physiological development of the EG, its assessment in pediatric clinical studies, and the challenges encountered in the pediatric population, particularly preterm newborns, including the physiology of the blood vessel development in the fetus and neonates. Disease processes reported in this review included cardiopulmonary bypass effects, trauma, infectious diseases, and chronic disorders such as diabetes mellitus. The authors conclude that damage to the EG is well documented in acute and chronic conditions within the adult population. The data for the pediatric and neonatal groups is scant at present. More investigations are needed to characterize the normal development of the EG from the fetus to adulthood, its contribution to a variety of disease processes, and the potential of developing target-specific therapies that can potentially preserve and heal the EG impacting clinical outcomes.
The current information related to HVM highlights the advances and challenges encountered in applying this technology to the neonatal and pediatric population. The evidence varies in the quality of information published and the number of patients used in each report, some of which do not have a control group. Using different techniques and devices resulted in obtaining measurement variables that require consistency among the reports. Videomicroscopy requires understanding the basic technical and photometric information for optimal image acquisition. Massey et al. described the parameters needed for high-quality data collection and the challenges with using them in the adult group (27). Unlike the pediatric population, a more standardized approach exists for the adult population. A task force involving international expert opinion published guideline recommendations for video microscopy targeting the critically ill adult population (10). The consensus discussed handheld videomicroscopy's technology, physiology, variable measurements, and clinical utility. This report has allowed reliable data collection for healthy individuals and patients with different disease processes. The resulting statements regarding the acquisition and interpretation of the microcirculatory images included databases of measurement variables recommended for the different types of shock and the type of evaluation needed for a variety of therapeutic interventions, mainly fluid administration, vasopressor administration, and weaning from ECMO or IABP. The extrapolation of these recommendations to the pediatric group does not apply to the nature of the device and the site needed to collect the data. The authors mention the need to describe normal values for different age groups in pediatrics and the variety of developmental changes occurring in the neonatal population, particularly during the first week of life, requiring further characterization.
The sublingual approach is the ideal location in the adult population to measure microcirculation (10). In a control group, Paize et al. (38) used awake patients older than six years of age for their control group and anesthetized patients younger than six years of age, demonstrating the difficulty of using sublingual SDF in the younger not-sedated population. The degree of abnormality in the microcirculatory flow pattern relates to the severity of the illness and improves over time as the clinical condition resolves. The standardized use of the sublingual approach to measuring microcirculation in the pediatric population and the validity of the norm values for neonates and children are areas of intense research (10, 50). Children require anesthesia or sedation to obtain meaningful measurements, and these values have greater reliability when pediatric patients are anesthetized, endotracheally intubated, and with mechanical ventilation. Cooperation from adult patients using this location is feasible and easily obtained.
The progression in our understanding of microcirculation and the endothelial system has led to the development of new approaches investigating the health of the endothelial glycocalyx (EG). The EG comprises proteoglycans and glycosaminoglycans and is crucial in maintaining a functional barrier and a healthy microcirculation (71). The disruption of this structure is currently recognized as having a central role in several critical diseases, such as sepsis and acute inflammation (72), acute respiratory distress syndrome (73), trauma (74), cardiopulmonary bypass (45), and ischemia/reperfusion (75). Jacobs et al. summarized the microcirculation's physiology and pathophysiology, emphasizing the EG's important role in hemodynamic responses (76). Several recent reviews address the importance of identifying biomarkers that can identify the shedding of the EG in disease processes leading to identifying, prognosticating, and ideally guiding clinical decision-making (68). Understanding the molecular basis of the EG in health and disease sets the possibility of developing new EG targeted therapies. The importance of the endothelium-specific Angiopoietin/Tie2 system controlling endothelial activation and its role in critical illness (77, 78) has received recent attention. The disruption of the endothelial Tie2 system appears associated with coagulopathy triggered by sepsis. Angiopoietin-1 (Angpt-1) binds to Tie2 at the endothelial surface and maintains adhesion between endothelial cells (Figure 1). During inflammation, Tie2 activation decreases, and TIE2 transcription is attenuated. Richter et al. described the association of plasma angiopoietin-1 and angiopoietin-2 levels and the Angiopoietin-2/-1 ratio in critically ill children with sepsis to measure organ injury (79). The authors found that in the acute phase of sepsis, angiopoietin-1 levels are decreased compared to controls, and angiopoietin-2 levels and ratio are elevated. These values correlated with organ injury, impacting mechanical ventilation duration and PICU length of stay. The authors suggest that angiopoietin dysregulation occurs early in sepsis and is potentially related to multiple organ dysfunction. In this review, the authors present data indicating that the Angpt/Tie2 system can be used to diagnose and treat critically ill patients. Few pediatric studies have used angiopoietin serum levels to assessEG degradation. Recently, Fernandez-Sarmient et al. used HVM and syndecan-1 to assess microcirculation and EG integrity along with angiopoietin-2 and annexin A5 levels to determine the effect of balanced and unbalanced crystalloid resuscitation during sepsis (49).
In the adult population, sepsis has been an area where microcirculation derangement has been well described in the literature (80). These data have shown that microcirculatory dysfunction is an early indicator of tissue hypoperfusion and precedes the onset of multiorgan failure and death (81). In 2018, four different types of alterations were described during the 2nd consensus on the assessment of sublingual microcirculation in critically ill patients. These included: Type 1, complete stagnated capillaries (cardiac arrest); Type 2, reduction in the number of flow in capillaries (hemodilution); Type 3, vessels with no flow next to vessels with flowing cells (sepsis, hemorrhage); Type 4, hyperdynamic flow within capillaries (hemodilution, sepsis) (10). For sepsis, the heterogeneity index is significantly elevated. Sepsis and septic shock show significant abnormalities in microperfusion parameters using HVM. In contrast, the data available for the pediatric population to assess microcirculation and endotheliopathy is limited.
Microcirculatory parameters show differences between patients with respiratory failure and patients with sepsis. In the presence of septic shock, a persistent decrease in functional vessel density (FVD) indicates poor survival when assessing with HVM (82, 83). Normal values for the adult population describe an MFI below 2.6 to differentiate normal from abnormal microcirculation (84). The extrapolation of these values to the pediatric population has yet to be validated.
Microcirculation monitoring could help assess fluid responsiveness. A report done in the adult population by Pranskunas et al. found that patients with clinical signs of impaired organ perfusion and an MFI less than 2.6 were fluid-responsive when MFI increased from 2.3 to 2.5, while patients with an MFI at 2.8 did not respond to fluid resuscitation indicating nonresponsiveness (85). The idea of incorporating microcirculatory targeted treatment in septic shock resuscitation guidelines was tested by van der Voort et al. (86). This group completed a randomized control pilot study to increase microcirculation variables using nitroglycerin, dopamine, enoximone, and dexamethasone. Although the results did not change end organ recovery compared to the control group, this clinical trial introduced the concept of microcirculatory assessment within treatment guidelines.
Data published for neonates and pediatric patients with respiratory failure and candidates for ECMO have also shown microcirculatory abnormalities. MFI values in respiratory failure are relatively high, and heterogeneity index (HI) values are relatively low (58). In these investigations, the findings did not correlate with mortality. In contrast and related to mortality, in pediatric patients (37) nonsurvivors of sepsis presented with elevated MFI and HI levels that did not decrease over time, contrasting with an initial reduced MFI in survivors. In adult patients with septic shock (83) nonsurvivors of sepsis presented with altered small vessel perfusion that did not improve, unlike the improvement noted in the survivor group over time. These microcirculatory findings were present despite similar hemodynamic and oxygenation parameters between survivors and non-survivors. The use of HVM to assess microcirculation over time can serve as a tool for risk stratification and prognostication and guide earlier intervention in sepsis management. The alterations in microvasculature, coupled with endothelial glycocalyx biomarkers, can lead to different management strategies. The emphasis on precision medicine for sepsis can determine which therapies in the future can work best individually (87).
In a recent data set related to other disease processes, the microcirculation assessment in the adult population with COVID-19 demonstrated a dysfunctional endothelial glycocalyx. Reports of elevated endothelial glycocalyx biomarkers (syndecan 1, chondroitin sulfate) (88) along with reduced heparanase-2, elevated ADAMTS13, and endothelial growth factor (89) led patients to a prothrombotic state. Rovas et al. combined the endothelial glycocalyx biomarkers with IDF videomicroscopy to quantify vascular density, red blood cell velocity, and glycocalyx dimensions for moderate-to-severe or critical COVID-19. The software used in this study calculates the dynamic lateral movement of RBCs into the permeable part of the glycocalyx layer expressed as the perfused boundary region (PBR in µm), inversely related to the endothelial glycocalyx dimension. The COVID-19 epidemic resulted in a clinical entity in the pediatric population termed multisystem inflammatory syndrome in children (MIS-C), a rare SARS CoV-2 virus infection complication. Fernandez et al. described two children with MIS-C where they investigated the endothelial function by combining videomicroscopy (microcirculation) and the biomarker Syndecan-1 to evaluate the endothelial Glycocalyx (48). In this report, the authors noted endothelial glycocalyx damage in a critically ill child with MIS-C. Degradation of the EG weakens the protection barrier covering the endothelial cells, favoring interstitial edema, capillary leak, fluid retention, and multiple organ dysfunction, progressing to failure with worsening clinical outcomes.
The technology to assess microcirculation continues to evolve with time. The recent investigations combining different strategies to evaluate the microcirculation, the endothelium, and EG suggest the potential benefit of collecting this information in critically ill patients. The routine use of videomicroscopy might provide the platform to assess therapeutic approaches for the resuscitation of the hemodynamically unstable pediatric patient. The data collected in the adult population with septic shock has led to the recognition of specific microcirculatory abnormalities these patients have and the resultant changes noted after therapeutic interventions (15). Despite these findings in microcirculation dysfunction, the information has yet to translate to clinical applicability for diagnoses or treatment assessments. Several studies have demonstrated that the lack of response of the microcirculation to resuscitation is associated with poor outcomes. A potential improvement in noninvasively assessing the microcirculation involves adding endothelial glycocalyx (EG) information. A significant number of biomarkers are available to evaluate both the quality of the glycocalyx and the vessel integrity to fluid extravasation.
The growth in physiological and pathophysiological knowledge has created potential treatment modalities to improve microcirculation. Clinical benefits have been observed in animal shock models where microcirculation and perfusion parameters (red blood cell velocity, functional capillary density) improve with the use of endothelial barrier modulators such as an angiopoietin-1 mimetic vasculotide or platelet-derived growth factor (16). A follow-up review from this group examined the current investigative therapies, including sex hormones and steroid use, proposed to prevent microvascular leakage related to endothelial glycocalyx damage targeting angiopoietin/Tie2 and sphingosine-1 phosphate signaling (90). Potential targeted therapies addressed in these models add understanding and novelty to future therapeutic interventions for critically ill patients.
Pediatric-specific work has been published indicating age-related developmental changes in the microcirculation (68) and the loss of hemodynamic coherence despite adequate macrocirculatory resuscitation (66) in the critically ill neonate. Despite advances in videomicroscopy, introducing these devices into the pediatric practice has yet to be incorporated. Technical shortcomings, inter-observer, and intra-observer variabilities challenge image analysis. Further, image analysis can be time-consuming (65). Future solutions to ease the assessment of microcirculation into clinical practice include the advancement in machine learning with the inclusion of software with appropriate algorithms allowing for instant analysis. Collecting quality information with the development of new algorithms can improve data processing, along with determining additional functional parameters of microvascular blood flow. Point-of-care assessment requires the development of integrated automated analysis software. There is a need to develop average values reference targeting different age groups. Ideally, integrating and evaluating pediatric clinical outcomes to microcirculatory guided therapies can add to the current hemodynamic assessments in critical care and anesthesiology practices.
Moreover, an essential role of the endothelium, particularly the endothelial glycocalyx, has emerged as having a central role in critical illness (71), and elevation of EG biomarkers such as Syndecan-1, heparan sulfate, hyaluronan can be related to EG-damage patient outcome, particularly with sepsis (91). In a recent review by Richter et al., the authors highlight the limited literature available describing EG pathophysiology in pediatric critical illness compared to adult data and analyze the current state of knowledge related to EG changes in age-maturation and EG alterations during pediatric acute critical illness (34). Fernandez et al. published a systematic review addressing EG alterations in sepsis assessed by biomarkers (92), including mostly adult and a few pediatric patients. The authors’ aimed to determine mortality risk as the primary outcome and respiratory failure and MODS as secondary outcomes. Inclusion criteria required patients with sepsis and abnormal biomarkers indicating glycocalyx injury, as determined by elevated glycocalyx biomarkers (Syndecan-1 and endocan) and clinical outcome descriptions. Their conclusions found a correlation between an abnormal result in biomarker levels and increased risk of death, respiratory failure, and MODS. These results raise the need to understand further the endothelial system's role in health and disease processes. The use of biomarkers in the critically ill pediatric and neonatal population remains an area requiring further investigation.
The microcirculatory alterations noted in the adult population have led investigators to attempt to combine videomicroscopy with biochemical elements that can provide added information on endothelial glycocalyx dysfunction. The MicroRESUS study (93), a prospective clinical trial to commence in 2023, including adult patients, will examine microcirculatory and mitochondrial function in human patients with circulatory shock undergoing cardiac bypass. The use of specific endothelial glycocalyx biomarkers, as described by Fernandez et al. (94) and Krispinsy et al. (95) in neonates, has opened the opportunity to understand microcirculatory abnormalities and endotheliopathy, escalating our understanding of the impact of critical illness at the end-organ perfusion. With our current state of knowledge, the use of biomarkers, and the technology available, the endothelial glycocalyx can become a treatment-targeted organ in managing critically ill patients. Future medical decision-making and prognostication algorithms can support clinical decision-making for children and neonates (Figure 3) by including microcirculation and endothelium glycocalyx assessments. Understanding epitheliopathy in critical illness and its impact on microcirculation and endothelial glycocalyx function holds promise for new therapeutic approaches to protect and repair the EG. The current understanding of the endothelial system's function in health and disease and its association with clinical outcomes is an ongoing area of investigation.
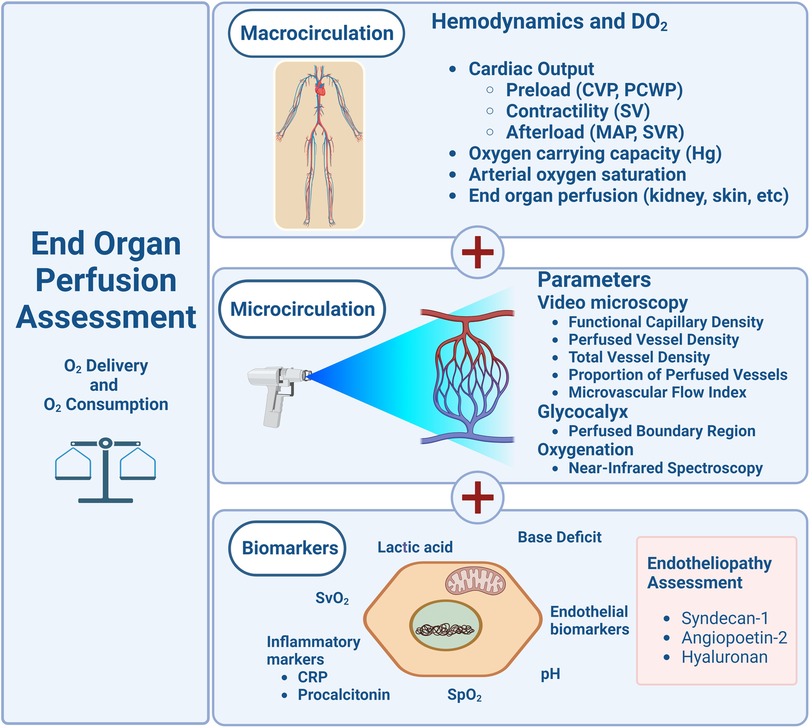
Figure 3. Schematic diagram proposing the evaluation of macro and micro circulations in the pediatric clinical assessment.
All authors contributed to the article and approved the submitted version.
We want to acknowledge Ms. Nina Arteaga's effort enhancing Figures 1,3 graphics.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. (2020) 21(2):e52–106. doi: 10.1097/PCC.0000000000002198
2. Vos JJ, Ellermann SF, Scheeren TWL. Journal of clinical monitoring and computing 2017/2018 end of year summary: monitoring-and provocation-of the microcirculation and tissue oxygenation. J Clin Monit Comput. (2019) 33(2):201–9. doi: 10.1007/s10877-019-00270-7
3. Scott HF, Brou L, Deakyne SJ, Fairclough DL, Kempe A, Bajaj L. Lactate clearance and normalization and prolonged organ dysfunction in pediatric sepsis. J Pediatr. (2016) 170:149–55.e1–4. doi: 10.1016/j.jpeds.2015.11.071
4. Schlapbach LJ, MacLaren G, Festa M, Alexander J, Erickson S, Beca J, et al. Prediction of pediatric sepsis mortality within 1 h of intensive care admission. Intensive Care Med. (2017) 43(8):1085–96. doi: 10.1007/s00134-017-4701-8
5. Marimón GA, Dockery WK, Sheridan MJ, Agarwal S. Near-infrared spectroscopy cerebral and somatic (renal) oxygen saturation correlation to continuous venous oxygen saturation via intravenous oximetry catheter. J Crit Care. (2012) 27(3):314.e13–8. doi: 10.1016/j.jcrc.2011.10.002
6. Phillips R, Acker SN, Shahi N, Meier M, Leopold D, Recicar J, et al. The ABC-D score improves the sensitivity in predicting need for massive transfusion in pediatric trauma patients. J Pediatr Surg. (2020) 55(2):331–4. doi: 10.1016/j.jpedsurg.2019.10.008
7. Hernandez G, Bellomo R, Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. (2019) 45(1):82–5. doi: 10.1007/s00134-018-5213-x
8. Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. (2019) 321(7):654–64. doi: 10.1001/jama.2019.0071
9. Bai Z, Zhu X, Li M, Hua J, Li Y, Pan J, et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatr. (2014) 14:83. doi: 10.1186/1471-2431-14-83
10. Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European society of intensive care medicine. Intensive Care Med. (2018) 44(3):281–99. doi: 10.1007/s00134-018-5070-7
11. Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. (2005) 12(1):33–45. doi: 10.1080/10739680590895028
12. Pierce RW, Giuliano JS Jr, Pober JS, Endothelial cell function and dysfunction in critically ill children. Pediatrics. (2017) 140(1):e20170355. doi: 10.1542/peds.2017-0355
13. Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. (2015) 19(Suppl 3):S8. doi: 10.1186/cc14726
14. Tachon G, Harrois A, Tanaka S, Kato H, Huet O, Pottecher J, et al. Microcirculatory alterations in traumatic hemorrhagic shock. Crit Care Med. (2014) 42(6):1433–41. doi: 10.1097/CCM.0000000000000223
15. Dubin A, Kanoore Edul VS, Caminos Eguillor JF, Ferrara G. Monitoring microcirculation: utility and barriers—a point-of-view review. Vasc Health Risk Manag. (2020) 16:577–89. doi: 10.2147/VHRM.S242635
16. van Leeuwen ALI, Dekker NAM, Jansma EP, Boer C, van den Brom CE. Therapeutic interventions to restore microcirculatory perfusion following experimental hemorrhagic shock and fluid resuscitation: a systematic review. Microcirculation. (2020) 27(8):e12650. doi: 10.1111/micc.12650
17. Greenwood JC, Jang DH, Spelde AE, Gutsche JT, Horak J, Acker MA, et al. Low microcirculatory perfused vessel density and high heterogeneity are associated with increased intensity and duration of lactic acidosis after cardiac surgery with cardiopulmonary bypass. Shock. (2021) 56(2):245–54. doi: 10.1097/SHK.0000000000001713
18. Spronk PE, Zandstra DF, Ince C. Bench-to-bedside review: sepsis is a disease of the microcirculation. Crit Care. (2004) 8(6):462–8. doi: 10.1186/cc2894
19. Yajnik V, Maarouf R. Sepsis and the microcirculation: the impact on outcomes. Curr Opin Anaesthesiol. (2022) 35(2):230–5. doi: 10.1097/ACO.0000000000001098
20. Eriksson S, Nilsson J, Sturesson C. Non-invasive imaging of microcirculation: a technology review. Med Devices (Auckl). (2014) 7:445–52. doi: 10.2147/MDER.S51426
21. Bol ME, Broddin BEK, Delhaas T, Sels JEM, van de Poll MCG. Variability of microcirculatory measurements in healthy volunteers. Sci Rep. (2022) 12(1):19887. doi: 10.1038/s41598-022-22947-x
22. Fagrell B, Intaglietta M. Microcirculation: its significance in clinical and molecular medicine. J Intern Med. (1997) 241(5):349–62. doi: 10.1046/j.1365-2796.1997.125148000.x
23. Bussau LJ, Vo LT, Delaney PM, Papworth GD, Barkla DH, King RG. Fibre optic confocal imaging (FOCI) of keratinocytes, blood vessels and nerves in hairless mouse skin in vivo. J Anat. (1998) 192(Pt 2):187–94. doi: 10.1046/j.1469-7580.1998.19220187.x
24. Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. (1995) 104(6):946–52. doi: 10.1111/1523-1747.ep12606215
25. van den Berg VJ, van Elteren HA, Buijs EA, Ince C, Tibboel D, Reiss IK, et al. Reproducibility of microvascular vessel density analysis in sidestream dark-field-derived images of healthy term newborns. Microcirculation. (2015) 22(1):37–43. doi: 10.1111/micc.12163
26. Dilken O, Ergin B, Ince C. Assessment of sublingual microcirculation in critically ill patients: consensus and debate. Ann Transl Med. (2020) 8(12):793. doi: 10.21037/atm.2020.03.222
27. Massey MJ, Shapiro NI. A guide to human in vivo microcirculatory flow image analysis. Crit Care. (2016) 20:35. doi: 10.1186/s13054-016-1213-9
28. Groner W, Winkelman JW, Harris AG, Ince C, Bouma GJ, Messmer K, et al. Orthogonal polarization spectral imaging: a new method for study of the microcirculation. Nat Med. (1999) 5(10):1209–12. doi: 10.1038/13529
29. Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream dark field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. (2007) 15(23):15101–14. doi: 10.1364/oe.15.015101
30. Obonyo NG, Fanning JP, Ng AS, Pimenta LP, Shekar K, Platts DG, et al. Effects of volume resuscitation on the microcirculation in animal models of lipopolysaccharide sepsis: a systematic review. Intensive Care Med Exp. (2016) 4(1):38. doi: 10.1186/s40635-016-0112-3
31. Aykut G, Veenstra G, Scorcella C, Ince C, Boerma C. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp. (2015) 3(1):40. doi: 10.1186/s40635-015-0040-7
32. Guven G, Hilty MP, Ince C. Microcirculation: physiology, pathophysiology, and clinical application. Blood Purif. (2020) 49(1–2):143–50. doi: 10.1159/000503775
33. Lee DD, Schwarz MA. Cell-cell communication breakdown and endothelial dysfunction. Crit Care Clin. (2020) 36(2):189–200. doi: 10.1016/j.ccc.2019.11.001
34. Richter RP, Payne GA, Ambalavanan N, Gaggar A, Richter JR. The endothelial glycocalyx in critical illness: a pediatric perspective. Matrix Biol Plus. (2022) 14:100106. doi: 10.1016/j.mbplus.2022.100106
35. Eickhoff MK, Winther SA, Hansen TW, Diaz LJ, Persson F, Rossing P, et al. Assessment of the sublingual microcirculation with the GlycoCheck system: reproducibility and examination conditions. PLoS One. (2020) 15(12):e0243737. doi: 10.1371/journal.pone.0243737
36. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151(4):264–9, W64. doi: 10.7326/0003-4819-151-4-200908180-00135
37. Top AP, Ince C, de Meij N, van Dijk M, Tibboel D. Persistent low microcirculatory vessel density in nonsurvivors of sepsis in pediatric intensive care. Crit Care Med. (2011) 39(1):8–13. doi: 10.1097/CCM.0b013e3181fb7994
38. Paize F, Sarginson R, Makwana N, Baines PB, Thomson AP, Sinha I, et al. Changes in the sublingual microcirculation and endothelial adhesion molecules during the course of severe meningococcal disease treated in the paediatric intensive care unit. Intensive Care Med. (2012) 38(5):863–71. doi: 10.1007/s00134-012-2476-5
39. Scolletta S, Marianello D, Isgrò G, Dapoto A, Terranova V, Franchi F, et al. Microcirculatory changes in children undergoing cardiac surgery: a prospective observational study. Br J Anaesth. (2016) 117(2):206–13. doi: 10.1093/bja/aew187
40. González Cortés R, Urbano Villaescusa J, Solana García MJ, López González J, Fernández Lafever SN, Ramírez Gómez B, et al. Microcirculatory differences in children with congenital heart disease according to cyanosis and age. Front Pediatr. (2019) 7:264. doi: 10.3389/fped.2019.00264
41. González R, López J, Urbano J, Solana MJ, Fernández SN, Santiago MJ, et al. Evaluation of sublingual microcirculation in a paediatric intensive care unit: prospective observational study about its feasibility and utility. BMC Pediatr. (2017) 17(1):75. doi: 10.1186/s12887-017-0837-5
42. González Cortés R, Urbano Villaescusa J, Solana García MJ, López González J, Fernández Lafever SN, Ramírez Gómez B, et al. Microcirculatory changes in pediatric patients during congenital heart defect corrective surgery. J Cardiovasc Transl Res. (2021) 14(6):1173–85. doi: 10.1007/s12265-021-10132-w
43. Erdem Ö, Kuiper JW, van Rosmalen J, Houmes RJ, Wildschut ED, Ince C, et al. The sublingual microcirculation throughout neonatal and pediatric extracorporeal membrane oxygenation treatment: is it altered by systemic extracorporeal support? Front Pediatr. (2019) 7:272. doi: 10.3389/fped.2019.00272
44. Erdem Ö, de Graaff JC, Hilty MP, Kraemer US, de Liefde II, van Rosmalen J, et al. Microcirculatory monitoring in children with congenital heart disease before and after cardiac surgery. J Cardiovasc Transl Res. (2023). doi: 10.1007/s12265-023-10407-4
45. Nussbaum C, Haberer A, Tiefenthaller A, Januszewska K, Chappell D, Brettner F, et al. Perturbation of the microvascular glycocalyx and perfusion in infants after cardiopulmonary bypass. J Thorac Cardiovasc Surg. (2015) 150(6):1474–81.e1. doi: 10.1016/j.jtcvs.2015.08.050
46. Buijs EA, Verboom EM, Top AP, Andrinopoulou ER, Buysse CM, Ince C, et al. Early microcirculatory impairment during therapeutic hypothermia is associated with poor outcome in post-cardiac arrest children: a prospective observational cohort study. Resuscitation. (2014) 85(3):397–404. doi: 10.1016/j.resuscitation.2013.10.024
47. Schinagl CM, Mormanova ZH, Puchwein-Schwepcke A, Schmid I, Genzel-Boroviczény O. The effect of red blood cell transfusion on the microcirculation of anemic children. Eur J Pediatr. (2016) 175(6):793–8. doi: 10.1007/s00431-016-2704-z
48. Fernández-Sarmiento J, Flórez S, Alarcón-Forero LC, Salazar-Peláez LM, Garcia-Casallas J, Mulett H, et al. Case report: endothelial glycocalyx damage in critically ill patients with SARS-CoV-2-related multisystem inflammatory syndrome (MIS-C). Front Pediatr. (2021) 9:726949. doi: 10.3389/fped.2021.726949
49. Fernández-Sarmiento J, Salazar-Peláez LM, Acevedo L, Niño-Serna LF, Flórez S, Alarcón-Forero L, et al. Endothelial and glycocalyx biomarkers in children with sepsis after one bolus of unbalanced or balanced crystalloids. Pediatr Crit Care Med. (2023) 24(3):213–21. doi: 10.1097/PCC.0000000000003123
50. Hilty MP, Akin S, Boerma C, Donati A, Erdem Ö, Giaccaglia P, et al. Automated algorithm analysis of sublingual microcirculation in an international multicentral database identifies alterations associated with disease and mechanism of resuscitation. Crit Care Med. (2020) 48(10):e864–75. doi: 10.1097/CCM.0000000000004491
51. Lyimo E, Haslund LE, Ramsing T, Wang CW, Efunshile AM, Manjurano A, et al. In vivo imaging of the buccal mucosa shows loss of the endothelial glycocalyx and perivascular hemorrhages in pediatric plasmodium falciparum malaria. Infect Immun. (2020) 88(3):e00679–19. doi: 10.1128/IAI.00679-19
52. Wagner M, Anzinger E, Hey F, Reiter K, Wermelt JZ, Pastor-Villaescusa B, et al. Monitoring of the microcirculation in children undergoing major abdominal and thoracic surgery: a pilot study. Clin Hemorheol Microcirc. (2023) 83(3):217–29. doi: 10.3233/CH-221617
53. Alba-Alejandre I, Hiedl S, Genzel-Boroviczeny O. Microcirculatory changes in term newborns with suspected infection: an observational prospective study. Int J Pediatr. (2013) 2013:768784. doi: 10.1155/2013/768784
54. Ergenekon E, Hirfanoglu IM, Turan O, Beken S, Gucuyener K, Atalay Y. Partial exchange transfusion results in increased cerebral oxygenation and faster peripheral microcirculation in newborns with polycythemia. Acta Paediatr. (2011) 100(11):1432–6. doi: 10.1111/j.1651-2227.2011.02358.x
55. Top AP, Ince C, Schouwenberg PH, Tibboel D. Inhaled nitric oxide improves systemic microcirculation in infants with hypoxemic respiratory failure. Pediatr Crit Care Med. (2011) 12(6):e271–4. doi: 10.1097/PCC.0b013e31820ac0b3
56. Ergenekon E, Hirfanoğlu I, Beken S, Turan O, Kulali F, Koç E, et al. Peripheral microcirculation is affected during therapeutic hypothermia in newborns. Arch Dis Child Fetal Neonatal Ed. (2013) 98(2):F155–7. doi: 10.1136/archdischild-2012-301647
57. Hiedl S, Schwepcke A, Weber F, Genzel-Boroviczeny O. Microcirculation in preterm infants: profound effects of patent ductus arteriosus. J Pediatr. (2010) 156(2):191–6. doi: 10.1016/j.jpeds.2009.08.034
58. Top AP, Buijs EA, Schouwenberg PH, van Dijk M, Tibboel D, Ince C. The microcirculation is unchanged in neonates with severe respiratory failure after the initiation of ECMO treatment. Crit Care Res Pract. (2012) 2012:372956. doi: 10.1155/2012/372956
59. Buijs EA, Reiss IK, Kraemer U, Andrinopoulou ER, Zwiers AJ, Ince C, et al. Increasing mean arterial blood pressure and heart rate with catecholaminergic drugs does not improve the microcirculation in children with congenital diaphragmatic hernia: a prospective cohort study. Pediatr Crit Care Med. (2014) 15(4):343–54. doi: 10.1097/PCC.0000000000000105
60. Schwepcke A, Weber FD, Mormanova Z, Cepissak B, Genzel-Boroviczény O. Microcirculatory mechanisms in postnatal hypotension affecting premature infants. Pediatr Res. (2013) 74(2):186–90. doi: 10.1038/pr.2013.78
61. Fredly S, Fugelseth D, Nygaard CS, Salerud EG, Stiris T, Kvernebo K. Noninvasive assessments of oxygen delivery from the microcirculation to skin in hypothermia-treated asphyxiated newborn infants. Pediatr Res. (2016) 79(6):902–6. doi: 10.1038/pr.2016.16
62. Fredly S, Nygaard CS, Skranes JH, Stiris T, Fugelseth D. Cooling effect on skin microcirculation in asphyxiated newborn infants with increased C-reactive protein. Neonatology. (2016) 110(4):270–6. doi: 10.1159/000446763
63. Puchwein-Schwepcke AF, Schottmayer K, Mormanová Z, Dreyhaupt J, Genzel-Boroviczeny O, Thome UH. Permissive hypercapnia results in decreased functional vessel density in the skin of extremely low birth weight infants. Front Pediatr. (2018) 6:52. doi: 10.3389/fped.2018.00052
64. Maitoza LA, Neeman E, Funaro M, Pierce RW. Relevance of microvascular flow assessments in critically ill neonates and children: a systematic review. Pediatr Crit Care Med. (2020) 21(4):373–84. doi: 10.1097/PCC.0000000000002201
65. Erdem Ö, Ince C, Tibboel D, Kuiper JW. Assessing the microcirculation with handheld vital microscopy in critically ill neonates and children: evolution of the technique and its potential for critical care. Front Pediatr. (2019) 7:273. doi: 10.3389/fped.2019.00273
66. Kuiper JW, Tibboel D, Ince C. The vulnerable microcirculation in the critically ill pediatric patient. Crit Care. (2016) 20(1):352. doi: 10.1186/s13054-016-1496-x
67. Top AP, Tasker RC, Ince C, The microcirculation of the critically ill pediatric patient. Crit Care. (2011). 15(2):213. doi: 10.1186/cc9995
68. Puchwein-Schwepcke A, Genzel-Boroviczeny O, Nussbaum C. The endothelial glycocalyx: physiology and pathology in neonates, infants and children. Front Cell Dev Biol. (2021) 9:733557. doi: 10.3389/fcell.2021.733557
69. Massey MJ, Hou PC, Filbin M, Wang H, Ngo L, Huang DT, et al. Microcirculatory perfusion disturbances in septic shock: results from the ProCESS trial. Crit Care. (2018) 22(1):308. doi: 10.1186/s13054-018-2240-5
70. Bruegger D, Brettner F, Rossberg I, Nussbaum C, Kowalski C, Januszewska K, et al. Acute degradation of the endothelial glycocalyx in infants undergoing cardiac surgical procedures. Ann Thorac Surg. (2015) 99(3):926–31. doi: 10.1016/j.athoracsur.2014.10.013
71. Jedlicka J, Becker BF, Chappell D. Endothelial glycocalyx. Crit Care Clin. (2020) 36(2):217–32. doi: 10.1016/j.ccc.2019.12.007
72. Cerny V, Astapenko D, Burkovskiy I, Hyspler R, Ticha A, Trevors MA, et al. Glycocalyx in vivo measurement. Clin Hemorheol Microcirc. (2017) 67(3–4):499–503. doi: 10.3233/CH-179235
73. Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. (2012) 18(8):1217–23. doi: 10.1038/nm.2843
74. Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. (2011) 254(2):194–200. doi: 10.1097/SLA.0b013e318226113d
75. Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. (2007) 116(17):1896–906. doi: 10.1161/CIRCULATIONAHA.106.684852
76. Jacob M, Chappell D, Becker BF. Regulation of blood flow and volume exchange across the microcirculation. Crit Care. (2016) 20(1):319. doi: 10.1186/s13054-016-1485-0
77. Sack KD, Kellum JA, Parikh SM. The angiopoietin-Tie2 pathway in critical illness. Crit Care Clin. (2020) 36(2):201–16. doi: 10.1016/j.ccc.2019.12.003
78. Richter RP, Ashtekar AR, Zheng L, Pretorius D, Kaushlendra T, Sanderson RD, et al. Glycocalyx heparan sulfate cleavage promotes endothelial cell angiopoietin-2 expression by impairing shear stress-related AMPK/FoxO1 signaling. JCI Insight. (2022) 7(15):e155010. doi: 10.1172/jci.insight.155010
79. Richter RP, Zheng L, Ashtekar AR, Walker SC, Pittet JF, Richter JR. Associations of plasma angiopoietins-1 and -2 and angiopoietin-2/-1 ratios with measures of organ injury and clinical outcomes in children with sepsis: a preliminary report. Pediatr Crit Care Med. (2020) 21(9):e874–8. doi: 10.1097/PCC.0000000000002508
80. De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. (2002) 166(1):98–104. doi: 10.1164/rccm.200109-016(c
81. Trzeciak S, Cinel I, Phillip Dellinger R, Shapiro NI, Arnold RC, Parrillo JE, et al. Resuscitating the microcirculation in sepsis: the central role of nitric oxide, emerging concepts for novel therapies, and challenges for clinical trials. Acad Emerg Med. (2008) 15(5):399–413. doi: 10.1111/j.1553-2712.2008.00109.x
82. De Backer D. Novelties in the evaluation of microcirculation in septic shock. J Intens Med. (2022) 3(2):124–30. doi: 10.1016/j.jointm.2022.09.002
83. Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. (2004) 32(9):1825–31. doi: 10.1097/01.ccm.0000138558.16257.3f
84. Vellinga NA, Ince C, Boerma EC. Microvascular dysfunction in the surgical patient. Curr Opin Crit Care. (2010) 16(4):377–83. doi: 10.1097/mcc.0b013e32833a0633
85. Pranskunas A, Koopmans M, Koetsier PM, Pilvinis V, Boerma EC. Microcirculatory blood flow as a tool to select ICU patients eligible for fluid therapy. Intensive Care Med. (2013) 39(4):612–9. doi: 10.1007/s00134-012-2793-8
86. van der Voort PH, van Zanten M, Bosman RJ, van Stijn I, Wester JP, van Raalte R, et al. Testing a conceptual model on early opening of the microcirculation in severe sepsis and septic shock: a randomised controlled pilot study. Eur J Anaesthesiol. (2015) 32(3):189–98. doi: 10.1097/EJA.0000000000000126
87. Coopersmith CM, Deutschman CS. The new sepsis definitions: implications for the basic and translational research communities. Shock. (2017) 47(3):264–8. doi: 10.1097/SHK.0000000000000763
88. Fraser DD, Patterson EK, Slessarev M, Gill SE, Martin C, Daley M, et al. Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: implications for microvascular platelet aggregation. Crit Care Explor. (2020) 2(9):e0194. doi: 10.1097/CCE.0000000000000194
89. Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. (2021) 24(1):145–57. doi: 10.1007/s10456-020-09753-7
90. van Leeuwen ALI, Borgdorff MP, Dekker NAM, van den Brom CE. Therapeutically targeting microvascular leakage in experimental hemorrhagic SHOCK: a systematic review and meta-analysis. Shock. (2021) 56(6):890–900. doi: 10.1097/SHK.0000000000001796
91. Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care. (2019) 23(1):16. doi: 10.1186/s13054-018-2292-6
92. Fernandez-Sarmiento J, Salazar-Pelaez LM, Carcillo JA. The endothelial glycocalyx: a fundamental determinant of vascular permeability in sepsis. Pediatr Crit Care Med. (2020) 21(5):e291–300. doi: 10.1097/PCC.0000000000002266
93. Greenwood JC, Talebi FM, Jang DH, Spelde AE, Kilbaugh TJ, Shofer FS, et al. Protocol for the MicroRESUS study: the impact of circulatory shock and resuscitation on microcirculatory function and mitochondrial respiration after cardiovascular surgery. PLoS One. (2022) 17(8):e0273349. doi: 10.1371/journal.pone.0273349
94. Fernández-Sarmiento J, Molina CF, Salazar-Pelaez LM, Flórez S, Alarcón-Forero LC, Sarta M, et al. Biomarkers of glycocalyx injury and endothelial activation are associated with clinical outcomes in patients with sepsis: a systematic review and meta-analysis. J Intensive Care Med. (2023) 38(1):95–105. doi: 10.1177/08850666221109186
Keywords: microcirculation, hemodynamic, videomicroscopy, neonate, children, critically ill
Citation: Arteaga GM and Crow S (2023) End organ perfusion and pediatric microcirculation assessment. Front. Pediatr. 11:1123405. doi: 10.3389/fped.2023.1123405
Received: 14 December 2022; Accepted: 5 September 2023;
Published: 29 September 2023.
Edited by:
Emmett E. Whitaker, University of Vermont, United StatesReviewed by:
Claudia Franziska Nussbaum, Ludwig Maximilian University of Munich, Germany© 2023 Arteaga and Crow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grace M. Arteaga YXJ0ZWFnYS5ncmFjZUBtYXlvLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.