
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 28 February 2023
Sec. Pediatric Surgery
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1122188
This article is part of the Research Topic Artificial Intelligence and Machine Learning in Pediatric Surgery View all 6 articles
 Otis C. van Varsseveld1*
Otis C. van Varsseveld1* Annebel ten Broeke2
Annebel ten Broeke2 Caspar G. Chorus2,3
Caspar G. Chorus2,3 Nicolaas Heyning2
Nicolaas Heyning2 Elisabeth M. W. Kooi4
Elisabeth M. W. Kooi4 Jan B. F. Hulscher1
Jan B. F. Hulscher1
Background: Critical decision making in surgical necrotizing enterocolitis (NEC) is highly complex and hard to capture in decision rules due to case-specificity and high mortality risk. In this choice experiment, we aimed to identify the implicit weight of decision factors towards future decision support, and to assess potential differences between specialties or centers.
Methods: Thirty-five hypothetical surgical NEC scenarios with different factor levels were evaluated by neonatal care experts of all Dutch neonatal care centers in an online environment, where a recommendation for surgery or comfort care was requested. We conducted choice analysis by constructing a binary logistic regression model according to behavioral artificial intelligence technology (BAIT).
Results: Out of 109 invited neonatal care experts, 62 (57%) participated, including 45 neonatologists, 16 pediatric surgeons and one neonatology physician assistant. Cerebral ultrasound (Relative importance = 20%, OR = 4.06, 95% CI = 3.39–4.86) was the most important factor in the decision surgery versus comfort care in surgical NEC, nationwide and for all specialties and centers. Pediatric surgeons more often recommended surgery compared to neonatologists (62% vs. 57%, p = 0.03). For all centers, cerebral ultrasound, congenital comorbidity, hemodynamics and parental preferences were significant decision factors (p < 0.05). Sex (p = 0.14), growth since birth (p = 0.25), and estimated parental capacities (p = 0.06) had no significance in nationwide nor subgroup analyses.
Conclusion: We demonstrated how BAIT can analyze the implicit weight of factors in the complex and critical decision for surgery or comfort care for (surgical) NEC. The findings reflect Dutch expertise, but the technique can be expanded internationally. After validation, our choice model/BAIT may function as decision aid.
While artificial intelligence as decision support is rapidly gaining ground in medicine (1–5), the use of artificial intelligence in the context of moral decisions is much less developed (6). Traditional rule-based decision support systems fail to capture the complexity and subtlety involved in medical decision making (2). Recently, we presented Behavioral Artificial Intelligence Technology (BAIT) as a novel approach to digitally capture expertise. The BAIT approach is a reconceptualization of econometric techniques, namely conjoint analysis and discrete choice theory, to generate decision transparency and support for medical experts (7). We have used BAIT in two single center pilot studies in the context of both an adult intensive care setting regarding COVID-19, and a neonatal intensive care setting regarding necrotizing enterocolitis (NEC) (7, 8). In both pilot studies BAIT provided insight into implicit decision trade-offs. In the present paper we will illustrate the use of this technique in cases of NEC on a nationwide and multicenter scale, as it may function as an important future adjunct in moral medical decision making.
NEC is a dreadful disease of the neonatal intestines, with an incidence varying between 3% and 17% in very low birth weight neonates (<1,500 g) (9–13). NEC incidence is increasing due to generally improved survival of the most preterm infants (14). Despite advances in neonatal care, mortality rates of NEC may still reach up to 40% (14, 15). For approximately one in three neonates with NEC, emergency laparotomy is necessary within hours to days after onset when conservative management does not suffice (surgical NEC) (16). However, perioperative mortality can reach 50%, and long-term morbidity, such as gastrointestinal complications and neurodevelopmental delay, occurs in over 75% (17). Hence, each case in which surgery becomes necessary poses both the treating medical team and the parents with the urgent dilemma whether surgery is still in the child’s best interest (10, 18).
The aim of the current study is to identify, interpret and further elucidate the implicit weights of decision factors in a national context and to identify possible between-group variations that contribute to critical decision making, in the context of one of the most difficult decisions in medicine: surgery versus comfort care for a critically ill preterm neonate with surgical NEC (18, 19). This may offer future decision support and educational insights to evaluate decision making and improve collaboration between stakeholders. Towards this goal, we assessed decision making in surgical NEC nationwide (the Netherlands), and subsequently focused on the differences between neonatologists and pediatric surgeons, between neonatal centers and between more and less experienced physicians, using the BAIT technology.
In a previous pilot study, we have developed a decision-analysis tool for NEC based on BAIT (7), which was employed on a larger scale for the current study. The BAIT technique comprises four steps: (1) definition of the expert decision and relevant factors; (2) determination of choice model structure; (3) design and execution of the choice experiment; (4) results analysis. The study was approved by the University Medical Center Groningen (UMCG) Ethical Board (METc 2020/310) and all methods were carried out in accordance with relevant guidelines and regulations.
First, an expert group of two senior neonatologists and two senior pediatric surgeons, defined the medical decision as follows: “to advise parents to proceed to surgery or to initiate comfort care (palliative care, resulting in death) for a critically ill infant with confirmed NEC and clear indication for surgery. This indication is a given fact for the sake of this experiment and is in daily practice always discussed within the multidisciplinary team treating the child (neonatologist, pediatric surgeon and pediatric anesthetist), and can consist of intestinal perforation confirmed by abdominal imaging and/or clinical deterioration despite maximum active conservative treatment”. This description captures the actual clinical situation as closely as possible.
The same expert group subsequently identified fourteen presumably relevant factors in the decision and their ranges (Table 1), also using the data from our pilot study. Certain factor levels were purposely formulated in a subjective fashion rather than based on instrumental measurements (e.g., cerebral ultrasound “good prognosis” rather than “no intraventricular hemorrhage”). This served two goals: (1) experts usually form a personal conclusion (good/intermediate/weak) for certain decision factors based on multiple clinical/objective inputs, so this resembles the clinical situation as closely as possible and; (2) we specifically aimed to capture subjective morality in our study. Constraining factor combinations in real life were specified for exclusion from the scenarios presented in the choice experiment as they will not occur in real-life cases. Excluded combinations were: (1) a gestational age of 24 or 26 weeks with a birth weight of 1,500 g; (2) gestational age of 30 weeks with a birth weight of 500 or 650 g; (3) no complications since birth in combination with poor lung function and/or poor neurodevelopmental prognosis from cerebral ultrasound.
Second, the choice model structure was defined. We opted for a binary logistic regression model, because of the positive and intuitive results achieved in our pilot study (7). For transparency and interpretability, the weight of factors was modelled linearly (e.g., a positive linear impact of increasing gestational age towards the decision to operate).
Third, the choice experiment was designed and executed. This consisted of scenarios mimicking real-life cases of surgical NEC patients, where a hypothetical yes/no choice should be made by the participating expert based on the provided factors (Figure 1). Efficient design techniques by Ngene software (version 1.2.1, ChoiceMetrics) ensured that the maximum amount of information regarding factor weights was obtained with each completed scenario. This entailed that a total of 35 scenarios were created for each participant. For each participant, the first two scenarios were extremes (i.e., maximum positive and negative values), functioning as a form of positive and negative control for general NEC expertise. Subsequent scenario order was randomized for each participant.
All neonatologists, pediatric surgeons and physician assistants of all tertiary neonatal care centers in the Netherlands were invited to participate, to allow representation of all Dutch care providers with expertise and involvement in NEC care. Participants were invited through e-mail to complete the experiment in an online application (WEM No-Code Platform).
The fourth step was analysis of choices that were observed in the choice experiment. For analysis we utilized Apollo (version 0.2.4, package in R) for logistic regression to estimate the importance weight of each factor, including their signs (positive or negative), with the maximum likelihood technique. We provide the logistic regression beta coefficient and odds ratio (OR) of each factor and its significance in regression analysis (p-value of factor in estimated model vs. null model). A two-tailed p-value <0.05 was considered significant. The attained choice model equipped with the estimated weights was subsequently used to assess particular hypothetical choice situations, including cases not presented in the actual choice experiment. By combining the estimated effect of different decision factors, the model forms a probability statement (percentage) that an expert that is randomly sampled from the expert group would advise to perform surgery on a patient with the given profile (Figure 2). Model fit is expressed as McFadden’s ρ2, calculated by the ratio of the maximized log likelihood (predictive model) and the null log likelihood (null model). Values between 0.2 and 0.4 indicate a good model fit (20), particularly for the type of choices made in experimental conditions with difficult trade-offs.
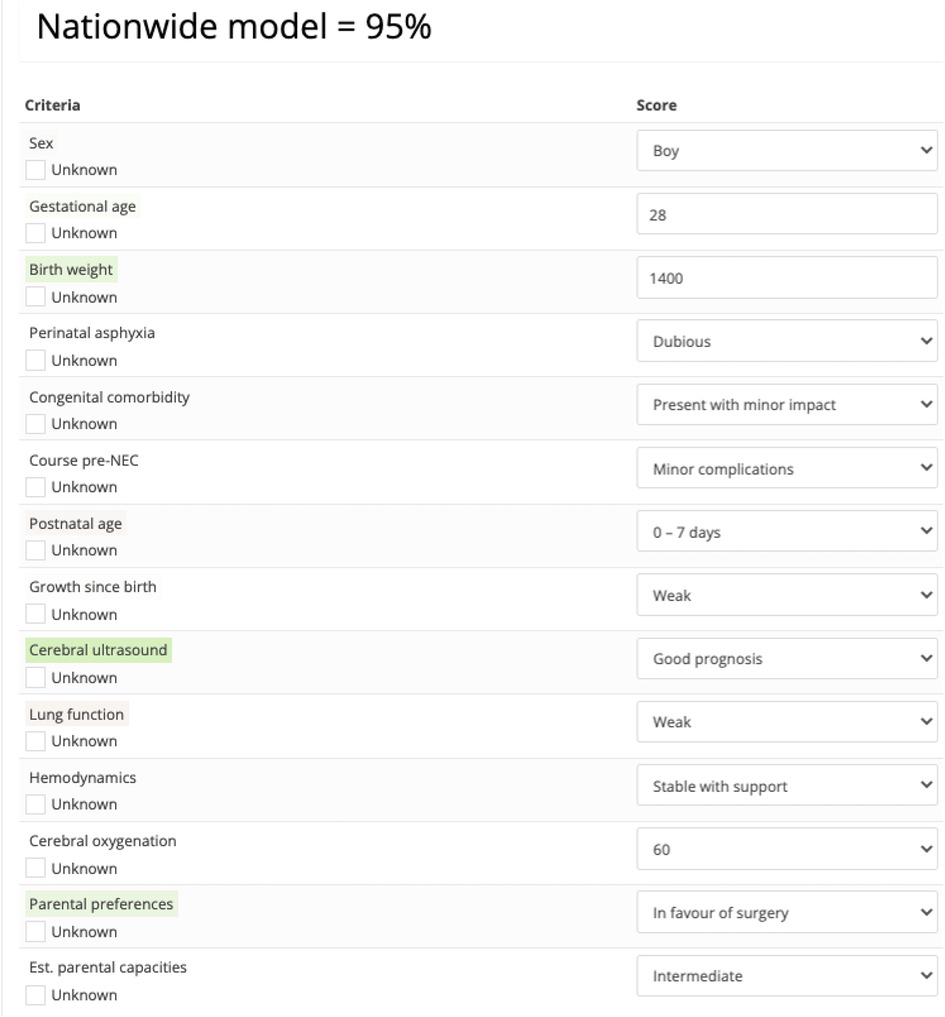
Figure 2. Example of an assessment generated by the nationwide choice model stating that the probability that a randomly sampled expert from the expert group would recommend to perform surgery on a patient with this profile equals 95%. Color coding highlights which factors had a positive or negative contribution to the assessment.
The relative importance (RI) was determined as follows: for each factor, the estimated weight is multiplied by the range of the factor (theoretical maximum effect of the factor). RI is then defined as the percentage contribution of each factor to the total theoretical maximum effect summed over all factors. To establish between-group significance of the difference in importance weights, we computed the standard error of the difference based on the standard errors of the weights and subsequently conducted standard t-tests. Between-group difference in the frequency to advise surgery or comfort care was assessed by the Chi2-test. We divided the participant—reported work experience subgroups in: less experienced (0–5 years), moderately experienced (5–15 years) and highly experienced (>15 years) physicians.
We invited 109 neonatal care experts, including 88 neonatologists and 21 pediatric surgeons. The choice experiment was completed by 45 neonatologists, 16 pediatric surgeons and one neonatology physician assistant. This amounted to a total of 62 participants out of 109 invitees (response rate 57%) (Table 2). Fourteen (23%) participants were excluded from subgroup analyses because they opted to omit occupational information: three from the specialty subanalysis, six from the center subanalysis and five from the work experience subanalysis.
Factor weights for nationwide choice analysis and for the subgroup of neonatologists and pediatric surgeons are displayed in Table 3. Model fit of the estimated nationwide model was calculated at a McFadden’s ρ2 value of 0.27. The mean absolute deviation was 5.8%, indicating that on average the predicted probability (percentage) of a recommendation to operate by the model was 5.8% higher or lower than the observed percentage of experts choosing for operation in the choice experiment. Out of the fourteen factors, eleven had significant impact on the decision. Sex (p = 0.14), growth since birth (p = 0.25) and estimated parental capacities (p = 0.06) did not significantly affect the decision in the nationwide model.
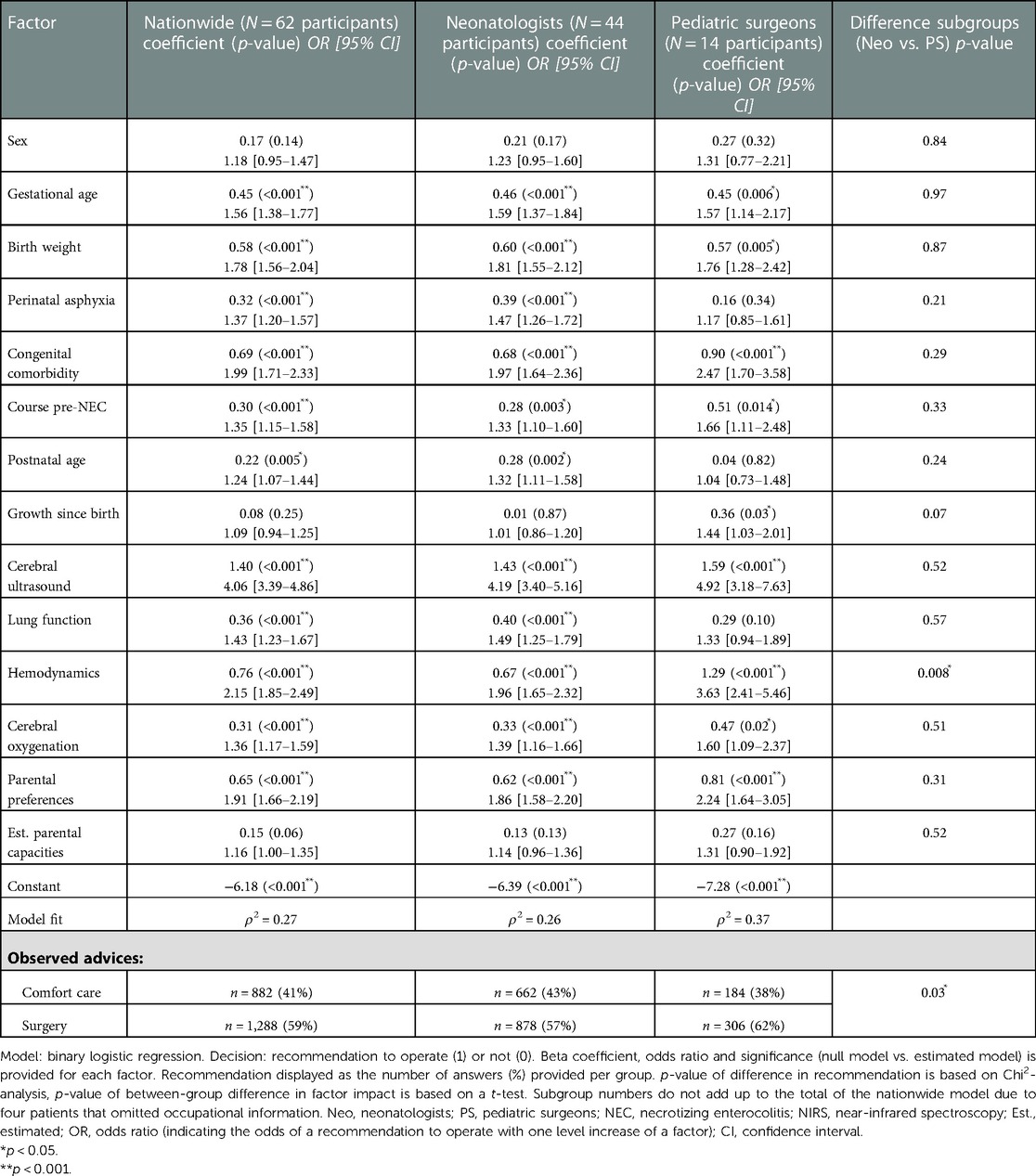
Table 3. Estimated linear weight (beta coefficient) of each factor per level, of the nationwide analysis and the neonatologist and pediatric surgeon subgroups.
Relatively, cerebral ultrasound [OR = 4.06, 95% confidence interval (CI) = 3.39–4.86] had the largest impact on the critical decision (Table 3). The most impactful (RI equal to or higher than 10%) and statistically significant factors were cerebral ultrasound (RI = 20%), birth weight (RI = 13%), hemodynamics (RI = 11%), gestational age (RI = 10%), congenital comorbidity (RI = 10%) (Figure 3). Having discussed the RI of individual factors, the probability of the decision to operate is the combination of all decision factors included in the choice analysis (Figure 2).
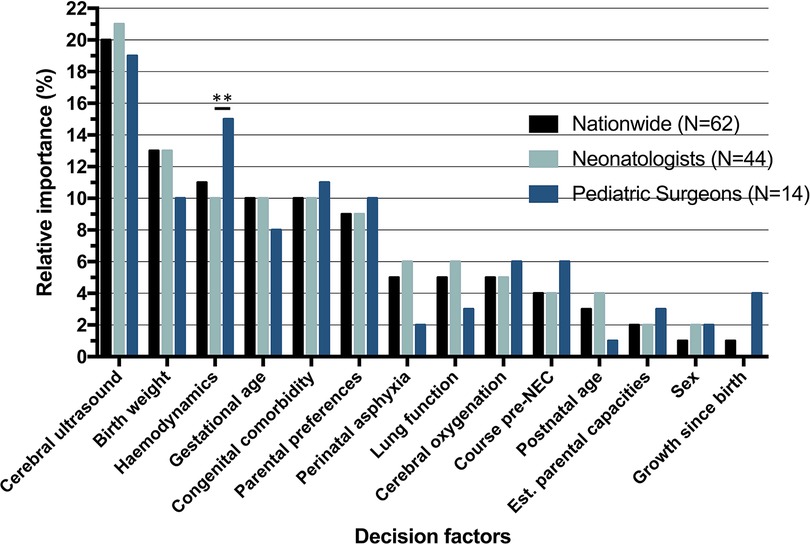
Figure 3. Relative importance (RI) of decision factors, nationwide and per specialty. RI determined by: . **Significant difference (p < 0.01) based on standard error of the difference between beta coefficients.
The McFadden’s ρ2 of the neonatologist group (n = 44) and the pediatric surgeon (n = 14) group were 0.26 and 0.37, respectively. Table 3 depicts choices and regression analysis outcomes of neonatologists and pediatric surgeons. Overall, pediatric surgeons were somewhat more inclined to advice surgery when compared to neonatologists (62% vs. 57% of scenario answers, p = 0.03). Cerebral ultrasound had the greatest RI for both neonatologists and pediatric surgeons (RI 21%, and 19% respectively, p-value of the difference: 0.52). Hemodynamics was the sole factor that differed significantly in impact on the decision between the two professions [RI 10% (neonatologists) vs. 15% (pediatric surgeons), p = 0.008].
RI of factors is displayed in Figure 4 for the four neonatal care centers with the most participants: center A (n = 17), center B (n = 12), center C (n = 7) and center D (n = 7). Other centers had participant numbers of <7 for subanalysis, resulting in limited value for additional analyses. The frequency of recommendation for surgery varied significantly between centers with 53% in center A, 63% in center B, 58% in center C and 57% in center D (p = 0.03). Interpretation of cerebral ultrasound was the factor with most impact on the decision in all four centers. In the estimated model for center A, birth weight (RI = 16%) had an equal impact on the decision as cerebral ultrasound (RI = 16%). Four factors consistently had a significant effect in per-center regression analysis of all centers, including: cerebral ultrasound, hemodynamics, congenital comorbidity and parental preferences. Factors with no significant impact on the decision in all four centers were sex, growth since birth and estimated parental capacities.
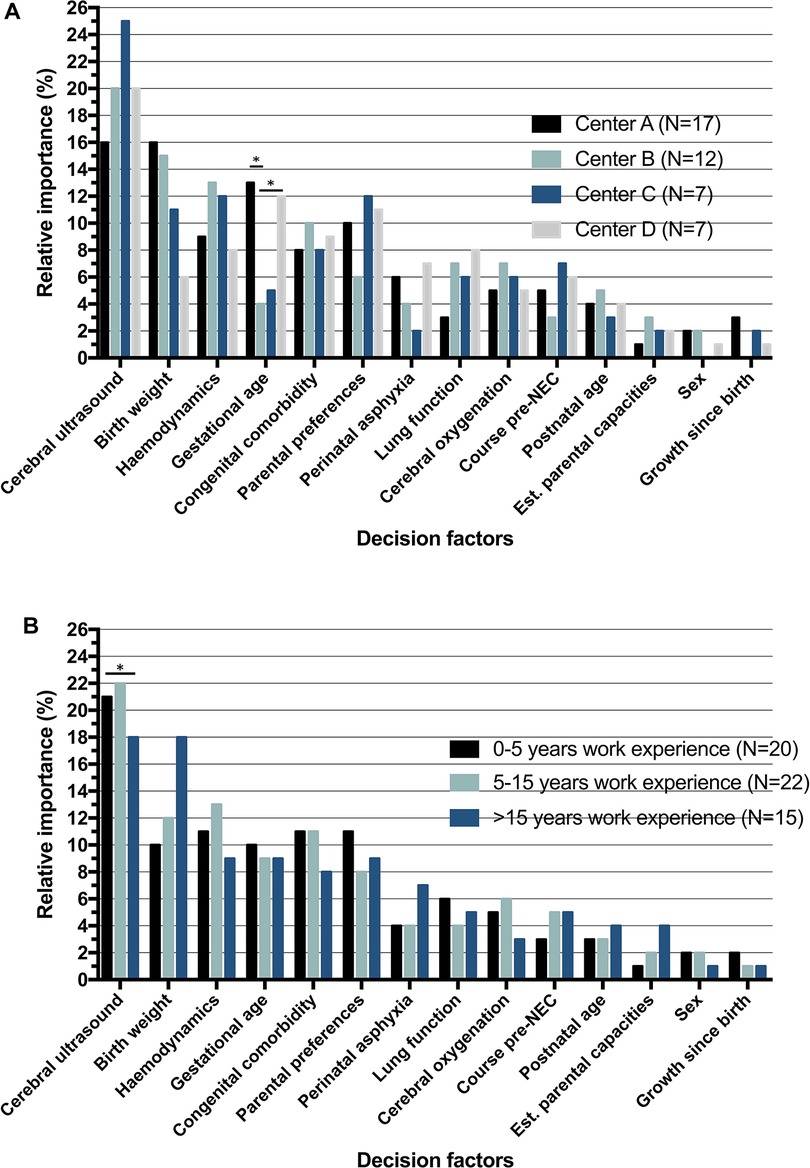
Figure 4. Relative importance (RI) of decision factors, (A) per center and (B) per work experience. RI determined by: . *Significant difference (p < 0.05) based on standard error of the difference between beta coefficients.
Subgroup analysis for years of working experience as a specialist is displayed in Table 4. The overall trend for all groups was more scenario answers in favor of surgery, with 398 (57%) answers in the 0–5 years, 476 (62%) answers in the 5–15 years and 289 (55%) answers in the >15 years work experience group. Comparing the linear weight of decision factors between the groups, there was only a significant difference in cerebral ultrasound between 5 and 15 and >15 years experience groups (beta coefficient 1.72 vs. 1.15, p = 0.02). In per-group analyses, course pre-NEC was not a significant factor in the decision for the 0–5 years work experience group (p = 0.09), whereas it was for the other two groups (5–15 years p = 0.02; >15 years p = 0.047). Conversely, cerebral oxygenation was not significant in the >15 years group (p = 0.18), while in the 0–5 years experience (p = 0.02) and 5–15 years experience (p = 0.001) groups it was.
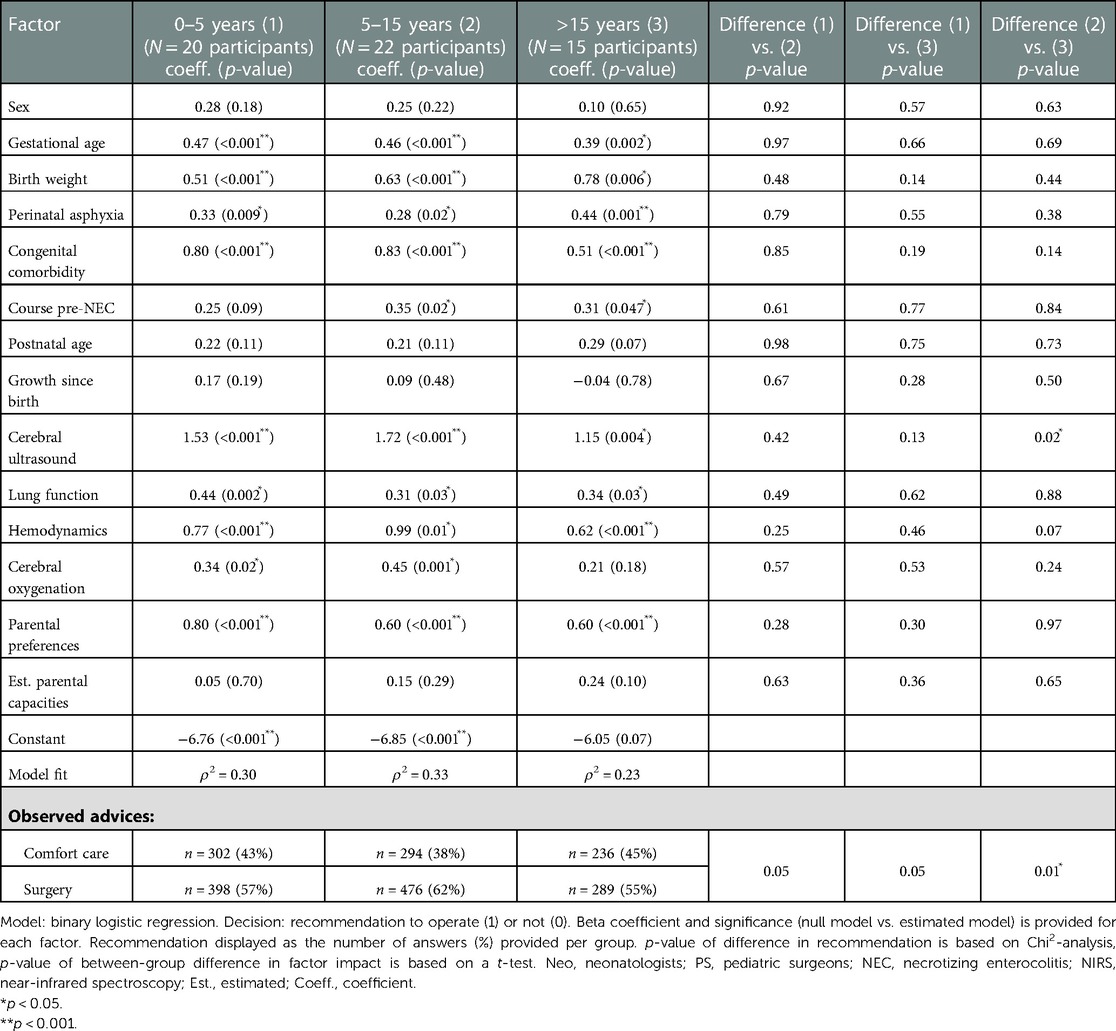
Table 4. Estimated linear weight of the work experience subgroup analysis, divided in three groups: 0–5 years (group 1), 5–15 years (group 2) and >15 years (group 3) of working experience as a neonatologist or pediatric surgeon.
In this study, we applied choice analysis techniques to identify, analyze and codify the weight of factors in the decision for comfort care or surgery in a critically ill neonate with surgical NEC. BAIT demonstrated that, both over the nationwide analysis and subanalyses, cerebral ultrasound was the factor with most impact on the decision. Notably, birth weight and gestational age were the second and third most important decision factors for neonatologists, whereas for pediatric surgeons these were hemodynamics and congenital comorbidity. Factors of significant impact in all centers were cerebral ultrasound, congenital comorbidity, hemodynamics and parental preferences and the maximum difference in number of recommendations for surgery between centers was 10 percentage points. We attained a choice model, equipped with the decision factor weights, with a mean absolute deviation of 5.8%.
Interpreted Cerebral ultrasound prognosis was the decision factor with most impact, in the nationwide model and all other subgroup models. Long-term neurodevelopmental impairment, including motor deficits, sensory deficits, behavioral issues and cognitive impairment, is a well-established association in infants suffering from both medical and surgical NEC (21–23). Meta-analysis has also established that infants surgically treated for NEC are even at a 16% higher risk for neurodevelopmental impairment (23). Brain injury visualized on cerebral ultrasonography is associated with neurodevelopmental delay (24, 25). Similarly, low birth weight and gestational age are well-known predictors of long-term neurodevelopmental impairment and had large RI in our study (26). Hence, the dominant weight of cerebral ultrasound and also the large impact of birth weight and gestational age may reflect the perceived importance of long-term neurodevelopmental function, i.e., the recommendation for NEC surgery becomes much less desirable for participants due to the long-term cognitive and functional prognosis after surgery.
In accordance with a recent study from the USA (27), we observed a significant difference between Dutch neonatologists (57% of cases) and pediatric surgeons (62% of cases) in recommending surgery. An explanation for a less pronounced difference in surgery recommendations in our study, may be the differences in the health care and insurance systems (28, 29). Factors with the second and third highest RI varied between neonatologists and pediatric surgeons. These findings are in accordance with two potentially different thought-processes between the two specialist groups: (1) neonatologists leaning more towards the consideration whether the child will be majorly impaired in the long-term (birth weight, gestational age) and; (2) pediatric surgeons leaning more towards the consideration whether the child may or may not survive NEC surgery (hemodynamics, congenital comorbidity). This is also in accordance with the recent USA survey study mentioned earlier (27).
Despite mainly congruent results in work experience subanalysis, course pre-NEC was not significant in the 0–5 years experience group (p = 0.09) and cerebral oxygenation was not significant in the >15 years experience group (p = 0.18). Association between low cerebral oxygenation (NIRS, near infrared spectroscopy) and NEC development, survival after NEC surgery and neurodevelopmental impairment has been found (10, 30–32). In the perspective of work experience, the findings possibly simulate a transition between the less and more experienced specialists: younger specialists focus more on more recently implemented monitor parameters (cerebral oxygenation), whereas more experienced specialists rely more on their clinical view (clinical course prior to NEC). As NIRS is not used in all centers, this relative lack of experience with this technique might also have influenced results due to certain participants blunting its relevance in the decision.
Between centers we noted a maximum difference of 10 percentage points in the frequency of surgery recommendation and a maximum difference of 9 percentage points between the RI of cerebral ultrasound between centers. Nevertheless, looking at the relatively small groups in per-center analyses, these differences could probably be explained by a single or a few participants, challenging their clinical significance and accentuating the relative intrinsic complexity of NEC decision making. Out of the total of fourteen factors, four were consistently significant and three consistently insignificant factors across the different center subgroups. This highlights the consonance of decision making in critically ill neonates within each center. The Dutch guidelines command that continuation of treatment for a critically ill neonate is conditional, tailored to the specific case (33). Our study reassuringly shows that, despite subtle differences, Dutch experts in (neonatal) health care settings have a relatively harmonious set of medical expertise, norms and values, to be tailored to a medical decision in a child’s best interest.
Non-significant factors nationwide were sex, growth since birth and estimated parental capacities. Sex was included as a factor based on evidence that extremely preterm females perform better in overall mortality and morbidity rates compared to males (34–37). Yet, an elaborate meta-analysis found no significant impact of sex on cognitive outcome of extreme/very preterm infants (38). Our study confirms that sex does not project significantly in decision making for surgical NEC. Growth since birth may be non-significant due to its multifactorial nature and the relatively early onset of NEC itself. The factor estimated parental capacities was not significant. Conversely, parental preferences was, which confirms that clinicians do find parental involvement important. Engagement of parents in NEC care and decisions also improves parental satisfaction (39). Hence, our study reflects that the desire of parents for treatment more accurately represents the perspectives for future care of an infant than our subjective estimation of parental capacities.
The BAIT decision-analysis tool enabled us to capture the expertise of a nationwide panel of neonatal and pediatric surgical specialists. It clearly provided insights into factors affecting their choices regarding one of the most difficult decisions in neonatology. A limitation is that captured factors are considered important by Dutch physicians and may not be generalizable, considering different ethical circumstances and attitudes surrounding the decision may exist worldwide. Moreover, it should be considered that our technique provides decision transparency but does not dictate which choice is “the best” in this nuanced critical decision. Nevertheless, this methodology can be applied to identify decision factor weights internationally or for parents. In the future, a more elaborate European or worldwide choice experiment could offer insight into factors that influence this decision in other countries, thereby shedding a light on the possible differences between countries and cultures.
Limited numbers of inclusions in the neonatal center subgroups did not allow for elaborate between-center comparison. In the nationwide model, as well as in the group of pediatric surgeons there was a relative overrepresentation of the UMCG. This might be due to the fact that the UMCG, a Center of Expertise for NEC as appointed by the Dutch Ministry of Health, initiated the study so that UMCG staff was more inclined to complete the study. Also, the 14 clinical variables and their ranges were determined by a UMCG expert group, which may have limited generalizability. However, given the nature of the experiment, potentially irrelevant variables added by the UMCG expert group would turn out to be insignificant in the overall study results, based on the input of all participants. Another limitation was that our choice experiment did not consist of real-life cases, but of hypothetical, computer-generated scenarios completed in a software environment. Hence, validation with real-life cases is required prior to its potential application in medical practice as a dynamic decision transparency tool. As the model can be updated based on encountered real-life cases, it will continuously progress alongside advances in neonatal and pediatric surgical care, harnessing the power of artificial intelligence (2).
As shown in this study, choice analysis may be utilized to educate us about the weight of factors in medical decision making and reflect upon them. Our methodology exposed between-group differences and showed that, despite some variation, Dutch neonatal care experts have a generally harmonious set of medical expertise, norms and values in critical decision making in surgical NEC. After validation, the model may serve as a decision aid for neonatologists, pediatric surgeons and parents of patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University Medical Center Groningen (UMCG) Ethical Board (METc 2020/310). The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the study conception and design. Design of the data collection instrument was performed by AB, CC and NH. Data collection, analysis and interpretation was performed by OV, AB, EK and JH. The first draft of the manuscript was written by OV and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Financial support for this study was provided in part by a grant from the European Research Council (ERC-Consolidator Grant BEHAVE, grant 724431). The funding agreement ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report. Financial support for publication of this study was provided by the For Wis(h)dom Foundation (Project 9, 2nd February 2022) (Baarn, Netherlands).
We thank Els L.M. Maeckelberghe (associate professor in bioethics and research ethics, University Medical Center Groningen, Groningen, the Netherlands) for her guidance in ethical considerations and critical revision of our manuscript.
The authors declare the following potential conflict of interest: AB, NH, and CC are associated with Councyl (the latter two as cofounders), a Delft University of Technology spin-off that develops and commercializes the behavioral artificial intelligence technology (BAIT) that is presented in this article. OV, JH and EK declare that no funding was received and that there are no competing interests.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gordon L, Grantcharov T, Rudzicz F. Explainable artificial intelligence for safe intraoperative decision support. JAMA Surg. (2019) 154(11):1064–5. doi: 10.1001/jamasurg.2019.2821
2. Loftus TJ, Tighe PJ, Filiberto AC, Efron PA, Brakenridge SC, Mohr AM, et al. Artificial intelligence and surgical decision-making. JAMA Surg. (2020) 155(2):148–58. doi: 10.1001/jamasurg.2019.4917
3. Ostropolets A, Zhang L, Hripcsak G. A scoping review of clinical decision support tools that generate new knowledge to support decision making in real time. J Am Med Inform Assoc. (2020) 27(12):1968–76. doi: 10.1093/jamia/ocaa200
4. Kruse CS, Ehrbar N. Effects of computerized decision support systems on practitioner performance and patient outcomes: systematic review. JMIR Med Inform. (2020) 8(8):e17283. doi: 10.2196/17283
5. Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. (2020) 3:17. doi: 10.1038/s41746-020-0221-y
6. Habli I, Lawton T, Porter Z. Artificial intelligence in health care: accountability and safety. Bull World Health Organ. (2020) 98(4):251–6. doi: 10.2471/blt.19.237487
7. Ten Broeke A, Hulscher J, Heyning N, Kooi E, Chorus C. Bait: a new medical decision support technology based on discrete choice theory. Med Decis Making. (2021) 41(5):614–9. doi: 10.1177/0272989(211001320
8. de Metz J, Thoral PJ, Chorus CG, Elbers PWG, van den Bogaard B. Behavioural artificial intelligence technology for COVID-19 intensivist triage decisions: making the implicit explicit. Intensive Care Med. (2021) 47(11):1327–8. doi: 10.1007/s00134-021-06453-8
9. Frost BL, Modi BP, Jaksic T, Caplan MS. New medical and surgical insights into neonatal necrotizing enterocolitis: a review. JAMA Pediatr. (2017) 171(1):83–8. doi: 10.1001/jamapediatrics.2016.2708
10. Kuik SJ, van der Heide M, Bruggink JLM, Bos AF, Verhagen AAE, Kooi EMW, et al. Intestinal oxygenation and survival after surgery for necrotizing enterocolitis: an observational cohort study. Ann Surg. (2022) 275(2):e503–10. doi: 10.1097/sla.0000000000003913
11. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20(1):344. doi: 10.1186/s12887-020-02231-5
12. Llanos AR, Moss ME, Pinzòn MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol. (2002) 16(4):342–9. doi: 10.1046/j.1365-3016.2002.00445.x
13. Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol. (2003) 23(4):278–85. doi: 10.1038/sj.jp.7210892
14. Heida FH, Stolwijk L, Loos MH, van den Ende SJ, Onland W, van den Dungen FA, et al. Increased incidence of necrotizing enterocolitis in the Netherlands after implementation of the new Dutch guideline for active treatment in extremely preterm infants: results from three academic referral centers. J Pediatr Surg. (2017) 52(2):273–6. doi: 10.1016/j.jpedsurg.2016.11.024
15. Bubberman JM, van Zoonen A, Bruggink JLM, van der Heide M, Berger RMF, Bos AF, et al. Necrotizing enterocolitis associated with congenital heart disease: a different entity? J Pediatr Surg. (2019) 54(9):1755–60. doi: 10.1016/j.jpedsurg.2018.11.012
16. Robinson JR, Rellinger EJ, Hatch LD, Weitkamp JH, Speck KE, Danko M, et al. Surgical necrotizing enterocolitis. Semin Perinatol. (2017) 41(1):70–9. doi: 10.1053/j.semperi.2016.09.020
17. Adams-Chapman I. Necrotizing enterocolitis and neurodevelopmental outcome. Clin Perinatol. (2018) 45(3):453–66. doi: 10.1016/j.clp.2018.05.014
18. Willems DL, Verhagen AA, van Wijlick E. Infants’ best interests in end-of-life care for newborns. Pediatrics. (2014) 134(4):e1163–8. doi: 10.1542/peds.2014-0780
19. Marlow N, Shaw C, Connabeer K, Aladangady N, Gallagher K, Drew P. End-of-life decisions in neonatal care: a conversation analytical study. Arch Dis Child Fetal Neonatal Ed. (2021) 106(2):184–8. doi: 10.1136/archdischild-2020-319544
20. McFadden D. Quantitative methods for analysing travel behaviour of individuals: some recent developments. In: Behavioural travel modelling. 707. New Haven, Connecticut, USA: Cowles Foundation Discussion Papers (1977). p. 279–318.
21. Roze E, Ta BD, van der Ree MH, Tanis JC, van Braeckel KN, Hulscher JB, et al. Functional impairments at school age of children with necrotizing enterocolitis or spontaneous intestinal perforation. Pediatr Res. (2011) 70(6):619–25. doi: 10.1203/PDR.0b013e31823279b1
22. Hickey M, Georgieff M, Ramel S. Neurodevelopmental outcomes following necrotizing enterocolitis. Semin Fetal Neonatal Med. (2018) 23(6):426–32. doi: 10.1016/j.siny.2018.08.005
23. Matei A, Montalva L, Goodbaum A, Lauriti G, Zani A. Neurodevelopmental impairment in necrotising enterocolitis survivors: systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed. (2020) 105(4):432–9. doi: 10.1136/archdischild-2019-317830
24. Gotardo JW, Volkmer NFV, Stangler GP, Dornelles AD, Bohrer BBA, Carvalho CG. Impact of peri-intraventricular haemorrhage and periventricular leukomalacia in the neurodevelopment of preterms: a systematic review and meta-analysis. PLoS One. (2019) 14(10):e0223427. doi: 10.1371/journal.pone.0223427
25. Dorner RA, Allen MC, Robinson S, Soares BP, Perin J, Ramos E, et al. Early neurodevelopmental outcome in preterm posthemorrhagic ventricular dilatation and hydrocephalus: neonatal icu network neurobehavioral scale and imaging predict 3-6-month motor quotients and capute scales. J Neurosurg Pediatr. (2019) 25(3):217–27. doi: 10.3171/2019.9.Peds19438
26. Luu TM, Rehman Mian MO, Nuyt AM. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol. (2017) 44(2):305–14. doi: 10.1016/j.clp.2017.01.003
27. Pet GC, McAdams RM, Melzer L, Oron AP, Horslen SP, Goldin A, et al. Attitudes surrounding the management of neonates with severe necrotizing enterocolitis. J Pediatr. (2018) 199:186–93. doi: 10.1016/j.jpeds.2018.03.074
28. Alker J, Corcoran A. Children’s uninsured rate rises by largest annual jump in more than a decade. Washington, DC: Georgetown University Center for Children and Families (2020).
29. Yannekis G, Passarella M, Lorch S. Differential effects of delivery hospital on mortality and morbidity in minority premature and low birth weight neonates. J Perinatol. (2020) 40(3):404–11. doi: 10.1038/s41372-019-0423-9
30. Schat TE, van Zoonen A, van der Laan ME, Mebius MJ, Bos AF, Hulzebos CV, et al. Early cerebral and intestinal oxygenation in the risk assessment of necrotizing enterocolitis in preterm infants. Early Hum Dev. (2019) 131:75–80. doi: 10.1016/j.earlhumdev.2019.03.001
31. Howarth C, Banerjee J, Leung T, Eaton S, Morris JK, Aladangady N. Cerebral oxygenation in preterm infants with necrotizing enterocolitis. Pediatrics. (2020) 146(3):e20200337. doi: 10.1542/peds.2020-0337
32. Verhagen EA, Van Braeckel KN, van der Veere CN, Groen H, Dijk PH, Hulzebos CV, et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Dev Med Child Neurol. (2015) 57(5):449–55. doi: 10.1111/dmcn.12622
33. Royal Dutch Medical Association (KNMG). Medische Beslissingen Rond Het Levenseinde Bij Pasgeborenen Met Zeer Ernstige Afwijkingen [Medical End-of-Life Decisions in Newborns with Severe Handicaps (2013). Available at: https://www.knmg.nl/download/knmg-standpunt-medische-beslissingen-rond-het-levenseinde-bij-pasgeborenen-met-zeer-ernstige-afwijkingen.htm.
34. Garfinkle J, Yoon EW, Alvaro R, Nwaesei C, Claveau M, Lee SK, et al. Trends in sex-specific differences in outcomes in extreme preterms: progress or natural barriers? Arch Dis Child Fetal Neonatal Ed. (2020) 105(2):158–63. doi: 10.1136/archdischild-2018-316399
35. Lee R, Williams EE, Dassios T, Greenough A. Influence of antenatal corticosteroids and sex on the mortality and morbidity of extremely prematurely born infants. J Matern Fetal Neonatal Med. (2022) 35(25):8062–5. doi: 10.1080/14767058.2021.1940941
36. Brawner KM, Yeramilli VA, Kennedy BA, Patel RK, Martin CA. Prenatal stress increases iga coating of offspring microbiota and exacerbates necrotizing enterocolitis-like injury in a sex-dependent manner. Brain Behav Immun. (2020) 89:291–9. doi: 10.1016/j.bbi.2020.07.008
37. Voskamp BJ, Peelen M, Ravelli ACJ, van der Lee R, Mol BWJ, Pajkrt E, et al. Association between fetal sex, birthweight percentile and adverse pregnancy outcome. Acta Obstet Gynecol Scand. (2020) 99(1):48–58. doi: 10.1111/aogs.13709
38. Twilhaar ES, Wade RM, de Kieviet JF, van Goudoever JB, van Elburg RM, Oosterlaan J. Cognitive outcomes of children born extremely or very preterm since the 1990s and associated risk factors: a meta-analysis and meta-regression. JAMA Pediatr. (2018) 172(4):361–7. doi: 10.1001/jamapediatrics.2017.5323
Keywords: necrotizing enterocolitis, decision making, artificial intelligence, choice analysis, critical care, comfort care, decision support
Citation: van Varsseveld OC, ten Broeke A, Chorus CG, Heyning N, Kooi EMW and Hulscher JBF (2023) Surgery or comfort care for neonates with surgical necrotizing enterocolitis: Lessons learned from behavioral artificial intelligence technology. Front. Pediatr. 11:1122188. doi: 10.3389/fped.2023.1122188
Received: 12 December 2022; Accepted: 31 January 2023;
Published: 28 February 2023.
Edited by:
Pietro Bagolan, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Erik David Skarsgard, British Columbia Children's Hospital, Canada© 2023 van Varsseveld, ten Broeke, Chorus, Heyning, Kooi and Hulscher. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Otis C. van Varsseveld by5jLnZhbi52YXJzc2V2ZWxkQHVtY2cubmw=
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.