- 1Center of Public Emergency Management, Ethiopian Public Health Institute, Addis Ababa, Ethiopia
- 2Health Financing Department, Clinton Health Access Initiative, Addis Ababa, Ethiopia
Introduction: Receiving at least four antenatal care (ANC) visits have paramount importance on the health of mothers and perinates. In Ethiopia, several studies were conducted on ANC service utilization; however, limited studies quantified the effect of care on maternal and perinate health. In response to this gap, this study is conducted to quantify the effect of optimal ANC care (≥4 visits) on maternal and perinatal health among women who received optimal care in comparison to women who did not receive optimal care.
Methods: The study utilized the Ethiopian perinatal death surveillance and response (PDSR) system dataset. A total of 3,814 reviewed perinatal deaths were included in the study. Considering the nature of the data, preferential within propensity score matching (PWPSM) was performed to determine the effect of optimal ANC care on maternal and perinatal health. The effect of optimal care was reported using average treatment effects of the treated [ATT].
Result: The result revealed that optimal ANC care had a positive effect on reducing perinatal death, due to respiratory and cardiovascular disorders, [ATT = −0.015, 95%CI (−0.029 to −0.001)] and extending intrauterine life by one week [ATT = 1.277, 95%CI: (0.563–1.991)]. While it's effect on maternal health includes, avoiding the risk of having uterine rupture [ATT = −0.012, 95%CI: (−0.018 to −0.005)], improving the utilization of operative vaginal delivery (OVD) [ATT = 0.032, 95%CI: (0.001–0.062)] and avoiding delay to decide to seek care [ATT = −0.187, 95%CI: (−0.354 to −0.021)].
Conclusion: Obtaining optimal ANC care has a positive effect on both maternal and perinatal health. Therefore, policies and interventions geared towards improving the coverage and quality of ANC services should be the top priority to maximize the benefit of the care.
1. Introduction
Antenatal care (ANC) is routine and regular maternity care provided for pregnant women from conception to the onset of labour (1). ANC is also considered as a precautionary measure for pregnant women to identify and manage pre-existing and potential causes of maternal and child health morbidity and mortality through supplementation of micronutrients, screening of infection, and health promotion (2, 3).
Globally, 87% of pregnant women had received ANC service at least once, by health professionals. However, not all women completed the bare minimum number of ANC visits (having at least four ANC visits). According to a similar study, only two-thirds (65%) of pregnant women receive four ANC visits. The proportion of complete ANC visits is different from region to region, Sub Sahara African and south Asian countries have the lowest proportion at 52% and 48% respectively (4). Ethiopia has made remarkable progress in improving the coverage of ANC service in the last two decades (5). The coverage has increased from 27% in 2000 to 74% in 2019. In line with this, the number of women who attend four and more ANC visits (ANC 4+) has also increased from 10% in 2000 to 43% in 2019 (5, 6). Similarly, the proportion of women who began ANC visits within the recommend period [between 8 and 12 gestational weeks] has also increased from 6% in 2000 to 28% in 2019 (5–7).
World Health Organization (WHO) has made continuous improvements to the protocol of ANC visits, and one of the recent recommendations was to increase the minimum number of ANC visits from four contacts to eight contacts (8). However, Ethiopia was stacked with the previous protocol of focused ANC care till 2021; however, lately, the country has accepted and adopted the recent recommendation of positive pregnancy experience for ANC visits in 2022 (9).
Ethiopia has taken various measures to address the barriers related to ANC service utilization. Those barriers are broadly classified into three categories: namely, perception, access, and quality (10). In response to this, the country has introduced a health extension program, and community-based health insurance to improve the service uptake. In addition, community health structures were organized to boost community engagement and awareness in the health system (11–13). Ensuring sustainable health financing and enacting measures aimed at installing quality care improvement (QIA) were some of the steps taken to improve access and quality of care (14, 15). Despite making the aforementioned efforts, the coverage and quality of the care remain unsatisfactory (9). The quality of care is compromised due to the lack of trained personnel, medical equipment, and physical health infrastructure (16–19). In addition, the presence of noticeable regional variation, lack of an effective monitoring mechanism for the proposed intervention, and lack of updated and data-driven evidence for decision-making were major barriers to realizing the target ascertained in the national strategy (20–23).
To address the gap in evidence generation and to produce a data-driven policy recommendation, The country has established maternal and perinatal death surveillance and response (MPDSR) system, to obtain updated information on the cause and contributing factors related to preventable maternal and perinatal death (24). As a result, both maternal and perinatal death are included under the surveillance system as compulsory events to be reported to the next level (25). ANC service utilization was included in the surveillance system as one component to evaluate and review maternal and perinatal death concerning ANC history (26). However, the system was not fully implemented due to the presence of limited community engagement, lack of trained personnel, lack of maternal health infrastructure, fear of legal repercussions, and defensive attitude towards the system (27, 28).
Having a proper ANC visit has a tremendously positive effect on the health of the mother and her child in addition to enhancing health promotion (29). Some of the benefits of complete ANC care on maternal health include reducing the probability of having maternal near miss, postpartum hemorrhage, preterm labour, and anemia (30–32). While dropping the risk of stillbirth, declining neonate death, improving the nutritional status of children, and reducing neonatal admission to neonatal intensive care (NICU) were included under neonatal positive effects of ANC care (33–38). Improving exclusive Breastfeeding practice, avoiding pre-lacteal feeding, enhancing the utilization of postnatal care, encouraging institutional delivery, augmenting the uptake of essential new care services, boosting the adherence to iron folate supplementation, ameliorating full vaccination of children, and increasing awareness of the danger sign of neonates are some major benefits in health promotion aspect (39–45). Considering all the health benefits stated above, the Ethiopian Federal Ministry of Health (FMOH) selected ANC as a priority area of research under the domain of improving maternal and child health (46).
Several studies were carried out, in Ethiopia, through the conventional regression analysis approach to assess the benefit of ANC care utilization without controlling potential confounders in the analysis (47–49). However, propensity score analysis (PSA) offers an alternative approach for program evaluation in a situation where randomized controlled trials are either infeasible or unethical through observational data (50). PSA has the potential to handle the drawback of conventional regression, which is the risk of bias on the exposures due to unobservable characteristics in the data set (51). PSA reduces this bias by matching women who attended 4 or more ANC visits (exposed) and those who attended less than 4 ANC visits (unexposed) with similar conditional probabilities to receive the treatment. The adjustment on the potential confounder makes PSA preferable to the traditional regression (52). The utilization of propensity score (PS) helps to make a balanced score within the potential cofounder between the exposed and unexposed groups based on surveillance data, hence resembling characteristics of randomized trials (53). To have a comparable balanced group of the respondent for the observed covariates, potential factors that influenced the utilization of ANC such as maternal age, maternal parity, maternal education status, religion, residence, ownership of the facility, type of health facility, and type of region were balanced accordingly (54–58). Considering the gaps in previous studies, and the benefit of the proposed approach, PSA was employed to determine the effect of optimal ANC care on maternal and perinatal health.
2. Methods
2.1. Study setting
Ethiopia has an estimated population of 117,876,000 in 2021, out of which 3,772,032 are under-one-year children (59). Administratively, Ethiopia has ten regions and two city administrations, namely Tigray, Afar, Amhara, Oromia, Somali, Benishangul-Gumuz, Southern Nations Nationalities, and Peoples Region (SNNPR), Sidama, Gambella, Harari, Addis Ababa city administration and Dire Dawa city administration (60). In 2020/21, there were 434 hospitals, 3,890 health centers, and 18,090 health posts with a health work density of 3.2 per 100,000 population (61). The country has high infant, under-five, and maternal mortality (47 per 1,000 LBs), (59 per 1,000 LBs), and (412 per 10,000 LBs), respectively (5, 6).
2.2. Data source and study participants
Epidemiological review of perinatal death was obtained from the Ethiopian Public Health Institutes (EPHI) during the four consecutive years of implementation (2018–2021). The data is collected and compiled from various health facilities across the country. The source population for this study is all perinate who died and were reviewed by the MPDSR committee during the study period. The system used facility-based abstraction format (FBAF) and verbal autopsy (VA) as data sources for the review process. Following the data extraction from the source, a review committee discuss and prepare the final report of the reviewed death. The final report will then be sent to the national data hub through perinatal death reporting format (PDRF) by the focal person assigned in each reporting facility. A total of 3,814 reviewed perinatal deaths were included in the study.
2.3. Study variables
Treatment (T) = 1 if woman had four or more ANC visits during the time of pregnancy; whereas women who had less than four ANC visits were labeled as “0”.
2.4. Outcome variables
Individual (maternal and perinatal) variables were selected after an extensive search of various works of literature. Thus, gestation age, place of delivery, cause of perinatal death, and mode of delivery were selected as perinatal variables, while maternal health condition, maternity outcome, and a score of delay one (delay to seek care) were considered as maternal health indicators. Cause of perinatal death and maternal health conditions were classified based on the International Classification of Diseases -Perinatal Mortality (ICD-PM) (62).
The score of delay one, which is a delay in deciding to seek care (63), was computed using the row sum of seven variables included under the domain; namely (1) family poverty, (2) bad experience with previous health care, (3) failed to recognize the danger signs of pregnancy,4) unaware where to go, (5) had no one take care of other children, (6) reliant on traditional practice and (7) lack of decision to a health facility. All of them were binary variables with “Yes” and “No” responses and after summation of the score and to keep the normality of the data a square root transformation was done (64). Finally, the transformed variable was treated as continuous variables to make a simple and parsimonious model (65).
2.5. Operational definition
2.5.1. Type of facility
Classified into three classes (primary, secondary, and tertiary health care facilities) according to their manpower, medical equipment, and service provision (61).
2.5.2. Optimal ANC visit
Women who had four and more ANC visits between conception up to the delivery of the perinate (66).
The cause of perinatal death was categorized by the time of death; antepartum, intrapartum stillbirth, unknown time of stillbirth, and neonatal death. The contributing maternal conditions were classified into five major categories (M1 to M4, with M5 representing no identified condition) per the guidance of ICD_PM_10 (Table 1) (67).
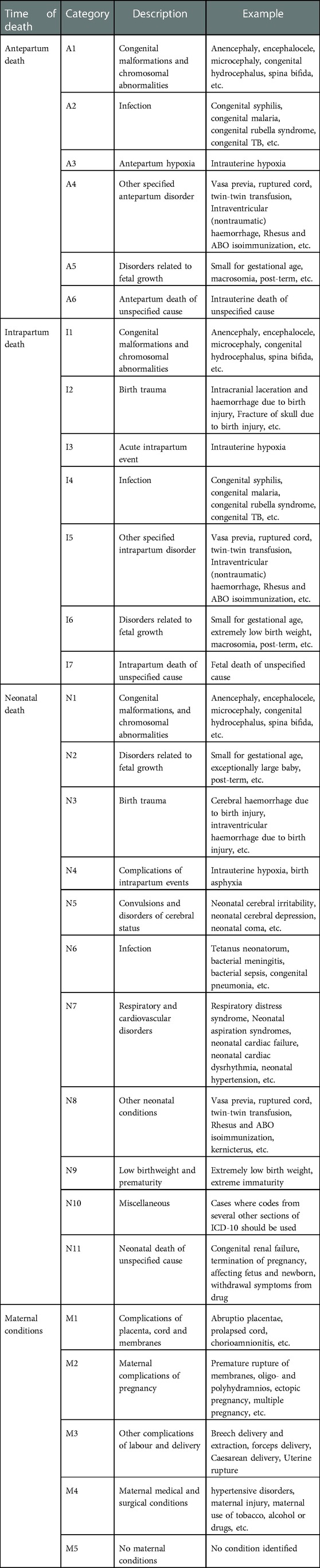
Table 1. ICD-PM categories with the specific cause of perinatal death and maternal health condition.
2.6. Data management and statistical analysis
For data cleaning and further analysis, the data was exported from Epi -info version 7.2 to Stata version 17, and both descriptive and analytical analyses were carried out. The descriptive analysis tables were presented using two-way tables, by classifying the table using the level of ANC care and whether the women received optimal ANC care or not. The analytical analysis was carried out using preferential within propensity score matching (PWPSM).
2.7. Model building strategy
To identify the final effect of treatment on the study outcome a series of statistical procedures were conducted. The following modeling building strategy was followed in estimating treatment effects which are: (1) “Estimating propensity score”, (2) “stratifying and balancing propensity score”, and (3) “estimating causal effect” (68, 69). Proper matching was also conducted since it is practically impossible to see the effect of optimal care on mothers and perinates without it.
2.7.1. Step one
The prosperity score (PS) was estimated for each observation, which indicates the probability of the individual unit experiencing the treatment (in this case receiving optimal ANC care). The score is computed using the potential individual and community level factors, which had a substantial role in the uptake of ANC.
2.7.2. Step two
After computing PS, the balance check was performed to look through the score if it is biased or not. As indicated in Supplementary Annex S1, the standardized biased was observed across the covariates with a range of −40 to 10.
2.7.3. Step three
To address the observed bias across the covariates, matching was performed using the nearest neighbor caliber matching with a radius of 0.25 standard deviation of the PS. After this arrangement, matching was carried out to avoid the bias observed in Supplementary Annex S1. As displayed in Supplementary Annex S2, a balance check of the PS was carried out to look at the quality of the matching, standardized bias was within an acceptable range of −10 to 10.
2.7.4. Step four
Selected an appropriate method of matching in considering the nature of the data. The surveillance data was hierarchal., i.e., the mother and deceased perinate were nested in 161 reporting health facilities and 45 provinces of the country. The data had both small and big clusters (reporting health facility) and by considering the nature of the cluster preferential within propensity score matching (PWPSM) was preferable and selected for the final PSA (70).
2.7.5. Step five
After model selection, the effect of the treatment was reported through the average treatment effect of treated (ATT) of the given covariate on selected outcome maternal and perinatal health interest (71).
3. Result
3.1. Selected characteristics of the reported facility
A total of 3,814 deceased perinates and their mothers were included in the study. Among mothers of the deceased perinate, only 33.9% of mothers received optimal ANC visits during their pregnancy. Among reporting facilities, 51.5% of mothers who were treated in tertiary health facilities received optimal ANC care. Region-wise, the proportion of mothers who received optimal ANC care was higher among mothers of a deceased perinate who resided in Addis Ababa (51.9%) than the mother of a deceased perinate who resided in the Gambella region (0.0%) (Table 2).
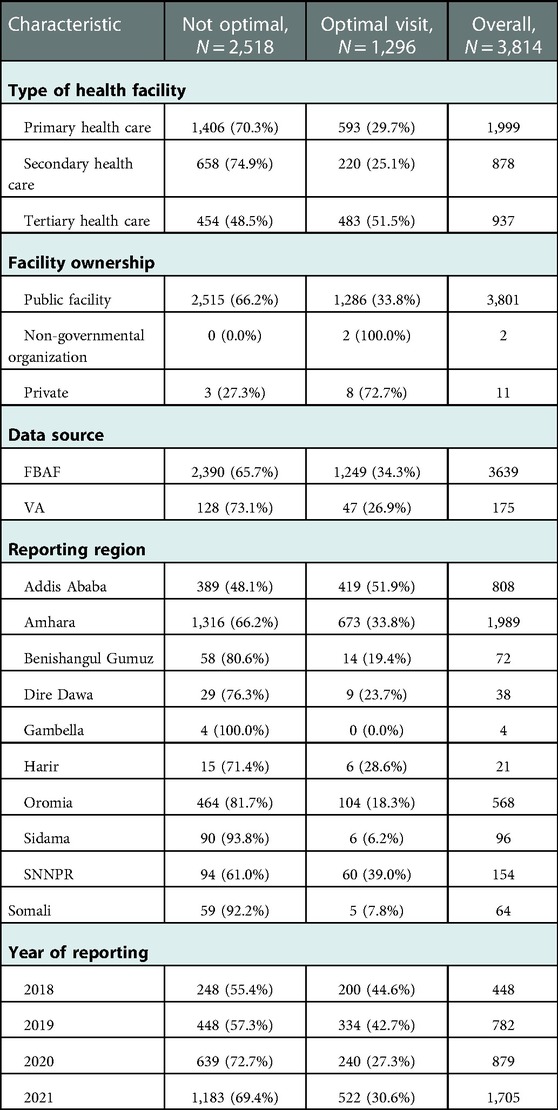
Table 2. Selected background characteristics of reporting facilities by the level of ANC follow-up in Ethiopia, 2021.
3.2. Selected characteristics of the mothers of the deceased perinate
The average maternal parity among mothers of deceased perinate was higher among mothers who did not receive optimal ANC care (2.4(with SD of 1.79) than mothers who received an optimal ANC visit (2.3(with SD of 1.62). The proportion of mothers who received optimal ANC care was higher among mothers who attend secondary education and above (47.9%) than mothers who had no education (27.1%). The proportion of mothers with a deceased perinate who received optimal care was higher among mothers who live in urban areas (41.1%) than mothers who live in rural areas (28.1%). Furthermore, women with no identified maternal complications had a higher proportion of receiving optimal ANC care (35.9%) than women with maternal complications of pregnancy (23.9%) (Table 3).
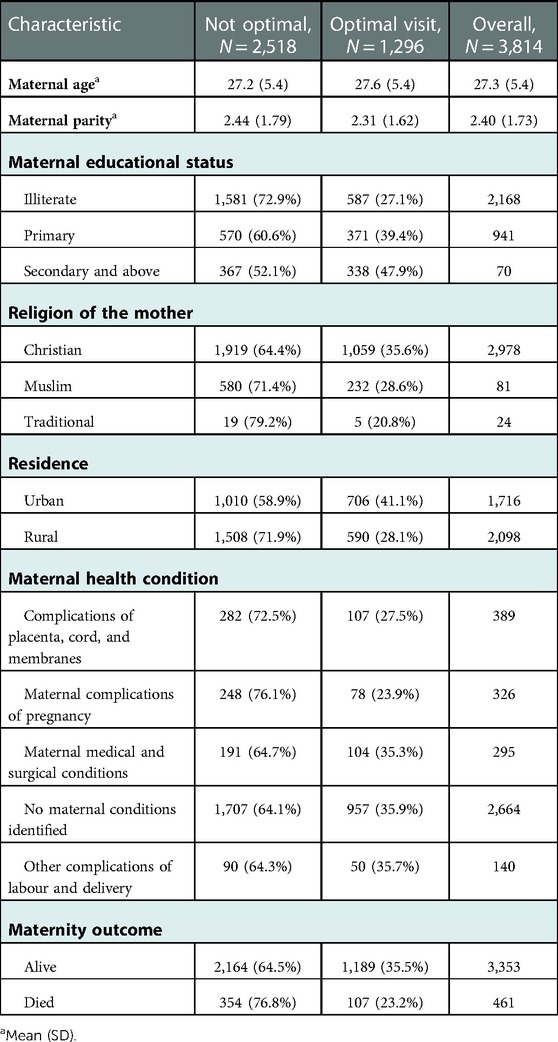
Table 3. Selected background characteristics of the deceased perinate's mother by the level of follow-up in Ethiopia, 2021.
3.3. Selected characteristics of the deceased perinate
The average gestational week of the perinate was higher among mothers who received optimal ANC care (36.1(with SD of 3.5) than mothers who did not receive optimal ANC care (35.2(with SD of 3.4). The proportion of mothers who received optimal ANC care was higher among mothers who give birth at health facilities (35.5%) than mothers who give birth at home (15.5%). Furthermore, the proportion of mothers who received optimal ANC care was higher among mothers who deliver through cesarean section (46.5%) than mothers who deliver through Spontaneous vaginal delivery (31.4%) (Table 4).
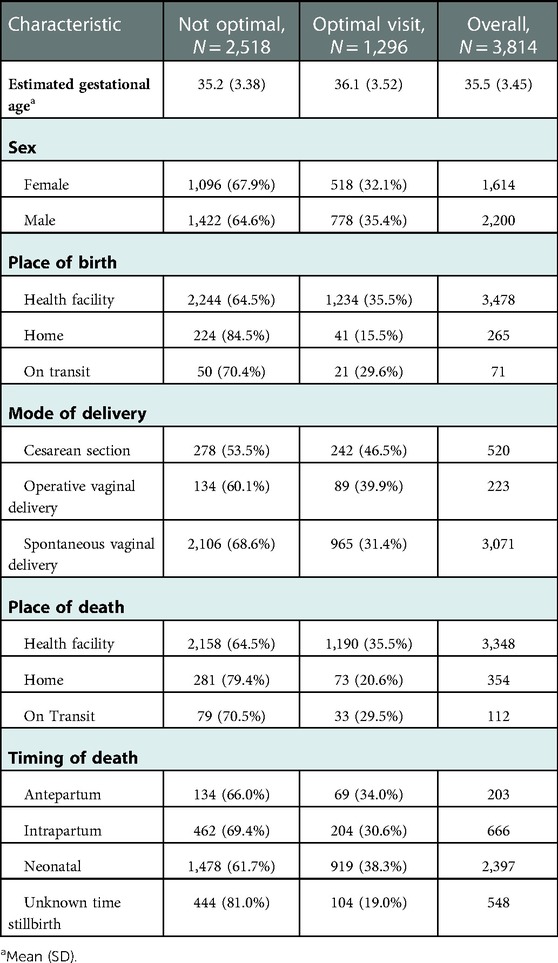
Table 4. Selected characteristics of the deceased perinate by the level of ANC follow-up in Ethiopia, 2021.
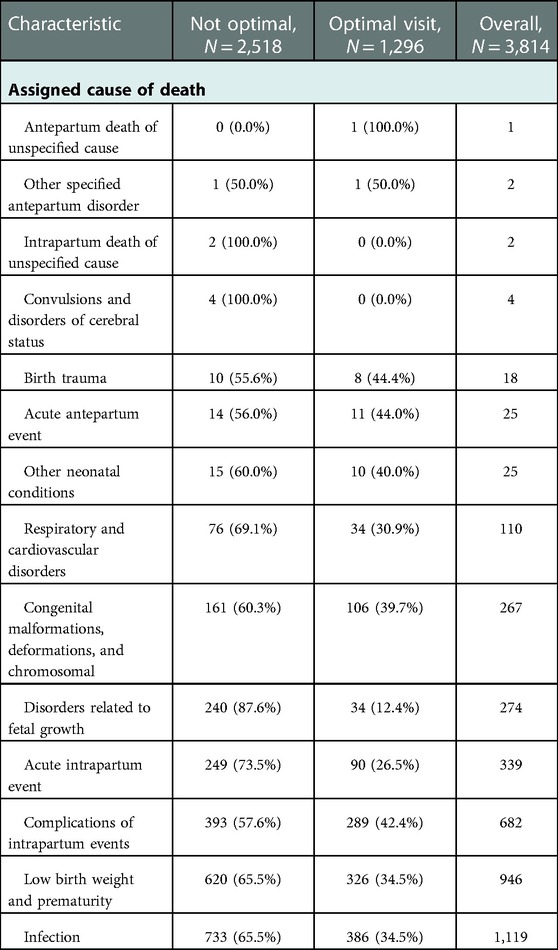
3.4. Assigned cause of death
The proportion of perinatal death due to disorders related to fetal growth was higher among mothers who received optimal ANC care (87.7%) than mothers who did not receive optimal ANC care (12.4%). The proportion of perinatal death due to Acute intrapartum events was higher among mothers who received optimal ANC care (73.5%) than mothers who did not receive optimal ANC care (26.5%). Furthermore, the proportion of perinatal death due to infection events was higher among mothers who received optimal ANC care (73.5%) than mothers who did not receive optimal ANC care (26.5%) (Table 5).
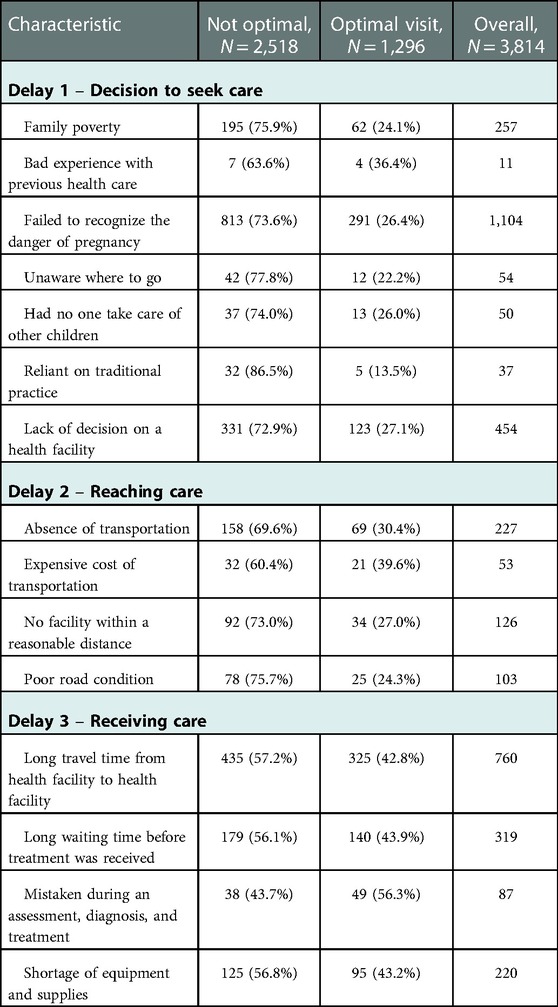
3.5. Delay factor by the level of ANC care
The proportion of perinatal death contributed by delay one was higher among mothers who received optimal ANC care due to previous bad experiences in health facilities (36.4%) than mothers who delayed seeking care due to relying on traditional remedies (13.5%). The proportion of perinatal death contributed by delay two was higher among mothers who received optimal ANC care due to the expensive cost of transportation (39.6%) than mothers who delayed reaching the health facility due to poor road conditions (24.3%). Furthermore, the proportion of perinatal death contributed by delay three was slightly higher among mothers who received optimal ANC care due to the wrong diagnosis and treatment (56.3%) than mothers who were delayed to receive the care due to multiple referrals (42.8%) (Table 6).
3.6. Propensity scores matching analysis
After matching and considering the cluster effect of the data, the probability of perinatal death due to respiratory and cardiovascular disorder among perinates born from mothers who received optimal ANC care was 2% [ATT = −0.015, 95%CI: (−0.029 to −0.001)] lower as compared to perinates who were born from the same mother who did not receive optimal ANC care. Furthermore, perinates who were born from mothers who received optimal ANC care had 1 week [ATT = 1.277, 95%CI: (0.563–1.991)] additional intrauterine life as compared to perinate who were born from the same mother who received less than four ANC visits. The likelihood of having uterine rupture among mothers who received optimal ANC care was 1% [ATT = −0.012, 95%CI: (−0.018 to −0.005)] lower as compared to the same women who did not receive optimal ANC care. The probability of giving birth through operative vaginal delivery (OVD) was 3% [ATT = 0.032, 95%CI: (0.001–0.062)] higher among mothers who received optimal ANC care as compared to the same mother who received less than four ANC visits. Additionally, the probability of delay to decide to seek care was 20% [ATT = −0.187, 95%CI: (−0.354 to −0.021)] lower among mothers who received optimal ANC care as compared to the same mothers who did not receive optimal ANC care (Table 7).

Table 7. Average treatment effects on the treated (ATT) 4 and more ANC visits on maternal and perinatal health in Ethiopia, 2021.
4. Discussion
The study aimed to quantify the effect of optimal ANC care on maternal and perinatal health. Per the final model optimal ANC care had both perinatal (gestational week and respiratory and cardiovascular disorders) and maternal (uterine rupture, operative vaginal delivery, and delay one) health outcomes.
Perinates who were born from mothers who received optimal ANC care had a lower chance of dying due to respiratory and cardiovascular disorders as compared to perinate who were born from mothers who did not receive optimal ANC visits. The finding was supported by the study conducted elsewhere (72, 73). This could be explained by the fact that mothers who received optimal ANC care had a better opportunity than mothers who do not receive the optimal care, in identifying and managing the potential predisposing factors leading to complications of respiratory and cardiovascular disorders. In Ethiopia, respiratory distress syndrome and neonatal aspiration syndromes are the commonest cause of perinate death under the domain of respiratory and cardiovascular disorders (74, 75). Uncontrolled gestational diabetes Mellitus (GDM), pregnancy-induced hypertension (PIH), short birth interval, home delivery, and preterm delivery are identified as modifiable risk factors for respiratory and cardiovascular disorders in Ethiopia (74–76). These predisposing factors could be managed by adhering to the protocol of ANC follow-up, which encourages dietary diversity and physical activity to reduce the risk of GDM and PIH (77, 78) and boost the utilization of postpartum contraceptives to improve the birth interval between consecutive pregnancies (79). On top of this, receiving optimal ANC care improves institutional delivery, which paves the way to access the best treatment modalities for managing respiratory and cardiovascular disorders including antenatal corticosteroids, surfactants, and advanced respiratory care (80). However, the availability of those advanced treatment modalities is limited in a resource-constrained setting (81). In response to this gap, Ethiopia has taken initiative in equipping selected health facilities with grade three neonatal intensive care equipment (82). Overall, the finding implied that optimal ANC care has a positive impact in reducing the death of perinates due to respiratory and cardiovascular disorders.
Perinates who were born from mothers who received optimal ANC care had one additional week of intrauterine life as compared to perinate who were born from mothers who did not receive optimal ANC visits. This indicated that receiving optimal ANC care had a role in the reduction of preterm delivery. The finding was comparable with studies conducted in Uganda (83), Bangladesh (84), Surinam (85), and Brazil (86). This might be explained by the role of optimal ANC care in creating a convenient environment to take precautionary measures such as obtaining multiple micronutrient supplementation, HIV treatment, adequate malaria prophylaxis, and counseling on a healthy lifestyle (87–90). In Ethiopia, experiencing premature rupture of the membrane (PROM), PIH, being HIV positive, urinary tract infection, and short birth interval were known modifiable factors prone to preterm delivery (91–93). In line with this, respiratory distress syndrome was the major cause of death among preterm perinate born in Ethiopia (94). Optimal ANC care enhances the utilization of postpartum contraceptives, obtaining nutritional supplementation, receiving pharmacological therapy, and conducting routine urine tests. All these measures reduce the risk of having preterm birth by improving birth interval, managing complications of PIH, and treating urinary tract infections (95–97). In general, the finding implied that receiving optimal ANC care could reduce the risk of having preterm delivery and complications that comes up with preterm delivery.
Women who received optimal ANC care had a lower risk of encountering uterine rupture as compared to the same women who did not receive optimal ANC care. The finding was aligned with studies conducted in different parts of Ethiopia (Adama, Dessie, Mizan Tepi, and Tigray) (98–101). This could be explained by optimal ANC's role in the identification of potential risk exposure to uterine rupture and bolster for immediate and planned intervention such as cesarean section(C/S) (102). Home delivery, obstructed labour, prolonged labour, severe anemia, previous history of C/S, and partograph utilization were identified as risk factors for uterine rupture in Ethiopia (103–105). All those risk factors are handled during ANC visits by providing iron folate, encouraging institutional delivery, and planning selective C/S (106, 107). Overall, the findings suggested that optimal ANC visits had an integral role in reducing the risk of having a uterine rupture. Hence, the effect of optimal visits should be maximized well by integrating it into other health programs for better maternal and perinatal outcomes.
The study also elucidated that those women who received optimal ANC care had a high probability of giving birth through operative vaginal delivery (OVD) as compared to similar women who attend less than 4 ANC visits. The finding was coherent with a study conducted in a different part of Ethiopia (Jimma and Hossana) (108, 109). The plausible explanation could be the fact that OVD is one of the packages in basic emergency obstetric care provided in health facilities, which is designed to shorten second-stage labour through the assistance of forceps and vacuums (110). On top of this, OVD is solely provided in the health facility by a trained health care provider, which makes it more accessible for women who received optimal ANC since they have a higher probability of having institutional delivery, compared to women who do not receive optimal care (111). The presence of fetal distress, prolonged stage of labour, maternal health condition (maternal cardiac disease and PIH), and previous C/S were the common indications of OVD in Ethiopia (112). However, the OVD procedure unless taken meticulously could result in unwanted maternal and child outcomes such as a post-partum hemorrhage to the mother and cerebral palsy for the neonate (113, 114). Overall, the finding suggested that receiving optimal ANC had a role in increasing the chance of obtaining basic obstetrics emergency services.
Receiving optimal ANC had a substantial role in avoiding delay one, which is a delay in deciding to seek care. The finding corresponded well with studies conducted in Ethiopia (Hawassa, Shashemene, South Gonder, and Jimma) (115–118) and Mali (119). This could be explained by the better birth preparedness that those who received optimal ANC have, making them more alert and ready for pregnancy-related emergencies (120). Delay in deciding to seek care is more common in women who live in rural areas with low educational status (121). Furthermore, the delay vitiated the completion of the continuum of maternity care (47). Considering all this gap, Ethiopia has designed a health extension program to provide basic health education in a way to improve the health-seeking behaviors of the rural community (122). In addition, community engagement and creating a woman-friendly environment were some of the measures taken to improve the utilization and quality of ANC care. To this effect, the coverage of ANC has remarkably improved in the last two decades (9). Overall, the finding implied that ANC could be used as one platform to enhance community health-seeking behaviors in coordination with other parallel programs.
The study had the following limitations that needs to be acknowledged. Firstly, almost all reviewed death come from the public facility with limited involvement from a private facility, which affects the inclusiveness of the study. Secondly, the surveillance system captured a small number of deaths as compared to the national estimate, which also affect the representativeness of the study.
5. Conclusion
The study has identified that optimal ANC care affects maternal and perinate health. Reduced risk of death due to respiratory and cardiovascular disorders and lower preterm birth were effects included under perinatal health, while reduced the risk of having uterine rupture, enhancing birth giving through OVD, and improving the health-seeking behavior of women were included under maternal health. Thus, an exerted effort is needed in improving the coverage and quality of optimal ANC care to maximize the benefit obtained from the care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors per the guidance of the data sharing policy of the institute.
Ethics statement
The studies involving human participants were reviewed and approved by The study was approved by EPHI scientific and ethical review office (SERO) with Ref. No. EPHI 6_5/437 and permission were obtained from Public Health Emergency Management. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
NT planned the study, GH coordinated the study, NT and FW cleaned and analyze data, NT analyzed the literature, NT and FW was major contributor in writing the manuscript study. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to acknowledge EPHI, particularly the Center of Public Health Emergency unit for their facilitation and support during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1120979/full#supplementary-material.
References
1. Moller AB, Petzold M, Chou D, Say L. Early antenatal care visit: a systematic analysis of regional and global levels and trends of coverage from 1990 to 2013. Lancet Glob Health. (2017) 5(10):e977–83. doi: 10.1016/S2214-109X(17)30325-X
2. World Health Organization. Strategies towards ending preventable maternal mortality (EPMM). Geneva (Switzerland): World Health Organization (2015).
3. Tesfu AA, Aweke AM, Gela GB, Wudineh KG, Beyene FY. Factors associated with timely initiation of antenatal care among pregnant women in Bahir Dar city, Northwest Ethiopia: cross-sectional study. Nurs Open. (2022) 9(2):1210–7. doi: 10.1002/nop2.1162
4. UNICEF D. Monitoring the situation of children and women. New York: United Nations Children’s Fund (2017).
5. Central Statistical Agency/CSA/Ethiopia and ICF. Ethiopia Demographic and health survey 2016. Addis Ababa, Ethiopia, and rockville, Maryland, USA: CSA and ICF (2016).
6. Ethiopian Public Health Institute (EPHI) [Ethiopia] and ICF. Ethiopia Mini demographic and health survey 2019: Final report. Rockville, Maryland, USA: EPHI and ICF (2021).
7. Sarker BK, Rahman M, Rahman T, Rahman T, Khalil JJ, Hasan M, et al. Status of the WHO recommended timing and frequency of antenatal care visits in Northern Bangladesh. PLoS One. (2020) 15(11):e0241185. doi: 10.1371/journal.pone.0241185
8. World Health Organization. New guidelines on antenatal care for a positive pregnancy experience. Geneva (Switzerland): World Health Organization (2016).
9. Ethiopian Minister of Health. National antenatal care guideline ensuring positive pregnancy experience. Addis Ababa (Ethiopia): FMOH (2022). Available at: https://tinyurl.com/3wta8529 (cited June 18, 2022).
10. Wilunda C, Scanagatta C, Putoto G, Montalbetti F, Segafredo G, Takahashi R, et al. Barriers to utilisation of antenatal care services in South Sudan: a qualitative study in Rumbek North County. Reprod Health. (2017) 14(1):1–10. doi: 10.1186/s12978-017-0327-0
11. Girmaye M, Berhan Y. Skilled antenatal care service utilization and its association with the characteristics of women’s health development team in Yeky District, South-West Ethiopia: a multilevel analysis. Ethiop J Health Sci. (2016) 26(4):369–80. doi: 10.4314/ejhs.v26i4.9
12. Yitbarek K, Abraham G, Morankar S. Contribution of women’s development army to maternal and child health in Ethiopia: a systematic review of evidence. BMJ Open. (2019) 9(5):e025937. doi: 10.1136/bmjopen-2018-025937
13. Shigute Z, Mebratie AD, Sparrow R, Alemu G, Bedi AS. The effect of Ethiopia’s community-based health insurance scheme on revenues and quality of care. Int J Environ Res Public Health. (2020) 17(22):8558. doi: 10.3390/ijerph17228558
14. Bradley E, Hartwig KA, Rowe LA, Cherlin EJ, Pashman J, Wong RE, et al. Hospital quality improvement in Ethiopia: a partnership–mentoring model. Int J Qual Health Care. (2008) 20(6):392–9. doi: 10.1093/intqhc/mzn042
15. Ali EE. Health care financing in Ethiopia: implications on access to essential medicines. Value Health Reg Issues. (2014) 4:37–40. doi: 10.1016/j.vhri.2014.06.005
16. Tadesse Berehe T, Modibia LM. Assessment of quality of antenatal care services and its determinant factors in public health facilities of Hossana town, Hadiya zone, Southern Ethiopia: a longitudinal study. Adv Public Health. (2020) 2020:1–11. doi: 10.1155/2020/5436324
17. Kassaw A, Debie A, Geberu DM. Quality of prenatal care and associated factors among pregnant women at public health facilities of Wogera District, Northwest Ethiopia. J Pregnancy. (2020) 2020:1–8. doi: 10.1155/2020/9592124
18. Hailu GA, Weret ZS, Adasho ZA, Eshete BM. Quality of antenatal care and associated factors in public health centers in Addis Ababa, Ethiopia, a cross-sectional study. PLoS One. (2022) 17(6):e0269710. doi: 10.1371/journal.pone.0269710
19. Seyoum T, Alemayehu M, Christensson K, Lindgren H. Provider-perceived benefits and constraints of complete adherence to antenatal care guideline among public health facilities, Ethiopia: a qualitative study. PLoS One. (2021) 16(8):e0255297. doi: 10.1371/journal.pone.0255297
20. Rono J, Kamau L, Mwangwana J, Waruguru J, Aluoch P, Njoroge M. A policy analysis of policies and strategic plans on Maternal, Newborn and Child Health in Ethiopia. Int J Equity Health. (2022) 21(1):1–8. doi: 10.1186/s12939-021-01581-5
21. Tessema ZT, Akalu TY. Spatial pattern and associated factors of ANC visits in Ethiopia: spatial and multilevel modeling of Ethiopian demographic health survey data. Adv Prev Med. (2020) 2020:1–13. doi: 10.1155/2020/4676591
22. Yeneneh A, Alemu K, Dadi AF, Alamirrew A. Spatial distribution of antenatal care utilization and associated factors in Ethiopia: evidence from Ethiopian demographic health surveys. BMC Pregnancy Childbirth. (2018) 18(1):1–2. doi: 10.1186/s12884-018-1874-2
23. Ethiopian Ministry of Health. National reproductive health strategy from 2016_to 2020. Addis Ababa, Ethiopia: FMOH (2022). Available at: https://tinyurl.com/mryvcs6w (Cited June 18, 2022).
24. Tesfay N, Tariku R, Zenebe A, Woldeyohannes F. Critical factors associated with postpartum maternal death in Ethiopia. PLoS One. (2022) 17(6):e0270495. doi: 10.1371/journal.pone.0270495
25. Melberg A, Mirkuzie AH, Sisay TA, Sisay MM, Moland KM. “Maternal deaths should simply be 0”: politicization of maternal death reporting and review processes in Ethiopia. Health Policy Plan. (2019) 34(7):492–8. doi: 10.1093/heapol/czz075
26. Ethiopian Public Health Institutes. Maternal and perinatal death surveillance and response technical guideline. Addis Ababa (Ethiopia): EPHI (2017). Available at: https://tinyurl.com/2p8f6rwz (cited June 27, 2022).
27. Ayele B, Gebretnsae H, Hadgu T, Negash D, Gsilassie F, Alemu T, et al. Maternal and perinatal death surveillance and response in Ethiopia: achievements, challenges and prospects. PLoS One. (2019) 14(10):e0223540. doi: 10.1371/journal.pone.0223540
28. Tura AK, Fage SG, Ibrahim AM, Mohamed A, Ahmed R, Gure T, et al. Beyond no blame: practical challenges of conducting maternal and perinatal death reviews in eastern Ethiopia. Glob Health Sci Pract. (2020) 8(2):150–4. doi: 10.9745/GHSP-D-19-00366
29. Al-Ateeq MA, Al-Rusaiess AA. Health education during antenatal care: the need for more. Int J Womens Health. (2015) 7:239. doi: 10.2147/IJWH.S75164
30. Kebede TT, Godana W, Utaile MM, Sebsibe YB. Effects of antenatal care service utilization on maternal near miss in Gamo Gofa zone, Southern Ethiopia: retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21(1):1–9. doi: 10.1186/s12884-021-03683-y
31. Haftu A, Hagos H, Mehari MA. Pregnant women adherence level to antenatal care visit and its effect on perinatal outcome among mothers in tigray public health institutions, 2017: cohort study. BMC Res Notes. (2018) 11(1):1–6. doi: 10.1186/s13104-017-3088-5
32. Tesfay N, Tariku R, Zenebe A, Firde H, Woldeyohannes F. Target areas to reduce the burden of maternal death due to obstetric hemorrhage in Ethiopia. PLoS One. (2022) 17(9):e0274866. doi: 10.1371/journal.pone.0274866
33. Oduse S, Zewotir T, North D. The impact of antenatal care on under-five mortality in Ethiopia: a difference-in-differences analysis. BMC Pregnancy Childbirth. (2021) 21(1):1–9. doi: 10.1186/s12884-020-03531-5
34. Tolossa T, Fekadu G, Mengist B, Mulisa D, Fetensa G, Bekele D. Impact of antenatal care on neonatal mortality among neonates in Ethiopia: a systematic review and meta-analysis. Arch Public Health. (2020) 78(1):1–11. doi: 10.1186/s13690-020-00499-8
35. Tekelab T, Chojenta C, Smith R, Loxton D. The impact of antenatal care on neonatal mortality in sub-Saharan Africa: a systematic review and meta-analysis. PLoS One. (2019) 14(9):e0222566. doi: 10.1371/journal.pone.0222566
36. Kuhnt J, Vollmer S. Antenatal care services and its implications for vital and health outcomes of children: evidence from 193 surveys in 69 low-income and middle-income countries. BMJ Open. (2017) 7(11):e017122. doi: 10.1136/bmjopen-2017-017122
37. Manjavidze T, Rylander C, Skjeldestad FE, Kazakhashvili N, Anda EE. The impact of antenatal care utilization on admissions to neonatal intensive care units and perinatal mortality in Georgia. PLoS One. (2020) 15(12):e0242991. doi: 10.1371/journal.pone.0242991
38. Woldeamanuel BT, Tesfaye TT. Risk factors associated with under-five stunting, wasting, and underweight based on Ethiopian demographic health survey datasets in Tigray region, Ethiopia. J Nutr Metab. (2019) 2019:1–11. doi: 10.1155/2019/6967170
39. Alebel A, Tesma C, Temesgen B, Ferede A, Kibret GD. Exclusive breastfeeding practice in Ethiopia and its association with antenatal care and institutional delivery: a systematic review and meta-analysis. Int Breastfeed J. (2018) 13(1):1–2. doi: 10.1186/s13006-018-0173-x
40. Temesgen H, Negesse A, Woyraw W, Getaneh T, Yigizaw M. Prelacteal feeding and associated factors in Ethiopia: systematic review and meta-analysis. Int Breastfeed J. (2018) 13(1):1–2. doi: 10.1186/s13006-018-0193-6
41. Geremew AB, Boke MM, Yismaw AE. The effect of antenatal care service utilization on postnatal care service utilization: a systematic review and meta-analysis study. J Pregnancy. (2020) 2020:1–7. doi: 10.1155/2020/7363242
42. Fekadu GA, Kassa GM, Berhe AK, Muche AA, Katiso NA. The effect of antenatal care on use of institutional delivery service and postnatal care in Ethiopia: a systematic review and meta-analysis. BMC Health Serv Res. (2018) 18(1):1–11. doi: 10.1186/s12913-018-3370-9
43. Amsalu ET, Kefale B, Muche A, Fentaw Z, Dewau R, Chanie MG, et al. The effects of ANC follow up on essential newborn care practices in east Africa: a systematic review and meta-analysis. Sci Rep. (2021) 11(1):1–21. doi: 10.1038/s41598-020-79139-8
44. Boti N, Bekele T, Godana W, Getahun E, Gebremeskel F, Tsegaye B, et al. Adherence to iron-folate supplementation and associated factors among pastoralist’s pregnant women in Burji districts, Segen area People’s zone, Southern Ethiopia: community-based cross-sectional study. Int J Reprod Med. (2018) 2018:1–8. doi: 10.1155/2018/2365362
45. Yadeta TA. Antenatal care utilization increases the odds of women knowledge on neonatal danger sign: a community-based study, eastern Ethiopia. BMC Res Notes. (2018) 11(1):1–5. doi: 10.1186/s13104-018-3957-6
46. Ethiopian Minister of Health. National reproductive, maternal, newborn, child, and adolescent health and nutrition research priorities 2019–2021. Addis Ababa (Ethiopia): FMOH (2021). Available at: https://tinyurl.com/ycycchn7 (cited June 18, 2022).
47. Dadi TL, Medhin G, Kasaye HK, Kassie GM, Jebena MG, Gobezie WA, et al. Continuum of maternity care among rural women in Ethiopia: does place and frequency of antenatal care visit matter? Reprod Health. (2021) 18(1):1–2. doi: 10.1186/s12978-020-01058-8
48. Mengist B, Desta M, Tura AK, Habtewold TD, Abajobir A. Maternal near miss in Ethiopia: protective role of antenatal care and disparity in socioeconomic inequities: a systematic review and meta-analysis. Int J Africa Nurs Sci. (2021) 15:100332. doi: 10.1016/j.ijans.2021.100332
49. Gashaye A, Kibret GD, Bazezew Y, Mengist B. Factors affecting institutional delivery in Ethiopia: a multi-level analysis. Int J Africa Nurs Sci. (2021) 15:100331. doi: 10.1016/j.ijans.2021.100331
50. Johara FT, Benedetti A, Platt R, Menzies D, Viiklepp P, Schaaf S, et al. Evaluating the performance of propensity score matching based approaches in individual patient data meta-analysis. BMC Med Res Methodol. (2021) 21(1):1–6. doi: 10.1186/s12874-021-01452-1
51. Guo S, Fraser MW. Propensity score analysis: Statistical methods and applications. Los Angeles: SAGE publications (2014).
52. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. (2011) 46(3):399–424. doi: 10.1080/00273171.2011.568786
53. Park K, Ewing R, Scheer BC, Ara Khan SS. Travel behavior in TODs vs. non-TODs: using cluster analysis and propensity score matching. Transp Res Rec. (2018) 2672(6):31–9. doi: 10.1177/0361198118774159
54. Azimi MW, Yamamoto E, Saw YM, Kariya T, Arab AS, Sadaat SI, et al. Factors associated with antenatal care visits in Afghanistan: secondary analysis of Afghanistan demographic and health survey 2015. Nagoya J Med Sci. (2019) 81(1):121. doi: 10.18999/nagjms.81.1.121
55. Tareke AA, Mittiku YM, Tamiru AT, Rade BK, Gudayu TW. Underutilization of the recommended frequency of focused antenatal care services in Northwest Ethiopia: using Andersen's healthcare service utilization model approach. Clin Epidemiol Glob Health. (2021) 11:100746. doi: 10.1016/j.cegh.2021.100746
56. Tenkorang EY. Type of health facility and utilization of antenatal care services among Ghanaian women. Popul Res Policy Rev. (2016) 35(5):631–50. doi: 10.1007/s11113-016-9406-0
57. Belay A, Astatkie T, Abebaw S, Gebreamanule B, Enbeyle W. Prevalence and factors affecting the utilization of antenatal care in rural areas of Southwestern Ethiopia. BMC Pregnancy Childbirth. (2022) 22(1):1–8. doi: 10.1186/s12884-021-04362-8
58. Miller P, Afulani PA, Musange S, Sayingoza F, Walker D. Person-centered antenatal care and associated factors in Rwanda: a secondary analysis of program data. BMC Pregnancy Childbirth. (2021) 21(1):1–11. doi: 10.1186/s12884-021-03747-z
59. United Nations. World population prospect. Population division (2022) Available at: https://population.un.org/wpp/ (Cited June 18, 2022).
60. Ethiopian Public Health Institutes. National maternal and perinatal death surveillance and response (MPDSR) system annual report of 2013EFY. Addis Ababa (Ethiopia): EPHI (2022). Available at: https://tinyurl.com/53ha8r5j (Cited June 18, 2022).
61. Federal Minister of Health of Ethiopia. Health and Health-Related Indicators 2020/2021. Addis Ababa (Ethiopia): FMOH (2021). Available at: https://tinyurl.com/2rvum73n (Cited March 1, 2022).
62. World Health Organization. The WHO application of ICD-10 to deaths during the perinatal period: iCD-PM. Geneva (Switzerland): World Health Organization (2016).
63. Callaghan-Koru JA, Nonyane BA, Guenther T, Sitrin D, Ligowe R, Chimbalanga E, et al. Contribution of community-based newborn health promotion to reducing inequities in healthy newborn care practices and knowledge: evidence of improvement from a three-district pilot program in Malawi. BMC Public Health. (2013) 13(1):1–2. doi: 10.1186/1471-2458-13-1
64. Jebena MG, Lindstrom D, Belachew T, Hadley C, Lachat C, Verstraeten R, et al. Food insecurity and common mental disorders among Ethiopian youth: structural equation modeling. PLoS One. (2016) 11(11):e0165931. doi: 10.1371/journal.pone.0165931
65. Uchechi OC. Assessing the impact of square root transformation on weibull-distributed error component of a multiplicative error model. Sci J Appl Math Stat. (2021) 9(4):94. doi: 10.11648/j.sjams.20210904.11
66. Mwebesa E, Kagaayi J, Ssebagereka A, Nakafeero M, Ssenkusu JM, Guwatudde D, et al. Effect of four or more antenatal care visits on facility delivery and early postnatal care services utilization in Uganda: a propensity score matched analysis. BMC Pregnancy Childbirth. (2022) 22(1):1–9. doi: 10.1186/s12884-021-04354-8
67. Aminu M, Mathai M, van den Broek N. Application of the ICD-PM classification system to stillbirth in four sub-Saharan African countries. PLoS One. (2019) 14(5):e0215864. doi: 10.1371/journal.pone.0215864
68. Li M. Using the propensity score method to estimate causal effects: a review and practical guide. Organ Res Methods. (2013) 16(2):188–226. doi: 10.1177/1094428112447816
69. Pan W, Bai H. Propensity score interval matching: using bootstrap confidence intervals for accommodating estimation errors of propensity scores. BMC Med Res Methodol. (2015) 15(1):1–9. doi: 10.1186/1471-2288-15-1
70. Arpino B, Cannas M. Propensity score matching with clustered data. An application to the estimation of the impact of caesarean section on the Apgar score. Stat Med. (2016) 35(12):2074–91. doi: 10.1002/sim.6880
71. Emaway Altaye D, Karim AM, Betemariam W, Fesseha Zemichael N, Shigute T, Scheelbeek P. Effects of family conversation on health care practices in Ethiopia: a propensity score matched analysis. BMC Pregnancy Childbirth. (2018) 18(1):43–52. doi: 10.1186/s12884-018-1672-x
72. Shiferaw K, Mengiste B, Gobena T, Dheresa M. The effect of antenatal care on perinatal outcomes in Ethiopia: a systematic review and meta-analysis. PLoS One. (2021) 16(1):e0245003. doi: 10.1371/journal.pone.0245003
73. Arunda M, Emmelin A, Asamoah BO. Effectiveness of antenatal care services in reducing neonatal mortality in Kenya: analysis of national survey data. Glob Health Action. (2017) 10(1):1328796. doi: 10.1080/16549716.2017.1328796
74. Fenta B, Yetwale A, Biyazin T, Dagnaw Y. Respiratory distress and its associated factors among preterm neonates admitted to Mizan Tepi University teaching hospital, Bench Maji Zone, South West Ethiopia, 2020. J Neonatal Nurs. (2022) 28(4):249–54. doi: 10.1016/j.jnn.2021.10.014
75. Abate E, Alamirew K, Admassu E, Derbie A. Prevalence and factors associated with meconium-stained amniotic fluid in a tertiary hospital, northwest Ethiopia: a cross-sectional study. Obstet Gynecol Int. (2021) 2021:1–8. doi: 10.1155/2021/5520117
76. Aynalem YA, Mekonen H, Habtewold TD, Endalamaw A, Petrucka PM, Shiferaw WS. Incidence of respiratory distress and its predictors among neonates admitted to the neonatal intensive care unit. Addis Ababa, Ethiopia: Black Lion Specialized Hospital. PLoS ONE. (2020) 15(7):e0235544. doi: 10.1371/journal.pone.0235544
77. Muche AA, Olayemi OO, Gete YK. Prevalence of gestational diabetes mellitus and associated factors among women attending antenatal care at gondar town public health facilities, Northwest Ethiopia. BMC Pregnancy Childbirth. (2019) 19(1):1–3. doi: 10.1186/s12884-019-2492-3
78. Belayhun Y, Kassa Y, Mekonnen N, Binu W, Tenga M, Duko B. Determinants of pregnancy-induced hypertension among mothers attending public hospitals in wolaita zone, south Ethiopia: findings from unmatched case-control study. Int J Hypertens. (2021) 2021:1–9. doi: 10.1155/2021/6947499
79. Mesfin Yesgat Y, Gultie Ketema T, Abebe Dessalegn S, Wallelign Bayabil A, Argaw Enyew M, Habte Dagnaw E. Extended post-partum modern contraceptive utilization and associated factors among women in Arba Minch town, Southern Ethiopia. PLoS One. (2022) 17(3):e0265163. doi: 10.1371/journal.pone.0265163
80. Neal S, Falkingham J. Neonatal death and national income in developing countries: will economic growth reduce deaths in the first month of life? Int J Popul Res. (2014) 2014:1–6. doi: 10.1155/2014/989485
81. Chanie ES, Alemu AY, Mekonen DK, Melese BD, Minuye B, Hailemeskel HS, et al. Impact of respiratory distress syndrome and birth asphyxia exposure on the survival of preterm neonates in East Africa continent: systematic review and meta-analysis. Heliyon. (2021) 7(6):e07256. doi: 10.1016/j.heliyon.2021.e07256
82. Ethiopian Ministry of Health. Annual Performance Report 2013EFY (2020/2021) (2022). Available at: https://tinyurl.com/28d4u55h (Cited June 1, 2022).
83. Bater J, Lauer JM, Ghosh S, Webb P, Agaba E, Bashaasha B, et al. Predictors of low birth weight and preterm birth in rural Uganda: findings from a birth cohort study. PLoS One. (2020) 15(7):e0235626. doi: 10.1371/journal.pone.0235626
84. Pervin J, Rahman SM, Rahman M, Aktar S, Rahman A. Association between antenatal care visit and preterm birth: a cohort study in rural Bangladesh. BMJ Open. (2020) 10(7):e036699. doi: 10.1136/bmjopen-2019-036699
85. Baldewsingh GK, Jubitana BC, Van Eer ED, Shankar A, Hindori-Mohangoo AD, Covert HH, et al. Adequate antenatal care and ethnicity affect preterm birth in pregnant women living in the tropical rainforest of Suriname. BMC Pregnancy Childbirth. (2020) 20(1):1–9. doi: 10.1186/s12884-020-03364-2
86. Ramos de Oliveira CV, Neves PA, Lourenço BH, Medeiros de Souza R, Malta MB, Fujimori E, et al. Prenatal care and preterm birth in the Western Brazilian Amazon: a population-based study. Glob Public Health. (2022) 17(3):391–402. doi: 10.1080/17441692.2020.1865429
87. Lancaster KE, Kwok C, Rinaldi A, Byamugisha J, Magwali T, Nyamapfeni P, et al. Incident pregnancy and pregnancy outcomes among HIV-infected women in Uganda and Zimbabwe. Int J Gynaecol Obstet. (2015) 131(3):255–9. doi: 10.1016/j.ijgo.2015.06.035
88. Orobaton N, Austin AM, Abegunde D, Ibrahim M, Mohammed Z, Abdul-Azeez J, et al. Scaling-up the use of sulfadoxine-pyrimethamine for the preventive treatment of malaria in pregnancy: results and lessons on scalability, costs and programme impact from three local government areas in Sokoto State, Nigeria. Malar J. (2016) 15(1):1–24. doi: 10.1186/s12936-016-1578-x
89. Wastnedge E, Waters D, Murray SR, McGowan B, Chipeta E, Nyondo-Mipando AL, et al. Interventions to reduce preterm birth and stillbirth, and improve outcomes for babies born preterm in low-and middle-income countries: a systematic review. J Glob Health. (2021) 11:2021. doi: 10.7189/jogh.11.04050
90. Berger R, Rath W, Abele H, Garnier Y, Kuon RJ, Maul H. Reducing the risk of preterm birth by ambulatory risk factor management. Dtsch Arztebl Int. (2019) 116(50):858. doi: 10.3238/arztebl.2019.0858
91. Sendeku FW, Beyene FY, Tesfu AA, Bante SA, Azeze GG. Preterm birth and its associated factors in Ethiopia: a systematic review and meta-analysis. Afr Health Sci. (2021) 21(3):1321–33. doi: 10.4314/ahs.v21i3.43
92. Fetene G, Tesfaye T, Negesse Y, Dulla D. Factors associated with preterm birth among mothers who gave birth at public hospitals in Sidama regional state, Southeast Ethiopia: unmatched case-control study. PLoS One. (2022) 17(4):e0265594. doi: 10.1371/journal.pone.0265594
93. Abadiga M, Wakuma B, Oluma A, Fekadu G, Hiko N, Mosisa G. Determinants of preterm birth among women delivered in public hospitals of Western Ethiopia, 2020: unmatched case-control study. PLoS One. (2021) 16(1):e0245825. doi: 10.1371/journal.pone.0245825
94. Muhe LM, McClure EM, Nigussie AK, Mekasha A, Worku B, Worku A, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): a prospective, cross-sectional, observational study. Lancet Glob Health. (2019) 7(8):e1130–8. doi: 10.1016/S2214-109X(19)30220-7
95. Rahnemaei FA, Fashami MA, Abdi F, Abbasi M. Factors effective in the prevention of preeclampsia: a systematic review. Taiwan J Obstet Gynecol. (2020) 59(2):173–82. doi: 10.1016/j.tjog.2020.01.002
96. Lori JR, Chuey M, Munro-Kramer ML, Ofosu-Darkwah H, Adanu RM. Increasing postpartum family planning uptake through group antenatal care: a longitudinal prospective cohort design. Reprod Health. (2018) 15(1):1–8. doi: 10.1186/s12978-017-0439-6
97. Kalinderi K, Delkos D, Kalinderis M, Athanasiadis A, Kalogiannidis I. Urinary tract infection during pregnancy: current concepts on a common multifaceted problem. J Obstet Gynaecol. (2018) 38(4):448–53. doi: 10.1080/01443615.2017.1370579
98. Abebe F, Mannekulih E, Megerso A, Idris A, Legese T. Determinants of uterine rupture among cases of adama city public and private hospitals, oromia, Ethiopia: a case control study. Reprod Health. (2018) 15(1):1–8. doi: 10.1186/s12978-018-0606-4
99. Workie A, Getachew Y, Temesgen K, Kumar P. Determinants of uterine rupture in dessie referral hospital, northeast Ethiopia, 2016: case control design. Int J Reprod Contracept Obstet Gynecol. (2018) 7(5):1712–7. doi: 10.18203/2320-1770.ijrcog20181900
100. Mengesha MB, Weldegeorges DA, Hailesilassie Y, Werid WM, Weldemariam MG, Welay FT, et al. Determinants of uterine rupture and its management outcomes among mothers who gave birth at public hospitals of tigrai, north Ethiopia: an unmatched case control study. J Pregnancy. (2020) 2020:1–9. doi: 10.1155/2020/8878037
101. Dadi TL, Yarinbab TE. Estimates of uterine rupture bad outcomes using propensity score and determinants of uterine rupture in Mizan-Tepi University teaching hospital: case control study. J Pregnancy. (2020) 2020:1–9. doi: 10.1155/2017/6517015
102. Desta M, Amha H, Anteneh Bishaw K, Adane F, Assemie MA, Kibret GD, et al. Prevalence and predictors of uterine rupture among Ethiopian women: a systematic review and meta-analysis. PLoS One. (2020) 15(11):e0240675. doi: 10.1371/journal.pone.0240675
103. Alemu AA, Bitew MS, Gelaw KA, Zeleke LB, Kassa GM. Prevalence and determinants of uterine rupture in Ethiopia: a systematic review and meta-analysis. Sci Rep. (2020) 10(1):1–11. doi: 10.1038/s41598-020-74477-z
104. Astatikie G, Limenih MA, Kebede M. Maternal and fetal outcomes of uterine rupture and factors associated with maternal death secondary to uterine rupture. BMC Pregnancy Childbirth. (2017) 17(1):1–9. doi: 10.1186/s12884-017-1302-z
105. Tesema O, Tilahun T, Kejela G. Determinants of uterine rupture at public hospitals of western Ethiopia: a case–control study. SAGE Open Med. (2022) 10:20503121221092643. doi: 10.1177/20503121221092643
106. Assefa EM, Janbo A, Ghiwot Y. Comparative analysis of cesarean section using the robson's ten-group classification system (RTCGS) in private and public hospitals, Addis Ababa, Ethiopia. Obstet Gynecol. (2021) 4:081–91. doi: 10.1186/2Fs12884-020-03474-x
107. Tarekegn M, Wubshet M, Atenafu A, Derso T, Woretaw A. Antenatal care and mothers’ education improved iron-folic acid adherence at Denbiya district health centers, Northwest Ethiopia: using pills count method. Arch Public Health. (2019) 77(1):1–6. doi: 10.1186/s13690-019-0356-y
108. Hubena Z, Workneh A, Siraneh Y. Prevalence and outcome of operative vaginal delivery among mothers who gave birth at Jimma University Medical Center, Southwest Ethiopia. J Pregnancy. (2018) 2018:1–12. doi: 10.1155/2018/7423475
109. Tamirat T, Abute L. Predictors of non-spontaneous vaginal delivery among mothers who gave birth in Wachemo University Specialized Hospital, Hossana, Ethiopia, 2021. Patient Relat Outcome Meas. (2022) 13:9. doi: 10.2147/PROM.S343866
110. Weldamanuel T, Mohammed A, Leta M. Outcomes of operative vaginal delivery and associated factors at Aksum Saint Mary Hospital, Tigray, Northern Ethiopia. East Afr Health Biomed Sci. (2020) 4(1):25–34.
111. Sewunet H, Abebe N, Zeleke LB, Aynalem BY, Alemu AA. Immediate unfavorable birth outcomes and determinants of operative vaginal delivery among mothers delivered in East Gojjam Zone Public Hospitals, North West Ethiopia: a cross-sectional study. PLoS One. (2022) 17(6):e0268782. doi: 10.1371/journal.pone.0268782
112. Abebaw Y, Kebede E. Trends in operative delivery in tikur anbessa specialized hospital, Addis Ababa, Ethiopia: a 5 years’ retrospective review. Ethiop J Health Sci. (2021) 31(6):1199–1206. doi: 10.4314/2Fejhs.v31i6.15
113. Harrison MS, Teshome B, Liyew T, Kirub E, Jimenez-Zambrano A, Muldrow M, et al. Prevalence of and characteristics associated with operative vaginal birth at Mizan-Tepi University teaching hospital. Int Health. (2021) 13(2):199–204. doi: 10.1093/inthealth/ihaa024
114. Ekanem PE, Nyaga AC, Tsegay N, Ebuy H, Imbusi EA, Ekanem R, et al. Determinants of cerebral palsy in pediatric patients in Northern Ethiopia: a hospital-based study. Neurol Res Int. (2021) 2021:1–10. doi: 10.1155/2021/9993912
115. Tsegaye B, Shudura E, Yoseph A, Tamiso A. Predictors of skilled maternal health services utilizations: a case of rural women in Ethiopia. PLoS One. (2021) 16(2):e0246237. doi: 10.1371/journal.pone.0246237
116. Terefe N, Nigussie A, Tadele A. Prevalence of obstetric danger signs during pregnancy and associated factors among mothers in Shashemene Rural District, South Ethiopia. J Pregnancy. (2020) 2020:1–7. doi: 10.1155/2020/6153146
117. Ayalew Tiruneh G, Melkamu Asaye M, Solomon AA, Tiruneh Arega D. Delays during emergency obstetric care and their determinants among mothers who gave birth in south gondar zone hospitals, Ethiopia. A cross-sectional study design. Glob Health Action. (2021) 14(1):1953242. doi: 10.1080/16549716.2021.1953242
118. Awel S, Bagilkar VV, Fekecha B. Delay in seeking institutional delivery service utilization and associated factors among mothers attending Jimma Medical Center, Jimma, Ethiopia. Risk Manag Healthc Policy. (2021) 14:1255. doi: 10.2147/RMHP.S295683
119. Mgawadere F, Unkels R, Kazembe A, van den Broek N. Factors associated with maternal mortality in Malawi: application of the three delays model. BMC Pregnancy Childbirth. (2017) 17(1):1–9. doi: 10.1186/s12884-017-1406-5
120. Limenih MA, Belay HG, Tassew HA. Birth preparedness, readiness planning and associated factors among mothers in Farta district, Ethiopia: a cross-sectional study. BMC Pregnancy Childbirth. (2019) 19(1):1–10. doi: 10.1186/s12884-019-2325-4
121. Tiruneh GA, Arega DT, Kassa BG, Bishaw KA. Delay in making decision to seek care on institutional delivery and associated factors among postpartum mothers in South Gondar zone hospitals, 2020: a cross-sectional study. Heliyon. (2022) 8(3):e09056. doi: 10.1016/j.heliyon.2022.e09056
Keywords: optimal ANC care, uterine rupture, delay one, operative vaginal delivery, intrauterine life, Ethiopia
Citation: Tesfay N, Hailu G and Woldeyohannes F (2023) Effect of optimal antenatal care on maternal and perinatal health in Ethiopia. Front. Pediatr. 11:1120979. doi: 10.3389/fped.2023.1120979
Received: 10 December 2022; Accepted: 9 January 2023;
Published: 7 February 2023.
Edited by:
Suksham Jain, Government Medical College and Hospital, IndiaReviewed by:
Adaobi Solarin, Lagos State University Teaching Hospital, NigeriaDewi Rokhmah, University of Jember, Indonesia
© 2023 Tesfay, Hailu and Woldeyohannes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neamin Tesfay bmVhbWludGVzZmF5ZTIxMjNAZ21haWwuY29t
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Abbreviations ATT, Average Treatment effects of the Treated; ENAP, Every Newborn Action Plan; GDW, Gestational Diabetics Mellitus; MDSR, Maternal Death Surveillance and Response; OVD, Operative Vaginal Delivery; PDSR, Perinatal Death Surveillance and Response; PIH, Pregnancy Induced Hypertension; PWPSM, Preferential Within Propensity Score Matching; SDG, Sustainable Development Goal.
 Neamin Tesfay
Neamin Tesfay Girmay Hailu1
Girmay Hailu1 Fitsum Woldeyohannes
Fitsum Woldeyohannes