- 1Department of Cardiovascular Medicine, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, China International Science and Technology Cooperation Base of Child Development and Critical Disorders, Children's Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Pediatrics, Sichuan Mianyang 404 Hospital, Mianyang, China
- 3Chongqing Key Laboratory of Pediatrics, Chongqing, China
Kawasaki disease (KD) is an acute, self-limited vasculitis, and the etiology is still unclear. Coronary arterial lesions (CALs) are a major complication of KD. Excessive inflammation and immunologic abnormities are involved in the pathogenesis of KD and CALs. Annexin A3 (ANXA3) plays crucial roles in cell migration and differentiation, inflammation, cardiovascular and membrane metabolic diseases. The purpose of this study was to investigate the effect of ANXA3 on the pathogenesis of KD and CALs. There were 109 children with KD in the KD group [which was divided into two groups: 67 patients with CALs in the KD-CAL group, and 42 patients with noncoronary arterial lesions (NCALs) in the KD-NCAL group] and 58 healthy children in the control (HC) group. Clinical and laboratory data were retrospectively collected from all patients with KD. The serum concentration of ANXA3 was measured by enzyme-linked immunosorbent assays (ELISAs). Serum ANXA3 levels were higher in the KD group than in the HC group (P < 0.05). There was a higher concentration of serum ANXA3 in the KD-CAL group than in the KD-NCAL group (P < 0.05). Neutrophil cell counts and serum ANXA3 levels were higher in the KD group than in the HC group (P < 0.05) and quickly decreased when the patients were treated with IVIG after 7 days of illness. Platelet (PLT) counts and ANXA3 levels concurrently exhibited significant increases 7 days after onset. Furthermore, ANXA3 levels were positively correlated with lymphocyte and PLT counts in the KD and KD-CAL groups. ANXA3 may be involved in the pathogenesis of KD and CALs.
Introduction
Kawasaki disease (KD), also known as mucocutaneous lymph node syndrome, is classified as acute systemic vasculitis, and approximately 80% of affected patients are children under 5 years old (1, 2). Thrombocytosis has been reported in KD patients at 7 days of illness (3, 4). Coronary arterial lesions (CALs) are a major complication of KD (5). Platelets play an important role in injuring the vessel wall by affecting leukocyte functions and recruitment (6, 7). Although a standard regimen with intravenous immunoglobulin (IVIG) and aspirin is used, 15%–25% of patients still have CALs, including coronary arterial aneurysm (CAA), stenosis, myocardial infarction, ischemia, and even sudden death. Furthermore, KD has now become the main cause of acquired heart disease in children in developed countries (3, 8–10). Nonetheless, the pathogenesis of this disease remains largely unclear, and it is well known that an unknown stimulus induces the immune-mediated inflammatory cascade within the innate and adaptive immune systems of genetically susceptible children (11, 12). Due to the lack of a specific diagnostic test, the diagnosis of KD relies on clinical criteria. IVIG, acetylsalicylic acid (ASA), corticosteroids, infliximab, and etanercept are used in the treatment of KD in the acute phase to reduce inflammation and arterial damage. IVIG can prevent the development of CAA; however, there is still a 1% risk of giant aneurysm and a 5% risk of CAA (13, 14). It is necessary to find potential biomarkers for the early diagnosis and timely treatment of KD.
Annexins constitute a group of Ca2+-dependent soluble and hydrophilic proteins that reversibly bind to negatively charged phospholipid head groups (15). Studies have shown that annexins are involved in many cellular functions, such as proliferation, differentiation, apoptosis, and signal transduction, and play a key role in the regulation of the inflammatory response and blood anticoagulation properties (16). Annexin A3 (ANXA3), also known as 35-alpha calcimedin, lipocortin III or placental anticoagulant protein III, is a member of the annexin family (17). Although the physiological functions of ANXA3 are still not entirely clear, an increasing number of studies have suggested that ANXA3 not only regulates inflammation, migration and differentiation of cells but also promotes angiogenesis, stimulating neutrophil aggregation (18–21). Furthermore, previous studies reported that ANXA3 is involved in tumor invasion, intracranial aneurysm, the immunopathology of sepsis, and cardiovascular disease (22–25). Whether ANXA3 participates in regulating abnormal inflammation and the immunologic response in KD and CALs has not been reported thus far.
Methods
Subjects and data collection
Participants were recruited from the Children's Hospital of Chongqing Medical University, Chongqing, China. All of the KD cases strictly met the criteria proposed by the Scientific Committee of the Japanese Circulation Society (26, 27). Healthy children were recruited from physical examinations. There were 109 KD patients (76 males and 33 females; average age, 3.334 ± 2.083 years old) in the KD group and 58 healthy children (38 males and 20 females; average age, 3.443 ± 2.363 years old) in the control group (HC).
The diameter and z value of coronary arteries were assessed in the acute phase of KD by echocardiography. According to the z value of the coronary artery, KD patients were divided into two groups: the KD with CALs group (KD-CALs, z value >2.0, n = 67) and the KD without CALs group (KD-NCALs, z value <2.0, n = 42).
All blood samples were obtained from KD patients during the first week of onset before treatment with anticoagulants and intravenous immunoglobulin (IVIG). Then, the samples were centrifuged at 3000 rpm for 10 min, and the serum was stored at −80°C.
All experiments complied with the ethics committee from Chongqing Medical University, and written informed consent for all participants was provided before the study by their parents or legal guardians.
Measurement of serum ANXA3 and clinical parameters
Serum concentrations of ANXA3 were quantified with a human ANXA3 ELISA kit (Cusabio, Wuhan, China) according to the manufacturer's instructions. The intra-assay and interassay precision were CV% < 8% and CV% < 10%, respectively.
The serum concentrations of ANXA3 were tested in KD and HC groups. The important clinical data of all the subjects with KD, including the duration days with fever and the time point of IVIG administration, were recorded. General laboratory data of all the subjects in KD were examined, including white blood cell counts (WBC), platelet counts (PLT), neutrophil counts, lymphocyte counts, alanine transaminase (ALT) levels, aspartate transaminase (AST) levels, albumin (ALB) levels, procalcitonin (PCT) levels, C-reactive protein (CRP) levels, the erythrocyte sedimentation rate (ESR) levels, and blood coagulation parameters such as thrombin time (TT), prothrombin time (PT), activated partial thromboplastin time (APTT) and plasma fibrinogen (FIB) levels. Eighty-eight KD patients had creatine kinase isoenzyme (CK-MB) results, 70 KD patients had brain natriuretic peptide (BNP) results, and 48 KD patients had cytokine results, including the levels of IL-6, IL-10, TNF-α, and INF-γ. Moreover, ANXA3 were tested in 15 paired KD-CAL patients both before and after day 7 disease onset.
Statistical analysis
All data in this study are described as the mean ± standard deviation (SED) or median and interquartile range (IQR). Statistical significance was defined as a P value (2-tailed) < 0.05. Student's t test was used to compare data from two cohorts that followed a normal distribution, while the Mann–Whitney U test was used to compare nonnormally distributed data. The Wilcoxon signed ranks test was used to test paired samples for which data failed to follow a normal distribution. Correlations between serum ANXA3 levels and various laboratory parameters were assessed via Spearman rank correlation analysis.
SPSS 23.0 was used to analyze all statistical results. GraphPad Prism 8.0 and Adobe Illustrator 2021 were used to create the figures.
Results
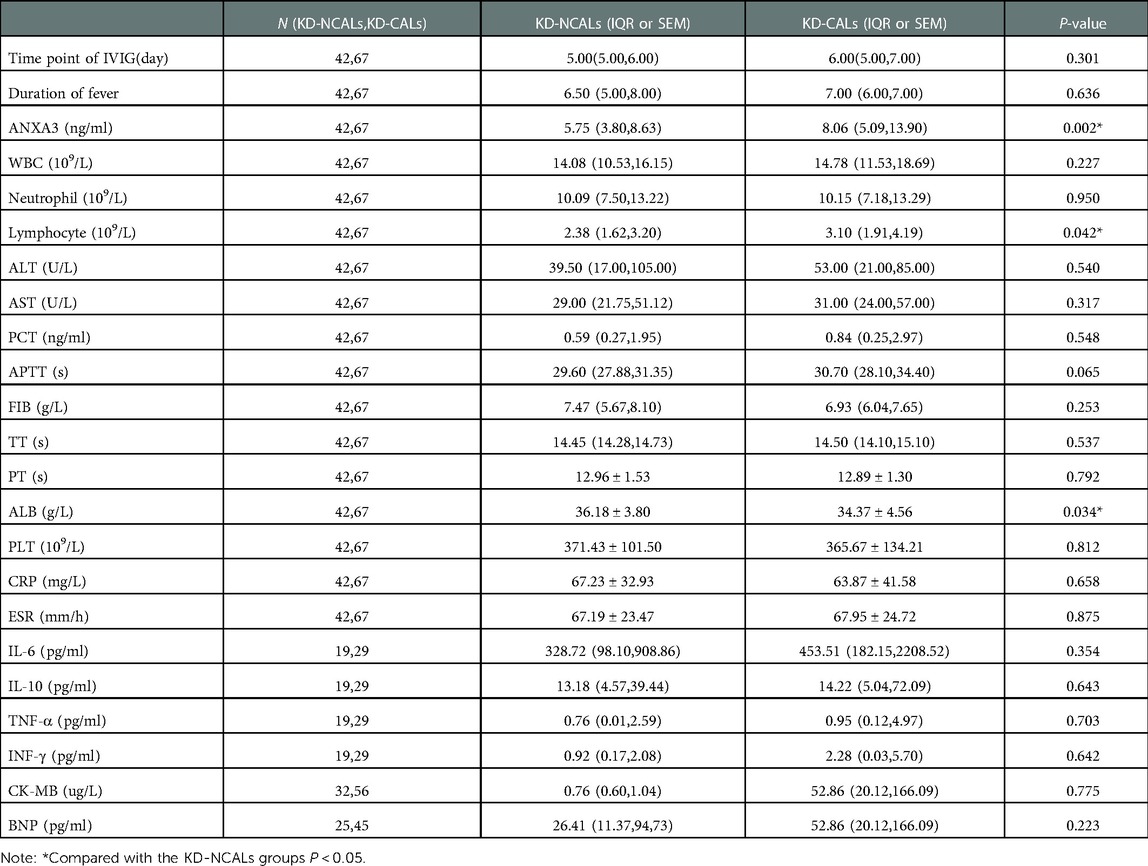
ANXA3 levels in the HC group and KD group
There were no differences in age or sex between the KD and HC groups (P > 0.05). The levels of serum ANXA3 in the KD group [6.617 pg/ml (4.045 pg/ml, 13.196 pg/ml) n = 109] were significantly higher than those in the HC group [1.775 pg/ml (0.866 pg/ml, 2.657 pg/ml), n = 58] (P < 0.001). In addition, there were significant differences in the serum levels of ANXA3 among the KD-CAL group, KD-NCAL group and HC group (P < 0.001). The serum levels of ANXA3 in the KD-NCAL group [5.754 ng/ml (3.796 pg/ml, 8.630 pg/ml), n = 42] were higher than those in the HC group [1.775 pg/ml (0.866 pg/ml, 2.657 pg/ml), n = 58] (P < 0.001) but lower than those in the KD-CAL group [8.060 ng/ml (5.089 pg/ml, 13.896 pg/ml), n = 67] (P = 0.033) (Figure 1).
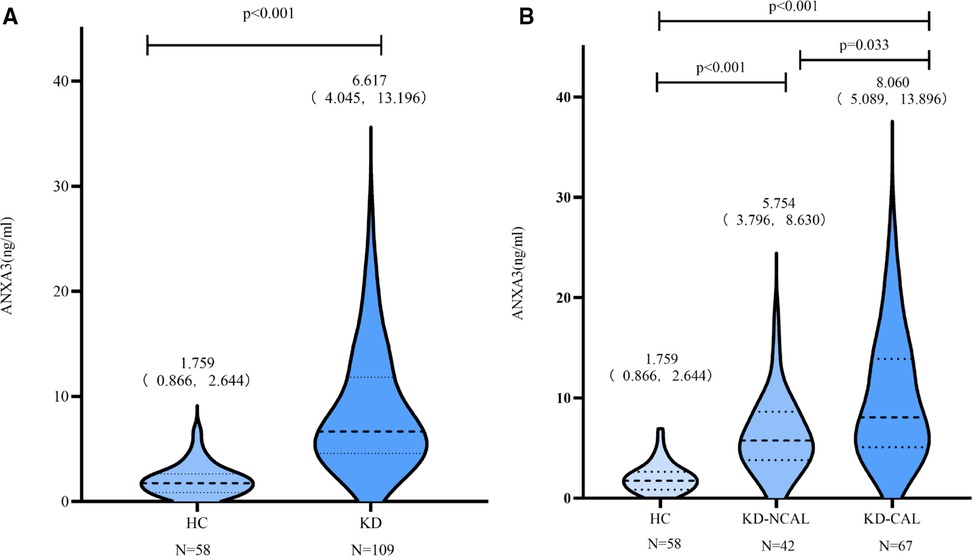
Figure 1. Serum levels of ANXA3 in the HC and KD groups A: ANXA3 levels in the HC and KD groups. B: ANXA3 levels in the HC, KD-NCALs and KD-CALs groups. Serum levels of ANXA3 was significant difference in the three groups (P < 0.001). The serum levels in HC group were significantly lower than that in KD-NCALs group and KD-CALs group (P < 0.001 both). The serum levels in KD-CALs group were significantly higher than that in KD-NCALs (P = 0.033). ANXA3: Annexin A3, HC: healthy children, KD: Kawasaki disease, KD-NCALs: Kawasaki disease non coronal arterial lesions, KD-CALs: Kawasaki disease with coronal arterial lesions.
Neutrophil cell counts in the HC and KD groups
The neutrophil cell counts were significantly elevated before day 7 of illness in the KD group [10.110 × 109/L (7.240 × 109/L, 13.410 × 109/L), n = 109] in comparison with the HC group [2.975 × 109/L (2.502 × 109/L, 4.080 × 109/L), n = 58] (P < 0.001). The neutrophil cell counts were markedly decreased when the patients were treated with IVIG after 7 days of illness [4.205 × 109/L (2.450 × 109/L, 6.168 × 109/L), n = 109] (Figure 2).
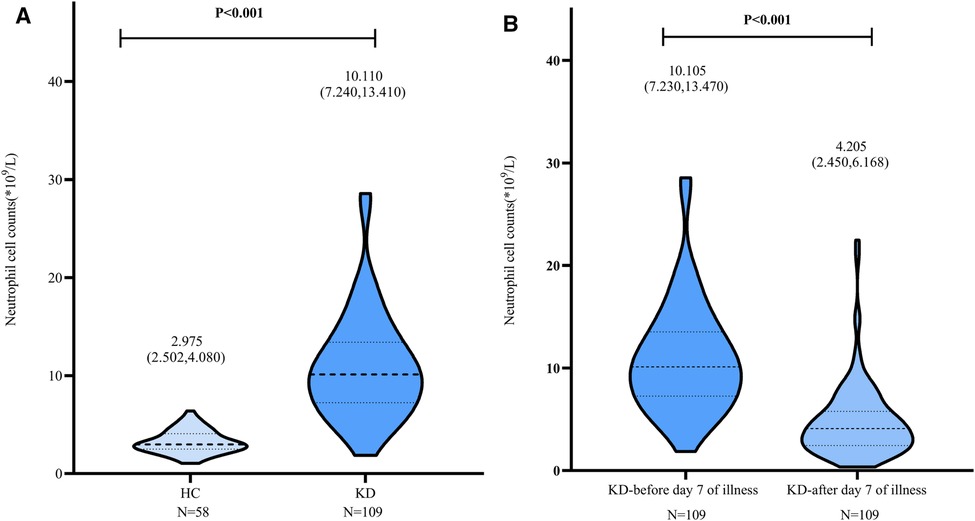
Figure 2. Neutrophil cell counts in HC group and KD group. A: The neutrophil cell counts in HC and KD groups. B: In KD group, the neutrophil cell counts decreased after day 7 of illness when treated with IVIG. IVIG: intravenous immunoglobulin.
Correlation between ANXA3 levels and clinical parameters
There were distinctly positive correlations between ANXA3 and WBC (r = 0.227, P = 0.018) in the KD group and significant positive correlations with the time point of IVIG (day) (r = 0.242, P = 0.049) in the KD-CAL group. In addition, lymphocyte and PLT counts showed significant positive correlations with ANXA3 in the KD and KD-CAL groups (r = 0.226, P = 0.018; r = 0.276, P = 0.004 and r = 0.349, P = 0.004; r = 0.375, P = 0.002, respectively) (Table 1).
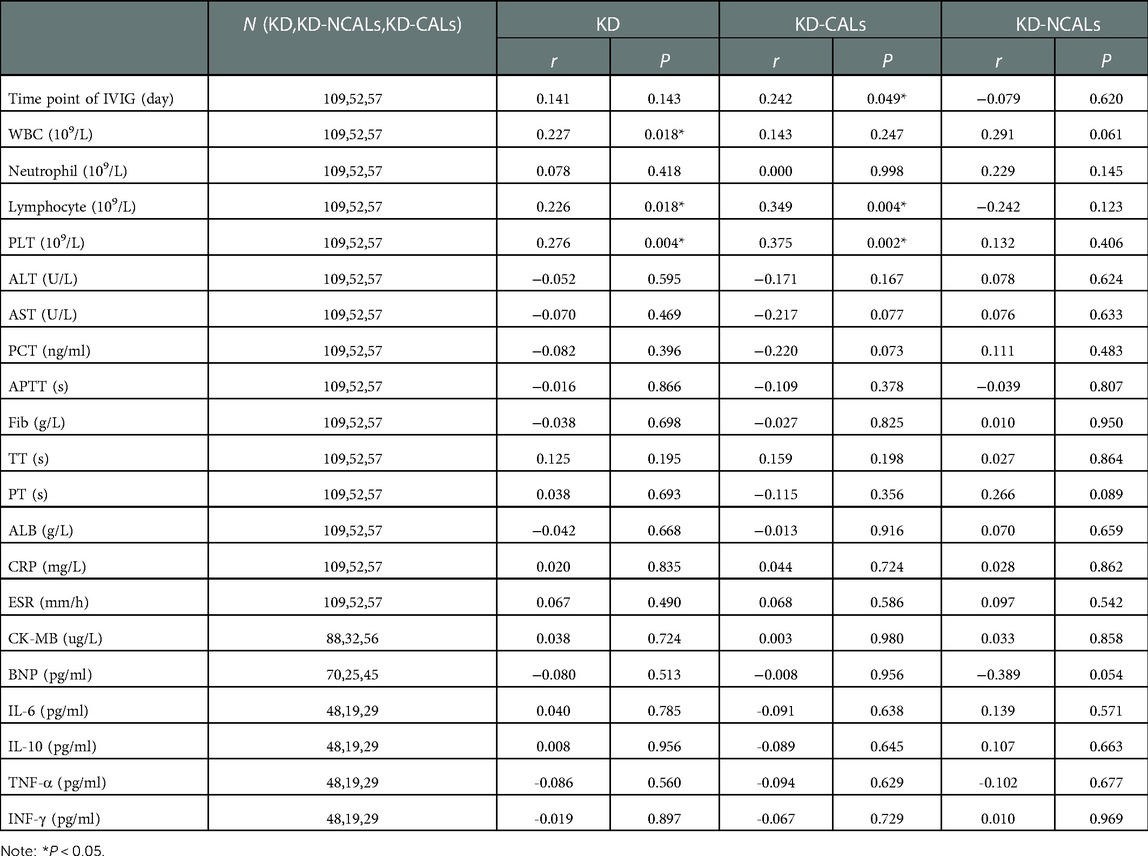
Table 1. Correlations between serum ANXA3 and clinical parameters in the KD, KD-CALs and KD-NCALs groups.
The relationship between serum ANXA3 and PLT counts in KD-CAL patients
Compared with the initial data obtained, the serum levels of ANXA3 and PLT counts showed a concurrent increase at day 7 after onset of illness in KD-CAL patients (Figure 3).
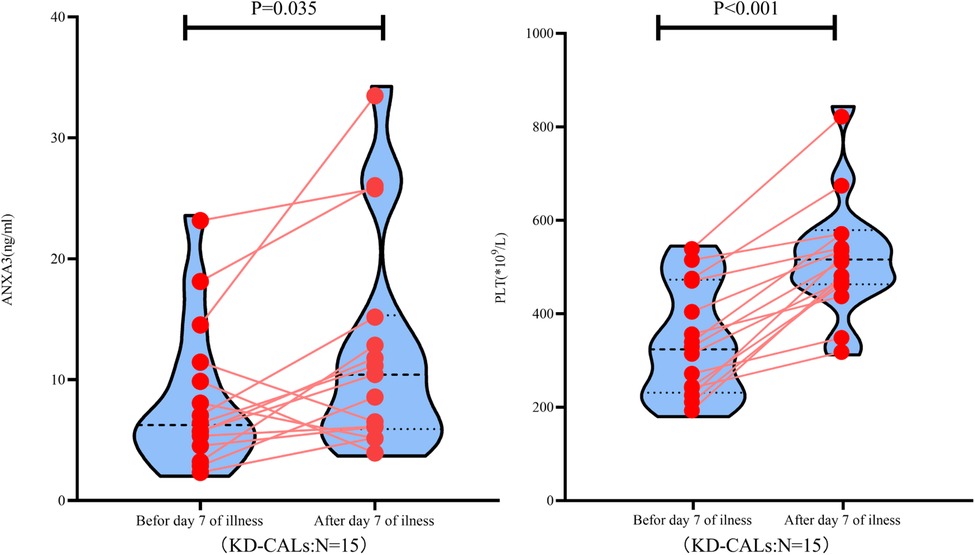
Figure 3. ANXA3 levels and PLT counts in paired KD patients (n = 15) both ANXA3 levels and PLT counts increased after day 7 of illness. ANXA3: Annexin A3, PLT: platelet counts, KD-CALs: Kawasaki disease with coronal arterial lesions.
The relationship between ANXA3 and other clinical and laboratory parameters in KD
There were significant differences in serum ANXA3, lymphocyte counts and serum albumin between the KD-NCAL and KD-CAL groups (P < 0.05) (Table 2).
The effect of ANXA3 in predicting KD and KD-CALs
The AUC values of the KD group and KD-CAL group were 0.930 and 0.679, respectively (95% confidence intervals: 0.892 to 0.969 and 0.578 to 0.779, respectively; P < 0.001 and P = 0.002, respectively). The cutoff values were 3.033 pg/ml and 9.846 pg/ml, respectively (Figure 4).
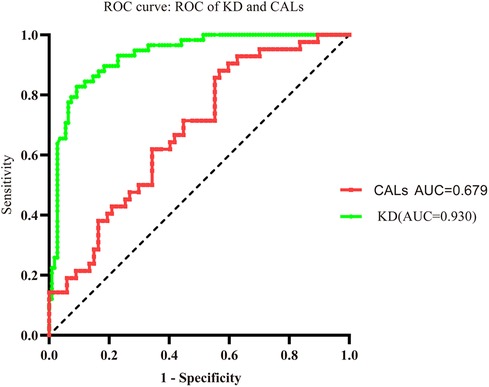
Figure 4. Evaluation of serum ANXA3 for diagnosis of patients with KD and KD-CALs. Receiver operating characteristic (ROC) curves of ANXA3 in KD and KD-CALs groups.
Discussion
KD as the main cause of acquired cardiovascular disease in children. It is presumably triggered by an infection-related abnormal inflammatory reaction in genetically susceptible children. The abnormal response of immune cells and vascular endothelial dysfunction are important causes of CALs in patients with KD (28). Finding a biomarker for the early diagnosis of KD has attracted much attention due to the lack of a “gold standard” for diagnosis. In our study, we examined the serum levels of ANXA3 in all subjects. The results showed that the serum levels of ANXA3 were increased in the KD group and were higher in the KD-CAL group than in the KD-NCAL group. Thus, we may predict that ANXA3 may be involved in the pathogenesis of KD and CALs.
First, studies showed that ANXA3 is expressed by neutrophils and monocytes, particularly in neutrophils (18, 29). ANXA3 consists of 323 amino acid residues and is encoded by a water-soluble protein-coding gene located on chromosome 4q13-q22 in the human genome. There are four conserved domains (I–IV) forming a circular structure. Each of the domains consists of five α-helices (helices A-E) and forms the C-terminal region of ANXA3. Two tryptophan residues are crucial for the interaction of calcium ions and phospholipid membranes in ANXA3 and are involved in various physiological events (30, 31). ANXA3 has been correlated with tumorigenesis, cell signal transduction, apoptosis, anticoagulation, and angiogenesis (21, 31, 32). Studies have examined the role of ANXA3 in immunity and inflammation and shown that ANXA3 promotes membrane-membrane contact in neutrophils, and furthermore, phagocytic vesicle membranes fuse with the membranes of neutrophil granules. Imaging data also demonstrated that in neutrophils, ANXA3 was redistributed to phagosomes (33, 34). In KD, neutrophils have been suggested to be among the first cells to invade the arterial wall and are the most important cells that participate in the first stage of arteritis (2, 35, 36). In our study, the serum levels of ANXA3 and neutrophil cell counts were increased in the acute phase of KD. ANXA3 is particularly abundant in neutrophils, accounting for ∼1% of all intracellular proteins. Increased serum levels of ANXA3 7 days before the onset of illness may be associated with neutrophil infiltration in KD. It can be speculated that ANXA3 and neutrophils may take part in the process of coronary arterial lesions in KD. Then, an increasing number of studies have confirmed the function of platelets not only in the regulation of hemostasis but also in immunity and inflammation (37, 38). Proteomic analysis has indicated that platelets can secrete more than 300 proteins to regulate inflammation and the immune response. Moreover, Joaquim, H.P.G et al. (39) reported that ANXA3 was expressed in platelets in schizophrenia patients. Additionally, Suades, R et al. (40) showed that Annexin family members were present in extracellular vesicles released from platelets. Laboratory tests confirmed that thrombocytosis occurs 7 days after illness and is a typical feature of the subacute phase of KD (2, 3). Platelets can act as the first responders in the recognition of pathogens. They rapidly generate host-defense peptides and then recruit and boost leukocytes to promote T cell-B cell crosstalk, which is necessary in adaptive immunity (41–43). In addition, platelets play an important role in injuring the vessel wall (4, 44). This study also demonstrated that there was a marked correlation between ANXA3 levels and PLTs, not only in the levels but also in the timing of increase during disease progression among KD patients. In addition, ANXA3 had a positive correlation with PLTs in the KD group and KD-CAL group. This suggests that ANXA3 and PLTs may be involved together in the pathogenesis of KD.
In this study, the higher level of serum ANXA3 was accompanied by an increase in the neutrophil cell count, and when KD patients were treated with IVIG, the neutrophil cell count decreased. However, serum ANXA3 remained at a higher level, so the higher levels of serum ANXA3 may come from PLTs. In addition, Meng H et al. demonstrated that the silencing of ANXA3 promotes the repair and healing of myocardial tissue through PI3K/Akt signaling pathway activation in rats with acute myocardial infarction (45). The ROC curves of ANXA3 showed that ANXA3 predicted KD and KD-CALs. Accordingly, we postulated that ANXA3 may be the intermediary agent between the neutrophils and PLTs involved in the pathogenesis of KD and further in the development of CALs. The pathogenesis of ANXA3 involvement in neutrophils and PLTs, which injure vascular endothelial cells in KD and CALs needs further study.
In conclusion, this study first verified that the serum concentration of ANXA3 was markedly higher in the KD group than in the HC group and higher in the KD-CAL group than in the KD-NCAL group. There was a positive relationship between ANXA3 and WBCs, lymphocytes and PLTs. The timing of the ANXA3 concentration increase coincides with neutrophil and PLT counts. Accordingly, we postulate that ANXA3 may be an intermediary agent between neutrophils and PLTs in the pathogenesis of KD and further in the development of CALs in different stages of KD. Therefore, ANXA3 may be a potential target in the prevention of KD and KD-CALs.
This study had limitations. One is due to the small number of KD samples, further studies are need to demonstrated the signaling pathway of ANXA3, neutrophil and PLTs that is involved in the pathogenesis of KD and CALs. The other is all the patients were enrolled from in a single center, multicenter experimental evidence were needed in the further.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Children's Hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
ML has a substantial contribution to the conceive and design this study, investigate, interpretation data and drafte the manuscript. DL: analysis data and interpretation data. FJ: interpretation data. RL: design this study and revise the article. QY: revise the article, make critical revision of the manuscript for key intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by The General Program of Natural Science Funding of Chongqing (grant number. cstc2021jcyj-msxmX0419).
Acknowledgments
We appreciate the recruited children for their kindness to support the development of medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen S, Dong Y, Kiuchi MG, Wang J, Li R, Ling Z, et al. Coronary artery complication in Kawasaki disease and the importance of early intervention: a systematic review and meta-analysis. JAMA Pediatr. (2016) 170(12):1156–63. doi: 10.1001/jamapediatrics.2016.2055
2. Newburger JW, Takahashi M, Burns JC. Kawasaki disease. J Am Coll Cardiol. (2016) 67(14):1738–49. doi: 10.1016/j.jacc.2015.12.073
3. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135(17):e927–999. doi: 10.1161/CIR.0000000000000484
4. Arora K, Guleria S, Jindal AK, Rawat A, Singh S. Platelets in Kawasaki disease: is this only a numbers game or something beyond? Genes Dis. (2020) 7(1):62–6. doi: 10.1016/j.gendis.2019.09.003
5. Rivas M N, Arditi M. Kawasaki disease: pathophysiology and insights from mouse models. Nat Rev Rheumatol. (2020) 16(7):391–405. doi: 10.1038/s41584-020-0426-0
6. Schulz C, Schäfer A, Stolla M, Kerstan S, Lorenz M, von Brühl ML, et al. Chemokine fractalkine mediates leukocyte recruitment to inflammatory endothelial cells in flowing whole blood: a critical role for P-selectin expressed on activated platelets. Circulation. (2007) 116(7):764–73. doi: 10.1161/CIRCULATIONAHA.107.695189
7. Sreeramkumar V, Adrover JM, Ballesteros I, Cuartero MI, Rossaint J, Bilbao I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. (2014) 346(6214):1234–8. doi: 10.1126/science.1256478
8. Noto N, Kamiyama H, Karasawa K, Ayusawa M, Sumitomo N, Okada T, et al. Long-term prognostic impact of dobutamine stress echocardiography in patients with Kawasaki disease and coronary artery lesions: a 15-year follow-up study. J Am Coll Cardiol. (2014) 63(4):337–44. doi: 10.1016/j.jacc.2013.09.021
9. Harnden A, Tulloh R, Burgner D. Kawasaki disease. Br Med J (Clin Res Ed). (2014) 349:g5336. doi: 10.1136/bmj.g5336
10. Burns JC, Glodé MP. Kawasaki syndrome. Lancet. (2004) 364(9433):533–44. doi: 10.1016/S0140-6736(04)16814-1
11. Ae R, Makino N, Kosami K, Kuwabara M, Matsubara Y, Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with Kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr. (2020) 225:23–29.e2. doi: 10.1016/j.jpeds.2020.05.034
12. Rife E, Gedalia A. Kawasaki disease: an update. Curr Rheumatol Rep. (2020) 22(10):75. doi: 10.1007/s11926-020-00941-4
13. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose [; meta-analysis]. J Pediatr. (1997) 131(6):888–93. doi: 10.1016/S0022-3476(97)70038-6
14. Ramphul K, Mejias SG. Kawasaki disease: a comprehensive review. Arch Med Sci Atheroscler Dis. (2018 2018) 3:e41–45. doi: 10.5114/amsad.2018.74522
15. Boye TL, Jeppesen JC, Maeda K, Pezeshkian W, Solovyeva V, Nylandsted J, et al. Annexins induce curvature on free-edge membranes displaying distinct morphologies. Sci Rep. (2018) 8(1):10309. doi: 10.1038/s41598-018-28481-z
16. Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. (2002) 82(2):331–71. doi: 10.1152/physrev.00030.2001
17. Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2 + signalling to membrane dynamics. Nat Rev Mol Cell Biol. (2005) 6(6):449–61. doi: 10.1038/nrm1661
18. Le Cabec V, Maridonneau-Parini I. Annexin 3 is associated with cytoplasmic granules in neutrophils and monocytes and translocates to the plasma membrane in activated cells. Biochem J. (1994) 303(Pt 2):481–7. doi: 10.1042/bj3030481
19. Mohr T, Haudek-Prinz V, Slany A, Grillari J, Micksche M, Gerner C. Proteome profiling in IL-1β and VEGF-activated human umbilical vein endothelial cells delineates the interlink between inflammation and angiogenesis. PLoS One. (2017) 12(6):e0179065. doi: 10.1371/journal.pone.0179065
20. Faugaret D, Chouinard FC, Harbour D, El azreq MA, Bourgoin SG. An essential role for phospholipase D in the recruitment of vesicle amine transport protein-1 to membranes in human neutrophils. Biochem Pharmacol. (2011) 81(1):144–56. doi: 10.1016/j.bcp.2010.09.014
21. Park JE, Lee DH, Lee JA, Park SG, Kim NS, Park BC, et al. Annexin A3 is a potential angiogenic mediator. Biochem Biophys Res Commun. (2005) 337(4):1283–7. doi: 10.1016/j.bbrc.2005.10.004
22. Guo C, Li N, Dong C, Wang L, Li Z, Liu Q, et al. 33-kDa ANXA3 isoform contributes to hepatocarcinogenesis via modulating ERK, PI3K/Akt-HIF and intrinsic apoptosis pathways. J Adv Res. (2021) 30:85–102. doi: 10.1016/j.jare.2020.11.003
23. Jin YF, Huang YT, Chen PF. ANXA3 Deletion inhibits the resistance of lung cancer cells to oxaliplatin. Eur Rev Med Pharmacol Sci. (2020) 24(7):3741–8. doi: 10.26355/eurrev_202004_20838
24. Wan X, Guo D, Zhu Q, Qu R. microRNA-382 suppresses the progression of pancreatic cancer through the PI3K/akt signaling pathway by inhibition of Anxa3. Am J Physiol Gastrointest Liver Physiol. (2020) 319(3):G309–322. doi: 10.1152/ajpgi.00322.2019
25. Wang Y, Wang C, Yang Q, Cheng YL. ANXA3 Silencing ameliorates intracranial aneurysm via inhibition of the JNK signaling pathway. Mol Ther Nucleic Acids. (2019) 17:540–50. doi: 10.1016/j.omtn.2019.06.005
26. Kobayashi T, Ayusawa M, Suzuki H, Abe J, Ito S, Kato T, et al. Revision of diagnostic guidelines for Kawasaki disease (6th revised edition). Pediatr Int. (2020) 62(10):1135–8. doi: 10.1111/ped.14326
27. Ogawa S, Ayusawa M, Fukazawa R, Hamaoka K, Ishii M, Nishigaki K, et al. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013) - digest version [editorial material]. Circ J. (2014) 78(10):2521–62. doi: 10.1253/circj.CJ-66-0096
28. Wang Y, Li T. Advances in understanding Kawasaki disease-related immuno-inflammatory response and vascular endothelial dysfunction. Pediatr Investig. (2022) 6(4):271–9. doi: 10.1002/ped4.12341
29. Ernst JD, Hoye E, Blackwood RA, Jaye D. Purification and characterization of an abundant cytosolic protein from human neutrophils that promotes Ca2(+)-dependent aggregation of isolated specific granules. J Clin Invest. (1990) 85(4):1065–71. doi: 10.1172/JCI114537
30. Toufiq M, Roelands J, Alfaki M, Syed Ahamed Kabeer B, Saadaoui M, Lakshmanan AP, et al. Annexin A3 in sepsis: novel perspectives from an exploration of public transcriptome data. Immunology. (2020) 161(4):291–302. doi: 10.1111/imm.13239
31. Sopkova J, Raguenes-Nicol C, Vincent M, Chevalier A, Lewit-Bentley A, Russo-Marie F, et al. Ca(2+) and membrane binding to annexin 3 modulate the structure and dynamics of its N terminus and domain III. Protein Sci. (2002) 11(7):1613–25. doi: 10.1110/ps.4230102
32. Yang L, Lu P, Yang X, Li K, Qu S. Annexin A3, a calcium-dependent phospholipid-binding protein: implication in cancer. Front Mol Biosci. (2021) 8:716415. doi: 10.3389/fmolb.2021.716415
33. Ernst JD. Annexin III translocates to the periphagosomal region when neutrophils ingest opsonized yeast [; research support, non-U.S. Gov't; research support, U.S. Gov't, P.H.S.]. J Immunol. (1991) 146(9):3110–4. doi: 10.4049/jimmunol.146.9.3110
34. Stoehr SJ, Smolen JE, Suchard SJ. Lipocortins are major substrates for protein kinase C in extracts of human neutrophils [; research support, non-U.S. Gov't; research support, U.S. Gov't, P.H.S.]. J Immunol. (1990) 144(10):3936–45. doi: 10.4049/jimmunol.144.10.3936
35. Kumrah R, Vignesh P, Rawat A, Singh S. Immunogenetics of Kawasaki disease. Clin Rev Allergy Immunol. (2020) 59(1):122–39. doi: 10.1007/s12016-020-08783-9
36. Shulman ST, Rowley AH. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat Rev Rheumatol. (2015) 11(8):475–82. doi: 10.1038/nrrheum.2015.54
37. Semple JW, Italiano JE Jr., Freedman J. Platelets and the immune continuum. Nat Rev Immunol. (2011) 11(4):264–74. doi: 10.1038/nri2956
38. Holinstat M. Normal platelet function. Cancer Metastasis Rev. (2017) 36(2):195–8. doi: 10.1007/s10555-017-9677-x
39. Joaquim HPG, Costa AC, Serpa MH, Talib LL, Gattaz WF. Reduced annexin A3 in schizophrenia. Eur Arch Psychiatry Clin Neurosci. (2020) 270(4):489–94. doi: 10.1007/s00406-019-01048-3
40. Suades R, Padró T, Vilahur G, Badimon L. Platelet-released extracellular vesicles: the effects of thrombin activation. Cell Mol Life Sci. (2022) 79(3):190. doi: 10.1007/s00018-022-04222-4
41. Shiari R, Jari M, Karimi S, Salehpour O, Rahmani K, Hassas Yeganeh M, et al. Relationship between ocular involvement and clinical manifestations, laboratory findings, and coronary artery dilatation in Kawasaki disease. Eye. (2020) 34(10):1883–7. doi: 10.1038/s41433-019-0762-y
42. Zhang C, Zhang X, Shen J, Lu X, Zhang J, Chen S. Changes in peripheral blood neutrophils, lymphocytes and IL-10 in children with Kawasaki disease from different age groups undergoing intravenous immunoglobulin: a retrospective study. Mediators Inflamm. (2020) 2020:5213451. doi: 10.1155/2020/5213451
43. Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol. (2014) 12(6):426–37. doi: 10.1038/nrmicro3269
44. Wang J, Jia X, Meng X, Li Y, Wu W, Zhang X, et al. Annexin A3 may play an important role in ochratoxin-induced malignant transformation of human gastric epithelium cells. Toxicol Lett. (2019) 313:150–8. doi: 10.1016/j.toxlet.2019.07.002
Keywords: Kawasaki disease (KD), coronary arterial lesions, Annexin A3 (ANXA3), platelet count (PLT), pathogenesis
Citation: Li M, Liu D, Jing F, Liu R and Yi Q (2023) The role of Annexin A3 in coronary arterial lesions in children with Kawasaki disease. Front. Pediatr. 11:1111788. doi: 10.3389/fped.2023.1111788
Received: 30 November 2022; Accepted: 18 January 2023;
Published: 14 February 2023.
Edited by:
David Alexander White, Children's Mercy Hospital, United StatesReviewed by:
Lovro Lamot, University of Zagreb, CroatiaAkihiro Nakamura, Kyoto Prefectural University of Medicine, Japan
© 2023 Li, Liu, Jing, Liu and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruixi Liu 270815992@qq.com Qijian Yi qjyi2003@hotmail.com
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Mengling Li1,2,3
Mengling Li1,2,3 Dong Liu
Dong Liu Qijian Yi
Qijian Yi