
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Pediatr. , 25 April 2023
Sec. Pediatric Immunology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1102354
 Nikolay N. Murashkin1,2,3,4*†
Nikolay N. Murashkin1,2,3,4*† Leyla S. Namazova-Baranova4,5
Leyla S. Namazova-Baranova4,5 Svetlana G. Makarova1
Svetlana G. Makarova1 Roman A. Ivanov1
Roman A. Ivanov1 Stepan G. Grigorev6,7
Stepan G. Grigorev6,7 Dmitri V. Fedorov1
Dmitri V. Fedorov1 Eduard T. Ambarchian1,4
Eduard T. Ambarchian1,4 Roman V. Epishev1,4
Roman V. Epishev1,4 Alexander I. Materikin1
Alexander I. Materikin1 Leonid A. Opryatin1
Leonid A. Opryatin1 Alena A. Savelova1
Alena A. Savelova1
Introduction: Epidermal barrier dysfunction in children with atopic dermatitis can cause transcutaneous sensitization to allergens and allergic diseases. We evaluated the effectiveness of an early-intervention algorithm for atopic dermatitis treatment, utilizing pimecrolimus for long-term maintenance therapy, in reducing transcutaneous sensitization in infants.
Method: This was a single-center cohort observational study that enrolled children aged 1-4 months with family history of allergic diseases, moderate-to-severe atopic dermatitis, and sensitization to ≥ 1 of the investigated allergens. Patients who sought medical attention at atopic dermatitis onset (within 10 days) were group 1 “baseline therapy with topical glucocorticoids with subsequent transition to pimecrolimus as maintenance therapy”; patients who sought medical attention later were group 2 “baseline and maintenance therapy with topical glucocorticoids, without subsequent use of pimecrolimus”. Sensitization class and level of allergen-specific immunoglobulin E were determined at baseline, and 6 and 12 months of age. Atopic dermatitis severity was evaluated using the Eczema Area and Severity Index score at baseline and 6, 9 and 12 months of age.
Results: Fifty-six and 52 patients were enrolled in groups 1 and 2, respectively. Compared with group 2, group 1 demonstrated a lower level of sensitization to cow's milk protein, egg white and house dust mite allergen at 6 and 12 months of age, and a more pronounced decrease in atopic dermatitis severity at 6, 9 and 12 months of age. No adverse events occurred.
Discussion: The pimecrolimus-containing algorithm was effective in treating atopic dermatitis and prophylaxis of early forms of allergic diseases in infants.
Trial registration: https://clinicaltrials.gov/ NCT04900948, retrospectively registered, 25 May 2021.
Atopic dermatitis (AD) is a relapsing, chronic inflammatory skin disease characterized by pronounced itching (1). It affects both children and adults and has a deleterious impact on patients’ quality of life (1). In the Russian Federation, the prevalence of AD in different regions is between 6.2–15.5%, and two epidemiological analyses (one conducted between 2005 and 2010 and one conducted between 2010 and 2015) have demonstrated a 1.9-fold increase in AD prevalence during these time periods in the pediatric population of the Russian Federation (2).
AD commonly occurs in early childhood and can become a starting point of the atopic march, which is a typical sequence of development of allergic diseases, such as food allergy (FA), asthma and allergic rhinitis (3). One of the earliest stages in the development of allergic diseases and antigen sensitization is the emergence of a FA against the background of AD (4–6).
It is increasingly recognized that the development and uncontrolled course of AD in young children results in a higher risk of FA owing to epidermal barrier dysfunction and development of transepidermal sensitization, leading to a pathological immune response (7, 8). Normally, upon passage of food proteins through the gastrointestinal tract, antigen sensitization does not develop due to oral tolerance mediated by a tolerogenic population of gastrointestinal dendritic cells and production of regulatory Treg cells by the gastrointestinal tract's immune system (9). However, transcutaneous allergen passage in the case of epidermal barrier dysfunction may lead to sensitization and development of allergic reactions (10). The mechanism of transcutaneous sensitization with development of FA and immediate or delayed hypersensitivity reaction is complex. Its underlying pathogenesis involves penetration of an antigen through the epidermal barrier, leading to production of proinflammatory cytokines and chemokines, a Th2-type immune response and generation of allergen-specific immunoglobulin E (IgE) (11–14).
There is a need for both specific treatment and prophylactic measures to prevent transcutaneous sensitization and formation of FA/other allergic diseases accompanied by epidermal barrier dysfunction in children with AD. Studies suggest that emollients used straight after birth are an effective and safe method for AD prevention, which may reduce occurrence of allergic disease (6), although a recent meta-analysis suggests that this effect may delay rather than prevent AD (15). Furthermore, antihistamine therapy in certain at-risk groups may reduce the probability of asthma in children with AD in whom asthma has an allergic component (16, 17). However, the role of anti-inflammatory agents, including topical calcineurin inhibitors (TCI), in reducing risk of allergic diseases and preventing the atopic march due to transcutaneous sensitization in young children with AD remains unclear (16, 18).
As the pivotal factor in transcutaneous sensitization in AD is epidermal barrier dysfunction, treatment should aim to restore the skin barrier. Current guidelines for AD recommend topical glucocorticoids (tGC) as first-line therapy for reducing inflammation, with subsequent transition to maintenance therapy using TCI until complete resolution and to extend the remission period (19). Compared with tGC, the TCI pimecrolimus 1% cream (PIM) has a more specific anti-inflammatory and immunosuppressive mechanism of action (20, 21). In addition, PIM, unlike tGC, does not cause skin atrophy (22, 23), and is recommended for sensitive skin areas (19, 23). Evidence also indicates that PIM restores the epidermal barrier in patients with AD by altering expression of genes responsible for the normal structure and functioning of the skin barrier (24, 25). There is consensus among experts that off-label use of PIM is preferable in the treatment of children under 2 years of age because of its more favorable tolerability profile (23); indeed, a 5-year study showed that PIM was effective and had a favorable long-term safety profile when used in children with AD under 2 years of age (26). It should be noted that in the Russian Federation, PIM is approved for use from the age of 3 months (27).
The purpose of this cohort observational study was to evaluate an early-intervention algorithm for topical treatment of AD utilizing PIM as long-term maintenance therapy, aimed at prevention of transcutaneous sensitization and allergic diseases in children during their first year of life.
This was a single-center cohort observational study (NCT04900948). The study was conducted at the Federal National State Institution “National Medical Research Center for Children's Health” of the Ministry of Healthcare of Russia from December 2017 to April 2020.
Patients were assigned to the groups according to the clinical situation and the time when they sought specialized medical attention: patients who sought medical attention immediately at the onset of AD (at the first signs of disease, with a maximum delay of 10 days) were included in group 1 “baseline therapy with tGC with subsequent transition to PIM as maintenance therapy according to the suggested regimen”; patients who sought medical attention later were included in group 2 “baseline therapy and maintenance therapy with tGC, without subsequent use of PIM” (Figure 1).
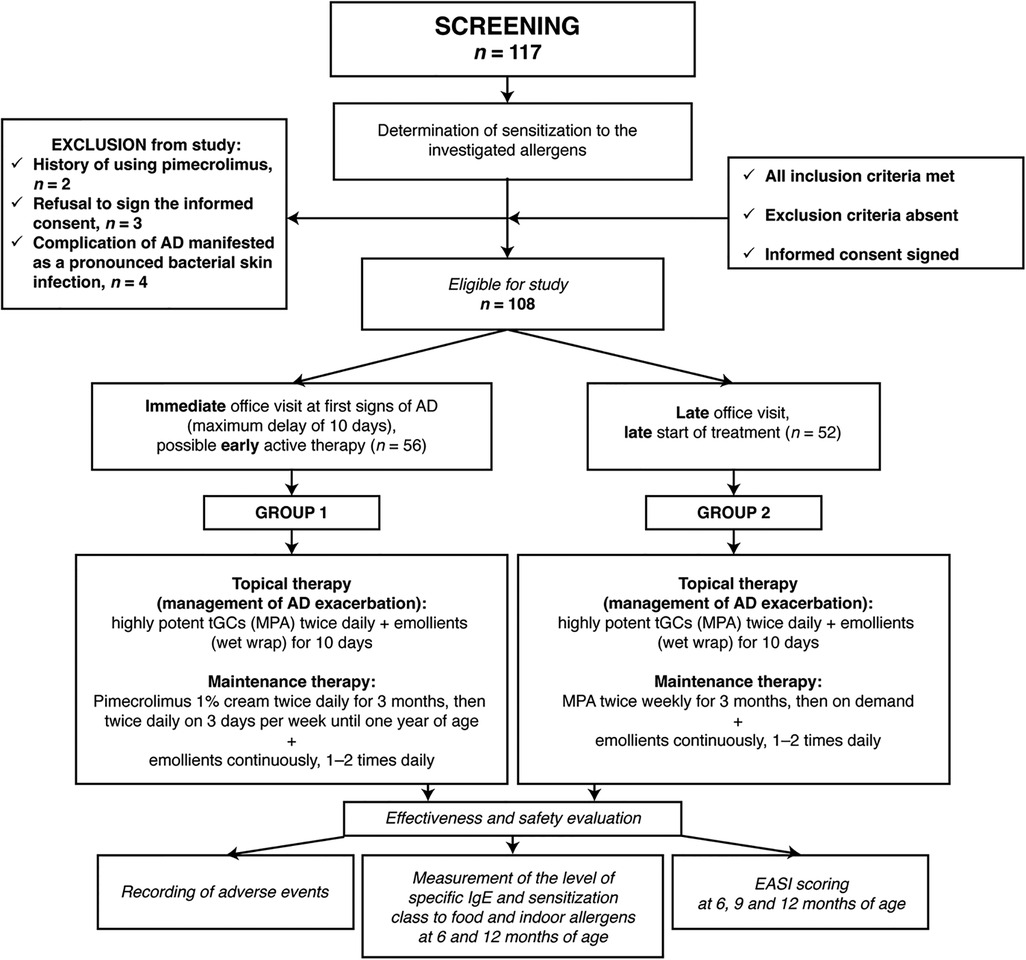
Figure 1. Study design flowchart. AD, atopic dermatitis; EASI, Eczema Area and Severity Index; tGC, topical glucocorticoids; MPA, methylprednisolone aceponate.
Patients were eligible for enrollment if they met the following inclusion criteria: aged 1–4 months; early onset of AD; Eczema Area and Severity Index (EASI) score ≥12; moderate or severe AD; a family history of allergies (presence of the following conditions in ≥1 parent: AD, FA, atopic asthma, allergic rhinitis); and sensitization to one or several of the investigated food and indoor allergens assessed by ImmunoCAP™ at the screening stage (cow's milk protein [CMP], egg white, house dust mite allergen [Dermatophagoides pteronyssinus], soy, wheat).
Patients were excluded if they had been treated with TCI (PIM) less than 30 days prior to enrollment; had a history of concomitant severe neurological, endocrine, cardiovascular, hepatic or renal diseases; or if acute bacterial or viral infections were present at enrollment.
The diagnosis of AD was based on clinical findings and medical history, and presence of three main, and at least three additional, diagnostic criteria (28). The severity of AD was evaluated using the EASI score (29, 30). The presence of sensitization to food and indoor allergens in a child was determined using ImmunoCAP. A family history of allergies was confirmed if ≥1 parent had AD, FA, atopic asthma, or allergic rhinitis.
All enrolled children with confirmed sensitization to one or several of the investigated allergens were recommended to follow a hypoallergenic lifestyle and prescribed a hypoallergenic diet and/or an individual elimination diet in accordance with the current clinical guidelines for the management of children with this pathology (31, 32). For instance, for breastfed children with sensitization to CMP, a hypoallergenic dairy-free diet was prescribed to the breastfeeding mother. If the child received formula or mixed nutrition, an extensively hydrolyzed milk protein formula or an amino acid-based formula was prescribed to the child in accordance with existing algorithms (31, 32).
Children from both groups were prescribed baseline therapy, which consisted of short-term treatment with a very potent topical tGC (methylprednisolone aceponate) twice daily combined with emollients (with wet wrap) for 10 days to relieve acute symptoms. This baseline therapy was also used to manage exacerbations when necessary.
After the baseline therapy, patients from group 1 were switched to a TCI: PIM was applied twice daily for 3 months, then twice daily on 3 days per week until one year of age, with concomitant continuous daily use of emollients (1–2 times daily).
Following the baseline therapy, children from group 2 were switched to methylprednisolone aceponate twice weekly for 3 months, then to an on-demand regimen (exacerbation of skin pathology). Patients from group 2 also used emollients continuously (1–2 times daily). PIM was not used by patients in group 2.
The main outcomes were the level of allergen-specific IgE (kUA/L) and the sensitization class (I–VI) to CMP, egg white, house dust mite allergen, soya, and wheat. These parameters were evaluated during examination of patients in order to determine the presence of sensitization to food and indoor allergens at the time of enrollment/screening, then at 6 and 12 months of age. The suggested therapeutic algorithm using PIM was deemed effective if the levels of allergen-specific IgE and sensitization class decreased during treatment and were lower in group 1 compared with group 2.
AD severity was assessed using the EASI score; the assessment took place at the time of enrollment/screening, then at 6, 9 and 12 months of age. The suggested therapeutic algorithm using PIM was deemed effective if it resulted in a more favorable and long-term stable course of AD with an EASI score ≤7 (mild AD) in group 1 compared with group 2.
The following adverse events were routinely monitored at 6, 9, and 12 months of age: local skin reactions (irritation, pruritus and skin redness, eruptions, exfoliation, dry skin, swelling); viral/bacterial infection; onset of allergic reactions (urticaria, angioedema); skin discoloration (hypopigmentation, hyperpigmentation). In an emergency, parents/legal guardians contacted investigators directly.
The presence of sensitization to food and indoor allergens was determined using an indirect immunofluorescence technique with the aid of the automated immunoassay analyzer ImmunoCAP250 (UniCAP® System, Thermo Fisher Scientific, formerly Phadia АВ), with the analytical sensitivity of 0.01 kUA/L. Sensitization to allergens was defined as a specific IgE concentration of >0.35 kUA/L in blood serum (according to the manufacturer's instructions). The between-assay coefficient of variation for the ImmunoCAP test system was 3.9–6.6% (according to the manufacturer's instructions). The class of sensitization was determined by the concentration of IgE: class I, 0.35–0.7 kUA/L; class II, 0.71–3.5; class III, 3.51–17.5 kUA/L; class IV, 17.51–50 kUA/L; class V, 50.01–100 kUA/L; class VI, >100 kUA/L.
The severity of AD was evaluated using the EASI score (29, 30). The severity of AD is recommended to be assessed using the following ranges on the EASI scale: 0, no signs of disease; 0.1–1.0, almost clear skin; 1.1–7.0, mild; 7.1–21.0, moderate; 21.1–50.0, severe; 50.1–72.0, very severe (29).
The required sample size was not calculated in advance.
Statistical analysis of the dynamics of the investigated parameters and their comparison between study groups was performed by multi-factor analysis of variance (ANOVA) using the STATISTICA™ software package version 10.0 (StatSoft, USA). The results are provided as tables and figures using the arithmetic mean. Variation of parameters was evaluated using the median, lower quartile (25th percentile, Q1), upper quartile (75th percentile, Q3) and interquartile range (Q1–Q3).
Quantitative values were compared in independent samples using least significant difference (LSD) test (ANOVA), and qualitative values were analyzed using Pearson chi-square test.
A total of 117 children were examined and 108 were eligible for the study (Figure 1). Based on the clinical situation and the time when they sought specialized medical attention, 56 children were assigned to group 1 (office visit at first signs of AD with a maximum delay of 10 days; baseline therapy followed by PIM) and 52 children to group 2 (late office visit; baseline therapy followed by tGC). The children in group 1 had a mean (standard deviation) age of 76 (11) days, EASI score of 33.3 (5.1) and delay in seeking medical attention of 4.8 (2.3) days (Table 1). The children in group 2 were slightly older (93 [14] days), had more severe AD at baseline (EASI score of 34.6 [3.7]) and a greater delay in seeking medical care (33.9 [9.1] days) (Table 1). All patients completed the study.
Sensitization to CMP was found in 19 and 17 patients from groups 1 and 2, respectively (Supplementary Table S1). Although the levels of CMP-specific IgE were greater in group 1 compared with group 2 at baseline, the levels remained relatively stable (mean values; Figure 2A) or decreased (median values; Supplementary Table S1) at 6 and 12 months of age in group 1. In contrast, CMP-specific IgE levels increased from baseline to 6 and 12 months of age in group 2, with mean levels much greater in group 2 compared with group 1 at 12 months of age (16.5 vs. 5.0 kUA/L, respectively, p = 0.024; Figure 2A).
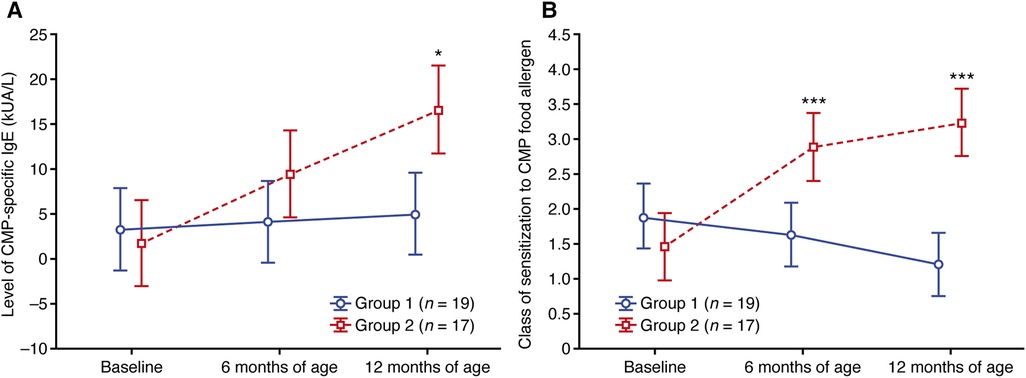
Figure 2. Change in (A) the level of CMP-specific IgE and (B) the class of sensitization to the CMP food allergen. Data shown are arithmetic mean ± 95% confidence interval. *p < 0.05; ***p < 0.001 vs. group 1. CMP, cow's milk protein.
A similar pattern was observed for the class of CMP sensitization (both median and mean values), with decreases observed at 6 and 12 months of age relative to baseline in group 1 compared with increases at 6 and 12 months of age vs. baseline in group 2 (Supplementary Table S2; Figure 2B). By 12 months of age, the mean class of sensitization was 1.21 in group 1 and 3.24 in group 2 (p < 0.001; Supplementary Table S2; Figure 2B).
Sensitization to egg white was found in 17 and 15 patients from groups 1 and 2, respectively (Supplementary Table S3). Similar to CMP sensitization, higher mean and median levels of egg white-specific IgE were observed in group 1 at baseline compared with group 2 (Supplementary Table S3), and the levels in group 1 remained relatively stable (mean values; Figure 3A) or decreased (median values; Supplementary Table S3) at 6 and 12 months of age. In group 2, however, the mean and median levels of egg-white specific IgE increased at 6 and 12 months of age vs. baseline (mean values; Figure 3A).
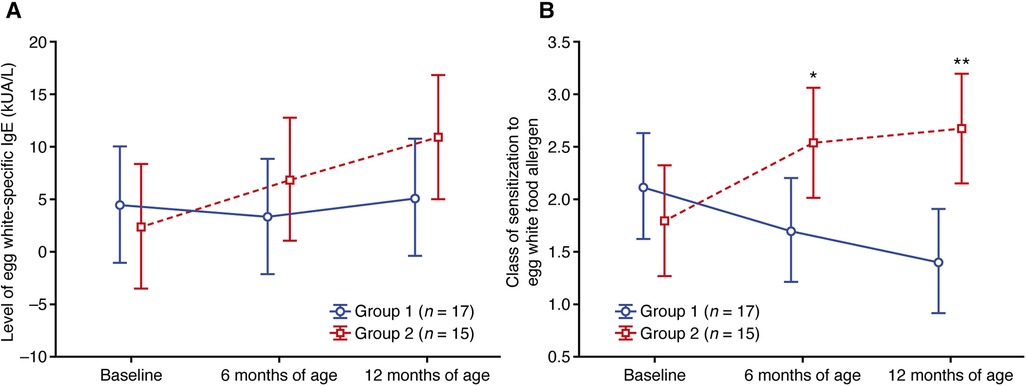
Figure 3. Change in (A) the level of egg white-specific IgE and (B) the class of sensitization to the egg white food allergen. Data shown are arithmetic mean ± 95% confidence interval. *p < 0.05; **p < 0.01 vs. group 1.
Starting from 6 months of age, the class of egg white sensitization was lower in group 1 compared with group 2 (Figure 3B; Supplementary Table S4), with a mean class of 1.41 in group 1 and 2.67 in group 2 at 12 months (p = 0.007; Supplementary Table S4).
Eleven patients from group 1 and 17 patients from group 2 were found to have sensitization to the indoor airborne allergen, house dust mite allergen (Supplementary Table S5). The levels of house dust mite allergen-specific IgE and class of house dust mite allergen sensitization remained relatively stable in group 1 throughout the study (Figure 4; Supplementary Tables S5, S6). In contrast, these parameters increased in group 2; both the level of house dust mite allergen-specific IgE and sensitization class were significantly greater in group 2 vs. group 1 at 12 months of age (Figure 4; Supplementary Tables S5, S6).
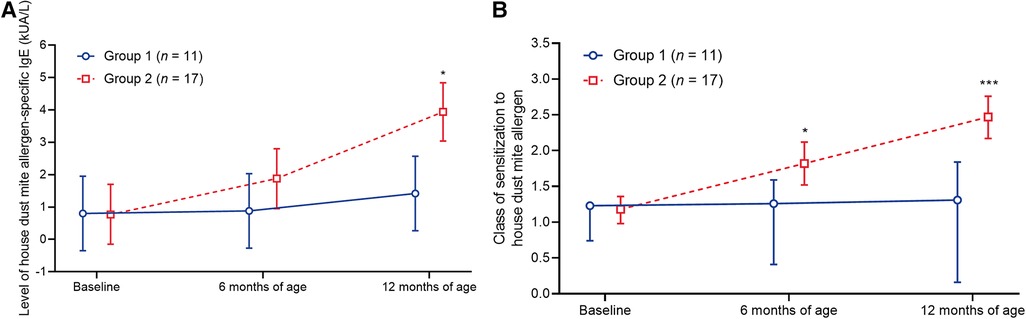
Figure 4. Change in (A) the level of house dust mite allergen-specific IgE and (B) the class of sensitization to the house dust mite allergen. Data shown are arithmetic mean ± 95% confidence interval. *p < 0.05; ***p < 0.001 vs. group 1.
Within groups, baseline levels of soya- and wheat-specific IgE were similar (Figure 5; Supplementary Tables S7, S8). Mean levels of soya- and wheat-specific IgE stayed relatively stable in both groups up to 12 months of age (Figure 5; Supplementary Tables S7, S8).
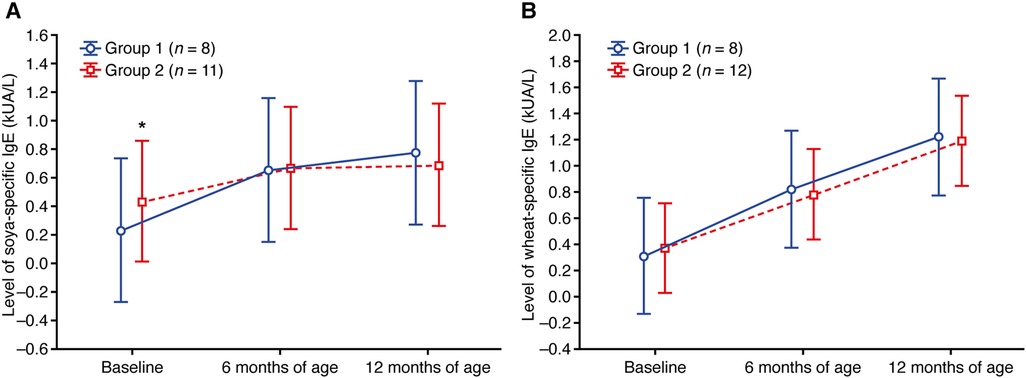
Figure 5. Change in the level of (A) soya-specific IgE and (B) wheat-specific IgE. Data shown are arithmetic mean ± 95% confidence interval. **p < 0.01 vs. group 1.
There was a more rapid and pronounced improvement in the severity of AD in group 1 compared with group 2, represented by a decrease in EASI scores at 6, 9 and 12 months of age (Figure 6; Supplementary Table S9).
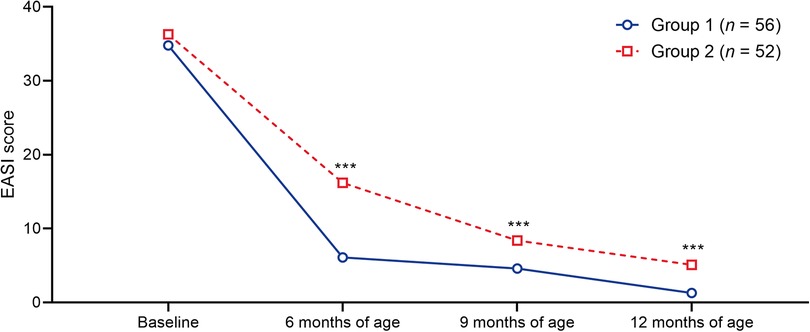
Figure 6. Timecourse of median EASI scores in the patient groups. ***p < 0.001 vs. group 1. EASI, Eczema Area and Severity Index.
No adverse events were reported.
The results of this study indicate that an algorithm using PIM for the treatment of AD, long-term maintenance therapy and prophylaxis of sensitization to food and indoor allergens was effective in preventing sensitization to CMP, egg white, and house dust mite allergen.
Group 1, which sought treatment early and received PIM as maintenance therapy, had lower values of the specific IgE level and sensitization class for CMP, egg white, and house dust mite allergen compared with group 2 (who received treatment later and did not receive PIM) at 6 and 12 months of age. Conversely, soya- and wheat-specific IgE levels were similar in group 1 and group 2 at baseline, 6 months, and 12 months of age, and IgE levels remained relatively stable throughout. This could be due to low baseline IgE levels and a low incidence of soya and wheat sensitization in the study participants. This is to be expected, considering the incidence of soya and wheat allergy in the general pediatric population is reported to be 0.5%, compared with 1.9% for milk (33).
Given that epidermal barrier dysfunction is a key factor in transcutaneous sensitization (11), the reduced levels of IgE and sensitization class for CMP, egg white, and house dust mite allergen in group 1 vs. group 2 could be due to effects of PIM on the skin barrier. Studies have shown that PIM restores the epidermal barrier in patients with AD by altering expression of genes responsible for synthesis of structural proteins that form part of a normal skin barrier (24, 25).
A previous study in children demonstrated that early treatment with PIM is associated with reduced incidence of major AD flares compared with vehicle (34). Similarly, our study suggests that early treatment with PIM is associated with a less severe course of AD. In addition, the effects of our PIM treatment algorithm on sensitization to food and indoor allergens indicate that the earlier treatment with PIM begins, the faster skin barrier function is restored and the less the risk of percutaneous allergen penetration.
There is convincing evidence for a favorable long-term safety profile for PIM when used by children under 2 years of age (23). For example, the 5-year long periodic use of PIM in the Petite study did not result in any safety signals in the form of an increased risk of infections, lymphomas or skin malignancies (26). Furthermore, when applied topically the systemic effect of PIM is minimal, likely due to the high molecular weight and lipophilicity of the compound (35, 36). The results of the current study support the safety profile for the long-term use of PIM, with no adverse events reported during treatment.
Although tGC are currently the first-line treatment of choice in inflammatory skin conditions (19), PIM has a more specific anti-inflammatory and immunosuppressive mechanism of action, exerting an effect on T lymphocytes and mast cells and thus minimizing skin atrophy (20, 21). As previously discussed, in contrast to corticosteroids, PIM has been shown to restore the epidermal barrier in patients with AD (24, 25). Together, these facts suggest that PIM may be considered for a more prominent role in the long-term management of such skin diseases (37).
A limitation of this study is that it was conducted at a single center in Russia with a relatively small sample size. However, we considered the patient population to be sufficient to draw general conclusions about the efficacy and safety of the treatment algorithms. Further study of PIM is warranted in larger patient populations to confirm our findings. As the patients in group 1 sought medical attention within 10 days of the onset of AD (mean delay 4.8 days) whereas patients in group 2 sought medical attention later (mean delay 33.8 days), the possibility that the effects of the PIM-containing treatment algorithm on sensitization to allergens and AD severity are primarily driven by early intervention rather than PIM cannot be excluded. Another limitation of the study includes the small number of food and indoor allergens investigated. While it was not within the scope of this study to investigate an exhaustive list of food and indoor allergens, future research should investigate use of pimecrolimus for the prevention of transcutaneous sensitization of other important allergens. Moreover, for clinical purposes, this study employed an open food challenge to confirm or rule out FAs, rather than an oral food challenge. Future studies are warranted investigating the effects of PIM using an oral food challenge, the gold standard for diagnosing FAs (38). Finally, calculation of the severity index on the EASI scale was carried out by several researchers, which may have led to distortions and differences in the results due to the subjectivity of the assessment.
The results of this study indicate that an early-intervention algorithm that utilizes PIM for long-term maintenance therapy reduces the development of transcutaneous sensitization, thus preventing the formation and progression of allergic diseases in children with AD, including FA. Maintaining skin barrier function, and ensuring an immediate suppression of inflammation, appear to be key aspects of AD treatment and prophylaxis of clinical manifestations of allergic diseases. Additional studies including larger numbers of patients are needed to confirm the benefits of PIM in this population.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the local independent Ethics Committee at the Federal State Autonomous Institution “National Medical Research Center for Children's Health” of the Ministry of Healthcare of Russia, and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
All authors contributed to the study conception and design. Data collection and analysis were performed by DVF, SGG and RAI. The first draft of the manuscript was written by NNM and RAI; all authors commented on this and subsequent versions of the manuscript. All authors contributed to the article and approved the submitted version.
Medical writing assistance in the preparation of this manuscript was funded by Meda Pharma S.p.A., a Viatris company.
Medical writing assistance in the preparation of this manuscript was provided by Jane Murphy, PhD, Molly Macpherson, BSc, and Bonnie Nicholson, PhD (Ashfield MedComms, an Inizio company), and funded by Meda Pharma S.p.A., a Viatris company.
NNM reports grants and personal fees from Jansen, grants from Eli Lilly, grants and personal fees from Novartis, personal fees from Galderma, personal fees from Pierre Fabre, personal fees from Bayer, personal fees from Leofarma, grants and personal fees from Pfizer, grants and personal fees from AbbVie, grants from Amryt Pharma, personal fees and non-financial support from Viatris, outside the submitted work; LSN reports grants and personal fees from MSD Pharmaceuticals LLC, grants and personal fees from FORT LLC, grants and personal fees from Shire Biotech Rus LLC, grants and personal fees from Pfizer Innovations LLC, grants and personal fees from Sanofi Aventis Group LLC, grants and personal fees from AbbVie LLC, grants and personal fees from Pierre Fabre LLC, outside the submitted work; SGM reports grants and personal fees from Nutricia, grants and personal fees from FrieslandCampina, outside the submitted work; ETA reports personal fees from Eli Lilly, personal fees from Novartis, personal fees from AbbVie, personal fees from Jansen, personal fees from Amryt Pharma, outside the submitted work; RVE reports personal fees from Eli Lilly, personal fees from Novartis, personal fees from AbbVie, personal fees from Amryt Pharma, outside the submitted work; AIM reports personal fees from Eli Lilly, personal fees from Novartis, personal fees from AbbVie, personal fees from Amryt Pharma, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construes as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1102354/full#supplementary-material.
1. Maliyar K, Sibbald C, Pope E, Gary Sibbald R. Diagnosis and management of atopic dermatitis: a review. Adv Skin Wound Care. (2018) 31:538–50. doi: 10.1097/01.ASW.0000547414.38888.8d
2. Namazova-Baranova LS, Baranov AA, Kubanova AA, Ilina NI, Kurbacheva OM, Vishneva EA, et al. Atopic dermatitis in children: current clinical guidelines for diagnosis and therapy. Curr Pediatr. (2016) 15:279–94. doi: 10.15690/vsp.v15i3.1566
3. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. (2014) 69:17–27. doi: 10.1111/all.12268
4. Zheng T, Yu J, Oh MH, Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. (2011) 3:67–73. doi: 10.4168/aair.2011.3.2.67
5. Delves PJ. Food Allergy. (2019) [updated October 2020; cited 2021 November]. Available at: https://www.msdmanuals.com/professional/immunology-allergic-disorders/allergic,-autoimmune,-and-other-hypersensitivity-disorders/food-allergy
6. Simpson EL, Chalmers JR, Hanifin JM, Thomas KS, Cork MJ, McLean WH, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. (2014) 134:818–23. doi: 10.1016/j.jaci.2014.08.005
7. Hill DA, Spergel JM. The Atopic March: Critical Evidence and Clinical Relevance. Ann Allergy Asthma Immunol. (2018) 120:131–7. doi: 10.1016/j.anai.2017.10.037
8. Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. (2012) 129:1187–97. doi: 10.1016/j.jaci.2012.02.036
9. Berin MC, Shreffler WG. Mechanisms underlying induction of tolerance to foods. Immunol Allergy Clin North Am. (2016) 36:87–102. doi: 10.1016/j.iac.2015.08.002
10. Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. (2008) 121:1331–6. doi: 10.1016/j.jaci.2008.04.032
11. Izadi N, Luu M, Ong PY, Tam JS. The role of skin barrier in the pathogenesis of food allergy. Children (Basel). (2015) 2:382–402. doi: 10.3390/children2030382
12. Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Invest. (2012) 122:440–7. doi: 10.1172/JCI57416
13. Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. (2008) 121:1311–20; quiz 1321-2. doi: 10.1016/j.jaci.2008.04.023
14. Valenta R, Hochwallner H, Linhart B, Pahr S. Food allergies: the basics. Gastroenterology. (2015) 148:1120–31. doi: 10.1053/j.gastro.2015.02.006
15. Zhong Y, Samuel M, van Bever H, Tham EH. Emollients in infancy to prevent atopic dermatitis: a systematic review and meta-analysis. Allergy. (2022) 77:1685–99. doi: 10.1111/all.15116
16. Han H, Roan F, Ziegler SF. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol Rev. (2017) 278:116–30. doi: 10.1111/imr.12546
17. Bustos GJ, Bustos D, Bustos GJ, Romero O. Prevention of asthma with ketotifen in preasthmatic children: a three-year follow-up study. Clin Exp Allergy. (1995) 25:568–73. doi: 10.1111/j.1365-2222.1995.tb01096.x
18. Lowe AJ, Leung DYM, Tang MLK, Su JC, Allen KJ. The skin as a target for prevention of the atopic march. Ann Allergy Asthma Immunol. (2018) 120:145–51. doi: 10.1016/j.anai.2017.11.023
19. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. (2018) 32:657–82. doi: 10.1111/jdv.14891
20. Gutfreund K, Bienias W, Szewczyk A, Kaszuba A. Topical calcineurin inhibitors in dermatology. Part I: properties, method and effectiveness of drug use. Postepy Dermatol Alergol. (2013) 30:165–9. doi: 10.5114/pdia.2013.35619
21. Reda AM, Elgendi A, Ebraheem AI, Aldraibi MS, Qari MS, Abdulghani MMR, et al. A practical algorithm for topical treatment of atopic dermatitis in the Middle East emphasizing the importance of sensitive skin areas. J Dermatolog Treat. (2019) 30:366–73. doi: 10.1080/09546634.2018.1524823
22. Nuutinen P, Riekki R, Parikka M, Salo T, Autio P, Risteli J, et al. Modulation of collagen synthesis and mRNA by continuous and intermittent use of topical hydrocortisone in human skin. Br J Dermatol. (2003) 148:39–45. doi: 10.1046/j.1365-2133.2003.05018.x
23. Luger T, Boguniewicz M, Carr W, Cork M, Deleuran M, Eichenfield L, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol. (2015) 26:306–15. doi: 10.1111/pai.12331
24. Grzanka A, Zebracka-Gala J, Rachowska R, Bozek A, Kowalska M, Jarzab J. The effect of pimecrolimus on expression of genes associated with skin barrier dysfunction in atopic dermatitis skin lesions. Exp Dermatol. (2012) 21:184–8. doi: 10.1111/j.1600-0625.2011.01417.x
25. Jensen JM, Scherer A, Wanke C, Brautigam M, Bongiovanni S, Letzkus M, et al. Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy. (2012) 67:413–23. doi: 10.1111/j.1398-9995.2011.02747.x
26. Sigurgeirsson B, Boznanski A, Todd G, Vertruyen A, Schuttelaar ML, Zhu X, et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics. (2015) 135:597–606. doi: 10.1542/peds.2014-1990
27. VIDAL. Elidel (Elidel) instructions for use Vidal's reference book “Medications in Russia”. [cited 2023 January 26]. Available at: https://www.vidal.ru/drugs/elidel__2979#dosage
28. Hanifin JRG. Diagnostic features of atopic dermatitis. Acta Derm Venereol. (1980) 92:44–7. doi: 10.2340/00015555924447
29. Leshem YA, Hajar T, Hanifin JM, Simpson EL. What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: an interpretability study. Br J Dermatol. (2015) 172:1353–7. doi: 10.1111/bjd.13662
30. Chalmers JR, Schmitt J, Apfelbacher C, Dohil M, Eichenfield LF, Simpson EL, et al. Report from the third international consensus meeting to harmonise core outcome measures for atopic eczema/dermatitis clinical trials (home). Br J Dermatol. (2014) 171:1318–25. doi: 10.1111/bjd.13237
31. Russian Association of Allergologists and Clinical Immunologists. Clinical guidelines. Atopic dermatitis in children: the union of pediatricians of Russia. Moscow: RAACI (2016)
32. Baranov AA, Namazova-Baranova LS. Federal clinical guidelines for provision of medical care to children with food allergy: the union of pediatricians of Russia. Moscow: RAACI (2015)
33. Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. (2018) 142:e20181235. doi: 10.1542/peds.2018-1235
34. Siegfried E, Korman N, Molina C, Kianifard F, Abrams K. Safety and efficacy of early intervention with pimecrolimus cream 1% combined with corticosteroids for major flares in infants and children with atopic dermatitis. J Dermatolog Treat. (2006) 17:143–50. doi: 10.1080/09546630600647297
35. Eichenfield LF, Thaci D, de Prost Y, Puig L, Paul C. Clinical management of atopic eczema with pimecrolimus cream 1% (Elidel) in paediatric patients. Dermatology. (2007) 215:3–17. doi: 10.1159/000102116
36. Billich A, Aschauer H, Aszodi A, Stuetz A. Percutaneous absorption of drugs used in atopic eczema: pimecrolimus permeates less through skin than corticosteroids and tacrolimus. Int J Pharm. (2004) 269:29–35. doi: 10.1016/j.ijpharm.2003.07.013
37. Korfitis C, Gregoriou S, Rallis E, Rigopoulos D. Pimecrolimus versus topical corticosteroids in dermatology. Expert Opin Pharmacother. (2007) 8:1565–73. doi: 10.1517/14656566.8.10.1565
Keywords: atopic dermatitis, children, sensitization, food allergens, indoor allergens, pimecrolimus
Citation: Murashkin NN, Namazova-Baranova LS, Makarova SG, Ivanov RA, Grigorev SG, Fedorov DV, Ambarchian ET, Epishev RV, Materikin AI, Opryatin LA and Savelova AA (2023) Observational study of pimecrolimus 1% cream for prevention of transcutaneous sensitization in children with atopic dermatitis during their first year of life. Front. Pediatr. 11:1102354. doi: 10.3389/fped.2023.1102354
Received: 12 December 2022; Accepted: 24 March 2023;
Published: 25 April 2023.
Edited by:
Stefania Arasi, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Burcin Beken, Acıbadem University, Türkiye© 2023 Murashkin, Namazova-Baranova, Makarova, Ivanov, Grigorev, Fedorov, Ambarchian, Epishev, Materikin, Opryatin and Savelova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolay N. Murashkin bV9ubjIwMDFAbWFpbC5ydQ==
†ORCID Nikolay N. Murashkin orcid.org/0000-0003-2252-8570
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
Abbreviations AD, Atopic dermatitis; CMP, Cow's milk protein; EASI, Eczema Area and Severity Index; FA, Food allergy; MPA, Methylprednisolone aceponate; PIM, Pimecrolimus; Q, Quartile; SD, Standard deviation; TCI, Topical calcineurin inhibitors; tGC, Topical glucocorticoids.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.