- 1Department of Otolaryngology-Head and Neck Surgery, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
- 2Department of Clinical Laboratory, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
Objective: Adenoid hypertrophy (AH) and otitis media with effusion (OME) are common pediatric otolaryngological diseases and often occur concurrently. The purpose of this study was to comprehensively analyze the factors that influence the occurrence of OME pediatric patients with AH.
Methods: Patients younger than 12 years with AH, who were hospitalized for treatment at Beijing Tsinghua Changgung Hospital in Beijing, China, between March 2018 and February 2022 were enrolled. The patients were divided into an AH group and an AH + OME group based on the presence of OME. The authors collected the following clinical data for univariable analysis: sex; age; body mass index (BMI); comorbid nasal congestion/rhinorrhea, recurrent tonsillitis, or allergic rhinitis (AR); adenoid and tonsil grade; tonsillar hypertrophy; food/drug allergy; history of adenoidectomy and congenital diseases; breastfeeding status; preterm birth; exposure to environmental tobacco smoke (ETS); family history of adenotonsillectomy, otitis media, and AR; main data of polysomnography and oropharyngeal conditional pathogen culture data of some patients. Univariate analysis was performed as a basis for logistic regression analysis.
Results: A total of 511 children (329 boys and 182 girls) were included, their mean age was 5.37 ± 2.10 years. Of them, 407 (79.6%) were in the AH group and 104 (20.4%) in the AH + OME group. Univariate analysis revealed statistically significant differences in age, BMI, adenoid grade, AR, breastfeeding status, and ETS exposure between the two groups. Multivariate stepwise logistic regression analysis showed that age, adenoid grade, AR, breastfeeding status, and ETS influenced the occurrence of OME in pediatric patients with AH. The risk of OME decreased with increasing age. High adenoid grade, ETS exposure, and comorbid AR were risk factors for OME in pediatric patients with AH, but breastfeeding was a protective factor. The final analytical results of the oropharyngeal conditional pathogen culture data showed that Streptococcus pneumoniae positivity was associated with OME in AH.
Conclusion: The pathogenesis of AH with OME is complex. Young age, high adenoid grade, ETS exposure, non-breastfed status, comorbid AR, and the presence of S. pneumoniae in the oropharynx are risk factors for OME in pediatric patients with AH.
1. Introduction
Adenoid hypertrophy (AH) and otitis media with effusion (OME) are common pediatric otolaryngological diseases. Repeated stimulation by bacteria, viruses, and allergens causes pathological AH, resulting in clinical symptoms (1), such as nasal congestion, rhinorrhea, open-mouthed breathing, obstructive sleep apnea (OSA), snoring, and “adenoid face” caused by chronic airway obstruction, which is an important factor that can induce or worsen OME, eustachian tube dysfunction (ETD), and acute/chronic rhinosinusitis in children (2).
OME is a middle ear effusion without an acute middle ear infection. It is most common in children aged six months to four years old. Approximately 90% of preschoolers and 25% of schoolchildren have had OME (3). The prevalence of OME ranges from 1.3% to 31.3% (4) depending on diagnostic methods, race, and environmental factors. However, the pathogenesis and etiology of OME remain unclear. Viral infection, bacterial colonization, allergies, and immune factors can promote the occurrence and progression of OME; mechanical obstruction and ETD also play critical roles. Adenoids obstruct the posterior nostrils and affect ventilation and drainage in the nasal cavity and sinuses, leading to chronic sinusitis. Pathogenic microorganisms and secretions pass through the eustachian tube (ET) and oropharynx to cause ET mucosal inflammation, congestion, edema, and retrograde infection, thereby aggravating or inducing OME (5). AH is the main predisposing factor for OME that often accompanies it in children (6). However, not all pediatric patients with AH develop OME. It is vital to understand the influencing factors associated with OME incidence in pediatric patients with AH. Previous studies have found that atopic or allergic rhinitis (AR), frequent tonsillitis, daycare attendance, exposure to smoke, and multiple family members were major risk factors for OME in pediatric patients with AH (7). Further and more comprehensive analysis of the risk factors for OME in children with AH is needed to provide a reference for the prevention and treatment of such cases and a basis for future in-depth mechanistic studies. Thus, the aim of this study was to comprehensively analyze the factors that influence the occurrence of OME in pediatric patients with AH.
2. Materials and methods
2.1. Study population
In this retrospective study, patients with AH aged ≤12 years old who were hospitalized for treatment at Beijing Tsinghua Changgung Hospital in Beijing, China, between March 2018 and February 2022 were enrolled. The reasons for hospitalization were related clinical symptoms and manifestations of AH, mainly nasal congestion, rhinorrhea, open-mouthed breathing, OSA, snoring, and “adenoid face” caused by chronic airway obstruction. All patients were admitted to the hospital for an adenoidectomy. Some surgeries were combined with tonsillectomy. Two major indications for tonsillectomy and/or adenoidectomy include obstruction and recurrent infection (8). The age limit of 12 years was chosen because the upper age limit in OME guidelines is 12 years (9). Children with OME underwent intraoperative tympanocentesis or tympanostomy tube insertion. During the study period, a total of 518 patients were hospitalized due to the above reasons in an ENT outpatient department at our hospital. Seven were excluded (one case of undetermined neurological disease, one case of nephroblastoma, one case of abnormal coagulation function, one case of hereditary deafness with intellectual disability, one case of middle ear malformation, one case of immune disease, and one case of severe heart disease combined with slow growth). Finally, a total of 511 patients were included as study participants.
The inclusion criteria were as follows: age ≤ 12 years; presence of adenoid tissue obstruction of the posterior nostril (>50%); and presence of nasal congestion, snoring, mouth breathing, and other clinical symptoms. Patients who had acute upper respiratory tract infection in the last 2 weeks that was treated using antibiotics or immune modulators, and patients with cleft palate and other craniofacial deformities; intellectual disability; immunodeficiency; cardiovascular, lung, genetic, autoimmune, and neuromuscular diseases; or other severe underlying diseases were excluded from this study. The included patients were subdivided into an AH group and an AH + OME group based on the presence of OME, and into three groups by age: 0–4 years, 5–8 years, and 9–12 years.
Diagnosis of AH was based on: signs, symptoms, and the results of fiberoptic nasopharyngoscopy, and that of OME was based on: signs, symptoms, and the results of auxiliary examinations (pure tone audiometry/behavioral audiometry, tympanometry, and ear endoscopy), and the intraoperative confirmation of tympanic effusion.
The clinical data of the pediatric patients, including sex; age; body mass index (BMI); comorbid nasal congestion/rhinorrhea (mucoid or mucopurulent), recurrent tonsillitis, or AR; adenoid grade; tonsil grade; tonsillar hypertrophy; food/drug allergy; history of adenoidectomy; history of congenital diseases; breastfeeding status; preterm birth; exposure to environmental tobacco smoke (ETS); family history of adenotonsillectomy, otitis media and AR; and main polysomnography (PSG) data, were collected for univariate analysis. In addition, upper respiratory tract (oropharynx) conditional pathogen culture data were collected to analyze the relationship between the presence of conditional pathogens and the occurrence of OME in pediatric patients with OME.
The study design was approved by Beijing Tsinghua Changgung Hospital Ethics Committee (NO.: 22538-6-01, Nov. 8th, 2022). The minor(s)' legal guardian/next of kin consented to the collection of medical history, and examination and operation information.
2.2. Grouping criteria
The key points of diagnosis of OME were as follows: (1) ear symptoms and signs without acute middle ear infection; (2) hearing loss, self-hearing enhancement, or hearing changes with posture changes occuring; (3) a tympanogram showed a “B” or “C” curve; (4) pure tone/behavioral audiometry indicating that the affected ear had mild to moderate conductive hearing loss; and (5) patients who showed tympanic effusion during the ear endoscopy before the operation which was confirmed intraoperatively. Patients diagnosed with OME according to the above criteria were included in the AH + OME group. Pediatric patients without OME were included in the AH group.
2.3. Criteria for collection of clinical data
Adenoid grading was based on endoscopic findings of the percentage of the posterior nostril blocked by the adenoid. Grades II–IV indicate a 26%–50%, 51%–75%, and ≥76% obstruction, respectively (10). Tonsil grading was performed according to Friedman's criteria. The tonsil grades include grade 0 (patients who have had their tonsils removed), grade 1 (the tonsils are inside the tonsillar fossa), grade 2 (the tonsils extend beyond the tonsillar pillars), grade 3 (the tonsils extend beyond the tonsillar pillars but do not reach the midline), and grade 4 (the tonsils extend as far as the midline (11). Tonsil hypertrophy was defined as tonsil grade II or higher. Regarding breastfeeding status, included children were those who were breastfed for more than six months after birth. Children in close contact with at least one active smoker (one or more cigarettes per day by any family member living with them) were considered to have ETS exposure (12). AR was diagnosed if a child showed excessive sneezing and at least one of the following symptoms: ocular pruritus, nasal pruritus, oropharyngeal pruritus, or clear nasal discharge (13). Preterm birth was defined as a gestational age of fewer than 37 weeks at the time of delivery. Family history was defined as the medical history of first-degree relatives. PSG monitoring data were collected, including obstructive apnea index (OAI) and apnea hypopnea index (AHI). An OAI > 1 time/h or AHI > 5 times/h for every nighttime sleep was considered abnormal (14). The severity of OSA was categorized as follows: mild, 5 times/h < AHI ≤10 times/h or 1 time/h < OAI ≤ 5 times/h; moderate, 10 times/h < AHI ≤ 20 times/h or 5 times/h < AHI ≤ 10 times/h; severe, AHI > 20 times/h or OAI > 10 times/h. Bacterial culture sampling was performed after general anesthesia and before surgery. For the collection of the samples, 0.9% sodium chloride solution was used to flush the oropharynx, and sterile pharyngeal swabs were used to swab the oropharynx repeatedly. The samples were then sent for bacterial culture. These strains were identified using MALDI-TOF MS (Bruker Dalton GmbH, Leipzig, Germany). The samples were cultured on Columbia agar supplemented with 5% sheep blood and Chocolate agar plates and incubated at 37°C for 48 h.
2.4. Statistical analysis
SAS Analysis Software (version 9.4, SAS Institute Inc, Cary, NC, USA) was used for data processing. P < 0.05 was considered to be statistically significant. The study was mainly divided into two parts for statistical and data analysis. Firstly, the influencing factors of the clinical data of AH complicated by OME were analyzed. Then the relationship between different pathogenic bacteria and AH complicated by OME was further analyzed based on a limited number of cases. In the process, the relevant clinical data of the AH group and AH + OME group were first analyzed by univariate analysis. Measurement data meeting the normality test and homogeneity analysis of variance were compared between the two groups by T-test; otherwise, Wilcoxon non-parametric test was used. Pearson's Chi-square test was used for enumeration data meeting the condition of the test; otherwise, a correction test or Fisher's exact test was used. Then, variables with P < 0.1 from the univariate analysis results were included in the multivariate logistic stepwise regression to screen out related factors affecting the pediatric patients with AH complicated by OME. In the analysis of correlation with pathogenic bacteria, the relevant pathogenic bacteria were screened out by univariate analysis. Furthermore, variables with P < 0.05 were taken as covariables, and the relationship between pathogenic bacteria and OME in pediatric patients with AH was further analyzed by multivariate logistic regression.
3. Results
3.1. Analysis of clinical data
A total of 511 pediatric patients with AH were included in this study. Of the 511 patients, 329 were boys, and 182 were girls. The age distribution of the patients was 5.37 ± 2.10 years. Regarding the two patient groups, 407 (79.6%) patients were included in the AH group, whereas 104 (20.4%) were included in the AH + OME group. Thirteen of the patients were missing PSG data owing to a lack of parental consent or lack of cooperation by the pediatric patients. Of the 498 patients with PSG data, 397 (79.7%) were in the AH group, and 101 (20.3%) were in the AH + OME group. The upper respiratory tract conditional pathogen culture data of 220 pediatric patients were collected. Of these, 178 (80.9%) were in the AH group, and 42 (19.1%) were in the AH + OME group.
Univariate analysis of the clinical data of the patients (Table 1) showed that there were statistically significant differences in age, BMI, adenoid grade, AR, breastfeeding status, and ETS between the AH and AH + OME groups (P < 0.05). The age distribution of the patients in the AH group was 5 (4–7) years, whereas that of those in the AH + OME group was 4 (4–5) years, and the difference between the two groups was statistically significant (P < 0.0001). The incidence of OME was higher in younger children and significantly higher in the 0–4 years age group than in the older age groups (P = 0.0002). The BMI (kg/m2) of the pediatric patients in the AH group was higher than that of the patients in the AH + OME group (P = 0.0030). The incidence of OME among patients in the adenoid grade IV group was 26.86%, which was higher than that among patients in the grade III group (14.5%) (P = 0.0005). The incidence of OME among pediatric patients exposed to ETS was 36.72%, which was higher than that among patients who were not exposed to ETS (11.68%) (P < 0.0001). The incidence of OME among breastfed pediatric patients was 17.32%, which was lower than that among those not breastfed (29.23%) (P = 0.0036). The incidence of OME among patients with AR was 25.31%, which was higher than that among those without AR (15.93%) (P = 0.0085). There were no statistically significant differences in sex, tonsil grade, comorbid nasal congestion/rhinorrhea, history of adenoidectomy, history of congenital diseases, preterm birth, history of food/drug allergy, family history of otitis media, adenotonsillectomy and AR, PSG monitoring results: AHI, OAI, and diagnosis and severity of OSA between the AH and AH + OME groups (P > 0.05).
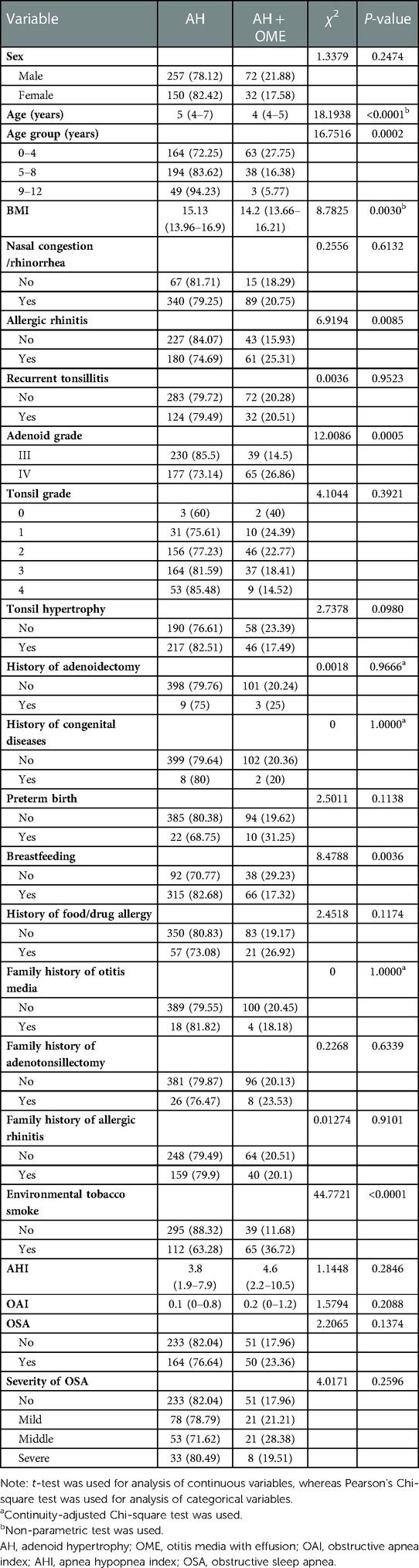
Table 1. Results of the univariate analysis of pediatric patients with adenoid hypertrophy with otitis media with effusion.
The factors with a Pvalue <0.1 in the univariate analysis were included in the multivariate stepwise logistic regression analysis (Table 2). The results showed that age, adenoid grade, AR, breastfeeding status, and ETS exposure were important factors that influence the occurrence of OME in pediatric patients with AH. The results also showed that the risk of OME decreases with age. The patients in the 5–8 years [P = 0.0062, OR: 0.494 (0.299–0.819)] and 9–12 years [P = 0.0055, OR: 0.169 (0.048–0.592)] age groups had lower risks for OME than those in the 0–4 years group. The results also showed that a high adenoid grade was a risk factor for OME in pediatric patients with AH. The incidence of OME among patients in the adenoid grade IV group was 1.662 times than those in the grade III group [P = 0.0438, OR: 1.662 (1.014–2.723)]. ETS exposure was a risk factor for OME in pediatric patients with AH. Exposure to ETS increased the risk for OME by 4.839 times compared with non-exposure [P < 0.0001, OR: 4.839 (2.994–7.819)]. Children with AR were 1.906 times more likely to develop OME than those without AR [P = 0.008, OR: 1.906 (1.183–3.071)]. Breastfeeding was a protective factor against OME in pediatric patients with AH [P = 0.0126, OR: 0.523 (0.314–0.870)].
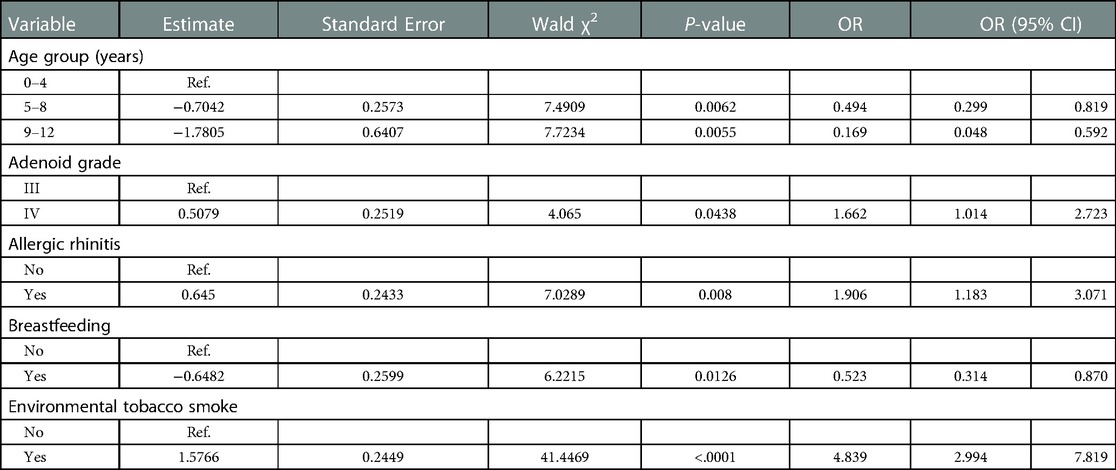
Table 2. Results of multivariate logistic regression analysis of pediatric patients with adenoid hypertrophy with otitis media with effusion.
3.2. Conditional pathogen culture analysis
The major pathogens identified in the upper respiratory tract (oropharynx) conditional pathogen culture analysis of the pediatric patients with AH included Staphylococcus aureus, Streptococcus pneumoniae, Moraxella catarrhalis, and Haemophilus influenzae (Table 3). Univariate analysis showed that the incidence of OME was 22.97% in the conditional pathogen-positive group, which was higher than that in the conditional pathogen-negative group (11.11%) (P = 0.0357). The incidence of OME was 34.88% in the S. pneumoniae-positive group, which was higher than that in the S. pneumoniae-negative group (15.25%) (P = 0.0033). The incidence of OME was 33.33% in the H. influenzae-positive group, which was higher than that in the H. influenzae-negative group (16.84%) (P = 0.0327). The difference between the two groups was statistically significant (P < 0.05).

Table 3. Relationship between conditional pathogen culture and patients with adenoid hypertrophy with otitis media with effusion.
The conditional pathogen culture analysis showed that age and adenoid grade were correlated with AH complicated by OME (P < 0.05) (Supplementary Table S1). Hence, after correcting for age and adenoid grade, the relationship between the presence of conditional pathogens and AH complicated by OME was analyzed (Table 4). Oropharyngeal S. pneumoniae positivity was found to be correlated with AH complicated by OME [P = 0.0161, OR: 2.647 (1.198–5.847)].
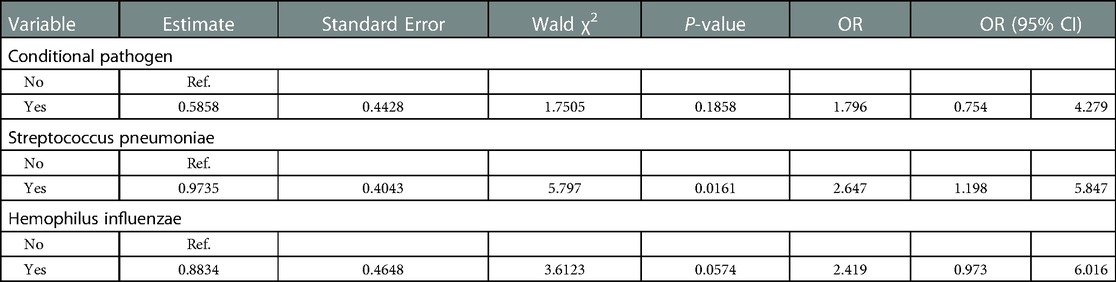
Table 4. Relationship between conditional pathogen culture and adenoid hypertrophy with otitis media with effusion (corrected for age and adenoid grade).
4. Discussion
OME is a major cause of hearing loss in children and can affect language and behavioral development (15). Epidemiological surveys have shown that more than 50% of children aged < 1 year and 60% of children aged <2 years have a history of OME (16). The high prevalence of OME among young patients is due to their incomplete structural and functional development of ET, which are affected by age-related ET factors, including length, angle, and closure capacity (17). In our study, age group analysis revealed that the proportion of pediatric patients with AH complicated by OME aged <4 years was higher than that of the other age groups. The results of the analysis also showed that the risk for OME decreased with increasing age. The difference between the two groups was statistically significant (P < 0.05). There is no consensus on the relationship between gender and OME. The disease is expected to be more common in boys as mastoid pneumatization is more rapid in girls and boys experience upper respiratory infection episodes more frequently. Some studies also showed that OME is more common in males (18). On the contrary, other studies showed no relationship between sex and the prevalence of OME (19–21). In our study, there was no statistical difference in sex distribution between the AH and AH + OME groups (P = 0.2475).
AH is the main cause of ETD, and OME is associated with ETD (10, 22). One study showed that 29.2% of children with adenoid enlargement had a co-morbidity of asymptomatic OME (23). The etiology of OME mainly includes anatomical, immune, microbiological, and environmental factors (6); however, its etiology is not completely clear. Hence, there is a need to examine the mechanisms and factors that influence AH complicated by OME. It is necessary to fully understand the risk factors related to OME incidence in pediatric patients with AH to better screen for and manage this disease. Our study fully collected various data from the etiology and mechanism. A comprehensive and in-depth analysis of these factors early can facilitate appropriate, timely intervention, thereby preventing disease progression. It provides a reference for the prevention and treatment of diseases. It also provides a basis and ideas for researching the deep mechanism and correlation of each influencing factor.
4.1. Mechanical obstruction
Hypertrophic adenoids, particularly tissues near the torus and pharyngeal opening of the ET, can directly compress and obstruct the ET, resulting in impaired middle ear drainage, negative middle ear pressure, mucosal exudation, and OME. Studies have shown that the degree of AH is significantly correlated with OME and that the greater the degree, the higher the incidence of OME. In addition, OME tends to persist, and conservative treatment tends to fail in cases of higher degrees of AH. Children with a higher grade of AH have a higher risk of OME persistence, leading to conservative treatment failure and requiring surgical intervention, but this study had a limited sample size of only 65 cases (24). The present study showed that the incidence of OME in the adenoid grade IV group was 26.86%, which was significantly higher than that in the grade III group (14.5%) (P = 0.0005). The incidence of OME in the adenoid grade IV group was 1.662 (1.014–2.723) times than that in the grade III group. A high adenoid grade is a risk factor for AH complicated by OME. The higher the adenoid grade, the greater the respiratory tract obstruction and the more severe the OSA in pediatric patients (25). However, PSG monitoring data analysis in this study revealed no significant correlation between the AHI, OAI, diagnosis and severity of OSA, and the incidence of OME in pediatric patients with AH (P > 0.05). This might be due to the population distribution in the present study and multiple potential factors. Further in-depth studies are required to clarify the relationship between these variables and the incidence of OME. Regardless, clinicians should be vigilant in managing pediatric patients with AH and OME who present with OSA. Timely screening and intervention should be performed in such cases.
4.2. Pathogenic microorganisms
OME may be a sequela of acute otitis media (AOM). OME tends to occur in cases of AH and ETD; however, the middle ear environment determines the occurrence of OME (26). The upper respiratory tract is an important region for the occurrence of otitis media. According to the pathogen reservoir theory, long-term retention of pathogen-carrying secretions in hypertrophic adenoid crypts can become a microorganism “reservoir”. Pathogens can reach the middle ear through the ET, causing disease. Bacteria detected in adenoid tests include normal bacteria and conditional pathogens in the nasopharynx. Conditional pathogens mainly include S. pneumoniae, H. influenzae, S. aureus, and M. catarrhalis. These pathogens can cause otitis media, nasal sinusitis, upper respiratory tract infections, pneumonia, and systemic diseases (26–30). Typical ear pathogens isolated from middle ear effusions, nasopharyngeal samples, and adenoid samples include S. pneumoniae, H. influenzae, and M. catarrhalis. In addition, multi-pathogen infections are often present in OME (31, 32). After viral infection of the upper respiratory tract occurs, the pathogens can ascend to the middle ear through the ET (33) and disrupt the respiratory tract flora. Viruses can cause bacteria to transform from commensal to pathogenic, disrupt the airway epithelial barrier, decrease mucus and cilia clearance, induce the host to provide nutrients to pathogens, and promote adhesion and virulence in ear pathogens (34). Oropharyngeal and nasopharyngeal pathogens in children tend to translocate to the middle ear owing to the structural characteristics of the ET and the middle ear negative pressure caused by AH (35). Some studies have compared the bacteriology of the adenoids and tonsils in children by culture, and found an overall similarity in the bacteria sampled from the surfaces of tonsils and adenoids of children (36, 37). Surface samples from the nasopharynx and oropharynx may easily be contaminated by saliva, tears, and other secretions. Other studies used 16S rRNA gene pyrosequencing. One study found that the microbiome differs between crypts of the adenoids and crypts of the tonsils, including the relative abundances of potential pathogens such as H. influenzae, S. pneumoniae, and M. catarrhalis (38). Another study has reported combined analyses of the adenoids and tonsils microbiome in pediatric. The microbiome was not significantly different at the phylum level between the adenoids and tonsils (39). In future studies, 16S rRNA gene pyrosequencing technology can be used to identify the differences in bacterial distribution. In addition, whole-genome sequencing should be conducted to analyze the specificity of bacterial capsules and virulence factors. In our study, conditional pathogen culture analysis of the oropharynx which represented the upper respiratory tract revealed that the conditional pathogens detected in the oropharynx of the pediatric patients mainly consisted of S. aureus, S. pneumoniae, M. catarrhalis, and H. influenzae. The results of the univariate analysis (Table 3) showed that the incidence of OME was higher in the conditional pathogen-positive group, S. pneumoniae-positive group, and H. influenzae-positive group than in the corresponding negative groups; the inter-group differences were statistically significant (P < 0.05). In addition, there were statistically significant differences in age and adenoid grade between the groups (P < 0.05) (Supplementary Table S1). After correcting for the effects of age and adenoid grade on the incidence of OME, the results showed that presence of S. pneumoniae in the oropharynx is a risk factor for the occurrence of OME in pediatric patients with AH. The incidence of OME in the S. pneumoniae-positive group was 2.647 times that in the S. pneumoniae-negative group [P = 0.0161, OR: 2.647 (1.198–5.847)] (Table 4). Future studies with larger sample sizes are needed for further analysis of these results.
4.3. Local immune dysregulation in adenoids and allergic reactions
The middle ear is an independent immune organ that is structurally connected to the ET and the upper respiratory tract. Based on the same airway principle, antigens that stimulate the nasal mucosa can also produce mucosal immune responses in the ET and the middle ear. Adenoids contain T and B lymphocytes in different developmental stages and are major secondary lymphoid organs that constitute Waldeyer's ring. They participate in innate and acquired immunity to resist upper respiratory tract infections in children. Local immune dysregulation decreases adenoid barrier function and increases the incidence of host OME. There are differences in adenoid lymphocyte subset distribution in pediatric patients with AH. Patients with AH + OME show higher T and B lymphocyte counts than those with AH only, which leads to increased local antigen-presenting dendritic cell count (40), increased capture of airway pathogens, decreased immune function, and increased host susceptibility. Immunoglobulins produced by B cells are an important defense mechanism against otitis media and other upper respiratory tract infections (41). The present study showed that AR increases the risk for OME in pediatric patients with AH. AR is an IgE-mediated type I hypersensitivity reaction that is characterized by increased vascular permeability, increased mast cells and related inflammatory cell secretion of histamine, leukotriene, and other inflammatory mediators, occurrence of mucosal edema and exudation, obstruction of the ET, and decreased cilia motility, resulting in OME. Allergies contribute to the occurrence and progression of AH and OME. The incidence of OME is significantly higher in pediatric patients with AR than in pediatric patients without AR (42, 43). However, the specific incidence of OME is determined by population characteristics and diagnostic criteria. A systematic review of the pathophysiology by which allergy increases the risk of otitis media showed that allergy is related to the occurrence and acute exacerbation of OME (44). Allergen stimulation causes local immune responses, elevated Th2 cytokine secretion, weakened upper respiratory tract mucosal barrier function, increased nasopharyngeal bacteria (45), and increased incidence of OME. IgE, IL-4, histamine, and eosinophil levels in middle ear effusions are increased in patients with OME. In patients with AR, IL-4, IL-5, IFN-γ, and histamine secretions in the nasal mucosa are increased (46). In a previous study of a mouse model of OME created by inducing middle ear infection, middle ear mucosal immune responses towards bacterial lipopolysaccharides were increased in the AR group (47).
Besides etiological factors, several immutable factors such as genetic factors and family history, and variable factors such as environment and lifestyle can affect the occurrence and progression of the disease (48). ETS exposure is also known as “secondhand smoking” or “passive smoking”. Animal studies have demonstrated that nicotine can stimulate the hypothalamus α3β4 nicotinic acetylcholine receptor to decrease appetite, energy intake, and body weight (49). Parental smoking is a common source of ETS exposure in children. Interventional measures should be employed to decrease ETS exposure in children and improve their health (50, 51). In the United States, 29.2% of adolescents are exposed to ETS (52).Thus, protocols for improving home-smoking behavior should be studied and implemented (53). ETS exposure and AR in children are associated with increased prevalence of eczema (54). Studies have shown that passive smoking and ETS exposure are environmental risk factors for OME in children (20, 55). Maternal smoking habits and the number of family members who are smokers are significantly correlated with the risk of otitis media in children aged < 4 years old (56). However, some studies did not show an association between ETS exposure and otitis media in children (19). Our study showed that ETS exposure is a risk factor for the occurrence of OME in pediatric patients with AH. The main harmful components of cigarettes, including nicotine, tar, carbon monoxide, and acrolein, can cause respiratory diseases (57) and destroy ET surfactants. Cilia toxins decrease ciliary beat frequency and cause the ET mucosa and cilia in children to be susceptible to damage. Thus, adult smoking can disrupt upper respiratory tract flora (58) and affect children.
Our study showed that the incidence of OME in breastfed pediatric patients was 17.32%, which was lower than that in the patients who were not (29.23%) (P = 0.0036). This indicates that breastfeeding is a protective factor against the occurrence of OME in pediatric patients with AH (Table 2). Breast milk not only contains antibacterial substances, but can also promote the development of healthy flora and is negatively correlated with respiratory tract infections (59, 60). In addition, breast milk can optimize immune function and decrease the risk of otitis media and respiratory tract infections in infants and toddlers (61–63). A Lancet paper analyzed the potential mechanisms underlying the effects of breastfeeding in terms of immunology, epigenetics, microbiomics, and stem cell research. Increased breastfeeding can decrease rate of mortality in children and prevent infectious diseases. In addition, the protective effects of breastfeeding against otitis media in children can extend up to age 2 and older (64). In addition to providing environmental pathogen-specific IgA, breast milk can develop non-specific defenses against bacterial pathogens. Besides preventing upper respiratory tract infections, maternal antibodies can also act on middle ear pathogens and interfere with bacterial adhesion to the nasopharyngeal epithelium to prevent acute otitis media (65). Antigen stimulation can result in the production of IgG antibodies against non-typeable H. influenzae (66) and regulate an infant's humoral immune responses towards common OME pathogens. Milk bottle suction and the swallowing pressure gradient can cause ETD and increase susceptibility to otitis media (67).
In summary, the pathogenesis of AH with OME is complex and is influenced by many factors. Younger age and a high adenoid grade are major risk factors for the occurrence of OME in pediatric patients with AH. ETS exposure, a non-breastfed status, AR, and presence of conditional pathogens (mainly S. pneumoniae) in the upper respiratory tract also influence the pathogenesis of AH complicated by OME. This study provides a foundation and basis for future etiological mechanism studies and treatments. It is necessary to study deep mechanisms and correlations in the future, such as the establishment of animal models related to the incidence of OME influencing factors and whole-genome sequencing for the analysis of the specificity of bacterial capsules and virulence factors.
The presented study still had some main limitations. The sample sizes were limited, especially the cases of conditioned pathogen culture. In addition, the study time range included the global COVID-19 pandemic, which involved Beijing's strict implementation of the dynamic zero-COVID policy during this period. The prevalence of the novel coronavirus did not affect the incidence and progression of the disease in the study population. However, the overall number of patients hospitalized for AH and related diseases decreased compared to before the epidemic. In particular, there were very few patients from other cities, which seemed to have a potentially unavoidable effect on the distribution of the study population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Beijing Tsinghua Changgung Hospital Ethics Committee. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
CWJ collected data, statistical analysis, and wrote the article. YGP revised the manuscript. CYJ, WYY, ZCM, and WW participated in data collection. WLJ proposed ideas for the experiment. YJY helped with study design and project administration. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the National Natural Science Foundation of China [No. 81873696], Tsinghua University Spring Breeze Fund [No. 20211080045], and the Beijing Municipal Administration of Hospitals Incubating Program [NO. PX2022040]
Acknowledgments
The authors would like to thank all the reviewers who participated in the review. We also would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1098067/full#supplementary-material.
References
1. Kheirandish-Gozal L, Gozal D. Obesity, asthma, and sleep-disordered breathing. J Pediatr. (2012) 160:713–4. doi: 10.1016/j.jpeds.2011.11.036
2. Dogru M, Evcimik MF, Calim OF. Does adenoid hypertrophy affect disease severity in children with allergic rhinitis. Eur Arch Otorhinolaryngol. (2017) 274:209–13. doi: 10.1007/s00405-016-4196-x
3. Rosenfeld RM, Culpepper L, Doyle KJ, Grundfast KM, Hoberman A, Kenna MA, et al. Clinical practice guideline: otitis media with effusion. Otolaryngol Head Neck Surg. (2004) 130:S95–118. doi: 10.1016/j.otohns.2004.02.002
4. Abdel Tawab HM, Tabook SMS. Correlation between adenoid hypertrophy, tympanometry findings, and viscosity of middle ear fluid in chronic otitis media with effusion, southern Oman. Ear Nose Throat J. (2021) 100:NP141–6. doi: 10.1177/0145561319875438
5. Teschner M. Evidence and evidence gaps in the treatment of eustachian tube dysfunction and otitis media. GMS Curr Top Otorhinolaryngol Head Neck Surg. (2016) 15:Doc05. doi: 10.3205/cto000132
6. Buzatto GP, Tamashiro E, Proenca-Modena JL, Saturno TH, Prates MC, Gagliardi TB, et al. The pathogens profile in children with otitis media with effusion and adenoid hypertrophy. PLoS One. (2017) 12:e0171049. doi: 10.1371/journal.pone.0171049
7. Songu M, Islek A, Imre A, Aslan H, Aladag I, Pinar E, et al. Risk factors for otitis media with effusion in children with adenoid hypertrophy. Acta Otorhinolaryngol Ital. (2020) 40:133–7. doi: 10.14639/0392-100X-2456
8. Ingram DG, Friedman NR. Toward adenotonsillectomy in children: a review for the general pediatrician. JAMA Pediatr. (2015) 169:1155–61. doi: 10.1001/jamapediatrics.2015.2016
9. Rosenfeld RM, Shin JJ, Schwartz SR, Coggins R, Gagnon L, Hackell JM, et al. Clinical practice guideline: otitis media with effusion executive summary (update). Otolaryngol Head Neck Surg. (2016) 154:201–14. doi: 10.1177/0194599815624407
10. Cassano P, Gelardi M, Cassano M, Fiorella ML, Fiorella R. Adenoid tissue rhinopharyngeal obstruction grading based on fiberendoscopic findings: a novel approach to therapeutic management. Int J Pediatr Otorhinolaryngol. (2003) 67:1303–9. doi: 10.1016/j.ijporl.2003.07.018
11. Friedman M, Tanyeri H, La Rosa M, Landsberg R, Vaidyanathan K, Pieri S, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. (1999) 109:1901–7. doi: 10.1097/00005537-199912000-00002
12. Evcimik MF, Dogru M, Cirik AA, Nepesov MI. Adenoid hypertrophy in children with allergic disease and influential factors. Int J Pediatr Otorhinolaryngol. (2015) 79:694–7. doi: 10.1016/j.ijporl.2015.02.017
13. Olusesi AD, Undie NB, Amodu JE. Allergy history as a predictor of early onset adenoids/adenotonsillar hypertrophy among Nigerian children. Int J Pediatr Otorhinolaryngol. (2013) 77:1032–5. doi: 10.1016/j.ijporl.2013.04.004
14. Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. (1992) 146:1235–9. doi: 10.1164/ajrccm/146.5_Pt_1.1235
15. Samuels TL, Yan JC, Khampang P, Dettmar PW, MacKinnon A, Hong W, et al. Association of gel-forming mucins and aquaporin gene expression with hearing loss, effusion viscosity, and inflammation in otitis media with effusion. JAMA Otolaryngol Head Neck Surg. (2017) 143:810–7. doi: 10.1001/jamaoto.2017.0386
16. Simon F, Haggard M, Rosenfeld RM, Jia H, Peer S, Calmels M-N, et al. International consensus (ICON) on management of otitis media with effusion in children. Eur Ann Otorhinolaryngol Head Neck Dis. (2018) 135:S33–9. doi: 10.1016/j.anorl.2017.11.009
17. Vanneste P, Page C. Otitis media with effusion in children: pathophysiology, diagnosis, and treatment. A review. J Otol. (2019) 14:33–9. doi: 10.1016/j.joto.2019.01.005
18. Ungkanont K, Charuluxananan S, Komoltri C. Association of otoscopic findings and hearing level in pediatric patients with otitis media with effusion. Int J Pediatr Otorhinolaryngol. (2010) 74:1063–6. doi: 10.1016/j.ijporl.2010.06.006
19. Gultekin E, Develioğlu ON, Yener M, Ozdemir I, Külekçi M. Prevalence and risk factors for persistent otitis media with effusion in primary school children in Istanbul, Turkey. Auris Nasus Larynx. (2010) 37:145–9. doi: 10.1016/j.anl.2009.05.002
20. Erdivanli OC, Coskun ZO, Kazikdas KC, Demirci M. Prevalence of otitis media with effusion among primary school children in eastern Black Sea, in Turkey and the effect of smoking in the development of otitis media with effusion. Indian J Otolaryngol Head Neck Surg. (2012) 64:17–21. doi: 10.1007/s12070-011-0131-z
21. Kucur C, Şimşek E, Kuduban O, Özbay İ. Prevalence of and risk factors for otitis media with effusion in primary school children: case control study in erzurum, Turkey. Turk J Pediatr. (2015) 57:230–5.26701940
22. Manno A, Iannella G, Savastano V, Vittori T, Bertin S, Pasquariello B, et al. Eustachian tube dysfunction in children with adenoid hypertrophy: the role of adenoidectomy for improving ear ventilation. Ear Nose Throat J. (2021):145561321989455. doi: 10.1177/0145561321989455
23. Sogebi OA, Oyewole EA, Ogunbanwo O. Asymptomatic otitis media with effusion in children with adenoid enlargement. J Natl Med Assoc. (2021) 113:158–64. doi: 10.1016/j.jnma.2020.08.005
24. Galić MZ, Klančnik M. Adenoid size in children with otitis media with effusion. Acta Clin Croat. (2022) 60:532–9. doi: 10.20471/acc.2021.60.03.25
25. Xiao L, Su S, Liang J, Jiang Y, Shu Y, Ding L. Analysis of the risk factors associated with obstructive sleep apnea syndrome in Chinese children. Front Pediatr. (2022) 10:900216. doi: 10.3389/fped.2022.900216
26. Chan CL, Wabnitz D, Bardy JJ, Bassiouni A, Wormald P-J, Vreugde S, et al. The microbiome of otitis media with effusion. Laryngoscope. (2016) 126:2844–51. doi: 10.1002/lary.26128
27. Johnston JJ, Douglas R. Adenotonsillar microbiome: an update. Postgrad Med J. (2018) 94:398–403. doi: 10.1136/postgradmedj-2018-135602
28. Johnston J, Hoggard M, Biswas K, Astudillo-García C, Radcliff FJ, Mahadevan M, et al. Pathogen reservoir hypothesis investigated by analyses of the adenotonsillar and middle ear microbiota. Int J Pediatr Otorhinolaryngol. (2019) 118:103–9. doi: 10.1016/j.ijporl.2018.12.030
29. Ren T, Glatt DU, Nguyen TN, Allen EK, Early SV, Sale M, et al. 16S rRNA survey revealed complex bacterial communities and evidence of bacterial interference on human adenoids. Environ Microbiol. (2013) 15:535–47. doi: 10.1111/1462-2920.12000
30. Huang C-C, Chang T-H, Lee C-Y, Wu P-W, Chen C-L, Lee T-J, et al. Tissue microbiota in nasopharyngeal adenoid and its association with pneumococcal carriage. Microb Pathog. (2021) 157:104999. doi: 10.1016/j.micpath.2021.104999
31. Silva MD, Lima A, Marçal N, Dias L, Gama M, Sillankorva S. Identification of the bacterial pathogens in children with otitis media: a study in the northwestern Portuguese district of Braga. Microorganisms. (2021) 10:54. doi: 10.3390/microorganisms10010054
32. Jörissen J, van den Broek M, De Boeck I, Beeck WV, Wittouck S, Boudewyns A, et al. Case-control microbiome study of chronic otitis media with effusion in children points at streptococcus salivarius as a pathobiont-inhibiting species. mSystems. (2021) 6:e00056–21. doi: 10.1128/mSystems.00056-21
33. Lappan R, Jamieson SE, Peacock CS. Reviewing the pathogenic potential of the otitis-associated bacteria Alloiococcus otitidis and Turicella otitidis. Front Cell Infect Microbiol. (2020) 10:51. doi: 10.3389/fcimb.2020.00051
34. Walker RE, Walker CG, Camargo CA Jr, Bartley J, Flint D, Thompson JMD, et al. Nasal microbial composition and chronic otitis media with effusion: a case-control study. PLoS One. (2019) 14:e0212473. doi: 10.1371/journal.pone.0212473
35. Minovi A, Dazert S. Diseases of the middle ear in childhood. Laryngorhinootologie. (2014) 93(Suppl 1):S1–23. doi: 10.1055/s-0033-1363213
36. Brook I, Shah K. Bacteriology of adenoids and tonsils in children with recurrent adenotonsillitis. Ann Otol Rhinol Laryngol. (2001) 110:844–8. doi: 10.1177/000348940111000908
37. Taylan I, Ozcan I, Mumcuoğlu I, Baran I, Murat Özcan K, Akdoğan O, et al. Comparison of the surface and core bacteria in tonsillar and adenoid tissue with Beta-lactamase production. Indian J Otolaryngol Head Neck Surg. (2011) 63:223–8. doi: 10.1007/s12070-011-0265-z
38. Fagö-Olsen H, Dines LM, Sørensen CH, Jensen A. The adenoids but not the palatine tonsils serve as a reservoir for bacteria associated with secretory otitis media in small children. mSystems. (2019) 4:e00169–18. doi: 10.1128/mSystems.00169-18
39. Kim KS, Min HJ. Correlations between the adenotonsillar microbiome and clinical characteristics of pediatric patients with snoring. Clin Exp Otorhinolaryngol. (2021) 14:295–302. doi: 10.21053/ceo.2020.01634
40. Kotowski M, Niedzielski A, Niedzielska G, Lachowska-Kotowska P. Dendritic cells and lymphocyte subpopulations of the adenoid in the pathogenesis of otitis media with effusion. Int J Pediatr Otorhinolaryngol. (2011) 75:265–9. doi: 10.1016/j.ijporl.2010.11.014
41. Jung SY, Kim D, Park DC, Lee EH, Choi Y-S, Ryu J, et al. Immunoglobulins and transcription factors in otitis media. Int J Mol Sci. (2021) 22:3201. doi: 10.3390/ijms22063201
42. Cheng X, Sheng H, Ma R, Gao Z, Han Z, Chi F, et al. Allergic rhinitis and allergy are risk factors for otitis media with effusion: a meta-analysis. Allergol Immunopathol (Madr). (2017) 45:25–32. doi: 10.1016/j.aller.2016.03.004
43. Pau BC, Ng DK. Prevalence of otitis media with effusion in children with allergic rhinitis, a cross sectional study. Int J Pediatr Otorhinolaryngol. (2016) 84:156–60. doi: 10.1016/j.ijporl.2016.03.008
44. De Corso E, Cantone E, Galli J, Seccia V, Lucidi D, Di Cesare T, et al. Otitis media in children: which phenotypes are most linked to allergy? A systematic review. Pediatr Allergy Immunol. (2021) 32:524–34. doi: 10.1111/pai.13431
45. Chawes BLK. Upper and lower airway pathology in young children with allergic- and non-allergic rhinitis. Dan Med Bull. (2011) 58:B4278.21535990
46. Steelant B, Seys SF, Van Gerven L, Van Woensel M, Farré R, Wawrzyniak P, et al. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. (2018) 141:951–63.e8. doi: 10.1016/j.jaci.2017.08.039
47. Kim DK, Park HE, Back SA, Park HR, Kim SW, Park Y, et al. Otitis media with effusion in an allergic animal model: a functional and morphological study. Int J Pediatr Otorhinolaryngol. (2016) 84:6–11. doi: 10.1016/j.ijporl.2016.02.018
48. Szőke H, Maródi M, Vagedes J, Székely B, Magyarosi I, Bedő A, et al. The P.E.A.N.U.T. method: update on an integrative system approach for the treatment of chronic otitis media with effusion and adenoid hypertrophy in children. Antibiotics (Basel). (2021) 10:134. doi: 10.3390/antibiotics10020134
49. Seeley RJ, Sandoval DA. Neuroscience: weight loss through smoking. Nature. (2011) 475:176–7. doi: 10.1038/475176a
50. Chiswell C, Akram Y. Impact of environmental tobacco smoke exposure on anaesthetic and surgical outcomes in children: a systematic review and meta-analysis. Arch Dis Child. (2017) 102:123–30. doi: 10.1136/archdischild-2016-310687
51. Behbod B, Sharma M, Baxi R, Roseby R, Webster P. Family and carer smoking control programmes for reducing children's Exposure to environmental tobacco smoke. Cochrane Database Syst Rev. (2018) 1:CD001746. doi: 10.1002/14651858.CD001746.pub4
52. Jain RB. Rates of exposure to environmental tobacco smoke from various indoor environments among US children and nonsmoker adolescents and adults. Environ Sci Pollut Res Int. (2018) 25:17002–11. doi: 10.1007/s11356-018-1891-8
53. Ferris E, Cummins C, Chiswell C, Jones L. Exploring stakeholder views on intervening in hospital around childhood secondhand smoke exposure (Precedent): a protocol for a qualitative study. BMJ Open. (2021) 11:e047817. doi: 10.1136/bmjopen-2020-047817
54. Singh S, Sharma BB, Salvi S, Chhatwal J, Jain KC, Kumar L, et al. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Respir J. (2018) 12:547–56. doi: 10.1111/crj.12561
55. Kırıs M, Muderris T, Kara T, Bercin S, Cankaya H, Sevil E. Prevalence and risk factors of otitis media with effusion in school children in eastern anatolia. Int J Pediatr Otorhinolaryngol. (2012) 76:1030–5. doi: 10.1016/j.ijporl.2012.03.027
56. Jensen RG, Koch A, Homøe P, Bjerregaard P. Tobacco smoke increases the risk of otitis media among Greenlandic inuit children while exposure to organochlorines remain insignificant. Environ Int. (2013) 54:112–8. doi: 10.1016/j.envint.2013.01.015
57. Kong S-K, Chon K-M, Goh E-K, Lee I-W, Lee J-W, Wang S-G. Histologic changes in the auditory tube mucosa of rats after long-term exposure to cigarette smoke. Am J Otolaryngol. (2009) 30:376–82. doi: 10.1016/j.amjoto.2008.07.009
58. Charlson ES, Chen J, Custers-Allen R, Bittinger K, Li H, Sinha R, et al. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS One. (2010) 5:e15216. doi: 10.1371/journal.pone.0015216
59. Biesbroek G, Bosch AATM, Wang X, Keijser BJF, Veenhoven RH, Sanders EAM, et al. The impact of breastfeeding on nasopharyngeal microbial communities in infants. Am J Respir Crit Care Med. (2014) 190:298–308. doi: 10.1164/rccm.201401-0073OC
60. de Steenhuijsen Piters WAA, Sanders EAM, Bogaert D. The role of the local microbial ecosystem in respiratory health and disease. Philos Trans R Soc Lond B Biol Sci. (2015) 370:20140294. doi: 10.1098/rstb.2014.0294
61. SŞ E, Öztürk M, Sevinç R, Derin S, Dinç AE, Erdem D. Risk factors for otitis media effusion in children who have adenoid hypertrophia. Int J Pediatr Otorhinolaryngol. (2015) 79:374–7. doi: 10.1016/j.ijporl.2014.12.030
62. Martines F, Salvago P, Ferrara S, Messina G, Mucia M, Plescia F, et al. Factors influencing the development of otitis media among Sicilian children affected by upper respiratory tract infections. Braz J Otorhinolaryngol. (2016) 82:215–22. doi: 10.1016/j.bjorl.2015.04.002
63. Lodge CJ, Bowatte G, Matheson MC, Dharmage SC. The role of breastfeeding in childhood otitis media. Curr Allergy Asthma Rep. (2016) 16:68. doi: 10.1007/s11882-016-0647-0
64. Victora CG, Bahl R, Barros AJD, França GVA, Horton S, Krasevec J, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. (2016) 387:475–90. doi: 10.1016/S0140-6736(15)01024-7
65. Abrahams SW, Labbok MH. Breastfeeding and otitis media: a review of recent evidence. Curr Allergy Asthma Rep. (2011) 11:508–12. doi: 10.1007/s11882-011-0218-3
66. Sabirov A, Casey JR, Murphy TF, Pichichero ME. Breast-feeding is associated with a reduced frequency of acute otitis media and high serum antibody levels against NTHi and outer membrane protein vaccine antigen candidate P6. Pediatr Res. (2009) 66:565–70. doi: 10.1203/PDR.0b013e3181b4f8a6
Keywords: adenoid hypertrophy, otitis media with effusion, influencing factor, obstructive sleep apnea, conditional pathogen
Citation: Chen W, Yin G, Chen Y, Wang L, Wang Y, Zhao C, Wang W and Ye J (2023) Analysis of factors that influence the occurrence of otitis media with effusion in pediatric patients with adenoid hypertrophy. Front. Pediatr. 11:1098067. doi: 10.3389/fped.2023.1098067
Received: 14 November 2022; Accepted: 7 February 2023;
Published: 22 February 2023.
Edited by:
Yanwei Dang, Zhengzhou Central Hospital Affiliated to Zhengzhou University, ChinaReviewed by:
Kate C Chan, The Chinese University of Hong Kong, ChinaVasile Valeriu Lupu, Grigore T. Popa University of Medicine and Pharmacy, Romania
© 2023 Chen, Yin, Chen, Wang, Wang, Zhao, Wang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingying Ye eWVqaW5neWluZ0BtYWlsLnRzaW5naHVhLmVkdS5jbg==
Specialty Section: This article was submitted to Pediatric Otolaryngology, a section of the journal Frontiers in Pediatrics
Abbreviations AH, adenoid hypertrophy; OME, otitis media with effusion; BMI, body mass index; AR, allergic rhinitis; ETS, environmental tobacco smoke; OSA, obstructive sleep apnea; ETD, eustachian tube dysfunction; ET, eustachian tube; PSG, polysomnography; OAI, obstructive apnea index; AHI, apnea hypopnea index; S. pneumoniae, Streptococcus pneumoniae; H. influenzae, Haemophilus influenzae; S. aureus, Staphylococcus aureus; M. catarrhalis, Moraxella catarrhalis.
 Wenjing Chen1
Wenjing Chen1 Guoping Yin
Guoping Yin Lijun Wang
Lijun Wang Jingying Ye
Jingying Ye