- Department of Neonatology, Fuzhou Children's Hospital of Fujian Medical University, Fuzhou, People's Republic of China
Objective: This study aims to review the evidence for the optimal regimen of probiotics for the prevention of necrotizing enterocolitis (NEC) in very low birth weight infants.
Design: Through searching PubMed, EMBASE, Cochrane Library, and Web of Science till September 30, 2022, only randomized controlled trials were included to evaluate the optimal regimen of probiotics for the prevention of NEC in very low birth weight infants. The methodological quality of the included studies was assessed by the Cochrane risk of bias assessment tool (RoB 2), and the collected data were analyzed accordingly using Stata software.
Results: Twenty-seven RCTs were included, and the total sample size used in the study was 529. The results of the network meta-analysis showed that Bovine lactoferrin + Lactobacillus rhamnosus GG (RR 0.03; 95% CI 0.00–0.35), Lactobacillus rhamnosus + Lactobacillus plantarum + Lactobacillus casei + Bifidobacterium lactis (RR 0.06; 95% CI 0.00–0.70), Bifidobacterium lactis + inulin (RR 0.16; 95% CI 0.03–0.91) were superior to the control group (Bifidobacterium lactis + Bifidobacterium longum) in reducing the incidence of NEC. The reduction in the incidence of NEC were as follows: Bovine lactoferrin + Lactobacillus rhamnosus GG (SUCRA 95.7%) > Lactobacillus rhamnosus + Lactobacillus plantarum + Lactobacillus casei + Bifidobacterium lactis (SUCRA 89.4%) > Bifidobacterium lactis + inulin (SUCRA 77.8%).
Conclusions: This network meta-analysis suggests that Lactobacillus rhamnosus GG combined with bovine lactoferrin maybe the most recommended regimen for the prevention of NEC in very low birth weight infants.
1. Introduction
Necrotizing enterocolitis (NEC) is a gastrointestinal disease that seriously threatens the life of newborns. Clinically the infant presents with feed intolerance, increased gastric aspirates, vomiting, blood in the stool which may progress to very severe illness including shock and perforation. It is a disease that has plagued neonatal care for a long time and is still relatively common in very low birth weight infants (1). NEC is associated with neurodevelopmental delays, growth retardation, intestinal strictures and adhesions, and short bowel syndrome with or without intestinal failure (2). The high incidence of NEC cannot be ignored, and according to large multi-center neonatal network databases in the United States and Canada (3–5), NEC may occur in 7 out of 100 very low birth weight infants over the decades. Despite overall improvement in survival of preterm infants, a recent review suggests that the mortality and prevalence of NEC in very low birth weight infants has barely changed (6).
NEC, once established, is challenging to stop and has limited and expensive treatments. Treatment methods for NEC include antibiotics, gastric decompression, parenteral nutrition, etc. (7). It is not clear whether NEC is a single entity or a combination of similar entities and while progress has been made in understanding the pathogenesis of NEC there is still lack of clarity on many fronts which has perhaps contributed to a lack of significant progress in the treatment of NEC over the last many decades (8). On the other hand, although very low birth weight infants account for only a small proportion of newborns, the cost of treatment is indeed disproportionate. It has been reported that NEC causes more than 1 billion dollars in losses to medical institutions (9). It is worth noting that about 40% of NEC cases require surgical intervention (10), and the cost of treatment for infants requiring surgery has also significantly increased. All these factors lead to a considerable economic burden on the family and society.
Multiple research studies have explored various interventions for prevention of NEC including the provision of human milk and microbial therapeutics, with probiotic therapy garnering the most attention. Shiloh R. Lueschow et al. found that Bifidobacterium infantis EVC001 can prevent NEC in mice through anti-inflammatory and epithelial barrier-restoring properties (11). The study by Xiu-Li Zhu et al. has shown that probiotics supplementation can reduce the incidence and severity of NEC in preterm neonates (12), which seems to be related to the functions of probiotics in regulating immunity and inhibiting the imbalance of intestinal flora.
Most studies in the past decade have suggested that probiotics can significantly reduce the risk of NEC; However, it is unclear which probiotic or combination of probiotics is more effective (13) and what the optimal dose is. Moreover data on VLBW are scarce, and other related studies have not reported on specific strains of probiotics (14). Therefore, this study aimed to compare the effects of various probiotic regimens on NEC through a network meta-analysis, with direct or indirect comparisons, and to estimate the rank order of each combination. This hopefully will help target further research as well as facilitate improvements in practice.
2. Materials and methods
2.1. Search strategy
This network meta-analysis was conducted following the PRISMA statement, and the protocol for this study was registered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (number INPLASY2022110001).
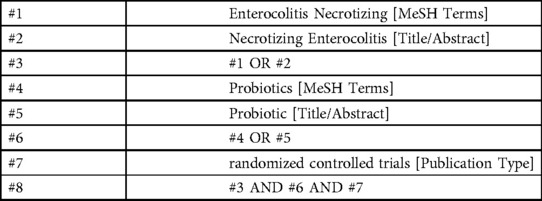
The researchers searched PubMed, EMBASE, Cochrane Library, and Web of Science till September 30, 2022. The search strategy was constructed around the PICOS tool: (P) Population: very low birth weight infants; (I) Intervention: probiotics; (C) Comparator: control group with only placebo or another probiotic usage; (O) Outcomes: necrotizing enterocolitis. (S) Study type: RCTs. The detailed search strategy is shown in (Table 1) (PubMed is used as an example).
2.2. Inclusion criteria
(1) Study designed as RCT; (2) Neonates with birth weight <1500 g; (3) Interventions included probiotics; (4) Reported outcomes included NEC stage ≥ II (Bell staging criteria); (5) The incidence of outcomes given by the study.
2.3. Exclusion criteria
(1) Studies from non-randomized controlled trials, including quasi-randomized controlled trials, non-human subjects, case reports, protocols, correspondence, or conference abstracts; (2) Studies with incomplete or unreported data.
2.4. Study selection
Literature was screened and excluded using the literature management software Endnote. Two researchers first screened papers by title to exclude duplication, non-randomized controlled trial studies, correspondence, review papers, and conference papers. Two researchers then read the abstracts to determine which studies to include and exclude. Finally, two researchers performed full-text readings to further identify the included literature. During this process, two researchers independently screened the literature and compared the remaining literature to determine whether they could be included in the study. Any conflicts were resolved by discussion with a third author.
2.5. Data extraction
A nine-item, standardized, and pre-selected data extraction form was used to record data from included studies under the following headings: (1) author, (2) year of publication, (3) country, (4) sample size, (5) mortality, (6) number of people progressing to NEC, (7) mean age, (8) details of the intervention, (9) overall risk of bias.
2.6. Risk of bias in individual studies
Two researchers independently assessed the risk of bias (RoB 2) according to the Cochrane Handbook version 6.1.0 tool for assessing RoB 2 in RCTs. Five items were considered: (1) randomization process, (2) deviations from intended interventions, (3) missing outcome date, (4) measurement of the outcome, and (5) selection of the reported result. The risk of bias for each domain can be classified into three levels: low risk, some concerns and high risk. If the risk of bias assessment for all domains is “low risk”, then the overall risk of bias is “low risk”; If the risk of bias assessment in some domains are “some concerns” and there is no “high risk” domain, then the overall risk of bias is “some concerns”; As long as the risk of bias assessment in one domain is “high risk”, the overall risk of bias is “high risk”.
2.7. Data analysis
In studies using probiotics as an intervention, outcome variables were dichotomized and expressed as risk ratios (RR) and 95% confidence intervals (CI). Due to potential differences between studies, we decided to use a random-effects model rather than a fixed-effects model to analyze the data.
Data were compiled and analyzed using Markov chain Monte Carlo simulation chains of Stata software (version 15.1) based on a Bayesian framework according to the PRISMA NMA instruction manual. To quantify and demonstrate the agreement between indirect and direct comparisons, we used the nodal method calculated according to the instructions in Stata. The consistency test was passed if the P-value was > 0.05.
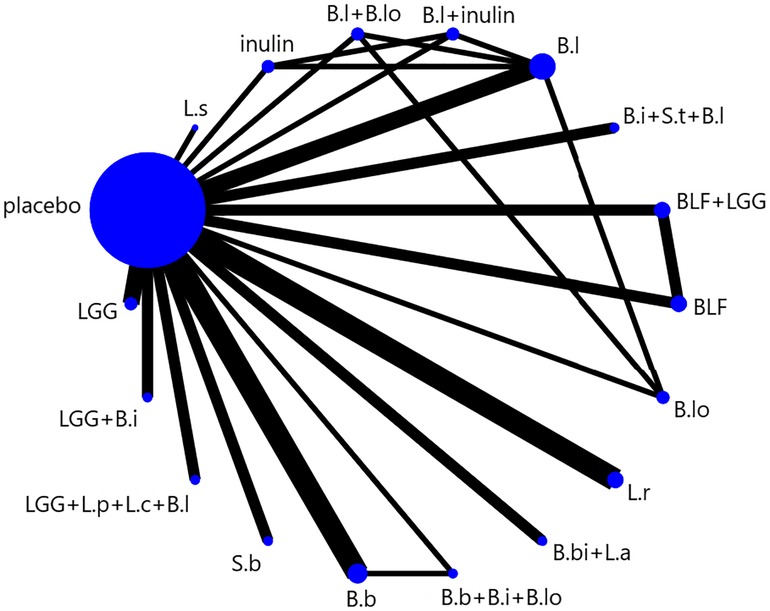
We presented and described network diagrams for different probiotic usage using Stata software. In the presented network diagrams, each node represents a different probiotic usage, and the lines connecting the nodes represent a direct comparison between interventions. The size of each node and the width of the connecting lines are proportional to the number of trials.
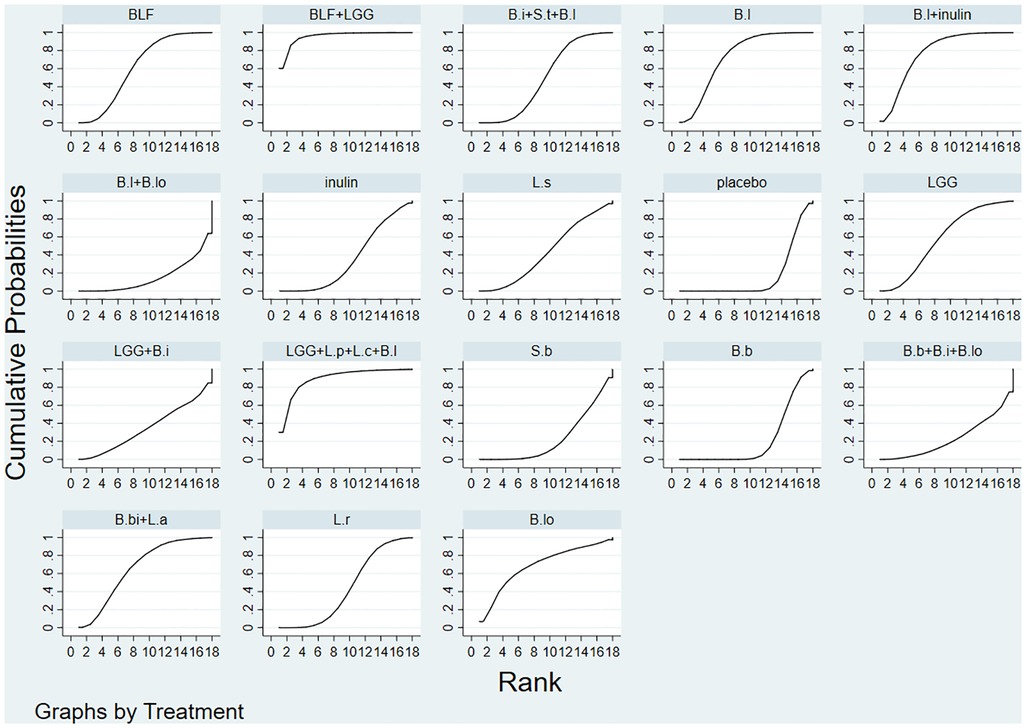
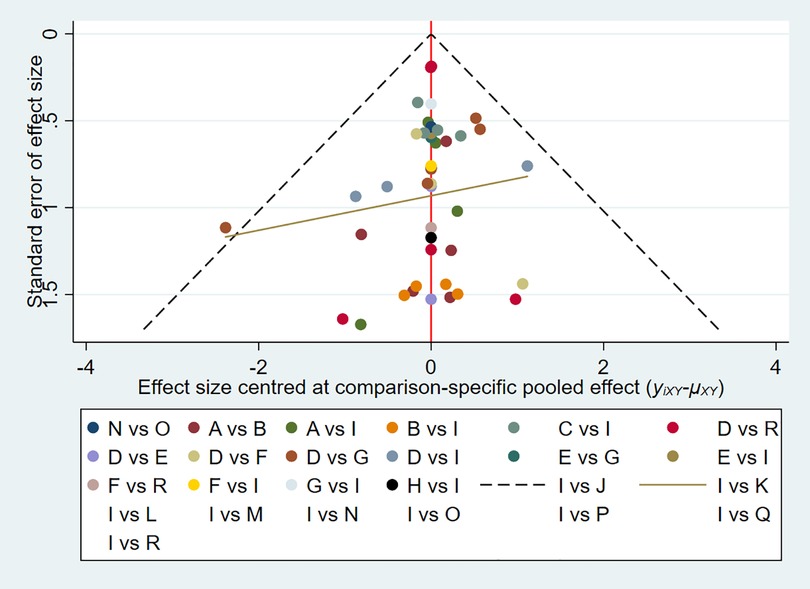
The evaluation of the intervention was summarized and presented in the form of a P score. The P score was considered as a frequency analog to surface under the cumulative ranking curve (SUCRA) values, a measure of the degree of certainty that one treatment is superior to another. The P score ranges from 0 to 1, with 1 representing the best treatment without uncertainty and 0 the worst treatment without uncertainty. The P score or SUCRA could be effectively expressed as a percentage of intervention effectiveness or acceptability, but this score should be interpreted with caution unless there is a genuine clinically meaningful difference between interventions. Small-scale studies could lead to publication bias in NMA, for which we created network funnel plots and checked them visually using symmetry criteria.
3. Results
3.1. Study and identification and selection
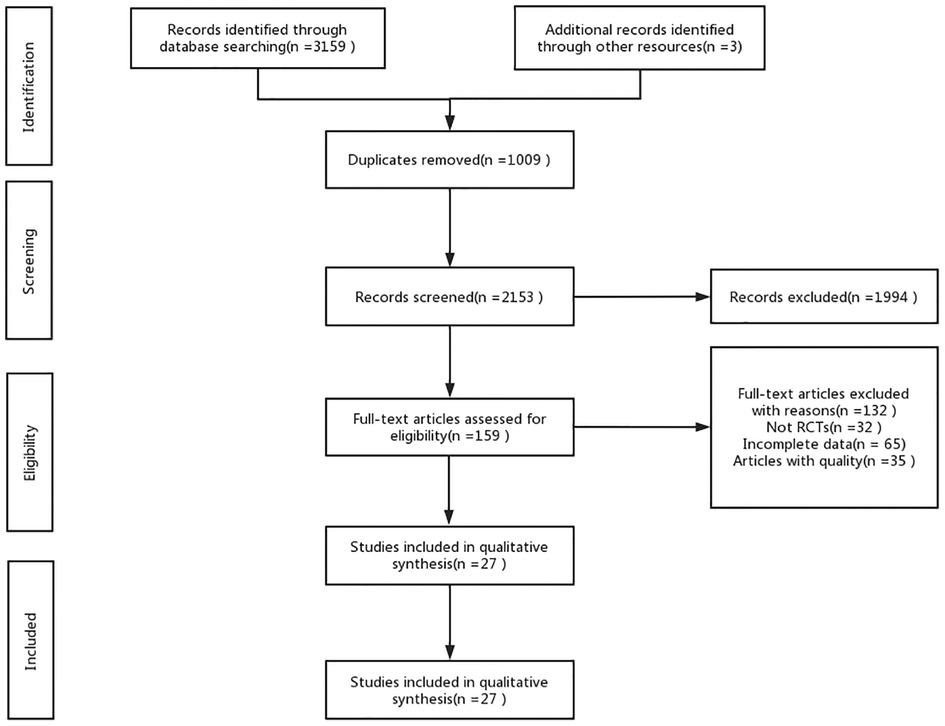
A total of 3,159 documents were retrieved from the electronic database, and an additional three documents were manually searched. After eliminating duplicates, the remaining 2,153 documents were read for titles and abstracts, and 1,994 documents were again excluded. The remaining 159 documents were read in full, and 132 documents were again excluded (for reasons including non-randomized controlled trials, incomplete data, conference papers, and failure to meet the interventions included in this review), leaving a final remaining 27 documents to be included in this study. (Figure 1).
3.2. Quality assessment of the included studies
Seventeen studies were defined as low risk, eight as some concerns, and two as high risk. Only three of these studies did not achieve simultaneous blinding of subjects and measures. Specific details are presented in (Supplementary Table S1).
3.3. Characteristics of the included studies
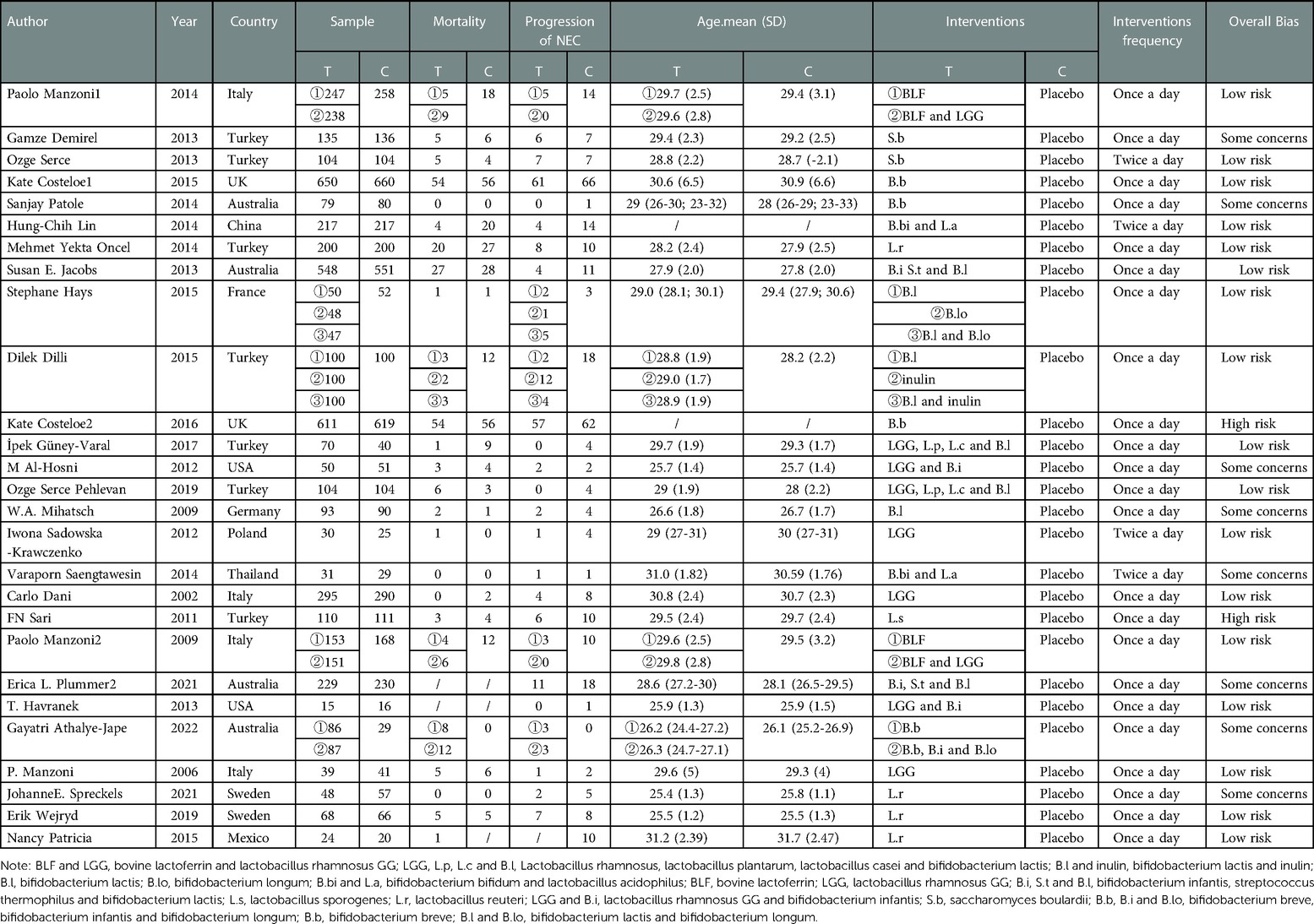
In total, we included studies from 27 randomized controlled trials, which included 529 patients diagnosed with NEC. Interventions included Bovine lactoferrin + Lactobacillus rhamnosus GG (2 studies) (15, 16), Bovine lactoferrin (2 studies) (15, 16), Lactobacillus rhamnosus + Lactobacillus planvtarum + Lactobacillus casei + Bifidobacterium lactis (2 studies) (17, 18), Bifidobacterium lactis + inulin (1 study) (19), inulin (1 study) (19), Bifidobacterium lactis + Bifidobacterium longum (1 study) (20), Bifidobacterium lactis (3 studies) (19–21), Bifidobacterium longum (1 study) (20), Bifidobacterium bifidum + Lactobacillus acidophilus (2 studies) (22, 23), Lactobacillus rhamnosus (3 studies) (24–26), Bifidobacterium infantis + Streptococcus thermophilus + Bifidobacterium lactis (2 studies) (27, 28), Lactobacillus sporogenes (1 study) (29), Lactobacillus reuteri DSM 17938 (4 studies) (30–33), Lactobacillus rhamnosus GG + Bifidobacterium infantis (2 studies) (34, 35), Saccharomyces boulardii (2 studies) (36, 37), Bifidobacterium breve + Bifidobacterium infantis + Bifidobacterium longum (1 study) (38) and Bifidobacterium breve (4 studies) (38–41). There were two studies from Asia, three studies from America, eighteen studies from Europe, and four studies from Oceania. The characteristics of the included studies are shown in (Table 2).
3.4. Network meta-analysis
The full NMA figure is shown in (Figure 2). All P-values for indirect and direct comparisons between all studies were tested for consistency and inconsistency, and most P-values were greater than 0.05, indicating that the effects of consistency between studies were acceptable. Details were shown in (Supplementary Table S2).
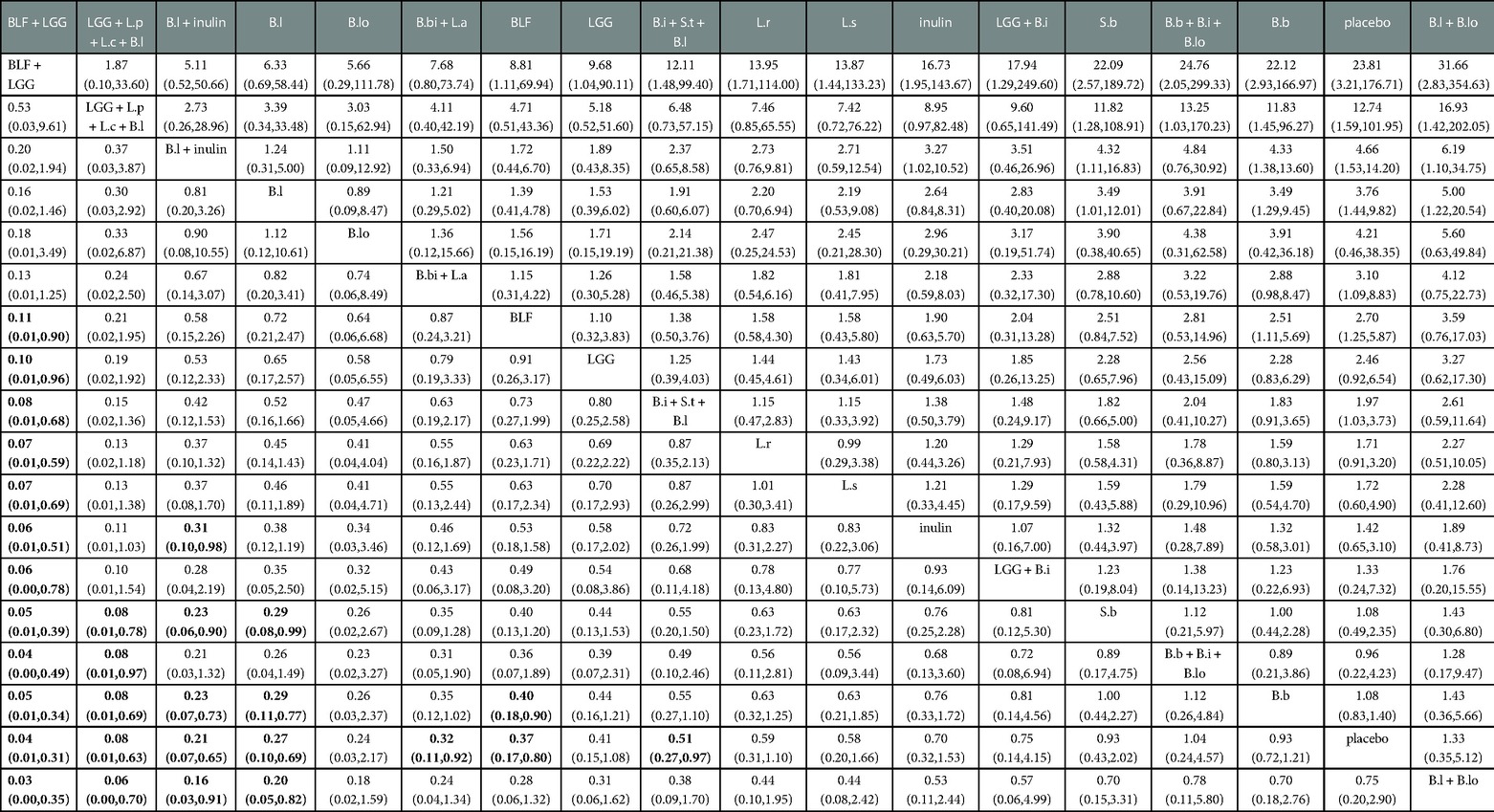
The results of the Network meta-analysis showed that Bovine lactoferrin + Lactobacillus rhamnosus (RR 0.03; 95% CI 0.00–0.35; Table 3), Lactobacillus rhamnosus + Lactobacillus plantarum + Lactobacillus casei + Bifidobacterium lactis (RR 0.06; 95% CI 0.00–0.70), Bifidobacterium lactis + inulin (RR 0.16; 95% CI 0.03–0.91), Bifidobacterium lactis (RR 0.20; 95% CI 0.05–0.82) were superior to the control group (Bifidobacterium lactis + Bifidobacterium longum) in reducing the incidence of NEC. Relative to the control group (placebo), Bifidobacterium bifidum + Lactobacillus acidophilus (RR 0.32; 95% CI 0.11–0.92) and Bifidobacterium infantis + Streptococcus thermophilus + Bifidobacterium lactis (RR 0.51; 95% CI 0.27–0.97) were superior to the control group (placebo) in reducing the incidence of NEC.
Bayesian Markov chain Monte Carlo modeling revealed that Bovine lactoferrin + Lactobacillus rhamnosus had the highest probability of having the lowest rate of NEC (SUCRA 95.7%; Figure 3), followed by Lactobacillus rhamnosus + Lactobacillus plantarum + Lactobacillus casei + Bifidobacterium lactis (SUCRA 89.4%), and Bifidobacterium lactis + inulin (SUCRA 77.8%).
3.5. Publication bias test
We constructed separate funnel plots for all outcome indicators to test for possible publication bias. Visual inspection of the funnel plots did not reveal any significant publication bias (42). Details were shown in (Figure 4).
4. Discussion
NEC is a gastrointestinal disorder that has plagued the field of neonatology for a long time. Considering the morbidity and mortality of NEC, as well as the high cost of treatment and socioeconomic loss, it is important to prioritize research on NEC prevention and treatment. This study included 27 trials with 18 interventions, including 9,501 very low birth weight infants. We aimed to investigate which probiotics effectively prevent NEC in very low birth weight infants. This network meta-analysis concluded that Lactobacillus rhamnosus plus bovine lactoferrin might be the most appropriate regimen for preventing NEC in very low birth weight infants compared to a placebo or other probiotic control group.
Lactobacillus rhamnosus GG belongs to the genus Lactobacillus, a naturally occurring gram-positive bacterium that was originally isolated from the healthy human intestine (43). Lactobacillus rhamnosus GG has strong adhesion to intestinal cells and can also exert its high immune activity in the acidic pH environment of the digestive tract (44), which are prerequisites for colonization in the human intestine. Lactoferrin is a transferrin-like protein with anti-infective and anti-inflammatory properties (45) and is found in high levels in human colostrum and low levels in breast milk, tears, saliva, and semen. Lactoferrin can be processed from bovine or human milk, with GM rice and GM corn currently under study as promising new sources (46). Since bovine lactoferrin is cheaper than human lactoferrin, it is more readily used.
The studies by Paolo Manzoni et al. (15, 16) have shown that bovine lactoferrin combined with Lactobacillus rhamnosus GG can significantly reduce the incidence of NEC in very low birth weight infants, which is consistent with the results of this network meta-analysis. They believe that this might be related to the ability of lactoferrin to provide some anti-infection, nutrition, and immune regulation activity in the intestine to synergize with the effect of Lactobacillus rhamnosus GG against NEC in premature infants. These findings are also supported by a retrospective cohort study by Michael P. Meyer et al., who also showed that the cost of prevention was significantly lower than the cost of treatment (47). Lactobacillus rhamnosus GG can adhere to the intestinal epithelium and generate biofilms and attenuate the pro-inflammatory effects of cytokines and protect the mucosal barrier (48, 49). In addition, Lactobacillus rhamnosus GG can play an anti-pathogen role by stimulating non-specific immunity, increasing the secretion of interleukin-6 and expressing over 90 antimicrobial or immunomodulatory proteins (43, 48–50). Bovine lactoferrin may provide a broad-spectrum anti-pathogen effect by directly lysing microbial cell membranes (51, 52). Moreover, lactoferrin can also protect intestinal epithelial cells by down-regulating the highly expressed pro-inflammatory cytokines in intestinal epithelial cells, inhibiting the activity of free radicals and reducing the levels of oxidative products (53, 54).
It is obvious that Lactobacillus rhamnosus GG and bovine lactoferrin have the similar effects and create good conditions for the growth of beneficial bacteria, and can also inhibit the colonization of pathogens. The combined use can enhance the overall effect (55). The study by Po-Wen Chen et al. has shown that when the growth of probiotics is not optimum, bovine lactoferrin provides a more substantial prebiotic effect and promotes the growth of probiotics, including Lactobacillus rhamnosus GG (56).
A Position Paper by the European Society for Pediatric Gastroenterology Hepatology and Nutrition Committee on Nutrition and the European Society for Pediatric Gastroenterology Hepatology and Nutrition Working Group for Probiotics and Prebiotics indicates that the question of which probiotic strain or combination to use is mainly based on known literature (mainly case series and author's expertise) (57), these recommendations are based on very low certainty of evidence. Compared with other studies, this study compared the effects of various probiotic regimens on NEC through network meta-analysis, and obtained the optimal probiotic regimen by ranking each intervention. In addition, this study also analyzed specific strains of probiotics. In the studies included in this network meta-analysis, the use of probiotics is described as being well tolerated and safe in very low birth weight infants. Feeding intolerance and clinical sepsis were significantly reduced in the probiotic group compared to the control group. Interestingly, these studies also suggest that different outcomes may be influenced by feeding type (human milk vs. formula). This appears to further support the benefits of lactoferrin in combination with probiotics. Combining probiotics and lactoferrin may be a good idea for future research studies.
5. Limitations of the study
This network meta-analysis also has some limitations. This study only discussed the selection of probiotics for the prevention of NEC in very low birth weight infants, while the questions of the dosage, the timing of the intervention, and when to start the intervention remains unresolved. Most interventions were evaluated in only one or two trials, and only a few options were tested in four randomized controlled trials. Therefore, most probiotic interventions were evaluated in small experimental populations. In conclusion, the results of this study should be interpreted with caution, as the number of included trials was insufficient, so there was limited evidence for direct comparisons of some interventions, and further related studies are needed.
6. Conclusions
Our analysis suggests that Lactobacillus rhamnosus GG combined with bovine lactoferrin is the most effective and recommended regimen for preventing NEC in very low birth weight infants. However further studies are required to confirm this and also answer questions about probiotic dosage, timing and duration of therapy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
KZZ: interpreted the data, wrote the initial manuscript, and was involved in the data analysis; KW, LXD, MH and YXL: were responsible for the collection of all relevant papers; LYZ: was responsible for the supervision of the study. Both authors have read and agreed to the published version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all the reviewers for their assistance and support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1095368/full#supplementary-material.
References
1. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. (2018) 103(2):F182–9. doi: 10.1136/archdischild-2017-31388029317459
2. Bazacliu C, Neu J. Necrotizing enterocolitis: long term complications. Curr Pediatr Rev. (2019) 15(2):115–24. doi: 10.2174/157339631566619031209311930864508
3. Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. (2006) 117(2):e137–42. doi: 10.1542/peds.2005-154316390920
4. Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. (2006) 20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x17052286
5. Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, LaCorte M, et al. Members of the Vermont Oxford, trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. (2002) 110(1 Pt 1):143–51. doi: 10.1542/peds.110.1.14312093960
6. Alsaied A, Islam N, Thalib L. Global incidence of necrotizing enterocolitis: a systematic review and meta-analysis. BMC Pediatr. (2020) 20(1):344. doi: 10.1186/s12887-020-02231-532660457
7. Neu J. Necrotizing enterocolitis: the future. Neonatology. (2020) 117(2):240–4. doi: 10.1159/00050686632155645
8. Neu J, Modi N, Caplan M. Necrotizing enterocolitis comes in different forms: historical perspectives and defining the disease. Semin Fetal Neonatal Med. (2018) 23(6):370–3. doi: 10.1016/j.siny.2018.07.00430100524
9. McElroy SJ. Unraveling the enigma that is neonatal necrotizing enterocolitis. J Perinatol. (2014) 34(10):729–30. doi: 10.1038/jp.2014.15525263723
10. Morgan RL, Preidis GA, Kashyap PC, Weizman AV, Sadeghirad B, P. McMaster Probiotic, G. Synbiotic Work. Probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: a systematic review and network meta-analysis of randomized trials. Gastroenterology. (2020) 159(2):467–80. doi: 10.1053/j.gastro.2020.05.09632592699
11. Lueschow SR, Boly TJ, Frese SA, Casaburi G, Mitchell RD, Henrick BM, et al. Bifidobacterium longum subspecies infantis strain EVC001 decreases neonatal murine necrotizing enterocolitis. Nutrients. (2022) 14(3):495. doi: 10.3390/nu14030495
12. Chandrashekar GS, Shettigar S, Varghese TC. Role of probiotics in prevention of necrotizing enterocolitis in preterm neonates. Indian J Child Health. (2018) 05(02):112–5. doi: 10.32677/IJCH.2018.v05.i02.010
13. Law JW-F, Thye AY-K, Letchumanan V, Tan LT-H, Kumari Y, Lee JK-F, et al. IDDF2022-ABS-0200 Probiotics To improve preterm babies’ health outcomes: research in recent 10 years (2012–2022). Gut. (2022) 71(Suppl 2):A52. doi: 10.1136/gutjnl-2022-IDDF.60
14. Sharif S, Meader N, Oddie SJ, Rojas-Reyes MX, McGuire W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst Rev. (2020) 10(10):CD005496. doi: 10.1002/14651858.CD005496.pub533058137
15. Manzoni P, Meyer M, Stolfi I, Rinaldi M, Cattani S, Pugni L, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev. (2014) 90:S60–5. doi: 10.1016/s0378-3782(14)70020-924709463
16. Manzoni P, Rinaldi M, Cattani S, Pugni L, Romeo MG, Messner H, et al. Italian task force for the, I.S.o.N. Prevention of neonatal fungal infections, bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. (2009) 302(13):1421–8. doi: 10.1001/jama.2009.140319809023
17. Guney-Varal I, Koksal N, Ozkan H, Bagci O, Dogan P. The effect of early administration of combined multi-strain and multi-species probiotics on gastrointestinal morbidities and mortality in preterm infants: a randomized controlled trial in a tertiary care unit. Turk J Pediatr. (2017) 59(1):13–9. doi: 10.24953/turkjped.2017.01.00329168358
18. Serce Pehlevan O, Benzer D, Gursoy T, Karatekin G, Ovali F. Synbiotics use for preventing sepsis and necrotizing enterocolitis in very low birth weight neonates: a randomized controlled trial. Clin Exp Pediatr. (2020) 63(6):226–31. doi: 10.3345/cep.2019.0038132023397
19. Dilli D, Aydin B, Fettah ND, Ozyazici E, Beken S, Zenciroglu A, et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr. (2015) 166(3):545–51 e1. doi: 10.1016/j.jpeds.2014.12.00425596096
20. Hays S, Jacquot A, Gauthier H, Kempf C, Beissel A, Pidoux O, et al. Probiotics and growth in preterm infants: a randomized controlled trial, PREMAPRO study. Clin Nutr. (2016) 35(4):802–11. doi: 10.1016/j.clnu.2015.06.00626220763
21. Mihatsch WA, Vossbeck S, Eikmanns B, Hoegel J, Pohlandt F. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology. (2010) 98(2):156–63. doi: 10.1159/00028029120234140
22. Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics. (2008) 122(4):693–700. doi: 10.1542/peds.2007-300718829790
23. Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai. (2014) 97(Suppl 6):S20–5.25391168
24. Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. (2002) 82(2):103–8. doi: 10.1159/00006309612169832
25. Manzoni P, Mostert M, Leonessa ML, Priolo C, Farina D, Monetti C, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis. (2006) 42(12):1735–42. doi: 10.1086/50432416705580
26. Sadowska-Krawczenko I, Korbal P, Polak A, Wietlicka-Piszcz M, Szajewska H. Ocena skuteczności Lactobacillus rhamnosus ATC A07FA w zapobieganiu martwiczego zapalenia jelit wcześniaków z bardzo małą urodzeniową masą ciała: badanie z randomizacją (wstępne wyniki). Pediatr Pol. (2012) 87(2):139–45. doi: 10.1016/s0031-3939(12)70608-x
27. Jacobs SE, Tobin JM, Opie GF, Donath S, Tabrizi SN, Pirotta M, et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics. (2013) 132(6):1055–62. doi: 10.1542/peds.2013-133924249817
28. Plummer EL, Danielewski JA, Garland SM, Su J, Jacobs SE, Murray GL. The effect of probiotic supplementation on the gut microbiota of preterm infants. J Med Microbiol. (2021) 70(8):001403. doi: 10.1099/jmm.0.001403
29. Sari FN, Dizdar EA, Oguz S, Erdeve O, Uras N, Dilmen U. Oral probiotics: lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr. (2011) 65(4):434–9. doi: 10.1038/ejcn.2010.27821245887
30. Hernández-Enríquez NP, Rosas-Sumano AB, Monzoy-Ventre MA, Galicia-Flores L. Lactobacillus reuteri DSM 17938 en la prevención de enterocolitis necrosante en recién nacidos prematuros. Estudio piloto de eficacia y seguridad. Revista Mexicana de Pediatría. (2016) 83(2):37–43.
31. Oncel MY, Sari FN, Arayici S, Guzoglu N, Erdeve O, Uras N, et al. Lactobacillus Reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2014) 99(2):F110–5. doi: 10.1136/archdischild-2013-30474524309022
32. Spreckels JE, Wejryd E, Marchini G, Jonsson B, de Vries DH, Jenmalm MC, et al. Lactobacillus reuteri colonisation of extremely preterm infants in a randomised placebo-controlled trial. Microorganisms. (2021) 9(5):915. doi: 10.3390/microorganisms905091533923278
33. Wejryd E, Marchini G, Frimmel V, Jonsson B, Abrahamsson T. Probiotics promoted head growth in extremely low birthweight infants in a double-blind placebo-controlled trial. Acta Paediatr. (2019) 108(1):62–9. doi: 10.1111/apa.1449729999201
34. Al-Hosni M, Duenas M, Hawk M, Stewart LA, Borghese RA, Cahoon M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol. (2012) 32(4):253–9. doi: 10.1038/jp.2011.5121546942
35. Havranek T, Al-Hosni M, Armbrecht E. Probiotics supplementation increases intestinal blood flow velocity in extremely low birth weight preterm infants. J Perinatol. (2013) 33(1):40–4. doi: 10.1038/jp.2012.3722441111
36. Demirel G, Erdeve O, Celik IH, Dilmen U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr. (2013) 102(12):e560–5. doi: 10.1111/apa.1241624028629
37. Serce O, Benzer D, Gursoy T, Karatekin G, Ovali F. Efficacy of saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev. (2013) 89(12):1033–6. doi: 10.1016/j.earlhumdev.2013.08.01324041815
38. Athalye-Jape G, Esvaran M, Patole S, Simmer K, Nathan E, Doherty D, et al. Effect of single versus multistrain probiotic in extremely preterm infants: a randomised trial. BMJ Open Gastroenterol. (2022) 9(1):e000811. doi: 10.1136/bmjgast-2021-00081135185013
39. Costeloe K, Bowler U, Brocklehurst P, Hardy P, Heal P, Juszczak E, et al. A randomized controlled trial of the probiotic Bifidobacterium breve BBG-001 in preterm babies to prevent sepsis, necrotising enterocolitis and death: the probiotics in preterm infantS (PiPS) trial. Health Technol Assess. (2016) 20(66):1–194. doi: 10.3310/hta2066027594381
40. Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet. (2016) 387(10019):649–60. doi: 10.1016/s0140-6736(15)01027-226628328
41. Patole S, Keil AD, Chang A, Nathan E, Doherty D, Simmer K, et al. Effect of Bifidobacterium breve M-16 V supplementation on fecal bifidobacteria in preterm neonates–a randomised double blind placebo controlled trial. PLoS One. (2014) 9(3):e89511. doi: 10.1371/journal.pone.008951124594833
42. Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. (2009) 9:80. doi: 10.1186/1471-2288-9-8019961608
43. Yan F, Liu L, Cao H, Moore DJ, Washington MK, Wang B, et al. Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol. (2017) 10(1):117–27. doi: 10.1038/mi.2016.4327095077
44. Capurso L. Thirty years of Lactobacillus rhamnosus GG: a review. J Clin Gastroenterol. (2019) 53(Suppl 1):S1–S41. doi: 10.1097/MCG.000000000000117030741841
45. Sanchez L, Calvo M, Brock JH. Biological role of lactoferrin. Arch Dis Child. (1992) 67(5):657–61. doi: 10.1136/adc.67.5.6571599309
46. Pammi M, Suresh G. Enteral lactoferrin supplementation for prevention of sepsis and necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. (2020) 3:CD007137. doi: 10.1002/14651858.CD007137.pub632232984
47. Meyer MP, Alexander T. Reduction in necrotizing enterocolitis and improved outcomes in preterm infants following routine supplementation with Lactobacillus GG in combination with bovine lactoferrin. J Neonatal Perinatal Med. (2017) 10(3):249–55. doi: 10.3233/NPM-1613028854514
48. Donato KA, Gareau MG, Wang YJJ, Sherman PM. Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling. Microbiology (Reading). (2010) 156(Pt 11):3288–97. doi: 10.1099/mic.0.040139-020656777
49. Segers ME, Lebeer S. Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microb Cell Fact. (2014) 13(Suppl 1):S7. doi: 10.1186/1475-2859-13-S1-S725186587
50. He F, Morita H, Kubota A, Ouwehand AC, Hosoda M, Hiramatsu M, et al. Effect of orally administered non-viable Lactobacillus cells on murine humoral immune responses. Microbiol Immunol. (2005) 49(11):993–7. doi: 10.1111/j.1348-0421.2005.tb03695.x16301810
51. Gifford JL, Hunter HN, Vogel HJ. Lactoferricin: a lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell Mol Life Sci. (2005) 62(22):2588–98. doi: 10.1007/s00018-005-5373-z16261252
52. Valenti P, Antonini G. Lactoferrin: an important host defence against microbial and viral attack. Cell Mol Life Sci. (2005) 62(22):2576–87. doi: 10.1007/s00018-005-5372-016261253
53. Berlutti F, Schippa S, Morea C, Sarli S, Perfetto B, Donnarumma G, et al. Lactoferrin downregulates pro-inflammatory cytokines upexpressed in intestinal epithelial cells infected with invasive or noninvasive Escherichia coli strains. Biochem Cell Biol. (2006) 84(3):351–7. doi: 10.1139/o06-03916936806
54. Raghuveer TS. Lactoferrin in the preterm Infants’ diet attenuates iron-induced oxidation products. Pediatr Res. (2002) 52(6):964–72. doi: 10.1203/01.Pdr.0000036362.22060.8e12438677
55. Deshpande G, Rao S, Patole S, Bulsara M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics. (2010) 125(5):921–30. doi: 10.1542/peds.2009-130120403939
56. Chen PW, Liu ZS, Kuo TC, Hsieh MC, Li ZW. Prebiotic effects of bovine lactoferrin on specific probiotic bacteria. Biometals. (2017) 30(2):237–48. doi: 10.1007/s10534-017-9999-828185076
57. van den Akker CHP, van Goudoever JB, Shamir R, Domellof M, Embleton ND, Hojsak I, et al. Probiotics and preterm infants: a position paper by the European society for paediatric gastroenterology hepatology and nutrition committee on nutrition and the European society for paediatric gastroenterology hepatology and nutrition working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr. (2020) 70(5):664–80. doi: 10.1097/MPG.000000000000265532332478
Keywords: necrotizing enterocolitis, very low birth weight infants, probiotics, network meta-analysis, prevention
Citation: Zhou K, Wu K, Deng L, Hu M, Luo Y and Zhang L (2023) Probiotics to prevent necrotizing enterocolitis in very low birth weight infants: A network meta-analysis. Front. Pediatr. 11:1095368. doi: 10.3389/fped.2023.1095368
Received: 11 November 2022; Accepted: 17 February 2023;
Published: 6 March 2023.
Edited by:
Minesh Khashu, University Hospitals Dorset NHS Foundation Trust, United KingdomReviewed by:
Giuseppe Lauriti, G. d'Annunzio University of Chieti and Pescara, ItalySilvia Salvatore, University of Insubria, Italy
© 2023 Zhou, Wu, Deng, Hu, Luo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Yan Zhang bGl5YW5uZW9AMTYzLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Ke-Zhao Zhou
Ke-Zhao Zhou Kang Wu
Kang Wu Li-Yan Zhang
Li-Yan Zhang