
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 15 March 2023
Sec. Pediatric Urology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1085143
This article is part of the Research TopicPosterior Urethral Valves: Advances in Diagnosis, Management, and Long-Term Follow UpView all 7 articles
 Davide Meneghesso1,†
Davide Meneghesso1,† Nicola Bertazza Partigiani1*†
Nicola Bertazza Partigiani1*† Rachele Spagnol1
Rachele Spagnol1 Alessandra Rosalba Brazzale2
Alessandra Rosalba Brazzale2 Alessandro Morlacco3
Alessandro Morlacco3 Enrico Vidal1,4
Enrico Vidal1,4
Background: Posterior urethral valves (PUVs) represent the most severe pediatric obstructive uropathy, responsible for chronic renal failure in up to 65% of cases and progression to end-stage kidney disease (ESKD) in about 8%–21% of patients. Unfortunately, renal outcomes have poorly improved over time. The key point is to identify patients at risk; thus, several prenatal and postnatal prognostic factors have been analyzed to improve clinical outcomes. Postnatal nadir creatinine seems to accurately predict long-term renal prognosis, but there is no definitive evidence to support this finding.
Objective: We performed a systematic review with meta-analysis to analyze the predictive value of nadir creatinine on long-term renal function in infants with PUVs.
Methods: We conducted this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. PubMed and Cochrane Library were systematically searched for studies published from January 2008 to June 2022. All the articles were checked independently by two reviewers in two steps.
Results: A total of 24 articles were screened, and 13 were included for data extraction. Data from 1,731 patients with PUVs were analyzed, with a mean follow-up of 5.5 years; of these, on average, 37.9% developed chronic kidney disease (CKD) and 13.6% developed ESKD. All the articles evaluated nadir creatinine as a predictor of CKD, most using a level of 1 mg/dL, with statistical significance at the 5% level. The relative risk of developing CKD in patients with creatinine values higher than the nadir cutoff considered was 7.69 (95% CI: 2.35–25.17, I2 = 92.20%, p < 0.001).
Conclusions: Nadir creatinine is the best prognostic factor for long-term renal function in patients affected by PUV. A value above the cutoff of 1 mg/dL should be considered a significant predictor for the risk of CKD and ESKD. Further studies are needed to define different nadir creatinine cutoffs for better stratification of the different CKD stages and for the development of reliable scores, which include the association of several variables.
Posterior urethral valves (PUVs) represent the most common cause of lower urinary tract obstruction (LUTO) in males with bilateral hydronephrosis (1, 2). PUVs can cause a decrease in fetal urinary output resulting in oligohydramnios with secondary pulmonary hypoplasia in the prenatal period, which is a major cause of perinatal death and associated with early mortality and morbidity by causing disorders of renal development (3–5). PUVs appear as intraluminal folds located immediately proximal to the verumontanum causing partial or complete obstruction to the urinary outflow. According to recent literature, infants with PUV may develop chronic kidney disease (CKD) in 20%–65% and progress to end-stage kidney disease (ESKD) in about 8%–21% of patients (6–8). Endoscopic resection of PUVs is the initial treatment in most patients; more rarely, an early temporary urinary diversion is required (9–12). Despite improving surgical techniques and early diagnosis, the renal outcome has poorly improved over time (13–15). Many prognostic factors have been analyzed both in prenatal and postnatal ages to identify children with PUVs at increased risk of progression to ESKD and dialysis. Predictive factors reported in the literature include nadir creatinine in the first year of life, which seems to be the most reliable marker, ultrasonographic kidney volume, and urinary markers, such as albuminuria, tubular markers, and new urinary molecules (16–22).
Although PUV is a well-known disease with a relevant impact on long-term renal prognosis, there is a lack of solid literature on predictors of CKD development. We therefore decided to perform a systematic review with meta-analysis analyzing the prognostic role of creatinine nadir during the first year of life in patients with PUVs.
We systematically reviewed studies reporting on nadir creatinine as a predictive factor of renal outcomes in PUVs. We considered all studies that refer to the long-term kidney function outcome in patients with PUVs and the possible role of nadir creatinine as a predictor of CKD and ESKD. Our study conforms to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (23, 24).
We searched PubMed and Cochrane Library to include citations published from January 2008 to June 2022 without any limitations (last research on October 1, 2022), with the assistance of a medical librarian. Details of the search terms and combinations are reported in the supplementary material. Furthermore, we manually reviewed the cited references of the selected studies to identify additional potentially relevant studies.
After the initial search, two investigators (NBP and DM) independently screened the identified titles and abstracts to exclude studies based on design (case reports, reviews and systematic reviews, meta-analyses, animal models, or editorials), method/intervention, or patient populations (patient > 18 years). Observational studies, both prospective and retrospective, and randomized controlled trials with more than 10 eligible patients were included.
The full text of each remaining article was manually reviewed by two investigators (NBP and DM) who were not blinded to the journal name, study authors, or institution. We excluded abstracts without full peer-reviewed publications and unavailable articles, as it was impossible to determine the clinical outcome of interest.
We selected all original research studies that include the following:
• patients aged 0–18 years who received a neonatal or antenatal diagnosis of PUV and underwent PUV correction, regardless of the type of intervention;
• collection of first year nadir creatinine data, defined as the lowest creatinine level during the first year of life of patients; and
• nadir creatinine being considered a possible predictor of CKD or ESKD development after PUV correction.
We abstracted the following data from each eligible study: study setting and design, journal, country, time period, population, nadir creatinine threshold in the first year, time of follow-up, incidence of CKD and ESKD, and other prognostic factors of long-term kidney function.
For relevant studies, when the required data were not presented or were unclear, we either excluded the article in its entirety or used the data only for outcomes that were clearly specified.
We assigned a quality measure to each included study using the 14-item National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies (Supplementary Table S1).
Using this rating system, two investigators (RS and NBP) rated each study as either poor, fair, or good. Then, we calculated the percentage of overall agreement between the two independent reviewers’ assessments of study quality. As for the data collection procedure, disagreements between different investigators about study quality were solved through consensus with a third investigator.
Six articles included suitable relative risk (RR) measures (HRs or ORs) to be included in a meta-analysis. As these two RR measures are asymmetric, all analyses were carried out on the logarithmic scale, that is, by referring to the log HRs and log ORs, although results will be reported on the original scale. A homogeneity test based on the Q statistic was performed to evaluate the between-study heterogeneity, which is summarized using the I2 index. Where significant at the 0.01 level, the summary effect, with a corresponding 95% confidence interval, was obtained from a random-effects model. A cumulative meta-analysis was carried out to assess the stability of the summary estimate. Meta-regression was used to account for possible factors of heterogeneity. Publication bias was assessed by an asymmetry of funnel plots. All analyses were carried out using the numerical computing environment R version 4.1.2 (2021-11-01) (25).
Our primary endpoint was to assess nadir creatinine in the first year of life as a prognostic factor for developing CKD and ESKD. Our secondary endpoint was the identification of a cutoff of nadir creatinine and its power as prognostic factor and the identification of other prognostic factors for developing CKD and ESKD in the population considered.
We identified 96 potentially relevant publications for screening (Figure 1). After screening the titles and abstracts, we identified 24 studies for full-text review. Following a full-text review and the manual search of the articles included in the reference lists, we identified 13 eligible studies meeting our inclusion criteria, which were included in the review and are presented in Table 1 (26–38).
All the studies were based on retrospective cohort data; two studies were multicentric (28, 35), while the remaining were monocentric. The study period differed among the studies, with a range between 1975 and 2020. Overall, the 13 eligible studies included 1,731 children, for an average follow-up time of 5.5 years (range 3.5–7.0 years). A prenatal diagnosis of PUV was performed in 49.9% of patients (range 13%–100%), and these data were available for eight studies (26, 28, 31, 33, 35–38). Table 2 presents the population characteristics of the considered studies.
The overall incidence of CKD was 37.9%, ranging 19.0%–63.0%, while the incidence of ESKD was 13.6%, ranging 8%–31%. In seven studies, newborns underwent endoscopic ablation of the urethral valves. Of these, two performed a temporary vesicostomy, and in other four studies, the type of intervention was not described (31, 34, 35, 37). Bilgutay et al. considered all the patients treated with at least one among transurethral resection of the PUV, vesicostomy, Foley placement, or bilateral cutaneous ureterostomy (28). Coquillette et al. considered newborns treated with a catheter or surgical urinary tract decompression (30).
Nadir creatinine was a significant prognostic factor for the development of CKD and ESKD in all the considered studies. The cutoff of nadir creatinine considered was between 0.7 and 2.7 mg/dL, with an average value of 1 mg/dL in the first year of life. A multivariable statistical analysis was performed in 11 studies, while Kaplan–Meier (30) and univariate (33) analyses were performed in the remaining two. The variables considered for the multivariate analysis differed among studies, and they are reported in Table 3.
Five studies analyzed the association between nadir creatinine and the development of CKD (27, 29, 33, 34, 36), five studies analyzed the association between nadir creatinine and the development of ESKD (26, 30, 31, 32, 35), and remaining three studies analyzed the association between nadir creatinine and the development of CKD and ESKD separately (28, 37, 38).
Eleven studies performed a multivariable analysis to screen potential confounders with the common result that nadir creatinine was an independent factor for the development of CKD. Other prognostic factors identified were proteinuria (27, 37), mild-to-severe bladder dysfunction (26, 31, 32), baseline creatinine (28, 37, 38), hypertension (37), recurrent urinary tract infection (UTI), polyuria (32), delay of intervention (27), high-grade vesico-ureteral reflux (VUR), prenatal diagnosis, renal echography abnormality, prematurity (28), creatinine at 6 weeks from the surgical ablation (38), and mechanical ventilation (30).
Figure 2 reports the forest plot that summarizes the results of the random-effects meta-analysis carried out on the selected six studies for the CKD endpoint. It was impossible to perform a meta-analysis for the relative risk of ESKD due to the lack of data from the selected studies. In all studies, nadir creatinine was revealed to be a significant prognostic factor for the development of CKD in patients affected by PUVs (Figure 2). A summary measure of 7.7 was estimated for the RR (RR = 7.69; 95% CI: 2.35–25.17, I2 = 92.20%, P < 0.001), which appeared to be stable in the cumulative meta-analysis. The funnel plot showed no publication bias (Figure 3). However, the very large values of the I2 index, supported by highly significant Q statistics, highlighted, as we may have expected, a substantial heterogeneity among the studies. Accounting for the different cutoffs used through a random-effects meta-regression did not succeed in explaining this heterogeneity, although the type of effect measure (HR or RR) seems to be relevant.
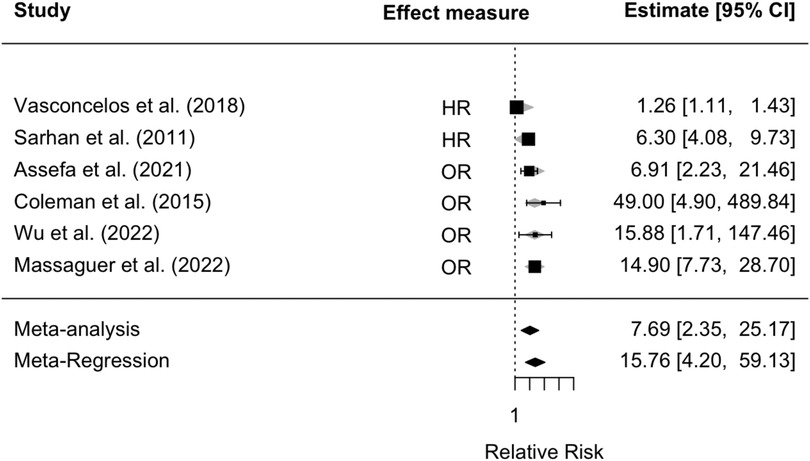
Figure 2. Forest plot for meta-analysis and meta-regression, accounting for the type of effect measure for the relative risk of developing CKD in patients with VUPs. CKD, chronic kidney disease; VUR, vesico-ureteral reflux.
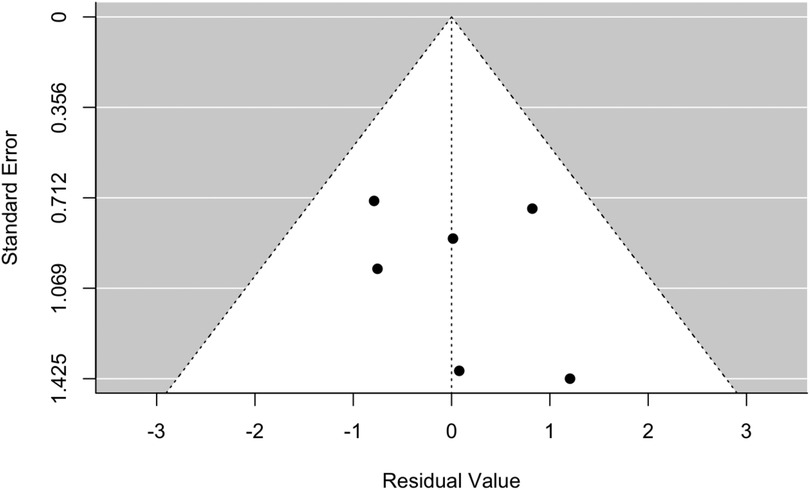
Figure 3. Funnel plot for meta-analysis and meta-regression, accounting for the type of effect measure, for the relative risk of developing CKD in patients with VUPs. CKD, chronic kidney disease.
Nadir creatinine is considered a predictor of CKD and ESKD in children affected by PUVs who underwent surgical correction, although strong literature about its reliability is still lacking (19, 39, 40).
We conducted a systematic review and meta-analysis of the risk of CKD and ESKD development based on the nadir creatinine measured in patients affected by PUVs. A previous systematic review in 2012 analyzed renal and bladder dysfunction after endoscopic treatment of LUTO, identifying nadir creatinine as the only predictive factor for the development of CKD and ESKD; however, considered studies contained heterogeneous patients and data, follow-up time, and control groups and lacked standardized reporting (14). Moreover, no nadir creatinine cutoff was identified as a significant factor in identifying patients who are at higher risk of developing CKD. Our systematic review seeks to address this literature gap. However, the clear majority of data resulted from retrospective and monocentric studies with intrinsic biases.
The most relevant result of our review is that nadir creatinine in the first year of life appears to be a reliable predictor of declining renal function in all the selected studies. The cutoff values ranged from 0.7 to 2.7 mg/dL, although most studies considered 1 mg/dL as a reference. A further important factor is that the statistical analysis, performed by multivariable analysis in most of the studies, led to highly significant results (average p-value of 0.005 for the development of CKD and average p-value of 0.0006 for ESKD). We estimated that, after about 5 years of follow-up, patients with a nadir creatinine above the threshold present a risk of developing CKD, which is 7.7 times higher than that of the other patients. This is the first study that provides a summary estimate for this type of risk. However, the meta-analysis and meta-regression suffer from the very high heterogeneity of the studies, both in terms of effect measures used (HRs and ORs) and the questionable precision of the corresponding estimates listed in the three studies.
An important topic is to stratify the risk of CKD development according to its staging. In fact, the management of children with kidney impairment depends on the stage of CKD, and the definition of a single nadir creatinine cutoff for all CKD stages is not sufficient. Wu et al. were the first to propose different cutoffs of creatinine for individual risk stratification of CKD severity; however, these data should be confirmed in further studies (40).
Moreover, nadir creatinine could be used in association with other prognostic factors to define a score for the stratification of the risk of chronic renal impairment. Coleman et al. suggested a PUV score composed of two variables: nadir creatinine and creatinine velocity, which can be calculated using simple linear regression applied to serum creatinine values during the first 5 days following bladder drainage, expressed in μmol/L/day (41). This is a new but not so diffused score, which should be validated on a larger population.
Other possible prognostic factors include echographic parameters, urinary and biochemical markers, prenatal parameters, and proteomic data. Klaus et al. concluded that the best postnatal predictor for progression to CKD was nadir serum creatinine after valve ablation, while the values of other urinary markers beyond microalbuminuria were poor, and novel postnatal biomarkers should be further investigated (42, 17). The final validation of the 12PUV signature is ongoing in the first clinical proteomics study in prenatal medicine. These fetal biomarkers showed promising results but require more extensive validation before being applied in clinical practice (43). Some genetic alterations are also related to a worse prognosis among patients with PUVs, with emphasis on renin–angiotensin system (RAS) polymorphisms, particularly those affecting the angiotensin-converting enzyme (ACE) and type II angiotensin receptor genes 1 and 2 (AGTR1 and AGTR2) (44, 45).
According to our results, bladder dysfunction and baseline creatinine are the second most frequent prognostic factor for the development of kidney impairment, identified as significant by three studies each through multivariable analysis, but the role and value of these prognostic factors are out of the scope of this paper.
Our results must be interpreted in the context of some limitations. Despite a rigorous search strategy and manually reviewed references in an endeavor to capture all the available data, the presence in literature of other studies not considered in the present review may not be excluded with absolute certainty. The systematic review of the literature was performed on two research systems (Medline and Cochrane Library); however, no studies of interest were identified in Cochrane Library. Moreover, the retrospective design of the studies analyzed and the lack of quality data in several fields of interest limit the evidence provided by most studies. Some articles reported missing information, and most of them were monocentric. Moreover, studies present HRs or ORs to estimate the relative risk of the development of CKD in patients with a certain cutoff of nadir creatinine. CKD is not a rare event in patients with PUVs, with an incidence of about 30% considering a mean follow-up time of only 5.5 years. Both the meta-analysis and meta-regression suffer from the very high heterogeneity of the studies, both in terms of the effect measures used (HRs and ORs) and the questionable precision of the corresponding estimates listed in three out of the six studies considered. Indeed, in these three studies, the estimated effect measures with their corresponding confidence intervals pinpoint a possible separation problem (46) that invalidates all estimation and inference. This aspect could be fixed by using a pooled analysis of all individual data retrieved from the different studies we analyzed.
Nadir creatinine is currently the best prognostic factor of long-term renal function in patients affected by PUVs. A value above the cutoff of 1 mg/dl should be considered at high risk for the development of CKD and ESKD in this population. Further studies are needed to define different nadir creatinine cutoffs for better stratification of the different CKD stages and the development of reliable scores, which include the association of several variables (i.e., bladder dysfunction and proteinuria).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
DM and NBP have equally contributed to the research, the reading of the articles, and the writing of the manuscript. RS oversaw the qualitative evaluation of the articles. ARB took care of the statistical part and, in particular, the meta-analysis of the data in the review. AM and EV reviewed and corrected the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1085143/full#supplementary-material.
1. Hodges SJ, Patel B, McLorie G, Atala A. Posterior urethral valves. Sci World J. (2009) 9:1119–26. doi: 10.1100/tsw.2009.127
3. Krishnan A, de Souza A, Konijeti R, Baskin LS. The anatomy and embryology of posterior urethral valves. J Urol. (2006) 175(4):1214–20. doi: 10.1016/S0022-5347(05)00642-7
4. Eckoldt F, Heling KS, Woderich R, Wolke S. Posterior urethral valves: prenatal diagnostic signs and outcome. Urol Int. (2004) 2004 73(4):296–301. doi: 10.1159/000081586
5. Lissauer D, Morris RK, Kilby MD. Fetal lower urinary tract obstruction. Semin Fetal Neonatal Med. (2007) 12(6):464–70. doi: 10.1016/j.siny.2007.06.005
6. Brownlee E, Wragg R, Robb A, Chandran H, Knight M, McCarthy L,BAPS-CASS. Current epidemiology and antenatal presentation of posterior urethral valves: outcome of BAPS CASS national audit. J Pediatr Surg. (2019) 54(2):318–21. doi: 10.1016/j.jpedsurg.2018.10.091
7. Thakkar D, Deshpande AV, Kennedy SE. Epidemiology and demography of recently diagnosed cases of posterior urethral valves. Pediatr Res. (2014) 76(6):560–3. doi: 10.1038/pr.2014.134
8. Tambo FFM, Tolefac PN, Ngowe MN, Minkande JZ, Mbouche L, Guemkam G, et al. Posterior urethral valves: 10 years audit of epidemiologic, diagnostic and therapeutic aspects in Yaoundé Gynaeco-Obstetric and Paediatric Hospital. BMC Urol. (2018) 18(1):46. doi: 10.1186/s12894-018-0364-1
9. Kihara T, Nakai H, Mori K, Sato R, Kitahara S, Yasuda K. Variety of congenital urethral lesions in boys with lower urinary tract symptoms and the results of endoscopic treatment. Int J Urol. (2008) 15(3):235–40. doi: 10.1111/j.1442-2042.2007.01968.x
10. Gupta A, Khosa J, Barker A, Samnakay N. Clinical spectrum and management options for prostatic utricle in children. J Pediatr Surg. (2022) 57(11):690–5. doi: 10.1016/j.jpedsurg.2022.01.013
11. Bajic P, Matoka D, Maizels M. Posterior urethral valves (PUV) in pediatric practice–promoting methods to understand how to diagnose and incise (PUV). J Pediatr Urol. (2016) 12(1):2–4. doi: 10.1016/j.jpurol.2016.02.001
12. Sarhan O, Zaccaria I, Macher MA, Muller F, Vuillard E, Delezoide AL, et al. Long-term outcome of prenatally detected posterior urethral valves: single center study of 65 cases managed by primary valve ablation. J Urol. (2008) 179(1):307–12. doi: 10.1016/j.juro.2007.08.160
13. Gatti JM, Kirsch AJ. Posterior urethral valves: pre- and postnatal management. Curr Urol Rep. (2001) 2(2):138–45. doi: 10.1007/s11934-001-0011-2
14. Hennus PM, van der Heijden GJ, Bosch JL, de Jong TP, de Kort LM. A systematic review on renal and bladder dysfunction after endoscopic treatment of infravesical obstruction in boys. PLoS One. (2012) 7:e44663. doi: 10.1371/journal.pone.0044663
15. Polak-Jonkisz D, Rehan LR, Fornalczyk K, Hackemer P, Zwolińska D. Valve bladder syndrome in children: on the trail of the best strategies to prevent chronic kidney disease. Adv Clin Exp Med. (2017) 26(8):1293–300. doi: 10.17219/acem/65782
16. Chevalier RL. Prognostic factors and biomarkers of congenital obstructive nephropathy. Pediatr Nephrol. (2016) 31(9):1411–20. doi: 10.1007/s00467-015-3291-3
17. Branco BC, Wilnes B, de Castro PASV, Vieira Leal CR, Simões e Silva AC. Novel biomarkers for posterior urethral valve. Curr Med Chem. (2022). [Epub ahead of print]. doi: 10.2174/0929867329666220803120302
18. Deshpande AV. Current strategies to predict and manage sequelae of posterior urethral valves in children. Pediatr Nephrol. (2018) 33(10):1651–61. doi: 10.1007/s00467-017-3815-0
19. Faure A, Bouty A, Caruana G, Williams L, Burgess T, Wong MN, et al. DNA copy number variants: a potentially useful predictor of early onset renal failure in boys with posterior urethral valves. J Pediatr Urol. (2016) 12(4):227.e1–227.e2277. doi: 10.1016/j.jpurol.2016.02.020
20. Odeh R, Noone D, Bowlin PR, Braga LH, Lorenzo AJ. Predicting risk of chronic kidney disease in infants and young children at diagnosis of posterior urethral valves: initial ultrasound kidney characteristics and validation of parenchymal area as forecasters of renal reserve. J Urol. (2016) 196(3):862–8. doi: 10.1016/j.juro.2016.03.137
21. Zhang W, Li P, Zhou H. Mid-short-term risk factors for chronic renal failure in children with posterior urethral valve. Pediatr Surg Int. (2022) 38(9):1321–6. doi: 10.1007/s00383-022-05154-7
22. Panigrahi P, Chowdhary S, Sharma SP, Kumar R, Agarwal N, Sharma SP. Role of urinary transforming growth factor beta-B1 and monocyte chemotactic protein-1 as prognostic biomarkers in posterior urethral valve. J Indian Assoc Pediatr Surg. (2020) 25(4):219–24. doi: 10.4103/jiaps.JIAPS_104_19
23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. (2009) 339:2700. doi: 10.1136/bmj.b2700
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
25. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: Foundation for Statistical Computing (2015). Available at: www.r-project.org (Accessed October 01, 2022).
26. Ansari MS, Gulia A, Srivastava A, Kapoor R. Risk factors for progression to end-stage renal disease in children with posterior urethral valves. J Pediatr Urol. (2010) 6(3):261–4. doi: 10.1016/j.jpurol.2009.09.001
27. Assefa HG, Getachew H, Tadesse A, Kiflu W, Temesgen F, Dejene B, et al. Outcome of PUV patients following ablation in a tertiary teaching hospital in Addis Ababa, Ethiopia. Res Rep Urol. (2021) 13:639–45. doi: 10.2147/RRU.S322822
28. Bilgutay AN, Roth DR, Gonzales ET Jr, Janzen N, Zhang W, Koh CJ, et al. Posterior urethral valves: risk factors for progression to renal failure. J Pediatr Urol. (2016) 2(3): 179.e1–179.e1797. doi: 10.1016/j.jpurol.2015.10.009
29. Coleman R, King T, Nicoara CD, Bader M, McCarthy L, Chandran H, et al. Nadir creatinine in posterior urethral valves: how high is low enough? J Pediatr Urol. (2015) 11(6): 356.e1–356.e3565. doi: 10.1016/j.jpurol.2015.06.008
30. Coquillette M, Lee RS, Pagni SE, Cataltepe S, Stein DR. Renal outcomes of neonates with early presentation of posterior urethral valves: a 10-year single center experience. J Perinatol. (2020) 40(1):112–7. doi: 10.1038/s41372-019-0489-4
31. DeFoor W, Clark C, Jackson E, Reddy P, Minevich E, Sheldon C. Risk factors for end stage renal disease in children with posterior urethral valves. J Urol. (2008) 180(4 Suppl):1705–8. doi: 10.1016/j.juro.2008.03.090
32. Kumar N, Yadav P, Jain S, Kumar G A, Kaushik VN, Ansari MS. Evaluation of polyuria and polydipsia along with other established prognostic factors in posterior urethral valves for progression to kidney failure: experience from a developing country. Pediatr Nephrol. (2021):1817–24. doi: 10.1007/s00467-020-04837-4
33. Lemmens AS, Mekahli D, Devlieger R, Levtchenko E, Allegaert K. Population-specific serum creatinine centiles in neonates with posterior urethral valves already predict long-term renal outcome. J Matern Fetal Neonatal Med. (2015) 36(7):1026–31. doi: 10.3109/14767058.2014.942278
34. Massaguer C, Martín-Solé O, Pérez-Bertólez S, Tarrado X, García-Aparicio L. Pop-off mechanisms as protective factors against chronic renal disease in children with posterior urethral valves. Cir Pediatr. (2022) 35(4):180–6. doi: 10.54847/cp.2022.04.18
35. McLeod DJ, Szymanski KM, Gong E, Granberg C, Reddy P, Sebastião Y, et al. Renal replacement therapy and intermittent catheterization risk in posterior urethral valves. Pediatrics. (2019) 143(3):e20182656. doi: 10.1542/peds.2018-2656
36. Sarhan OM, El-Ghoneimi AA, Helmy TE, Dawaba MS, Ghali AM, Ibrahiem E-HI. Posterior urethral valves: multivariate analysis of factors affecting the final renal outcome. J Urol. (2011) 185(6 Suppl):2491–5. doi: 10.1016/j.juro.2011.01.023
37. Vasconcelos MA, e Silva ACS, Gomes IR, Carvalho RA, Pinheiro SV, Colosimo EA, et al. A clinical predictive model of chronic kidney disease in children with posterior urethral valves. Pediatr Nephrol. (2019) 34(2):283–94. doi: 10.1007/s00467-018-4078-0
38. Wu CQ, Blum ES, Patil D, Shin HS, Smith EA. Predicting childhood chronic kidney disease severity in infants with posterior urethral valve: a critical analysis of creatinine values in the first year of life. Pediatr Nephrol. (2022) 37(6):1339–45. doi: 10.1007/s00467-021-05271-w
39. Harper L, Waubant A, Vignes J, Amat S, Dobremez E, Lefevre Y, et al. Can quantity of amniotic fluid reliably predict postnatal renal function in boys with posterior urethral valves: a decision curve analysis. Prenat Diagn. (2017) 37(9):931–4. doi: 10.1002/pd.5120
40. Wu CQ, Blum ES, Patil D, Smith EA. Posterior urethral morphology on initial voiding cystourethrogram correlates to early renal outcomes in infants with posterior urethral valves. J Pediatr Urol. (2022) 18(6):813–9. doi: 10.1016/j.jpurol.2022.06.002
41. Coleman R, King T, Nicoara CD, Bader M, McCarthy L, Chandran H, et al. Combined creatinine velocity and nadir creatinine: a reliable predictor of renal outcome in neonatally diagnosed posterior urethral valves. J Pediatr Urol. (2015) 11(4):214.e1–214.e2143. doi: 10.1016/j.jpurol.2015.04.007
42. Klaus R, Lange-Sperandio B. Chronic kidney disease in boys with posterior urethral valves-pathogenesis, prognosis and management. Biomedicines. (2022) 10(8):1894. doi: 10.3390/biomedicines10081894
43. Buffin-Meyer B, Klein J, Breuil B, Muller F, Moulos P, Groussolles M, et al. Definition, diagnosis and management of fetal lower urinary tract obstruction: consensus of the ERKNet CAKUT-obstructive uropathy work group. Nat Rev Urol. (2022) 19(5):295–303. doi: 10.1038/s41585-022-00563-8
44. Buffin-Meyer B, Klein J, Breuil B, Muller F, Moulos P, Groussolles M, et al. Combination of the fetal urinary metabolome and peptidome for the prediction of postnatal renal outcome in fetuses with PUV. J Proteomics. (2018) 184:1–9. doi: 10.1016/j.jprot.2018.06.012
45. Klein J, Lacroix C, Caubet C, Siwy J, Zürbig P, Dakna M, et al. Fetal urinary peptides to predict postnatal outcome of renal disease in fetuses with posterior urethral valves (PUV). Sci Transl Med. (2013) 5(198):198ra106. doi: 10.1126/scitranslmed.3005807
Keywords: nadir creatinine, chronic kidney disease, posterior urethral valves, predictive factor, posterior urethral valve (PUV)
Citation: Meneghesso D, Bertazza Partigiani N, Spagnol R, Brazzale AR, Morlacco A and Vidal E (2023) Nadir creatinine as a predictor of renal outcomes in PUVs: A systematic review and meta-analysis. Front. Pediatr. 11:1085143. doi: 10.3389/fped.2023.1085143
Received: 31 October 2022; Accepted: 16 February 2023;
Published: 15 March 2023.
Edited by:
Massimo Garriboli, Guy's and St Thomas’ NHS Foundation Trust, United KingdomReviewed by:
Alina Christine Hilger, University of Erlangen Nuremberg, Germany© 2023 Meneghesso, Bertazza Partigiani, Spagnol, Brazzale, Morlacco and Vidal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Bertazza Partigiani bmljb2xhLmJlcnRhenphcGFydGlnaWFuaUBhb3BkLnZlbmV0by5pdA==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Urology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.