- 1Department of Paediatrics, Division of Paediatric Emergency Medicine, Inselspital, Bern University Hospital, University of Bern, Switzerland
- 2Faculty of Medicine and Medical Sciences, University of Balamand, Beirut, Lebanon
Background: Nurse-directed pain protocols for intranasal fentanyl administration are not widely implemented in European (EU) pediatric emergency departments (PED). Barriers include perceived safety concerns for intranasal (IN) fentanyl. The aim of this study is to describe our experience with a nurse-directed triage IN fentanyl protocol with a focus on safety in a tertiary EU PED.
Methods: We conducted a retrospective analysis of patient records of children aged 0–16 years who received nurse-directed IN fentanyl between January 2019 and December 2021 at the PED of the University Children's Hospital of Bern, Switzerland. Extracted data points included demographics, presenting complaint, pain score, IN fentanyl dosage, concomitant pain medication use, and adverse events.
Results: A total of 314 patients were identified with ages ranging from 9 months to 15 years. The main indication for nurse-directed fentanyl administration was musculoskeletal pain due to trauma (n = 284, 90%). Mild adverse events (vertigo) were reported in two patients (0.6%), without a correlation to concomitant pain medication or protocol violation. The only reported severe adverse event of syncope and hypoxia in a 14-year-old adolescent occurred in a setting where the institutional nurse-directed protocol was violated.
Conclusion: In accordance with previous studies outside of Europe, our data support the case that when appropriately used, nurse-directed IN fentanyl is a safe potent opioid analgesic for pediatric acute pain management. We strongly encourage the introduction of nurse-directed triage fentanyl protocols Europe-wide in order to provide effective and adequate acute pain management in children.
Introduction
Nurse-directed triage pain protocols (protocols enabling nurses to give analgesics at admission without any medical prescription) have been shown to improve the time to analgesia in emergency medicine (1). Nurse-directed triage pain protocols are essential, especially in pediatric patients, in whom pain is often underrecognized and not adequately addressed before physician contact (2).
However, as recently shown by a survey study conducted by the Research in European Pediatric Emergency Medicine (REPEM) network, in European (EU) pediatric emergency departments (PEDs), triage nurse-directed pain protocols are still not widely implemented (3). Indeed, among those EU PEDs that already have nurse-directed pain protocols in place, only a minority of sites adopt in their protocols intranasal (IN) fentanyl for severe pain management (3), although IN fentanyl represents a potent opioid analgesic providing rapid relief for severe pain without the need for venous access in the PED setting (4, 5). Opioid prescription remains a “physician territory” in Europe, and there are often historical concerns around safety and the apparent lack of specific safety data in the EU PED setting (4, 6).
The standard IN dose of 1.5 µg/kg fentanyl has been shown to provide effective analgesia comparable to the analgesic potency of IV morphine (7, 8). As a selective mu-receptor agonist, fentanyl is safer than other opioids with fewer cardiovascular effects due to a lack of histamine release (9). In standard doses of 1–2 µg/kg, there is no sedative effect and therefore there is a low risk for respiratory depression (5, 8). In addition, when administered IN, fentanyl shows a rapid onset and short duration of action, making it also attractive for prehospital use in children by paramedics (10).
The aim of this retrospective observational study is to describe our experience with a nurse-directed triage IN fentanyl protocol with a focus on safety in a tertiary EU PED.
Methods
The PED in this study is part of a level 1 pediatric trauma center and university children's hospital with an average of 25,000–30,000 annual presentations of children aged 0–16 years. A nurse-directed triage IN fentanyl protocol has been established since 2013. The protocol allows triage nurses to administer IN fentanyl (standard dose 1.5 µg/kg, max. 100 µg) in patients older than 12 months without prior physician prescription, when severe pain is present. Pain assessment occurs using one of three different pain score scales dependent on age. These are the “Kindliche Unbehagen und Schmerz Skala—KUSS” (<4 years), the Wong–Baker Faces Pain Scale (4–7 years), and the Visual Analog Scale (>8 years). After IN fentanyl administration, oxygen saturation is monitored continuously for 30 min and patients are clinically reassessed by nurses every 15 min (see Supplementary Materials). The nurse-directed IN fentanyl protocol is part of the institutional pain management concept, which is based on the WHO analgesic ladder approach, and allows nurses the administration of non-opioid analgesics for mild and moderate pain or as first-line medication in combination with IN fentanyl.
We retrospectively analyzed the electronic medical records of all children <16 years who received IN fentanyl at triage from January 2019 to December 2021. We extracted data pertaining to demographics, presenting complaint, pain score, concurrent pain medication use, effectively administered IN fentanyl dose, and adverse events. Pain scores were corrected to the Visual Analog Scale, with severe pain defined by a score of ≥6. When adverse events were reported in the electronic medical record, a more detailed chart review was completed in order to identify the relationship with fentanyl administration and to assess the severity of the event (according to fentanyl reference safety information provided by the European Medicine Agency). The vital signs monitoring the record of all included patients were manually screened for any atypical observations that may indicate an adverse event, which was not previously captured. A further search for adverse events was conducted by checking the medication administration record for rescue treatments (e.g., naloxone, volume bolus, etc.).
Continuous variables were described as means ± standard deviations (SD) or median and interquartile range (IQR) and categorical variables as percentages (STATA®.Statistics/Data Analysis, StataCorp LLC).
Results
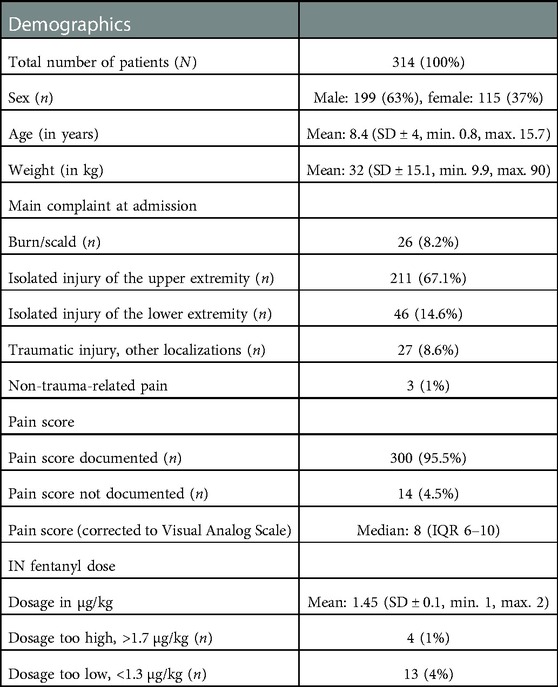
A total of 314 patients were identified who received nurse-directed IN fentanyl at triage. During the investigated period, our PED reported 76,043 visits. Table 1 summarizes patient characteristics.
In terms of age, the nurse-directed protocol was violated in two cases, in a 9-month-old infant and an 11-month-old one treated for a scald burn. No adverse events were reported in these patients.
Pain in musculoskeletal injury due to trauma was the main indication for nurse-directed IN fentanyl, with isolated injury of the upper extremity being the most reported complaint. Non-trauma-related pain (headache, testicular torsion, torticollis) accounted for only 1% of all treated patients. Interestingly, despite most of the patients receiving IN fentanyl with high pain scores according to the institutional protocol (corrected to Visual Analog Scale scores ≥6), in 28 patients (8.9%), IN fentanyl was administered with lower pain scores. This phenomenon seemed to occur especially when fracture was clinically suspected in anticipation of an x-ray.
One-third of all patients (n = 117, 37.3%) had already received analgesics in the 2 h prior admission, of which only three patients (1%) had already received intranasal fentanyl by paramedics. None of these had adverse events. No patient had received other opioids before admission. All patients received IN fentanyl within 10 min from the beginning of triage. Dosing errors with doses exceeding >1.7 µg/kg occurred in four patients (1%), with the maximal administered fentanyl dose being 2 µg/kg. Underdosing (<1.3 µg/kg) in relation to the institutional protocol occurred in 13 patients (4%).
A total of 152 patients (48.4%) received a non-opioid analgesic in addition to IN fentanyl at triage, with non-steroidal anti-inflammatory drugs (NSAIDs) being the most used comedication (n = 105, 33.4%) vs. acetaminophen (n = 46, 14.6%) and metamizol (n = 1, 0.3%).
A second dose of fentanyl was administered in 63/314 (20%) of patients during their PED stay.
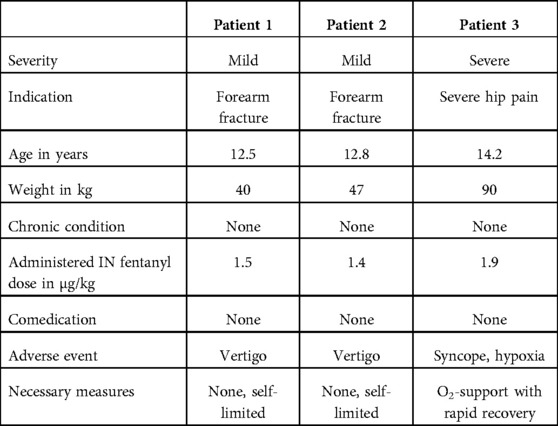
Only three adverse events were reported, one of which was categorized as “severe.” The details of the three patients experiencing adverse events are summarized in Table 2.
One severe adverse event was reported in a 14-year-old, previously healthy patient. IN fentanyl was used to mobilize the patient with severe hip pain out of the parent's car. The patient received two consecutive doses: 120 µg (1.3 µg/kg) and an additional 50 µg (0.6 µg/kg) within 5 min. After a further 10 min, the patient could be mobilized but experienced a syncope and transient desaturation on pulse oximetry requiring short-term oxygen support (description in the electronic medical record, minimum level of SpO2 not specified). The patient showed rapid recovery, and the administration of naloxone or volume bolus was not needed. Globally seen, this severe adverse event occurred in an atypical setting and the IN fentanyl administration breached the conditions of our institutional nurse-directed pain protocol (first dose exceeding the maximum absolute dose of 100 µg, second dose after 5 min and administered via medical prescription and not nurse-directed).
Discussion
We presented our experience of IN fentanyl use at triage through a nurse-directed pain protocol in an EU PED setting. In our retrospective cohort study, we could show that IN doses of 1.3–1.7 µg/kg are not associated with relevant adverse events in accordance with previous evidence outside of EU PEDs (4, 5, 11, 12).
Our findings are supported by two previous studies on nurse-initiated IN fentanyl protocols in American and Australian PEDs (11, 12). In these settings, no safety concerns were reported and IN fentanyl was found to be effective for early pain relief. Numerous studies have demonstrated the safety and efficacy of IN fentanyl in physician-directed protocols, including procedural sedation (8, 9, 13, 14). However, previous studies reported data limited to children >3 years. We included a total of 34 patients aged ≤36 months, two of whom were younger than 12 months (protocol violation). No adverse events were observed in this younger age group. IN fentanyl use in children less than 3 years has also been reported to be safe in prehospital settings (10).
Our protocol uses an IN fentanyl dose of 1.5 µg/kg. A total of 20% of all patients required repeated IN fentanyl doses during the course of their stay at PED, which suggests that this dose is generally efficacious. This is especially true when IN fentanyl is used in combination with over-the-counter analgesics such as NSAIDs and acetaminophen. In our cohort, 36.3% of patients had received non-opioid analgesics prior to arrival at the PED and 48.4% of them received first-line medications in addition to fentanyl at triage. This is in accordance with the WHO analgesic ladder approach and with international pediatric pain management guidelines, which recommend the use of fentanyl as a co-analgesia for moderate and severe pain with first-line medications, in order to sustain pain relief and to achieve an opioid-sparing effect with reduced adverse events for the patient (15).
Dosing errors occurred in 5% of patients, and only 1% were administered a dose that exceeded the protocol-defined dosage. Except for one case of overdosing due to a protocol violation, all dosing errors occurred because of incongruence between the patient's weight that was verbally reported by parents in triage and the actual patient's weight measured later on in the consultation. A reported weight is often used in triage in order to not delay time to drug administration and to avoid further pain during the weighting process. Although weight is often underestimated in children, as reflected by more underdosing errors in our cohort, IN fentanyl seems to have the advantage over other opioid agents with a narrower therapeutic index, in that safety is maintained even with higher-than-target doses and repeat dosing (10, 11).
The unique reported severe adverse event in our cohort occurred in a patient where the protocol was violated with the administration of repeated doses of fentanyl within 5 min and with the first administration exceeding the absolute dose of 100 µg. While adverse events from fentanyl can include hypotonia and respiratory depression, the rapid spontaneous resolution of the symptoms without the need for the administration of a volume bolus or of naloxone in our patient supports the hypothesis that vasovagal syncope occurred possibly because of pain. In previous studies, repeated IN fentanyl doses exceeding the total dose of 2 µg/kg have not been shown to be associated with severe adverse events (11).
In this regard, one limitation of our study is the lack of a systematic assessment of adverse events. This is principally due to the retrospective nature of our study, but also due to a lack of a digitalized safety reporting system specific for medications in our institution. In consideration of the fact that the review process for adverse events consisted in the analysis of the patients' electronic medical records as well as of the monitoring and medication administration records, we expect that no severe adverse events were missed in our cohort. However, mild adverse events may be underrepresented in our study if they had not been properly documented.
Another limitation of our study is selection bias: the main indication for nurse-directed fentanyl administration in our cohort was musculoskeletal pain due to trauma in previously healthy patients. Although IN fentanyl has been shown to be effective and safe in patients with chronic illness in the case of vaso-occlusive pain in sickle-cell disease, further research is needed to expand the use of nurse-directed intranasal fentanyl protocols to pediatric patients with acute severe pain in underlying chronic disease (16).
An interesting finding of our study was that 9% of all patients received IN fentanyl despite reporting low pain scores (Visual Analog Scale <6). A detailed chart review of these patients showed that IN fentanyl was given in anticipation of expected pain during x-ray. Anticipatory pain treatment appears to be an important indication in nurse-directed pain management protocols (1). In comparison with adults, pain in children is often undertreated, especially because of communication challenges and out of fear of adverse effects from analgesic medications (2). The availability of IN fentanyl protocols has been shown to increase the awareness of the need for providing pain relief in children, resulting in a reduction in time to pain treatment (17). Holdgate et al. also demonstrated that it also leads to an increase in the proportion of young children treated for severe pain overall (17).
Although nurse-directed pain protocols in general have the potential for providing more effective pain relief in the PED setting and sensitization of caregivers toward adequate pain management for their children, these protocols are still not widely implemented. In a survey among 171 university hospitals and tertiary care centers of 19 EU countries, nurse-directed pain protocols were available only in 53% of all sites and IN fentanyl only in 11 sites (6%) (2). Important barriers to implementation include the perceived safety of nurse-directed IN fentanyl protocols (18).
In conclusion, on the basis of our data on nurse-directed IN fentanyl protocol in an EU PED setting, combined with previous evidence on this topic outside Europe, as well as the evidence available on the efficacy and safety of IN fentanyl in general, we encourage the implementation of nurse-directed IN fentanyl pain protocols in EU PED to improve pediatric acute pain management.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: Institutional and privacy restrictions. Requests to access these datasets should be directed to Fabrizio Romano,ZmFicml6aW8ucm9tYW5vQGluc2VsLmNo.
Ethics statement
The studies involving human participants were reviewed and approved by the Bern Cantonal Ethics Committee (Kantonale Ethikkommission für die Forschung des Kantons Bern). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with national legislation and institutional requirements.
Author contributions
All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. FR conducted the project, analyzed the data, and wrote the manuscript. MW conducted the literature research and contributed to data analysis and to the writing of the manuscript. During her research sabbatical at our institution, RM carried out data sorting and contributed to literature research. OA-M, CS, and FH as members of our quality improvement team provided important help in data extraction from digital patient records. IS supported the study as a clinical expert. KK supervised the whole project. All authors contributed to the article and approved the submitted version.
Funding
Open access funding by University Of Bern.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1070685/full#supplementary-material.
References
1. Cabilan CJ, Boyde M. A systematic review of the impact of nurse-initiated medications in the emergency department. Australas Emerg Nurs J. (2017) 20:53–62. doi: 10.1016/j.aenj.2017.04.001
2. American Academy of Pediatrics. Committee on Psychosocial Aspects of Child and Family Health, Task Force on Pain in Infants, Children, and Adolescents. The assessment and management of acute pain in infants, children, and adolescents. Pediatrics (2001) 108:793–7. doi: 10.1542/peds.108.3.793
3. Sahyoun C, Catais A, Gervaix A, Bressan S, Löllgen R, Krauss B. Pediatric procedural sedation and analgesia in the emergency department: surveying the current European practice. Eur J Pediatr. (2021) 180(6):1799–813. doi: 10.1007/s00431-021-03930-6
4. Pansini V, Curatola A, Gatto A, Lazzareschi I, Ruggiero A, Chiaretti A. Intranasal drugs for analgesia and sedation in children admitted to pediatric emergency department: a narrative review. Ann Transl Med. (2021) 9:189. doi: 10.21037/atm-20-5177
5. Mudd S. Intranasal fentanyl for pain management in children: a systematic review of the literature. J Pediatr Health Care. (2011) 25(5):316–22. doi: 10.1016/j.pedhc.2010.04.011
6. Nemeth M, Jacobsen N, Bantel C, Fieler M, Sümpelmann R, Eich C. Intranasal analgesia and sedation in pediatric emergency care—a prospective observational study on the implementation of an institutional protocol in a tertiary children’s hospital. Pediatr Emerg Care. (2019) 35:8. doi: 10.1097/PEC.0000000000001017
7. Borland M, Jacobs I, King B, O’Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. (2007) 49:335–40. doi: 10.1016/j.annemergmed.2006.06.016
8. Roback MG, Carlson DW, Babl FE, Kennedy RM. Update on pharmacological management of procedural sedation for children. Curr Opin Anaesthesiol. (2016) 29(Suppl 1):S21–35. doi: 10.1097/ACO.0000000000000316
9. Krauss B, Green SM. Procedural sedation and analgesia in children. Lancet. (2006) 367(9512):766–80. doi: 10.1016/S0140-6736(06)68230-5
10. Murphy AP, Hughes M, Mccoy S, Crispino G, Wakai A, O’Sullivan R. Intranasal fentanyl for the prehospital management of acute pain in children. Eur J Emerg Med. (2017) 24(6):450–4. doi: 10.1097/MEJ.000000000000038910
11. Boreland M, Jacobs I, Geelhoed G. Intranasal fentanyl reduces acute pain in children in the emergency department: a safety and efficacy study. Emerg Med (Fremantle). (2002) 14(3):275–80. doi: 10.1046/j.1442-2026.2002.00344.x
12. Schoolman-Anderson K, Lane RD, Schunk JE, Mecham N, Thomas R, Adelgais K. Pediatric emergency department triage-based pain guideline utilizing intranasal fentanyl: effect of implementation. Am J Emerg Med. (2018) 36(9):1603–7. doi: 10.1016/j.ajem.2018.01.042
13. Ryan PM, Kienstra AJ, Cosgrove P, Vezzetti R, Wilkinson M. Safety and effectiveness of intranasal midazolam and fentanyl used in combination in the pediatric emergency department. Am J Emerg Med. (2019) 37:237–40. doi: 10.1016/j.ajem.2018.05.036
14. Seiler M, Staubli G, Landolt MA. Combined nitrous oxide 70% with intranasal fentanyl for procedural analgosedation in children: a prospective, randomised, double-blind, placebo-controlled trial. Emerg Med J. (2019) 36(3):142–7. doi: 10.1136/emermed-2018-207892
15. Canadian Pediatric Society—Position Statement: Best practices in pain assessment and management for children. (2022). Available from: https://cps.ca/documents/position/pain-assessment-and-management
16. Akinsola B, Hagbom R, Zmitrovich A, Kavanagh PL, Ashkouti A, Simon HK, et al. Impact of intranasal fentanyl in nurse initiated protocols for sickle cell vaso-occlusive pain episodes in a Pediatric Emergency Department. Am J Hematol. (2018) 93:E205–7. doi: 10.1002/ajh.25144
17. Holdgate A, Cao A, Lo KM. The implementation of intranasal fentanyl for children in a mixed adult and pediatric emergency department reduces time to analgesic administration. Acad Emerg Med. (2010) 17:214–7. doi: 10.1111/j.1553-2712.2009.00636.x
Keywords: pediatric emergency medicine, analgesia, pain management, nurse-directed triage protocol, intranasal fentanyl, safety
Citation: Romano F, Wendelspiess M, Mansour R, Abplanalp-Marti O, Starvaggi C, Holzner F, Steiner I and Keitel K (2023) Safety of nurse-directed triage intranasal fentanyl protocol for acute pain management in a European pediatric emergency department: A retrospective observational analysis. Front. Pediatr. 11:1070685. doi: 10.3389/fped.2023.1070685
Received: 15 October 2022; Accepted: 18 January 2023;
Published: 13 February 2023.
Edited by:
Silvia Bressan, University of Padua, ItalyReviewed by:
Jennifer Thull-Freedman, University of Calgary, CanadaChris A. Rees, Emory University, United States
© 2023 Romano, Wendelspiess, Mansour, Abplanalp-Marti, Starvaggi, Holzner, Steiner and Keitel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: K. Keitel a3Jpc3RpbmEua2VpdGVsQGluc2VsLmNo
Specialty Section: This article was submitted to General Pediatrics and Pediatric Emergency Care, a section of the journal Frontiers in Pediatrics
 F. Romano
F. Romano M. Wendelspiess
M. Wendelspiess R. Mansour
R. Mansour O. Abplanalp-Marti1
O. Abplanalp-Marti1 K. Keitel
K. Keitel