- 1Hospital Pediatrics Department, Saint-Petersburg State Pediatric Medical University, Saint-Petersburg, Russia
- 2Pediatric Orthopedics and Surgery Department, Saint-Petersburg Research Institute of Phthisiopulmonology, Saint-Petersburg, Russia
- 3Traumatology and Orthopedic Department, Pavlov First Saint Petersburg State Medical University, Saint-Petersburg, Russia
Purpose: Osteomyelitis is a group of bone infectious (bacterial osteomyeilitis—BO) and noninfectious inflammatory diseases (nonbacterial osteomyelitis—NBO) with similar clinical, radiology, and laboratory features. Many patients with NBO are misdiagnosed as BO and receive unnecessary antibiotics and surgery. Our study aimed to compare clinical and laboratory features of NBO and BO in children, to define key discriminative criteria, and to create an NBO diagnostic score (NBODS).
Methods: The retrospective multicenter cohort study included clinical, laboratory, and instrumental information about histologically confirmed NBO (n = 91) and BO (n = 31). The variables allowed us to differentiate both conditions used to construct and validate the NBO DS.
Results: The main differences between NBO and BO are as follows: onset age—7.3 (2.5; 10.6) vs. 10.5 (6.5; 12.7) years (p = 0.03), frequency of fever (34.1% vs. 90.6%, p = 0.0000001), symptomatic arthritis (67% vs. 28.1%, p = 0.0001), monofocal involvement (28.6% vs. 100%, p = 0.0000001), spine (32% vs. 6%, p = 0.004), femur (41% vs. 13%, p = 0.004), foot bones (40% vs. 13%, p = 0.005), clavicula (11% vs. 0%, p = 0.05), and sternum (11% vs. 0%, p = 0.039) involvement. The following four criteria are included in the NBO DS: CRP ≤ 55 mg/l (56 points), multifocal involvement (27 points), femur involvement (17 points), and neutrophil bands ≤ 220 cell/μl (15 points). The sum > 17 points allowed to differentiate NBO from BO with a sensitivity of 89.0% and a specificity of 96.9%.
Conclusion: The diagnostic criteria may help discriminate NBO and BO and avoid excessive antibacterial treatment and surgery.
Introduction
Osteomyelitis is a group of bone infectious and noninfectious inflammatory diseases with similar clinical, radiology, and laboratory features. Nonbacterial osteomyelitis (NBO), known as chronic recurrent multifocal osteomyelitis (CRMO) or chronic nonbacterial osteomyelitis (CNO), is an immunomediated disease that primarily affects children and adolescents and is characterized by recurrent progression as spontaneous remission (1–3). The clinical pattern of the disease is variable. The disease may proceed as a focal bone lesion with local pain accompanied by swelling and hyperthermia above the damaged area, usually without severe general well-being suffering (4). Monofocal or multifocal osteomyelitis lesions affect the axial and peripheral skeleton, combined with fever and different comorbid immunomediated diseases such as juvenile arthritis, ankylosing spondylitis, and inflammatory bowel disease, psoriasis, and uveitis (5, 6).
Routine inflammatory markers—white blood cell count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP)—are usually normal or slightly elevated but may be increased significantly in some cases (7, 8). The radiological features include bone marrow edema and bone sclerosis surrounding the osteolytic lesions (9–11). In some immunocompromised patients, NBO may develop after the onset of the disease and requires differentiation with bone infections, which may be related to both immune dysregulation and immunosuppressive treatment.
Bacterial osteomyelitis (BO) represents an acute septic bone inflammation predominantly involving children and adolescents. Usually, BO presents as monofocal bone disease, but in the septic process in immunocompromised children, multifocal involvement is possible (12, 13). The main features of BO are related to severe inflammation, such as fever, acute pain in the injured part of the skeleton, swelling, skin hyperemia, and local hyperthermia (12, 13). Laboratory changes reflect the inflammation and are usually characterized by an increased leukocyte level, neutrophilia, increased ESR, and increased CRP (12, 13). Similar clinical and laboratory features of NBO and BO and the absence of diagnostic procedures and clear diagnostic criteria lead to delayed diagnostics of NBO and inappropriate treatment. Many patients with NBO are misdiagnosed as BO and receive unnecessary antibiotics and surgery (11, 14).
Our study aimed to define key clinical and laboratory discriminative criteria between NBO and BO in children.
Materials and methods
Study design and patients
Written consent was obtained according to the Declaration of Helsinki. The Ethics Committee of St. Petersburg State Pediatric University (protocol number 10/8 from 23.10.2017) has approved the study. The data of 122 patients (91 NBO and 31 BO) under 18 years were included in the retrospective case–control study. The information was extracted from the clinical charts of patients with CNO and BO from pediatric and surgery departments both.
Inclusion criteria
We included only patients with histologically confirmed NBO and certain NBO (scores 39 or higher) according to Jansson (2, 15) and Roderick et al. criteria (1). Acute BO was diagnosed in the presence of culturally confirmed bone infection (obligatory), acute onset, fever, intensive pain, acute phase reactants, and successful treatment with antibiotics.
Exclusion criteria
We did not include in the analysis patients with culture-negative BO. We excluded patients older than 18 years or with other bone diseases (oncology, tuberculosis, fractures).
Patients’ demographics
For each patient, the initial (i) demographic data, including gender, age of disease onset, and time from the first symptom to diagnosis; (ii) clinical data, including the presence of fever, bone pain, painful lesions, painful swelling and their intensity, local hyperthermia and hyperemia, foci number, presence of arthritis, and concomitant immunomediated diseases; and (iii) radiological data, including the number of foci, their location, and presence of surrounding sclerosis, were evaluated. Bone destruction is confirmed by different imaging techniques (x-ray, computed tomography, and/or magnetic resonance tomography). Also, (iv) inflammatory markers, such as hemoglobin, white blood cells (WBC) and differential blood count, platelets, ESR, and CRP, were determined; and (v) bacteriology assays, including blood, synovial fluid, and abscess puncture assays, were performed for confirmation of infection etiology of bone inflammation. We compared the earliest possible presentations of both diseases, which were available in the patients’ charts.
Statistics
The sample size was not calculated. Software Statistica (release 10.0, StatSoft Corporation, Tulsa, OK, USA), Biostat, and MedCalc were used for data analyses. The descriptive statistics were reported in medians and interquartile ranges (IQRs) for continuous variables and absolute frequencies and percentages for categorical variables. We used the Mann–Whitney U-test to compare quantitative variables in two groups and the chi-square test to compare qualitative data or Fisher's exact test in the case of expected frequencies <5. A logistic regression analysis (Backward Stepwise) was performed to identify the initial clinical and laboratory features distinguishing NBO from BO patients. The ability of each variable to differentiate NBO from BO was evaluated with sensitivity and specificity analysis, AUC-ROC (area under the receiver operating curve) with 95% confidence interval (CI), and the odds ratio (OR) for the detection of the best cutoffs of continuous variables. The higher values of OR of variables interfere with better discriminatory ability. For each categorical variable, the sensitivity and specificity analysis was performed. We avoided using the known “standard” threshold (i.e., threshold reported in the literature before or judged as clinically meaningful). We used the “best” threshold obtained through the ROC curve analysis of our data because it provides the most appropriate means between sensitivity and specificity. By univariate analysis, each variable of interest was associated with the positive diagnosis of NBO, with a p-value of < 0.05. The variables were therefore included in a multivariate logistic model to assess their independent contribution to the outcome. Binary variables included in the model (e.g., femur involvement) were coded as present or absent. The threshold value was based on a receiver operating characteristic (ROC) curve analysis, retaining the value at which sensitivity plus specificity was maximized. No interaction terms were included in the model. The pseudo-R2 statistic was used for assessing the goodness of fit of the model. The coefficients resulting from this multiple logistic regression analysis were used to assign score points for constructing the NBO diagnostic score. For each variable significantly associated with the outcome in the logistic regression, the rule was to multiply the beta value for each range by 100 and round off to the nearest integer.
Results
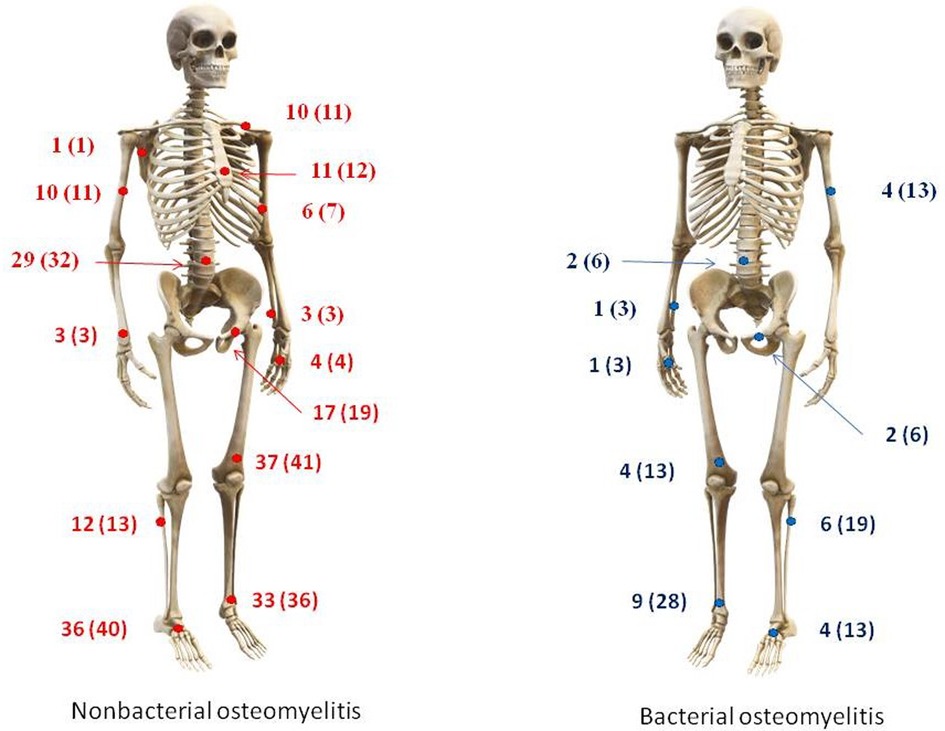
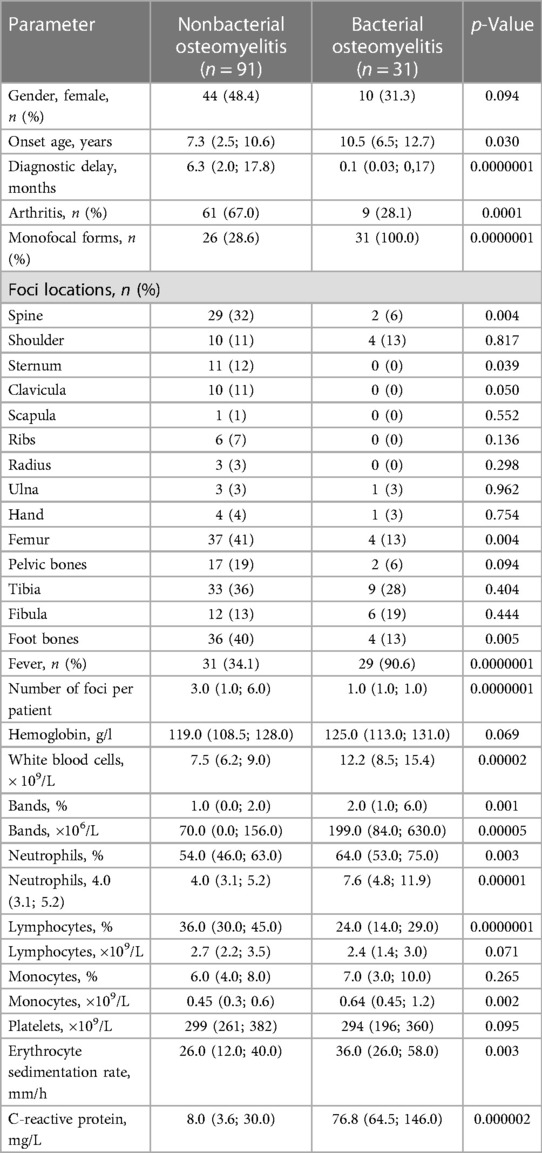
A total of 122 participants were enrolled in the present study: 91 patients with NBO and 31 with BO. The main patterns typical for BO were sole monofocal involvement (100% vs. 28.6%), older onset age—10.5 (6.5; 12.7) vs. 7.3 (2.5; 10.6) years, increased inflammation (more frequent and more intensive fever (90.6% vs. 34.1%), and increased inflammatory markers: CRP—76.8 (64.5; 146.0) vs. 8.0 (3.6; 30.0) mg/l, ESR—36.0 (26.0; 58.0) vs. 26.0 (12.0; 40.0) mm/h, WBC—12.2 (8.5; 15.4) vs. 7.5 (6.2; 9.0) ×109/L, neutrophilia—7.6 (4.8; 11.9) vs. 4.0 (3.1; 5.2) х 109/L). Nonbacterial osteomyelitis was characterized by association with arthritis (67% vs. 28.1%) and more frequent involvement of the spine (32% vs. 6%), femur (41% vs. 13%), clavicula (11% vs. 0%), sternum (12% vs. 0%), foot bones (40% vs. 13%), and long diagnostic delay—6.3 (2.0; 17.8) vs. 0.1 (0.03; 0,17) months. Data are in Table 1 and Figure 1.
Patients with NBO had comorbidities (n = 62; 68.1%): enthesitis-related arthritis (n = 34; 54.8%), polyarticular or oligoarticular categories of juvenile idiopathic arthritis (n = 21; 33.9%), psoriatic arthritis (n = 3; 4.8%), inflammatory bowel disease (n = 1; 1.6%), SAPHO syndrome (n = 2; 3.2%), and Behcet's disease (n = 1; 1.6%). The concomitant treatment for NBO and its comorbidity included sulfasalazine (n = 12; 13.2%), methotrexate (n = 18; 19.8%), pamidronate (n = 23; 25.2%), and tumor necrosis factor-α inhibitors (n = 27; 29.7%). Nobody from the BO group had significant comorbidities and immunosuppressive treatment.
Discriminative criteria between NBO and BO (creation of the NBO diagnostic model)
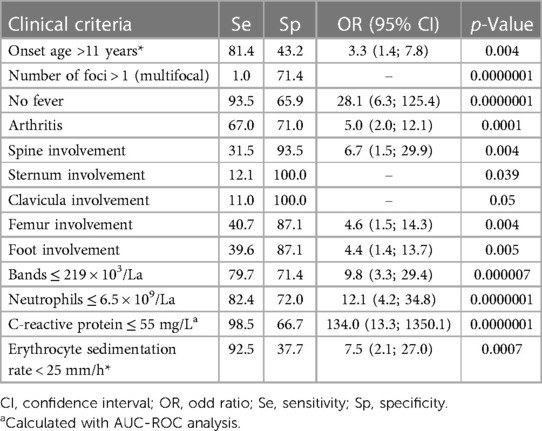
In the next step, we selected continuous and categorical variables with statistical significance, and an analysis of sensitivity and specificity with odds ratio was done. Data are in Table 2. Then, we extracted parameters with the highest sensitivity, specificity, odds ratio, and clinical meaning. We excluded duplicated parameters, and multivariate analysis allowed extracting four criteria: femur involvement, multifocal involvement, CRP ≤ 55 mg/L, and neutrophil bands ≤ 220 cell/μl. In the multivariate analysis, only four variables from the initial 13 included in the regression model remained significantly associated with the probability of being classified as having NBO. The optimal cutoff was selected as the threshold giving the highest value for the sum of sensitivity and specificity. The area under the curve (AUC) = 0.948 (0.893; 0.980), diagnostic score (DS) for NBO > 17 points, allowed to differentiate NBO from BO with 89.0% sensitivity and 96.9% specificity (Table 3 and Figure 2). The pseudo-R2 statistic for the model was 0.61 (p < 0.00001). Missing data were scored as 0. According to the analysis, CRP ≤ 55 mg/L and multifocal involvement were found as the major criteria, and femur involvement and neutrophil bands < 220 cells/μl appeared to be minor criteria. The decision rule is the following: discrimination of NBO from BO required having at least one major criterion or at least a combination of two minor criteria.
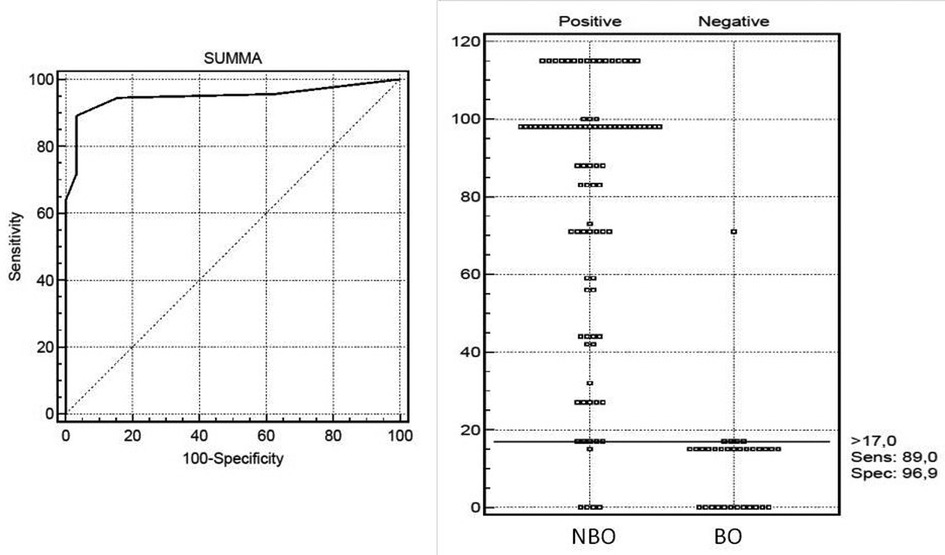
Figure 2. Receiver operating characteristic (ROC) curve analysis for diagnosis NBO in children with diagnostic scores computed with the developmental data set. The optimal cutoff was selected as the threshold giving the highest value for the sum of sensitivity and specificity. Area under the curve (AUC) = 0.948 (0.893; 0.980), NBO DS >17 points with 89.0% sensitivity and 96.9% specificity.
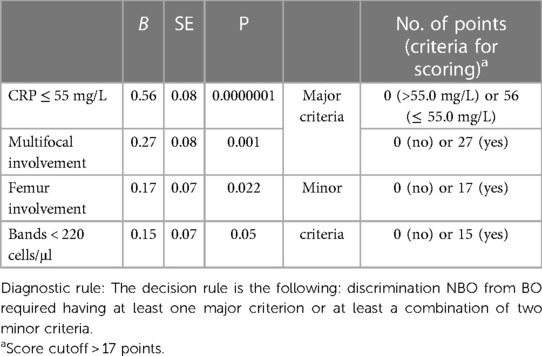
Table 3. Variables included in the development of the diagnostic set and diagnostic score calculation.
Discussion
Inflammatory bone diseases are not rare among children and adolescents and are the most frequent type of autoinflammatory diseases in the same ages (11). One of the challenging problems is the proper diagnosis of NBO and its correct discrimination from other bone-destructive diseases, especially from BO. In our study, the main discriminative criteria between these two diseases were CRP ≤ 55.0 mg/L, multifocal bone involvement, femur involvement, and neutrophil bands ≤ 220 cell/μl. The annual incidence rate of acute hematogenous osteomyelitis in children in developed countries is about 10–80:100,000, with more frequent involvement in boys (12, 16, 17). The data on the incidence of nonbacterial osteomyelitis are scarce, and approximately 2%–5% among all osteomyelitis cases with an incidence rate in Germany of 0.4:100.000 are reported (18). According to the author's opinion, the data were underestimated due to a lack of unique diagnostic criteria and low physician's awareness of this problem (18). In the study conducted by Schnabel et al., a similar prevalence of both diseases was shown, despite the differences (up to 100 folds) in the estimated rates between diseases. Our clinic has a prevalence of patients with nonbacterial osteomyelitis over bacterial osteomyelitis. Many cases of negative bone culture (approximately 50%) and similarities in the bone morphology between NBO and subacute and chronic forms of BO make the differential diagnosis difficult (12, 14, 19). Radiological features are sometimes similar in both types of bone involvement (20). Many NBO patients misevaluated as cultural-negative forms of BO and received unnecessary antibiotics and surgery (12, 17, 19). The key features of NBO are mild-to-moderate inflammation, usually without fever, normal or slight elevated CRP, associations with (auto)inflammatory disorders, and multifocal pattern of bone involvement (1, 21).
In our cohort, all patients with BO were monofocal, but 28.6% of NBO patients had monofocal involvement, making the proper diagnosis difficult. Rarely patients with BO might have multifocal involvement too (12). High inflammation is typical for BO, but 6% of our NBO cohort had CRP > 55 mg\L. In 2016, Roderick et al. suggested NBO diagnostic criteria where a key role referred to the number of lesion foci, C-reactive protein level, and clavicula involvement (1). NBO might be confirmed by multifocal lesions with CRP levels less than 30 mg/L, and morphological and bacteriological data are considered with monofocal lesions or CRP levels of more than 30 mg/L (1). In our cohort calculated CRP, the cutoff level was higher (55 mg/L), but the diagnostic concept was the same: monofocal lesions or increased CRP > 55 mg/L is typical for BO. Multifocal lesions, mild-to-moderate laboratory activity, and negative results of bacteriological studies are more typical for NBO. Applying different imaging tools may explain the high frequency of patients with NBO with monofocal involvement. Not all NBO patients had whole-body MRI, and BO patients usually did not have whole-body MRI at all (11, 20). Clavicula involvement is a typical location for NBO and was positioned by Roderick et al. as an additional criterion. In our cohort, clavicula and sternum involvement was only in NBO patients with 100% sensitivity, but the incidence of these locations was not so frequent, 11% and 12%, relatively. Both predictors (clavicula and sternum involvement) initially confirmed by the univariate analysis had not reached the level of significance (p-value > 0.05) and were not included in the final multiple regression model. Fever, asymmetry of bone lesions, and age of onset of less than 3 years were typical for acute osteomyelitis, fever and symmetrical lesions in young children with intensive periosteal lesions were typical for juvenile osteoperiostitis, and the symmetrical lesions, absence of fever, and onset age > 3 years were hallmarks of chronic nonbacterial osteomyelitis (21). Chronic nonbactrial osteomyelitis, an autoinflammatory disease, is often associated with other immunomediated comorbidities, which took place in two first sets of criteria for CNO (2, 15). The presence of psoriasis, arthritis, and inflammatory bowel diseases makes the differential diagnosis easier (22). In our cohort, 68% of NBO patients had other autoinflammatory diseases, and nobody was from the BO group. Physicians should be aware of cases of bacterial osteomyelitis development in immunomediated (immunosuppressive/immunocompromised) children and not misunderstand them as NBO. The therapeutic tactic is almost different in such cases: bacterial osteomyelitis in immunocompromised children requires active antibiotic treatment, decreasing immunosuppression and surgery assistance, but developing NBO in the same cases usually requires increasing/modulating immunosuppression. In these two scenarios, CRP and neutrophilia might explain either infection conditions or flare of underlined immunomediated diseases. In every doubtful case, the biopsy and empirical antibacterial treatment seem safer initial options before the final diagnosis. In previous studies, multifocal NBO patients had more chance of having immunomediated comorbidities compared to monofocal ones, but in our cohort, the same rate of immunomediated comorbidities was observed in the patients with monofocal (61.5%) and multifocal (69.2%) involvement (22). Monofocal bone destruction requires bone biopsy in all cases, independent of CRP levels, to exclude “silent bone infections” such as tuberculosis or fungi, especially in patients with concomitant immunomediated diseases compared to the recommendation to perform a biopsy in monofocal lesions (except clavicular involvement alone) if CRP is above 30 mg/L (1, 23). In the cases of multifocal involvement in patients with immunomediated diseases, bone metastasis should be excluded from NBO (24, 25).
It is necessary to note the differences in the arthritis presentation between NBO and BO: chronic with mild/moderate pain in NBO and acute, warm, red, and painful in BO, usually accompanied by fever. Synovitis in NBO is close to resembling enthesitis-related arthritis from a pathological point of view, but synovitis in BO is part of a joint–bone infection. Several patients from our NBO cohort further developed arthritis, similar to the enthesitis-related JIA category or ankylosing spondyloarthropathy with a small proportion of HLA B27-positive patients, which was also published in the literature (26).
Study limitations
Several limitations of the study should be mentioned: only two centers were included in the present study and the study's retrospective design led to missing some data. The low sensitivity and specificity of conventional radiographs using different diagnostic modalities at different time points might decrease the strength of the study. To decrease the bias, we excluded patients with culture-negative clinically confirmed BO, which decreased the sample size and might influence the final results. The retrospective study design might misinterpret subacute or chronic culture-negative monofocal BO as NBO due to previous antibacterial treatment or other issues making the bone culture negative in routine practice. The high level of comorbidities and concomitant treatment with immunosuppressive drugs might influence the results of the study.
Conclusion
The diagnosis of nonbacterial osteomyelitis is a diagnosis of exclusion. A number of laboratory and instrumental examinations are required with obligatory morphological and bacteriological analyses from bone lesion focus in all cases of BO and all doubtful cases. Application of the obtained diagnostic criteria might help practicing physicians decrease the risk of misdiagnosing, avoid improper treatment and procedures, and have better outcomes. Further prospective large cohort studies are required to make the best diagnostic and treatment protocol for patients with bone inflammation disorders.
Data availability statement
The original contributions presented in the study are included in the article/further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the local ethics committee of St. Petersburg State Pediatric University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and institutional requirements.
Author contributions
All authors were involved in drafting the article or revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC-ROC, area under receiver operating curve; BO, bacterial osteomyelitis; CNO, chronic nonbacterial osteomyelitis; CI, confidence interval; CRMO, chronic recurrent multifocal osteomyelitis; IQR, interquartile ranges; NBO, nonbacterial osteomyelitis; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; OR, odds ratio; Se, sensitivity; Sp, specificity; WBC, white blood cell.
References
1. Roderick MR, Shah R, Rogers V, Finn A, Ramanan AV. Chronic recurrent multifocal osteomyelitis (CRMO)—advancing the diagnosis. Pediatr Rheumatol Online J. (2016) 14(1):47. doi: 10.1186/s12969-016-0109-1
2. Jansson AF, Müller TH, Gliera L. Clinical score for non-bacterial osteitis in children and adults. Arthritis Rheum. (2009) 60(4):1152–9. doi: 10.1002/art.24402
3. Ferguson PJ, Sandu M. Current understating of the pathogenesis and management of chronic reccurent multifocal osteomyelitis. Curr Rheumatol Rep. (2012) 14(2):130–41. doi: 10.1007/s11926-012-0239-5
4. Wintrich S, Horneff G. Characteristics and outcomes of chronic non-bacterial osteitis in children. Eur J Rheumatol. (2015) 2(4):139–42. doi: 10.5152/eurjrheum.2015.0020
5. Queiroz RM, Rocha PHP, Lauar LZ, Costa MJBD, Laguna CB, Oliveira RGG. Chronic recurrent multifocal osteomyelitis exhibiting predominance of periosteal reaction. Rev Assoc Med Bras (1992). (2017) 63(4):303–6. doi: 10.1590/1806-9282.63.04.303
6. Hofmann SR, Kapplusch F, Girschick HJ, Morbach H, Pablik J, Ferguson PJ, et al. Chronic recurrent multifocal osteomyelitis (CRMO): presentation, pathogenesis, and treatment. Curr Osteoporos Rep. (2017) 15(6):542–54. doi: 10.1007/s11914-017-0405-9
7. Borzutzky A, Stern S, Reiff A, Zurakowski D, Steinberg EA, Dedeoglu F, et al. Pediatric chronic nonbacterial osteomyelitis. Pediatrics. (2012) 130(5):e1190–7. doi: 10.1542/peds.2011-3788
8. Miettunen P, Lafay-Cousin L, Guilcher G, Nettel-Aguirre A, Moorjani V. Widespread osteonecrosis in children with leukemia revealed by whole-body MRI. Clin Orthop Relat Res. (2012) 470(12):3587–95. doi: 10.1007/s11999-012-2579-x
9. Kalle T, Heim N, Hospach T, Langendörfer M, Winkler P, Stuber T. Typical pattern of bone involvement in whole-body MRI of patients with chronic reccurent multifocal osteomyelitis (CRMO). Rofo. (2013) 185(7):655–61. doi: 10.1055/s-0033-1335283
10. Arnoldi AP, Schlett CL, Douis H, Geyer LL, Voit AM, Bleisteiner F. Whole-body MRI in patients with non-bacterial osteitis: radiological findings and correlation with clinical data. Eur Radiol. (2016) 27(6):2391–9. doi: 10.1007/s00330-016-4586-x
11. Hedrich CM, Morbach H, Reiser C, Girschick HJ. New insights into adult and paediatric chronic non-bacterial osteomyelitis CNO. Curr Rheumatol Rep. (2020) 22(9):52. doi: 10.1007/s11926-020-00928-1
12. Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med. (2014) 370(4):352–60. doi: 10.1056/NEJMra1213956
13. Chiappini E, Camposampiero C, Lazzeri S, Indolfi G, De Martino M, Galli L. Epidemiology and management of acute haematogenous osteomyelitis in a tertiary paediatric center. Int J Environ Res Public Health. (2017) 14(5):477. doi: 10.3390/ijerph14050477
14. Schnabel A, Range U, Hahn G, Siepmann T, Berner R, Hedrich CM. Unexpectedly high incidences of chronic non-bacterial as compared to bacterial osteomyelitis in children. Rheumatol Int. (2016) 36(12):1737–45. doi: 10.1007/s00296-016-3572-6
15. Jansson A, Renner ED, Ramser J, et al. Classification of non-bacterial osteitis: retrospective study of clinical, immunological and genetic aspects in 89 patients. Rheumatology (Oxford). (2007) 46(1):154–60. doi: 10.1093/rheumatology/kel190
16. Arnold JC, Bradley JS. Osteoarticular infections in children. Infect Dis Clin North Am. (2015) 29(3):557–74. doi: 10.1016/j.idc.2015.05.012
17. Street M, Puna R, Huang M, Crawford H. Pediatric acute hematogenous osteomyelitis. J Pediatr Orthop. (2015) 35(6):634–9. doi: 10.1097/BPO.0000000000000332
18. Jansson AF, Grote V. ESPED Study group. Nonbacterial osteitis in children: data of a German incidence surveillance study. Acta Paediatr. (2011) 100(8):1150–7. doi: 10.1111/j.1651-2227.2011.02205.x
19. Russell CD, Ramaesh R, Kalima P, Murray A, Gaston MS. Microbiological characteristics of acute osteoarticular infections in children. J Med Microbiol. (2015) 64(Pt 4):446–53. doi: 10.1099/jmm.0.000026
20. Khanna G, Sato TSP, Ferguson P. Imaging of chronic recurrent multifocal osteomyelitis. RadioGraphics. (2009) 29(4):1159–77. doi: 10.1148/rg.294085244
21. Zulian F, Marigo E, Ardenti-Morini F, Vittadello F, Zuliani M, Giraudo C, et al. Osteoperiostitis in children: proposal for a diagnostic algorithm. Eur J Pediatr. (2021) 180(10):3229–35. doi: 10.1007/s00431-021-04058-3
22. Gamalero L, Belot A, Zajc Avramovic M, Giani T, Filocamo G, Guleria S, et al. Chronic non-bacterial osteomyelitis: a retrospective international study on clinical manifestations and response to treatment. Clin Exp Rheumatol. (2020) 38(6):1255–62. PMID: 32828142.32828142
23. Rawat A, Vignesh P, Sudhakar M, Sharma M, Suri D, Jindal A, et al. Clinical, immunological, and molecular profile of chronic granulomatous disease: a multi-centric study of 236 patients from India. Front Immunol. (2021) 12:625320. doi: 10.3389/fimmu.2021.625320
24. Ferris B, Gonzalez MD, Fox T, Kao CM. Multifocal osteomyelitis in a child presenting with a mediastinal mass. Clin Pediatr (Phila). (2020) 59(13):1199–201. doi: 10.1177/0009922820941235;
25. Grote V, Silier CCG, Voit AM, Jansson AF. Bacterial osteomyelitis or non-bacterial osteitis in children: a study involving the German surveillance unit for rare diseases in childhood. Pediatr Infect Dis J. (2017) 36(5):451–6. doi: 10.1097/INF.0000000000001469
26. Girschick H, Finetti M, Orlando F, Schalm S, Insalaco A, Ganser G. The multifaceted presentation of chronic recurrent multifocal osteomyelitis: a series of 486 cases from the eurofever international registry. Paediatric rheumatology international trials organisation (PRINTO) and the eurofever registry. Rheumatology. (2018) 57:1203–11. doi: 10.1093/rheumatology/key058
Keywords: nonbacterial osteomyelitis, chronic recurrent multifocal osteomyelitis, bacterial osteomyelitis, hematogenous osteomyelitis, diagnostic criteria
Citation: Kostik MM, Maletin AS, Petukhova VV and Mushkin AY (2023) Nonbacterial and bacterial osteomyelitis in children: a case–control retrospective study. Front. Pediatr. 11:1067206. doi: 10.3389/fped.2023.1067206
Received: 11 October 2022; Accepted: 10 April 2023;
Published: 3 May 2023.
Edited by:
Andrea Taddio, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Roberta Caorsi, Giannina Gaslini Institute (IRCCS), ItalyMeinrad Beer, Ulm University Medical Center, Germany
© 2023 Kostik, Maletin, Petukhova and Mushkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikhail M. Kostik a29zdC1taWtoYWlsQHlhbmRleC5ydQ==; bWlraGFpbC5rb3N0aWtAZ21haWwuY29t
 Mikhail M. Kostik
Mikhail M. Kostik Alexey S. Maletin2
Alexey S. Maletin2