- 1Department of Obstetrics, Tongde Hospital of Zhejiang Province, Hangzhou, China
- 2Department of Gynaecology, Tongde Hospital of Zhejiang Province, Hangzhou, China
Objective: The objective of this study was to systematically determine the benefits of Kangaroo-Mother Care (KMC) on the clinical outcomes of low birthweight (LBW) and preterm infants.
Methods: For this study, the following databases were retrieved for articles published until November 2021: PubMed, Web of Science, EBSCO, and the Cochrane library. The primary clinical outcome was mortality between enrollment and 28 days. The secondary clinical outcomes were the mean duration of hospital stay, hypothermia, sepsis, exclusive breastfeeding at the end of the neonatal period, and exclusive breastfeeding at discharge.
Results: We conducted a meta-analysis, which included 17 RCTs, involving overall 17,668 participants. The results of this meta-analysis showed that KMC could reduce the primary clinical outcome of mortality between enrollment and 28 days (RR: 0.80, 95% Cl: 0.71–0.91, p < 0.01). For the secondary clinical outcomes, KMC had a varying degree of benefits on the mean duration of hospital stay (SMD: −0.96, 95% Cl: −1.02–0.90, p < 0.001), hypothermia (RR: 0.45, 95% Cl: 0.27–0.75, p < 0.01), and sepsis (RR: 0.79, 95% Cl: 0.70–0.89, p < 0.001). The exclusive breastfeeding at the end of the neonatal period and exclusive breastfeeding at discharge of KMC had benefits, which was not statistically different though (OR: 2.16, 95% Cl: 0.55–8.41, p = 0.27; OR: 1.16, 95% Cl: 0.82–1.64, p = 0.39, respectively).
Conclusions: KMC was decreased mortality in LBW and premature infants between enrollment and 28 days. In addition, KMC also had a favorable effectiveness on the secondary clinical outcomes, such as mean duration of hospital stay, hypothermia, sepsis. Moreover, KMC also had a slight effectiveness on exclusive breastfeeding at the end of the neonatal period and exclusive breastfeeding at discharge.
Introduction
Kangaroo Mother Care (KMC), originally defined as skin-to-skin contact between mother and newborn, frequent exclusive or almost exclusive breastfeeding, and early discharge, has been proposed as an alternative to traditional interventions of care for low birthweight (LBW) infants (1, 2) and is a multifaceted intervention for LBW infants, preterm infants and their parents (3). In the early 1970s, researchers studied the impact of extra contact between mothers and babies in the early stages of life. This entailed skin-to-skin contact with the mother's bare chest as quickly as possible after birth (4). This became known as “Kangaroo Care” (KC) (4). KC, is known as KMC or skin-to-skin contact (4). As a human-centered care intervention (5), KMC is effective in improving the survival of LBW and premature infants (6), whether KMC is initiated after the LBW infant's vital signs (34038632) are stabilized or is received prior to stabilization (2, 6). WHO has recommended that health facilities use KMC for LBW infants for more than a decade, and some studies have shown that in low- and middle-income countries, KMC for all LBW infants, regardless of birthplace, can significantly reduce neonatal mortality (7, 8). In addition, Charpak et al. found that KMC still had significant, enduring social and behavioral protective effects 20 years after the intervention, indicating that KMC was effective in promoting neurological development in newborns (3). KMC has many benefits not only for the newborns, but also for the mothers. In low- and middle-income countries, approximately 1 in 5 women experience postpartum depression, and mothers of LBW infants are at higher risk (9). Through a randomized clinical trial, Sinha et al. discovered that KMC significantly reduced the risk of moderate to severe depressive symptoms in the postpartum period (9). Based on this, this study attempted to evaluate the benefits of KMC systematically and intuitively from the effectiveness of KMC on the clinical outcomes (mortality, hospital stay, sepsis, hypothermia and exclusive breastfeeding) of LBW and preterm infants in the first 28 days, aiming to make it more widely used in clinical practice.
Methods
Search strategy
From its inception to November 6, 2021, PubMed, Web of Science, EBSCO, and Cochrane were searched to identify relevant research for this meta-analysis. Literature search was conducted combining the use of free-text terminology and medical subject headings. Search terms included “Kangaroo-Mother Care” or “Kangaroo Care”, and “low birthweight infant” or “the infant with low birthweight” or “LBW infant”, and “premature” or “premature infant.” In addition, we manually searched the references of included studies. This meta-analysis was instructed according to the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 statement (10).
Inclusion and exclusion criteria
Inclusion criteria were: (1) English reports were randomized controlled trials assessing the effectiveness of KMC on LBW and preterm infants; (2) studies compared KMC to standard care; (3) studies provided data on the clinical outcomes of LBW and preterm infants. Here, KMC is different from SSC. KMC is that the mother sits in a comfortable position wearing a loose top, the baby is held upright between the breasts with all limbs relaxed and their head turned to one side. During KMC, there is no feeding. Whereas SSC is that the dry, naked baby is lying prone on the mother's naked chest, usually covered with a warm blanket, from the inception of birth to the end of the first breastfeeding.
Exclusion criteria were: (1) participants were neither LBW nor premature infants; (2) single-arm studies or studies did not have suitable subgroup analysis; (3) either participants in the experimental group did not use KMC or participants in the control group did not use standard care; (4) research data could not be extracted. If there were continual publications or ongoing updates of studies, we would incorporate the latest study to ensure the credibility of this review.
Clinical outcomes measure
The primary clinical outcome of this study was mortality between enrollment and 28 days. The secondary clinical outcomes were other relevant results (the mean duration of hospital stay, hypothermia, sepsis, exclusive breastfeeding at the end of the neonatal period, and exclusive breastfeeding at discharge).
Assessment of the risk of bias and data extraction
The Cochrane Collaboration Risk of Bias Assessment tool (11) was used to assess the potential risk of bias in trials. Two investigators performed the review independently, and a third investigator resolved the disagreements. Baseline information included author, publication year, country, KMC vs. standard care, and number of participants; the primary clinical outcome included mortality between enrollment and 28 days; the secondary clinical outcomes included mean duration of hospital stay, hypothermia, sepsis, exclusive breastfeeding at the end of the neonatal period and exclusive breastfeeding at discharge. In addition, studies were included if they reported one or more clinical outcomes and not necessarily all.
Statistical analysis
The primary clinical outcome and most secondary clinical outcomes were analysed by risk ratio (RR) and a 95% confidence interval (95% Cl). Among the secondary clinical outcomes, the mean duration of hospital stay was expressed by standardized mean difference (SMD) and a 95% Cl. This study selected the random-effects models when I2 was higher 50 and fixed-effects models when I2 was lower than or equal to 50. The Begg's and Egger's test was employed to evaluate publication bias, and p < 0.05 was considered statistically significant. The sensitivity analysis was performed using the Stata 12.0 Software (12).
Results
Eligible studies and inclusion characteristics
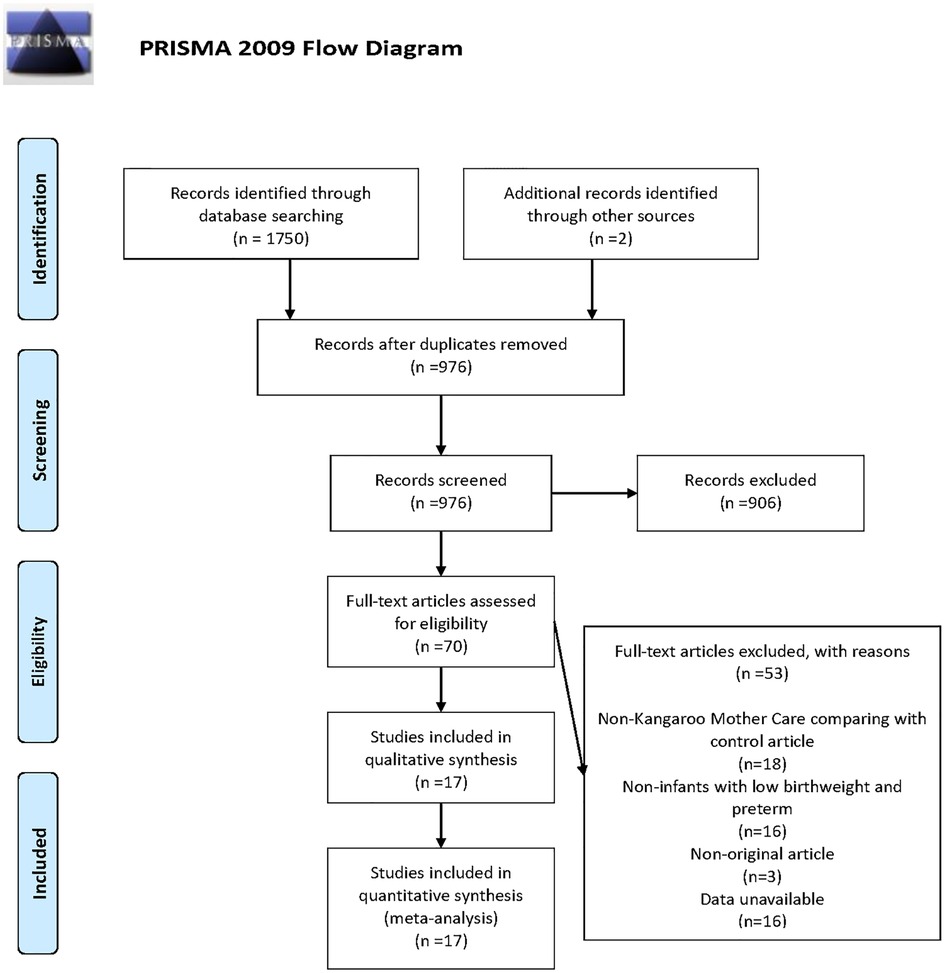
According to the search strategy, 1,752 clinical trials from the database were preliminarily retrieved, including two manually retrieved studies. Due to the rigorous selection criteria, we excluded single-arm studies, studies where participants were not LBW or premature infants, or studies that did not fulfil all the inclusion criteria. Figure 1 shows the detailed search and screening process. In this study, 17 RCTs involving overall 17,668 participants were included, of which 14 studies (2, 13–15, 17, 19–26, 28) targeted at LBW infants and 3 studies (16, 18, 27) focused on premature infants. The quality of studies was detailed in Supplementary Table S2. Among the 17 studies, standard care used as the control group. The use of skin-to-skin contact was identified in one study (27). Apart from one study (16) where Kangaroo Care was applied, KMC was used in the remaining 15 studies (2, 13–15, 17–26, 28) among which one study (24) evaluated the efficacy of Community-Based Kangaroo Mother Care. As for the clinical outcomes of the newborns, 5 studies (2, 15, 21, 24, 28) mentioned the primary clinical outcome, and 15 studies (2, 13–23, 25–27) mentioned the secondary clinical outcomes, which were mean duration of hospital stay (n = 12), hypothermia (n = 7), sepsis (n = 4), exclusive breastfeeding at discharge (n = 3), exclusive breastfeeding at the end of neonatal period (n = 2), respectively. The characteristics of included studies were presented in Supplementary Table S1.
The primary clinical outcome
Among the included studies, five studies described mortality between enrollment and 28 days, including 16,162 neonates with 8,400 neonates in the experimental group and 7,762 neonates in the control group respectively. The comparison of KMC and the control group found that KMC could decrease the mortality of LBW and premature infants (RR: 0.80, 95% Cl: 0.71–0.91, p < 0.01; Figure 2) with a low heterogeneity.
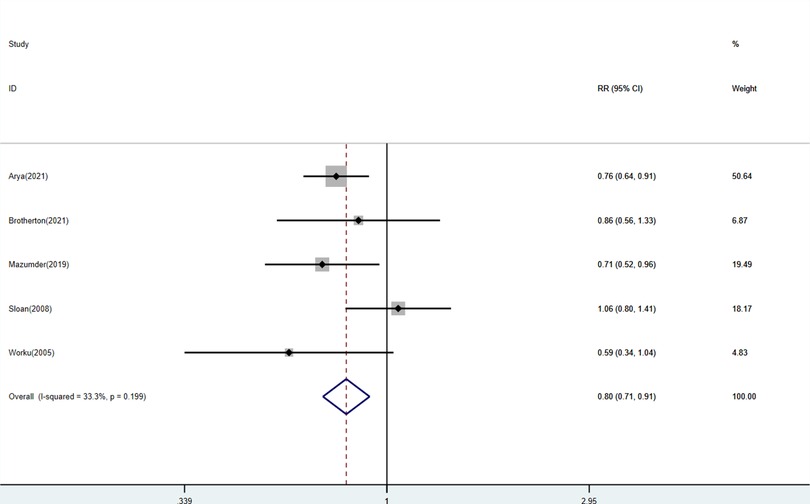
Figure 2. Forest plots of the mortality between enrollment and 28 days for KMC versus control group.
The secondary clinical outcomes
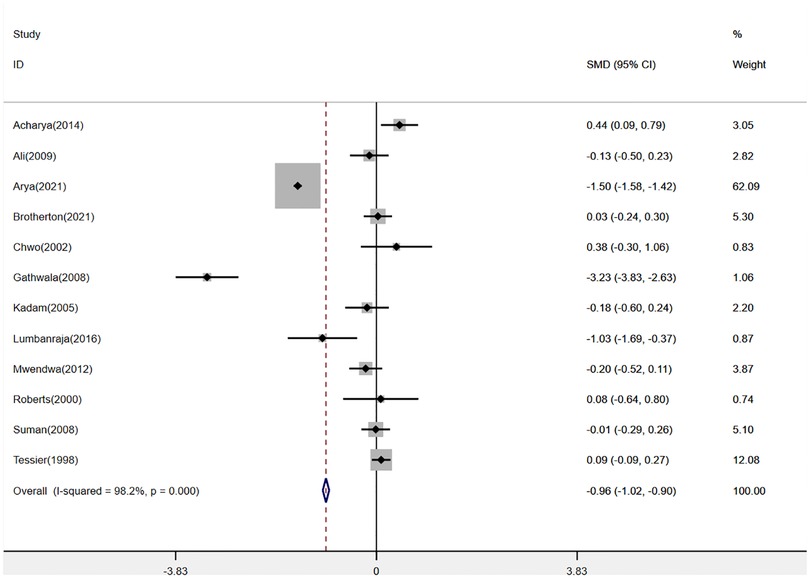
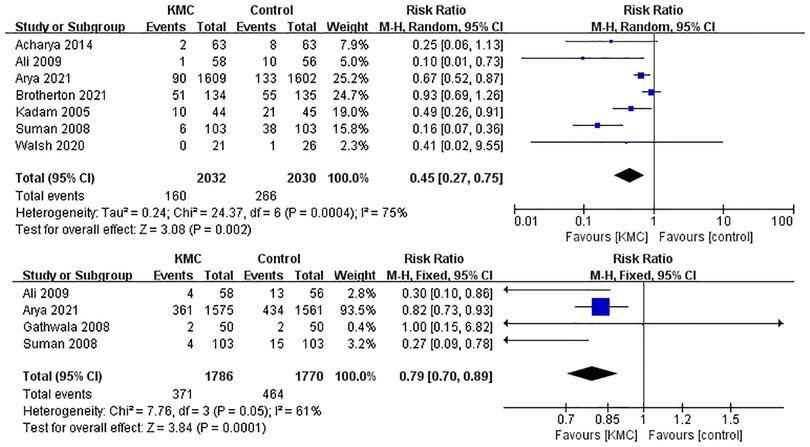
Twelve studies (2, 14–17, 19, 20, 22, 23, 25–27) were pooled when analyzing the outcome of the mean duration of hospital stay. The analysis found that KMC could shorten the mean duration of hospital stay in LBW and premature infants (SMD: −0.96, 95% Cl: −1.02–0.90, p < 0.001, Figure 3). There was a total of 8 studies (2, 13–15, 17, 19, 25, 27) reporting hypothermia and sepsis. KMC could effectively reduce the incidence of hypothermia and sepsis (RR: 0.45, 95% Cl: 0.27–0.75, p < 0.01; RR: 0.79, 95% Cl: 0.70–0.89, p < 0.001; respectively, Figure 4). In addition, four studies (2, 15, 18, 21) mentioned exclusive breastfeeding at discharge and exclusive breastfeeding at the end of the neonatal period. There was a trend for KMC to increase exclusive breastfeeding at the end of the neonatal period and exclusive breastfeeding at discharge, however, this did not reach statistical significance (OR: 2.16, 95% Cl: 0.55–8.41, p = 0.27; OR: 1.16, 95% Cl: 0.82–1.64, p = 0.39, respectively, Supplementary Figure S1).
Publication bias and sensitivity analysis of the secondary clinical outcomes
Given that we included 17 studies in this analysis, publication bias test was conducted to reassess the effectiveness of KMC. The results showed no publication bias, Egger's p = 0.135 and Begg's p = 0.881, both p > 0.05. Moreover, sensitivity analysis showed that the result remained consistent every time each study was removed consecutively (Supplementary Figures S2, S3).
Discussion
From a general perspective, KMC was beneficial to the clinical outcomes (mortality, hospital stay, sepsis, hypothermia and exclusive breastfeeding) of LBW and premature infants in the first 28 days. Studies have shown that premature birth was the leading cause for morbidity and mortality in children under five years of age (29), and there were approximately 1 million children died of the complications of premature birth such as hypothermia each year (30). Hospital-based KMC could save the lives of LBW and premature infants (31). Salim et al. showed that KMC could reduce the mortality of newborns in the stable period (32), especially in sanitary environments with limited resources (31). KMC has been recommended by the World Health Organization for the care of LBW infants whose weight was 2,000 g or less (33). However, KMC has not been integrated into health systems worldwide (34), and the implementation of this method has been restricted (27). Further research found that medical care, time, social support, and family acceptance were the obstacles to the application of KMC (35). Therefore, this study attempted to evaluate the benefits of KMC systematically and intuitively centering around LBW and preterm infants to make it more widely applied in clinical practice.
This study showed that KMC could significantly reduce mortality between enrollment and 28 days compared with standard care. We speculated that it might be related to the position of KMC. Charpak et al. (5) have shown that the position of KMC (skin-to-skin contact to the mother's chest) could enhance the bond between the baby and its mother, increasing the baby's dependence on its mother (36), and give appropriate stimulation to protect against apnea events (37), meanwhile provide enough warmth (38) to prevent hypothermia-related mortality in premature infants (31). In addition, studies have shown that during KMC, the respiratory patterns of the parents could make babies more comfortable (39). Moreover, among premature infants, KMC could also increase weight (40), accelerate skin maturation (41), improve cerebral blood flow, affect brain structure and promote the development of the nervous system (42). Besides, babies fed by KMC had a lower heart rate (42). These positive effectiveness ensured that LBW and premature infants consumed less energy and were less stressed (43), thereby ensuring LBW and premature infants improved more rapidly. Garg et al. (44) pointed out that KMC was correlated with lower bilirubin levels in newborns, which may be explained by improved gastro-intestinal peristalsis during KMC. With increased defecation, the hepatoenteral circulation of bilirubin may decrease.
Moreover, parents of LBW or premature babies may also suffer psychologically since they were often not prepared for their babies' unexpected birth. By implementing KMC, nurses could help parents prepared and adapted to changes in their lives within a short of time and boost self-confidence (45). Some studies have shown that KMC could decrease mothers’ negative emotions (such as anxiety or depression) and promote positive parent–child interaction (46). We hypothesized that all the reasons mentioned above might explain the decreased mortality of LBW and premature infants.
For secondary clinical outcomes, KMC could shorten the median hospital stay of LBW and premature infants and reduce the incidence of hypothermia and sepsis. It might be related to the fact that KMC can promote the growth and development of LBW and premature infants (40). Studies have shown that KMC could effectively reduce the pain response during heel blood collection in premature infants, increase the length of sleeping time, and promote newborns' growth and development, thereby shortening the median length of hospital stay (43, 47). In addition, KMC was an effective and low-cost technique to prevent hypothermia in newborns (30). Since KMC could stabilize the heart and lungs through the autonomic nervous system (48) and promote the microcirculation, KMC could effectively increase the tissue temperature with increased blood flow among LBW and premature infants (49).
Interestingly, Hucklenbruch-Rother et al. found that KMC could significantly reduce the mRNA expression of six important stress response genes such as corticotropin-releasing hormone receptor 2 (CRH-R2), glucocorticoid receptor gene (NR3C1), and serotonin of the transporter gene (SLC6A4) in LBW and preterm infants, which affected the long-term expression of stress response related genes in premature infants (50). We speculated that the mechanism of action might explain the reason why KMC could reduce the incidence of sepsis. Nevertheless, the underlying molecular mechanism still needs to be further explored in depth.
In this meta-analysis, KMC could increase exclusive breastfeeding both at discharge and at the end of the neonatal period in LBW and premature infants to some extent. However, the difference did not reach statistical significance. We considered that the small number of included studies might be to blame. Yilmaz et al. showed that KMC could increase the self-efficacy of breastfeeding mothers and reduce the perceived insufficient milk supply, which indicated that KMC might have a great influence on breastfeeding perception (51). Furthermore, studies have also shown that the prolonged time of skin contact between mothers and newborns was related to the early initialization of exclusive breastfeeding (52). We hypothesized that it might be because the longer duration of skin contact yields better mother-infant interaction that significantly reduces the mother's emotional stress (53) and promotes the secretion of prolactin, thereby stimulating milk secretion. However, this hypothesis still needs to be further confirmed by future research.
From an overall perspective, this study has so far been the most comprehensive analysis of KMC on clinical outcomes of LBW and premature infants with a high level of evidence in that the included studies were all randomized controlled trials. Still, this study also had some shortcomings. This analysis mainly focused on outcomes that threat the newborns' health most and thus did not evaluate the effectiveness of KMC on other clinical outcomes such as weight gain, etc. Moreover, given the long time span of the included studies, there existed significant heterogeneity when analyzing secondary clinical outcomes. Hence, conclusions about secondary clinical outcomes in this study need to be further confirmed in the future.
Conclusion
In summary, this study showed that KMC was beneficial to LBW and premature infants in any case, ranging from decreasing the incidence of hypothermia and sepsis, shortening the mean duration of hospital stay to reducing neonatal mortality. Therefore, KMC should be vigorously promoted, popularized and standardized in clinical practice (54). However, clinical outcomes of KMC involved in this study among LBW and preterm infants were not comprehensive enough. More in-depth and comprehensive randomized clinical experiments need to be conducted to better guide the clinical practice of KMC.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
All authors helped to perform the research. ZZ, GZ, and XW manuscript writing; WC and SP performing procedures and data analysis; QW contribution to writing the manuscript; HG contribution to drafting conception and design. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1067183/full#supplementary-material.
References
1. Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. (2016) 2016(8):Cd002771. doi: 10.1002/14651858.CD002771.pub4
2. Arya S, Naburi H, Kawaza K, Newton S, Anyabolu CH, Bergman N, et al. Immediate “kangaroo mother care” and survival of infants with low birth weight. N Engl J Med. (2021) 384(21):2028–38. doi: 10.1056/NEJMoa2026486
3. Charpak N, Tessier R, Ruiz JG, Hernandez JT, Uriza F, Villegas J, et al. Twenty-year follow-up of kangaroo mother care versus traditional care. Pediatrics. (2017) 139(1):1–11. doi: 10.1542/peds.2016-2063
4. Kostandy RR, Ludington-Hoe SM. The evolution of the science of kangaroo (mother) care (skin-to-skin contact). Birth Defects Res. (2019) 111(15):1032–43. doi: 10.1002/bdr2.1565
5. Charpak N, Ruiz JG. Latin American clinical epidemiology network series - paper 9: the kangaroo mother care method: from scientific evidence generated in Colombia to worldwide practice. J Clin Epidemiol. (2017) 86:125–8. doi: 10.1016/j.jclinepi.2016.05.019
6. Mekonnen AG, Yehualashet SS, Bayleyegn AD. The effects of kangaroo mother care on the time to breastfeeding initiation among preterm and LBW infants: a meta-analysis of published studies. Int Breastfeed J. (2019) 14:12. doi: 10.1186/s13006-019-0206-0
7. Mazumder S, Taneja S, Dube B, Bhatia K, Ghosh R, Shekhar M, et al. Effect of community-initiated kangaroo mother care on survival of infants with low birthweight: a randomised controlled trial. Lancet (London, England). (2019) 394(10210):1724–36. doi: 10.1016/S0140-6736(19)32223-8
8. Nyondo-Mipando AL, Kinshella MW, Salimu S, Chiwaya B, Chikoti F, Chirambo L, et al. “It brought hope and peace in my heart:” caregivers perceptions on kangaroo mother care services in Malawi. BMC Pediatr. (2020) 20(1):541. doi: 10.1186/s12887-020-02443-9
9. Sinha B, Sommerfelt H, Ashorn P, Mazumder S, Taneja S, More D, et al. Effect of community-initiated kangaroo mother care on postpartum depressive symptoms and stress among mothers of low-birth-weight infants: a randomized clinical trial. JAMA Network Open. (2021) 4(4):e216040. doi: 10.1001/jamanetworkopen.2021.6040
10. Du N, Chen M, Shen Z, Li S, Chen P, Khadaroo PA, et al. Comparison of quality of life and nutritional Status of between roux-en-Y and billroth-I reconstruction after distal gastrectomy: a systematic review and meta-analysis. Nutr Cancer. (2020) 72(5):849–57. doi: 10.1080/01635581.2019.1656262
11. Gu L, Chen M, Guo D, Zhu H, Zhang W, Pan J, et al. PD-L1 and gastric cancer prognosis: a systematic review and meta-analysis. PLoS One. (2017) 12(8):e0182692. doi: 10.1371/journal.pone.0182692
12. Gu L, Chen B, Du N, Fu R, Huang X, Mao F, et al. Zhao S: relationship between bariatric surgery and gastroesophageal reflux disease: a systematic review and meta-analysis. Obes Surg. (2019) 29(12):4105–13. doi: 10.1007/s11695-019-04218-3
13. Acharya N, Singh R, Bhatta N, Poudel P. Randomized control trial of kangaroo mother care in low birth weight babies at a tertiary level hospital. J Nepal Paediatr Soc. (2014) 34(1):18–23. doi: 10.3126/jnps.v34i1.8960
14. Ali SM, Jyoti S, Rajyashree S, Seema A. Kangaroo mother care as compared to conventional care for low birth weight babies. Dicle Med J. (2009) 36(3):155–60. https://www.researchgate.net/profile/S-Ali-8/publication/242271796_Kangaroo_Mother_Care_as_compared_to_conventional_care_for_low_birth_weight_babies_Du_uk_do_gum_a_gorloklo_bebekler_icin_Kanguru_anne_bakomonon_geleneksel_bakomla_kar_olatorolmaso/links/00b7d53c546458b4ac000000/Kangaroo-Mother-Care-as-compared-to-conventional-care-for-low-birth-weight-babies-Due-uek-do-gum-a-gorloklo-bebekler-icin-Kanguru-anne-bakomonon-geleneksel-bakomla-kar-olatorolmaso.pdf
15. Brotherton H, Gai A, Kebbeh B, Njie Y, Walker G, Muhammad AK, et al. Impact of early kangaroo mother care versus standard care on survival of mild-moderately unstable neonates <2000 grams: a randomised controlled trial. EClinicalMedicine. (2021) 39:101050. doi: 10.1016/j.eclinm.2021.101050
16. Chwo MJ, Anderson GC, Good M, Dowling DA, Shiau SH, Chu DM. A randomized controlled trial of early kangaroo care for preterm infants: effects on temperature, weight, behavior, and acuity. J Nurs Res. (2002) 10(2):129–42. doi: 10.1097/01.JNR.0000347592.43768.46
17. Gathwala G, Singh B, Balhara B. KMC Facilitates mother baby attachment in low birth weight infants. Indian J Pediatr. (2008) 75(1):43–7. doi: 10.1007/s12098-008-0005-x
18. Hake-Brooks SJ, Anderson GC. Kangaroo care and breastfeeding of mother-preterm infant dyads 0-18 months: a randomized, controlled trial. Neonatal Netw. (2008) 27(3):151–9. doi: 10.1891/0730-0832.27.3.151
19. Kadam S, Binoy S, Kanbur W, Mondkar JA, Fernandez A. Feasibility of kangaroo mother care in Mumbai. Indian J Pediatr. (2005) 72(1):35–8. doi: 10.1007/BF02760578
20. Lumbanraja SN. Influence of maternal factors on the successful outcome of kangaroo mother care in low birth-weight infants: a randomized controlled trial. J Neonatal Perinatal Med. (2016) 9(4):385–92. doi: 10.3233/NPM-161628
21. Mazumder S, Taneja S, Dube B, Bhatia K, Ghosh R, Shekhar M, et al. Effect of community-initiated kangaroo mother care on survival of infants with low birthweight: a randomised controlled trial. Lancet. (2019) 394(10210):1724–36. doi: 10.1016/S0140-6736(19)32223-8
22. Mwendwa AC, Musoke RN, Wamalwa DC. Impact of partial kangaroo mother care on growth rates and duration of hospital stay of low birth weight infants at the kenyatta national hospital, Nairobi. East Afr Med J. (2012) 89(2):53–8. doi: 10.3233/NPM-161628
23. Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonatal Netw. (2000) 19(4):31–5. doi: 10.1891/0730-0832.19.4.31
24. Sloan NL, Ahmed S, Mitra SN, Choudhury N, Chowdhury M, Rob U, et al. Community-based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics. (2008) 121(5):e1047–1059. doi: 10.1542/peds.2007-0076
25. Suman RP, Udani R, Nanavati R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr. (2008) 45(1):17–23. doi: 10.1891/0730-0832.19.4.31
26. Tessier R, Cristo M, Velez S, Giron M, de Calume ZF, Ruiz-Palaez JG, et al. Kangaroo mother care and the bonding hypothesis. Pediatrics. (1998) 102(2):e17. doi: 10.1542/peds.102.2.e17
28. Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis Ababa, Ethiopia. J Trop Pediatr. (2005) 51(2):93–7. doi: 10.1093/tropej/fmh085
27. Walsh RS, Payne A, Cossler NJ, Thompson CL, Bhola M. Safety of immediate skin-to-skin contact after vaginal birth in vigorous late preterm neonates - A pilot study. J Neonatal Perinatal Med. (2021) 14(1):95–100. doi: 10.3233/NPM-190311
29. Lewis TP, Andrews KG, Shenberger E, Betancourt TS, Fink G, Pereira S, et al. Caregiving can be costly: a qualitative study of barriers and facilitators to conducting kangaroo mother care in a US tertiary hospital neonatal intensive care unit. BMC Pregnancy Childbirth. (2019) 19(1):227. doi: 10.1186/s12884-019-2363-y
30. Olawuyi O, Ezenwa BN, Fajolu IB, Onwuama M, Ezeaka CV. Knowledge, attitude and practice of kangaroo mother care among mothers in the neonatal wards of a tertiary care center. Pan Afr Med J. (2021) 38(364):1–9. doi: 10.11604/pamj.2021.38.364.22833
31. Nyondo-Mipando AL, Kinshella MW, Hiwa T, Salimu S, Banda M, Vidler M, et al. Mothers’ quality of life delivering kangaroo mother care at Malawian hospitals: a qualitative study. Health Qual Life Outcomes. (2021) 19(1):186. doi: 10.1186/s12955-021-01823-8
32. Salim N, Shabani J, Peven K, Rahman QS, Kc A, Shamba D, et al. Kangaroo mother care: eN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. (2021) 21(Suppl 1):231. doi: 10.1186/s12884-020-03423-8
33. Kinshella MW, Hiwa T, Pickerill K, Vidler M, Dube Q, Goldfarb D, et al. Barriers and facilitators of facility-based kangaroo mother care in sub-saharan Africa: a systematic review. BMC Pregnancy Childbirth. (2021) 21(1):176. doi: 10.1186/s12884-021-03646-3
34. Ding X, Makino T, Koezuka S, Azumi T, Otsuka H, Hata Y, et al. Primary extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue with multiple pure ground-glass opacities: a case report. J Cardiothorac Surg. (2017) 12(1):2. doi: 10.1186/s13019-017-0565-9
35. Chan GJ, Labar AS, Wall S, Atun R. Kangaroo mother care: a systematic review of barriers and enablers. Bull World Health Organ. (2016) 94(2):130–141j. doi: 10.2471/BLT.15.157818
36. Zhang SH, Yip WK, Lim PF, Goh MZ. Evidence utilization project: implementation of kangaroo care at neonatal ICU. Int J Evid Based Healthc. (2014) 12(2):142–50. doi: 10.1097/XEB.0000000000000009
37. Montealegre-Pomar A, Bohorquez A, Charpak N. Systematic review and meta-analysis suggest that kangaroo position protects against apnoea of prematurity. Acta Paediatr. (2020) 109(7):1310–6. doi: 10.1111/apa.15161
38. Nimbalkar S, Sadhwani N. Implementation of kangaroo mother care - challenges and solutions. Indian Pediatr. (2019) 56(9):725–9. doi: 10.1007/s13312-019-1635-y
39. Schets MW, Chen W, S BO. Design of a breathing mattress based on the respiratory movement of kangaroo mother care for the development of neonates. Annu Int Conf IEEE Eng Med Biol Soc. (2015) 2015:6764–7. doi: 10.1109/EMBC.2015.7319946
40. Evereklian M, Posmontier B. The impact of kangaroo care on premature infant weight gain. J Pediatr Nurs. (2017) 34:e10–6. doi: 10.1016/j.pedn.2017.02.006
41. Park HK, Choi BS, Lee SJ, Son IA, Seol IJ, Lee HJ. Practical application of kangaroo mother care in preterm infants: clinical characteristics and safety of kangaroo mother care. J Perinat Med. (2014) 42(2):239–45. doi: 10.1515/jpm-2013-0066
42. Korraa AA, El Nagger AA, Mohamed RA, Helmy NM. Impact of kangaroo mother care on cerebral blood flow of preterm infants. Ital J Pediatr. (2014) 40(83):1–6. doi: 10.1186/s13052-014-0083-5
43. Özdel D, Sarı HY. Effects of the prone position and kangaroo care on gastric residual volume, vital signs and comfort in preterm infants. Jpn J Nurs Sci. (2020) 17(1):e12287. doi: 10.1111/jjns.12287
44. Garg BD, Bansal A, Kabra NS. Role of kangaroo mother care in the management of neonatal hyperbilirubinemia in both term and preterm neonates: a systematic review. J Perinat Educ. (2020) 29(3):123–33. doi: 10.1891/J-PE-D-18-00043
45. Mu PF, Lee MY, Chen YC, Yang HC, Yang SH. Experiences of parents providing kangaroo care to a premature infant: a qualitative systematic review. Nurs Health Sci. (2020) 22(2):149–61. doi: 10.1111/nhs.12631
46. Athanasopoulou E, Fox JR. Effects of kangaroo mother care on maternal mood and interaction patterns between parents and their preterm, low birth weight infants: a systematic review. Infant Ment Health J. (2014) 35(3):245–62. doi: 10.1002/imhj.21444
47. Andreoli SP. 50 Years ago in the journal of pediatrics: congenital abnormalities of the urinary system, IV: valvular obstruction of the posterior urethra. J Pediatr. (2013) 163(1):186. doi: 10.1016/j.jpeds.2013.01.047
48. Sehgal A, Nitzan I, Jayawickreme N, Menahem S. Impact of skin-to-skin parent-infant care on preterm circulatory physiology. J Pediatr. (2020) 222:91–97.e92. doi: 10.1016/j.jpeds.2020.03.041
49. Miranda RM, Cabral Filho JE, Diniz KT, Clough GF, Alves JGB, Lima GMS, et al. Effect of kangaroo position on microcirculation of preterm newborns: a controlled randomized clinical trial. J Pediatr (Rio J). (2021) 98:196–203.
50. Hucklenbruch-Rother E, Vohlen C, Mehdiani N, Keller T, Roth B, Kribs A, et al. Delivery room skin-to-skin contact in preterm infants affects long-term expression of stress response genes. Psychoneuroendocrinology. (2020) 122:104883. doi: 10.1016/j.psyneuen.2020.104883
51. Yilmaz F, Küçükoğlu S, Aytekin Özdemir A, Oğul T, Aşki N. The effect of kangaroo mother care, provided in the early postpartum period, on the breastfeeding self-efficacy level of mothers and the perceived insufficient milk supply. J Perinat Neonatal Nurs. (2020) 34(1):80–7. doi: 10.1097/JPN.0000000000000434
52. Oras P, Thernström Blomqvist Y, Hedberg Nyqvist K, Gradin M, Rubertsson C, Hellström-Westas L, et al. Skin-to-skin contact is associated with earlier breastfeeding attainment in preterm infants. Acta Paediatr. (2016) 105(7):783–9. doi: 10.1111/apa.13431
53. Tallandini MA, Scalembra C. Kangaroo mother care and mother-premature infant dyadic interaction. Infant Ment Health J. (2006) 27(3):251–75. doi: 10.1002/imhj.20091
Keywords: Kangaroo-Mother care (KMC), efficacy, low birthweight (LBW) infants, premature infants, meta-analysis
Citation: Zhu Z, Wang X, Chen W, Pei S, Wang Q, Guan H and Zhu G (2023) The efficacy of Kangaroo-Mother care to the clinical outcomes of LBW and premature infants in the first 28 days: A meta-analysis of randomized clinical trials. Front. Pediatr. 11:1067183. doi: 10.3389/fped.2023.1067183
Received: 11 October 2022; Accepted: 31 January 2023;
Published: 27 February 2023.
Edited by:
Joemer Maravilla, The University of Queensland, AustraliaReviewed by:
Maria Esterlita T. Villanueva-Uy, University of the Philippines Manila, PhilippinesGiuseppe Lauriti, G. d'Annunzio University of Chieti and Pescara, Italy
© 2023 Zhu, Wang, Chen, Pei, Wang, Guan and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Zhu WFpIdGRja0AxNjMuY29t
Specialty Section: This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
Abbreviations KMC, Kangaroo-Mother Care; LBW, low birthweight; RCTs, randomized controlled trials; RR, risk ratio; SMD, standardized mean difference; 95%CI, confidence interval of 95%.
 Zhen Zhu
Zhen Zhu Xinchen Wang1
Xinchen Wang1 Guang Zhu
Guang Zhu