
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 06 April 2023
Sec. Neonatology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1063558
This article is part of the Research Topic Neonatal Infections and the Developing Neonatal Immune System: Current Evidence and Research Gaps to Fill View all 9 articles
A commentary has been posted on this article:
Commentary: Risk factors and early markers for echovirus type 11 associated haemorrhage-hepatitis syndrome in neonates, a retrospective cohort study
 Ping Wang1,†
Ping Wang1,† Yi Xu2,†
Yi Xu2,† Ming Liu3,†
Ming Liu3,† Huixian Li4
Huixian Li4 Hui Wang5
Hui Wang5 Yumei Liu6
Yumei Liu6 Bin Wang7
Bin Wang7 Shiwen Xia5
Shiwen Xia5 Heng Su1
Heng Su1 Mou Wei1
Mou Wei1 Li Tao1
Li Tao1 Xiaowen Chen1
Xiaowen Chen1 Bingtai Lu8
Bingtai Lu8 Xiaoqiong Gu9
Xiaoqiong Gu9 Hui Lyu3
Hui Lyu3 Wei Zhou1*
Wei Zhou1* Huayan Zhang1,10*
Huayan Zhang1,10* Sitang Gong11*
Sitang Gong11*
Background: Echovirus type 11(E-11) can cause fatal haemorrhage-hepatitis syndrome in neonates. This study aims to investigate clinical risk factors and early markers of E-11 associated neonatal haemorrhage-hepatitis syndrome.
Methods: This is a multicentre retrospective cohort study of 105 neonates with E-11 infection in China. Patients with haemorrhage-hepatitis syndrome (the severe group) were compared with those with mild disease. Clinical risk factors and early markers of haemorrhage-hepatitis syndrome were analysed. In addition, cytokine analysis were performed in selective patients to explore the immune responses.
Results: In addition to prematurity, low birth weight, premature rupture of fetal membrane, total parenteral nutrition (PN) (OR, 28.7; 95% CI, 2.8–295.1) and partial PN (OR, 12.9; 95% CI, 2.2–77.5) prior to the onset of disease were identified as risk factors of developing haemorrhage-hepatitis syndrome. Progressive decrease in haemoglobin levels (per 10 g/L; OR, 1.5; 95% CI, 1.1–2.0) and platelet (PLT) < 140 × 10⁹/L at early stage of illness (OR, 17.7; 95% CI, 1.4–221.5) were associated with the development of haemorrhage-hepatitis syndrome. Immunological workup revealed significantly increased interferon-inducible protein-10(IP-10) (P < 0.0005) but decreased IFN-α (P < 0.05) in peripheral blood in severe patients compared with the mild cases.
Conclusions: PN may potentiate the development of E-11 associated haemorrhage-hepatitis syndrome. Early onset of thrombocytopenia and decreased haemoglobin could be helpful in early identification of neonates with the disease. The low level of IFN-α and elevated expression of IP-10 may promote the progression of haemorrhage-hepatitis syndrome.
Human echovirus, an enterovirus subgroup B virus, is an important cause of perinatal infection in the neonates. Among the various subtypes of echoviruses, echovirus type 11 (E-11) is an important cause of perinatal infection in neonates, which spreads rapidly over large geographic areas (1–3). It has been identified as the causal agent of haemorrhage-hepatitis syndrome (hepatitis with liver dysfunction and coagulopathy) in neonates and of outbreaks in the neonatal intensive care units (NICUs) (4–11). Data from the United States National Enterovirus Surveillance System reported significantly higher mortality from E-11 infection in neonates as compared with infants older than 1 month (19% vs. 3.2%, respectively) (12). However, current data specific to neonatal haemorrhage-hepatitis syndrome owing to E-11 infection are limited and mostly from single case reports or small number cases series. Specific risk factors and early markers associated with haemorrhage-hepatitis syndrome in neonates and the immune response remain unknown. More data is needed to better characterize the neonatal disease and help to elucidate why some neonates are prone to haemorrhage-hepatitis syndrome.
The data from an E-11 infection outbreak in neonates in China in 2019 were collected. The main objectives of this study were as follows: (1) to summarize the clinical features of neonates with haemorrhage-hepatitis syndrome and (2) to explore immunological parameters associated with haemorrhage-hepatitis syndrome. These data may help clinicians to identify and manage severe E-11 infection in neonates in a timely manner and may bring some insights on the immunological responses in neonates with E-11 associated haemorrhage-hepatitis syndrome.
This was a multicentre retrospective cohort study. Neonates with E-11 infection who were admitted to one NICU in Hubei province and three NICUs in Guangdong province in China between April and October 2019 were included. A confirmed neonatal case was defined with laboratory evidence of E-11 infection detected by reverse transcription polymerase chain reaction (RT-PCR). Investigators at each participating hospital reviewed the medical records of the included patients and obtained the following data: (1) basic demographic data and perinatal history; (2) clinical course, treatments and outcomes; (3) laboratory and immunological testing. Complete blood counts were all obtained in the first three days of E-11 infection onset, and the other laboratory tests were obtained within the first week. Blood samples for immunological testing were taken between seven and ten days of illness.
Patients were categorised into two groups (mild and severe) based on the absence or presence of haemorrhage-hepatitis syndrome. Patients who were asymptomatic or with mild symptoms without hepatic dysfunction (elevated liver enzymes) and coagulopathy were categorised as the mild group. Patients in the severe group had hepatic dysfunction and coagulopathy, with the most severe ones having liver failure (defined as elevated liver enzymes with an international normalized ratio ≥2) and disseminated intravascular coagulation (DIC) (13). We reviewed the disease course from the onset of illness to up to 4 months after discharge. Patient characteristics were compared to identify risk factors and early markers associated with the development of haemorrhage-hepatitis syndrome.
This study was approved by the Institutional Review Board of Guangzhou Women and Children's Medical Centre [GWCMC- (2020)63201]. Written informed consent was collected from the parents of the participants before their inclusion in the study.
E-11 infection was identified using RT-PCR (14, 15). E-11 RNA was isolated from the throat and rectal swabs, blood specimens and/or cerebrospinal fluid of the patients (16), The extracted RNA was digested and fragmented, end repaired, and followed by adaptor ligation and PCR amplification to construct the library. The Agilent 2,100 Bioanalyzer quality control library fragment size was about 300 bp, and the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific Inc.) was used. The concentration of DNA libraries is controlled, and the constructed libraries are pooled in equal masses according to the detected concentration. The pooled libraries are looped to form a single-stranded loop structure. After rolling circle replication (RCA), DNB nanospheres are generated. The prepared DNB nanospheres are loaded onto the sequencing chip, and BGISEQ-50/MGISEQ-2000 is used for sequencing. The whole-genome sequence was compared and analysed in the National Centre of Biotechnology Information (NCBI) database.
A total of 13 cytokines and lymphocyte subsets were analysed in plasma samples obtained from six patients in one NICU in Guangdong and were compared to age-matched neonatal controls that had no symptoms of any infection. The tested cytokines included interleukin (IL)-1β, IL-6, tumour necrosis factor-alpha (TNF-α), interferon (IFN)-inducible protein-10 (IP-10), IFN-λ1, IL-8, IL-12p70, IFN-α2, IFN-λ2/3, granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-β, IL-10 and IFN-γ). Two blood samples were collected from E-11 infected infants between seven and ten days of their illness and plasma were stored at −80°C. The protein levels of cytokines were tested. For each patient, the time point with the highest inflammatory cytokine levels was considered an acute inflammatory state and thus selected for presentation.
Cytokine levels in plasma were measured using LEGENDplex™ Human Anti-Virus Response Panel (Biolegend, Cat#740118) according to the manufacturer's instructions. Samples were read by flow cytometry. LEGENDplex v8.0 was used to analyze the results. For results under the limits of detection (LOD), the lowest value of the standard for each of the cytokines was used for statistical analysis.
Peripheral blood mononuclear cells (PBMCs) were extracted from peripheral blood of the six patients and sub-populations of PBMCs were identified by using flow cytometry (FACS Aria Sorp, BD Biosciences, San Jose, CA, USA) and analysed with Flowjo 10.4 software (FlowJo LLC, Ashland, OR, USA). Peripheral blood samples were also stimulated with Phorbol-12-myristate-13-acetate (PMA). Neutrophil ROS production was measured by flow cytometry using the Dihydroethidium, DHE dye.
All antibodies used in this study were purchased from BioLegend, San Diego, CA, USA. These included BV785 anti-human CD3 (Cat # 344842), PerCP/Cy5.5 anti-human CD235 (Cat # 349110), BV510 anti-human CD8 (Cat # 344732), BV650 anti-human CD4 (Cat # 300536), FITC anti-human CD25 (Cat # 356108), PE_Dazzle594 anti-human CD127 (Cat # 351336), BV785 anti-human CD19 (Cat # 302240), PE_Dazzle594 anti-human CD269 (Cat # 357512), PE anti-human CD38 (Cat # 303506), BV510 anti-human CD138 (Cat # 356518).
Standard descriptive statistics were used to summarise the demographic and clinical data. Categorical variables were expressed as counts and percentages. The Cochran-Armitage trend or Kruskal-Wallis tests were performed to determine if significant differences existed among patients in different groups. Continuous variables that did not follow a normal distribution, as determined by the Shapiro-Wilk test, were expressed as the median and interquartile range (IQR) and were compared using the Kruskal-Wallis test. Univariate logistic regression models were used to assess the risk factors for severe infection with liver dysfunction and poor outcomes. Variables with statistical significance were further analysed using a multivariate logistic regression model. A complete-case analysis was initially performed, followed by a multiple imputation sensitivity analysis. Multiple imputation sensitivity analyses based on multistage imputation were performed to assess whether our results were biased because of missing data. The multistage imputation used the Markov Chain Monte Carlo (MCMC) and monotone regression methods to achieve approximate stationarity in fewer iterations (17). Variables selected in any one of the five imputed datasets were aggregated using PROC MIANALYZE. All probability values were two-sided, and overall statistical significance was defined as P < 0.05. For post-hoc pairwise comparisons, the Bonferroni's method was used, and the significance levels were 0.0167 (three pairwise comparisons). Analyses were performed using SAS 9.4 Windows software (SAS Institute, Inc., Cary, NC, USA, 2015).
A total of 105 neonates with E-11 infection were included in the study; 59 were male patients and 46 were female patients. These infants were born at a median gestational age of 38+3 (37–39+1) weeks with a median birth weight of 3100 g (2950–3285 g). E-11 infection occurred at a median age of 13 (3–20) days after birth. Of these 105 infants, 71% (75/105) of infants had mild disease, 29% (30/105) had severe disease. The case-fatality rate was 6% (6/105). Compared with the mild groups, the severe group had significantly higher percentages of infants born at <37 weeks (12% vs. 50%), with <2500 g birth weight (8% vs. 30%), and had onset of the disease at <3 days of life (21% vs. 33%) (P < 0.05). We found a statistically significant difference in the percentage of patients receiving parenteral nutrition (PN) prior to the onset of E-11 infection between the mild and severe groups (22% vs. 67%, respectively) (P < 0.001). (Table 1). The occurrence of E-11 infection peaked in June and July, followed by a decline in August in this cohort from two provinces that are approximately 1,000 km (approximately 600 miles) apart in China.
In this cohort, the most common initial symptoms of E-11 infection were fever (52%) and tachypnoea (21%). During the course of illness, haemorrhage (including petechiae, gastric bleeding or bloody stools) was the most common clinical symptoms (46%). The most common complications included acute myocardial injury (41%, as evidenced by elevated myocardial enzyme levels and decreased cardiac function on echocardiography), pneumonia (22%), acute respiratory distress syndrome (ARDS; 16%, excluding RDS owing to surfactant deficiency), DIC (10%), acute renal injury (9%, with elevated creatinine levels with or without oliguria/anuria) and shock (9%). However, in the severe group, all patients presented with hepatic dysfunction and coagulopathy (P < 0.001) (Supplementary Table S1).
The whole-genome RNA of E-11 from patients in Guangzhou was examined. We named the virus as E-11 strain GWCMC01/GZ/CHN/2019, whose sequence information was 99% consistent with the E-11 strain D207 (GenBank No. EF634316) that was isolated in Slovakia in 2007 (Figure 1).
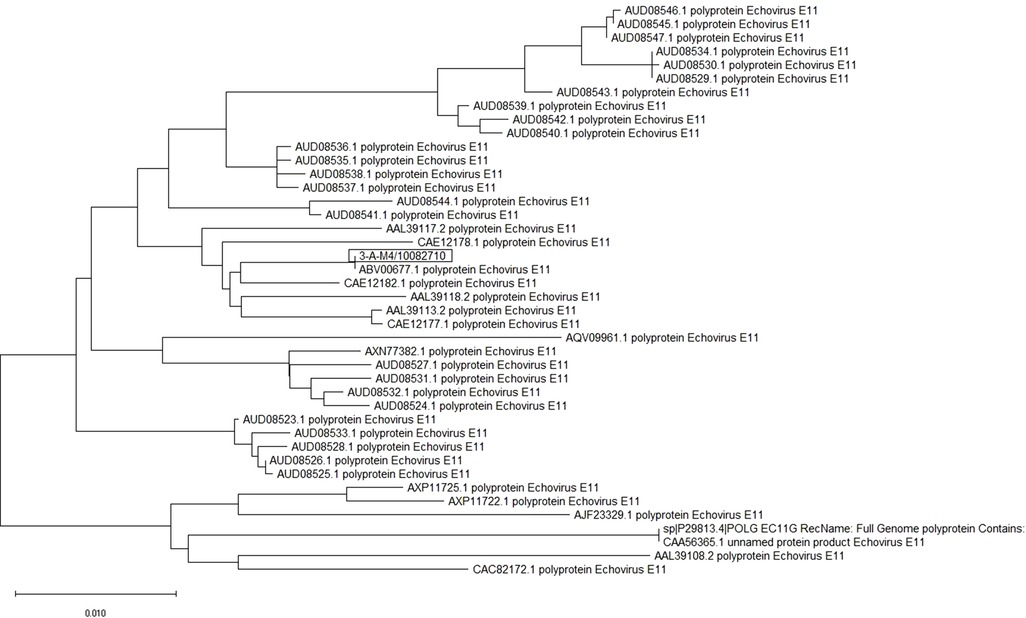
Figure 1. E-11 sequence information and phylogenetic analysis. Sequence of E-11 isolated from two patients in Guangzhou Women and Children's Medical Center was shown. It revealed that GWCMC01/GZ/CHN/2019 is 99% consistent with E-11 strain D207 (GenBank No. EF634316).
Within the first three days of the disease onset, white blood cell (WBC) counts were mostly within the normal range. Abnormalities seen within the first few days of the disease onset included decreased platelet (PLT; 16%) and lymphocyte counts (10%) and increased serum creatine kinase-MB (CK–MB) levels (39%), which were substantially elevated (50%, 14% and 55%, respectively) in the severe group from the beginning of disease onset (Table 1). Thrombocytopenia (PLT < 140 × 10⁹/L) was rare in the mild group(1%). However, half of patients in the severe group (50%) had an early onset of thrombocytopenia within the first three days (P < 0.001) (Table 1).
Most patients in the mild group only required routine care. In severe cases, transfusion (60%) was the most common treatment, followed by mechanical ventilation (57%), vasopressors (53%) and intravenous immunoglobulin (47%). In addition, some patients in the severe group were critically ill and received extensive multi-organ/system support including steroids (27%), plasmapheresis (10%) and/or continuous renal replacement therapy (CRRT; 7%). There was no death in the mild group. The median duration of hospitalisation in mild and severe groups were 7 (6–9) and 14 (8–21) days, respectively.
Patients who survived were followed up after discharge until approximately 4 months of age. All patients in the mild group remained healthy with normal development. However, there were six death and two cases of prolonged hepatic dysfunction after haemorrhage-hepatitis syndrome in the severe group (Supplementary Table S1).
Risk factors and early markers associated with haemorrhage-hepatitis syndrome and poor outcomes are demonstrated in Table 2. Infants born prematurely, had premature rupture of foetal membrane (PROM), receiving PN (TPN) at the disease onset had increased risk of developing haemorrhage-hepatitis syndrome, while age at the disease onset ≥8 days may be a protective factor. PLT < 140 × 10⁹/L within the first three days of onset, alanine transaminase (ALT) > 50 U/L and aspartate transaminase (AST) > 60 U/L in the first week of disease onset, lactic dehydrogenase (LDH) > 322 U/L and CK-MB > 40 U/L were associated with haemorrhage-hepatitis syndrome. We also found that the degree of change in several factors was associated with the severity of the disease, which include decreased haemoglobin levels (per 10 g/L; odds ratio [OR], 1.2; 95% confidence interval [CI], 1.0–1.4), increased PT (OR, 1.9; 95% CI, 1.3–2.8), activated partial thromboplastin time (APTT) (OR, 1.1; 95% CI, 1–1.2), D-dimer (OR, 1.2; 95% CI, 1–1.3), and direct bilirubin levels (OR, 1.2; 95% CI, 1–1.3).
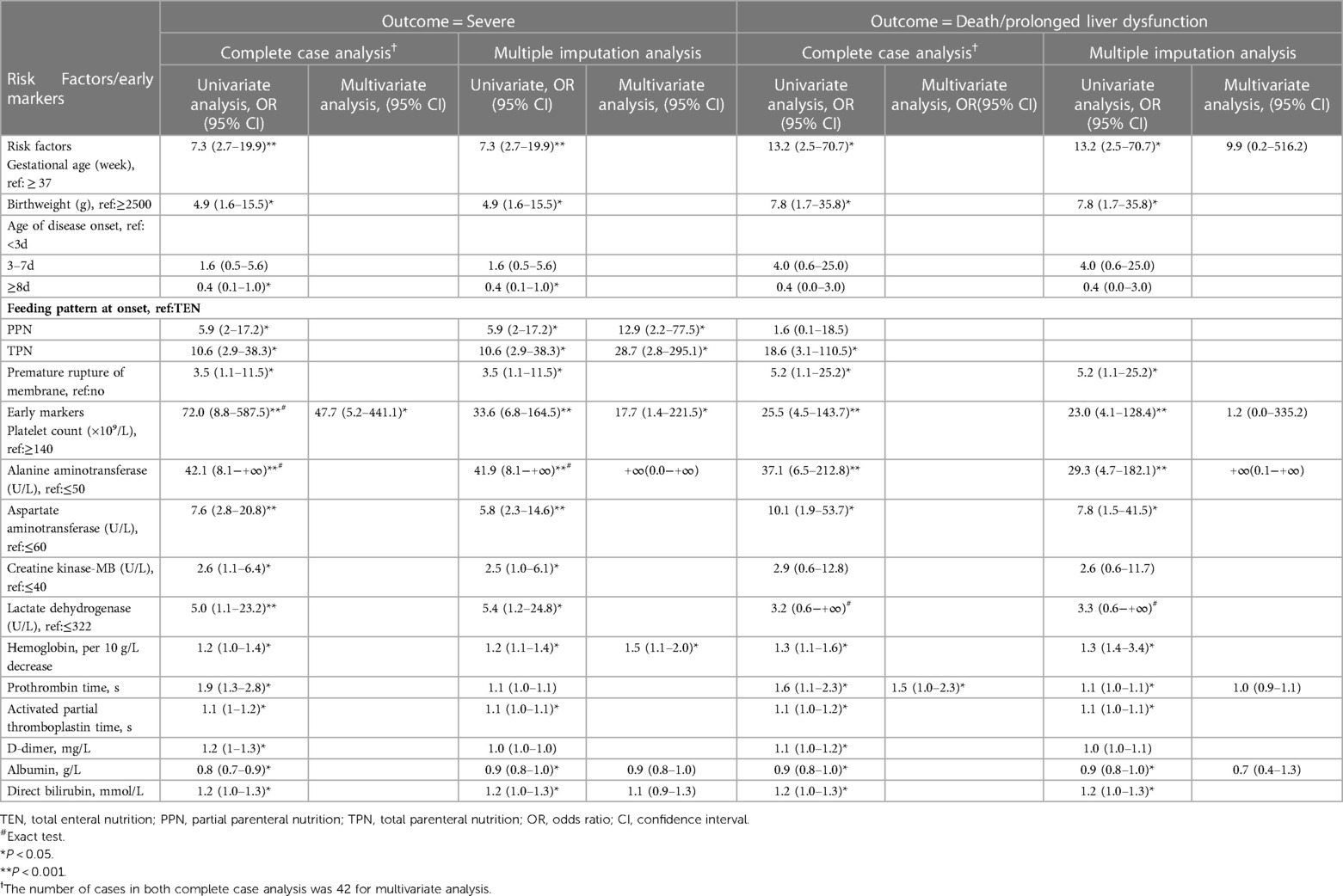
Table 2. Univariate and multivariate logistic regression analysis of risk factors/early markers for severe E-11 infection or death/prolonged liver dysfunction.
In the multiple imputation analysis, the results of the univariate analysis were similar to those of complete case analysis. According to the aggregation model, TPN (OR, 28.7; 95% CI, 2.8–295.1) and PPN (OR, 12.9; 95% CI, 2.2–77.5) at the onset of disease, decreased haemoglobin levels (per 10 g/L; OR, 1.5; 95% CI, 1.1–2.0) and PLT <140 × 10⁹/L (OR, 17.7; 95% CI, 1.4–221.5) were associated with haemorrhage-hepatitis syndrome. However, for the outcome of death/prolonged liver dysfunction, there were no statistical significance in multivariate regression for any variables (Table 2).
There were six patients with nosocomial E-11 infection from the same ward in Guangdong province. Of the six patients, four had severe disease and two had mild infection. The levels of proinflammatory cytokines (IP-10, IL-1β, IL-6, IL-8, TNF-α and GM-CSF) and anti-virus-related factors (IFN-α, IFN-λ1 and IFN-γ) were upregulated in the blood samples of the six patients as compared those of the control patients without infection. Patients with haemorrhage-hepatitis syndrome had lower concentrations of IFN-α, IFN-λ2/3, IL-1β and IFN-γ and higher levels of IP-10, IFN-λ1, IL-6, IL-10, TNF-α and GM-CSF. Among these, the difference in the protein levels of IFN-α (P < 0.05) and IP-10 (P < 0.0005) reached statistical significance (Figure 2A).
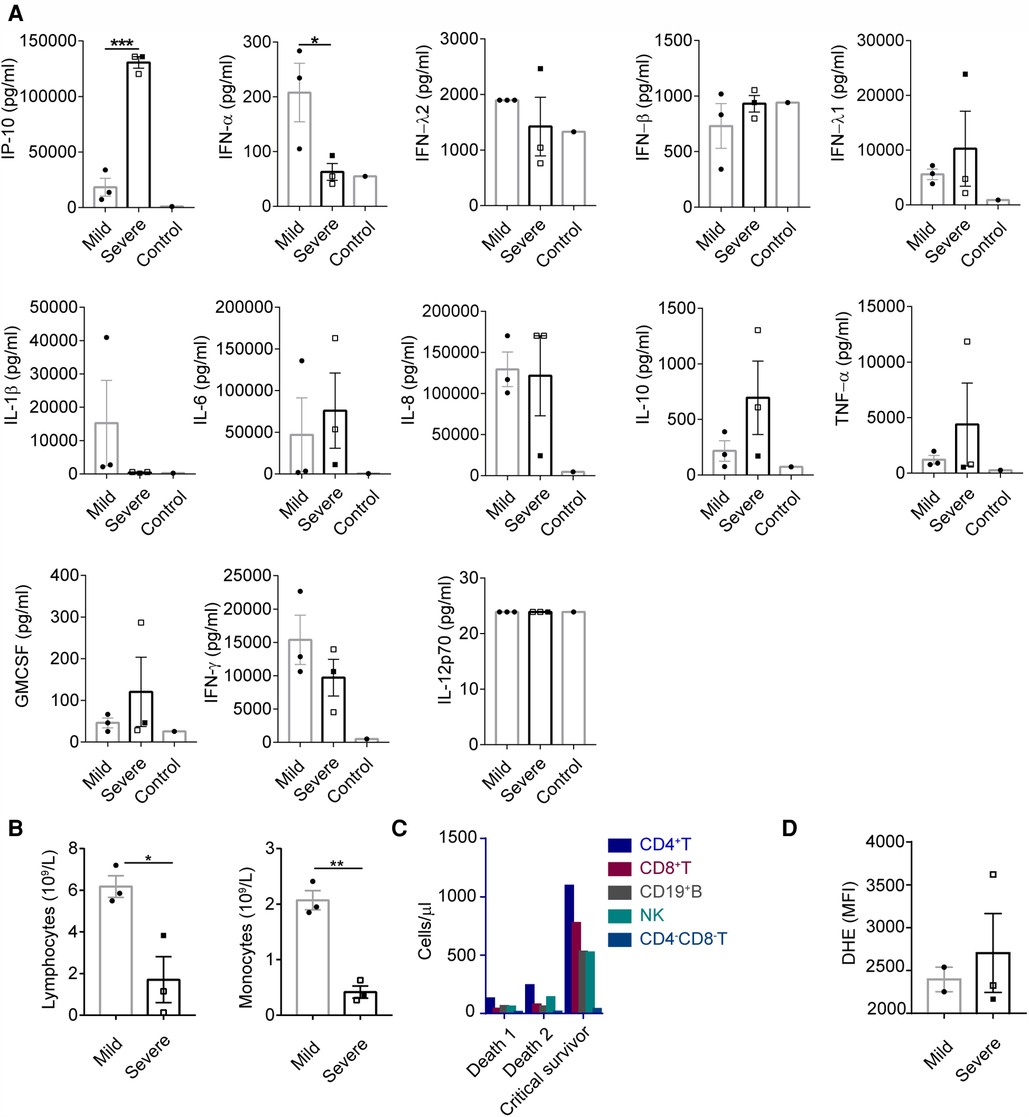
Figure 2. Impaired anti-viral capacity and increased inflammation were observed in patients with haemorrhage-hepatitis syndrome associated with severe E-11 infection. (A) Concentrations of maximum cytokine levels of plasma from E-11 infected subjects during hospitalization (severe, n = 3; mild, n = 3) and healthy control (n = 1). Cytokine levels were determined using LEGENDplex™ Human Anti-Virus Response Panel (13-plex). (A–C) Empty square represented patients that had passed away. P values were calculated by unpaired t test. *, P < 0.05; **, P < 0.01; ***, P < 0.0005; ****, P < 0.0001. (B) Lymphocyte and monocyte count from blood of E-11 infected subjects between 7 and 10 days during the course of infection (severe, n = 3; mild, n = 3). (C) CD4 + T, CD8 + T, CD19 + B, NK and CD4-CD8-T cell count from blood of E-11 infected subjects in the 10th day in the course of infection. (D) Peripheral blood samples from E-11 infected subjects post-diagnosis (severe, n = 3; mild, n = 2) were stimulated with Phorbol-12-myristate-13-acetate (PMA). Neutrophil ROS production was measured by flow cytometry using the Dihydroethidium, DHE dye. PMA induced neutrophil ROS production measured by the DHR MFI in were shown as the median (IQR).
As demonstrated in Figure 2B, the absolute lymphocyte and monocyte counts were significantly lower in the severe group than those in the mild group. The subsets of CD3+, CD4+, CD8+, CD19 + and natural killer (NK) cells were higher in patients with severe disease who survived than in those who died (Figure 2C). No difference was observed in the ability of neutrophils to generate reactive oxygen species (ROS) between the groups (Figure 2D).
Despite being an important cause of haemorrhage-hepatitis syndrome or death, E-11 infection in neonates has not been sufficiently studied (18–20). With a larger sample size than previous studies, our study compared the characteristics of patients with and without E-11 associated haemorrhage-hepatitis syndrome.
Data from previous studies suggest that more than 90% of patients with E-11 infection are asymptomatic or present with mild fever (2). However, in our cohort of neonates, we found that 29% of the infected neonates were severe cases and the case-fatality rate was 6%. Consistent with some other studies, severe E-11 infection in our neonates was associated with haemorrhage-hepatitis syndrome that causes hepatitis with liver dysfunction and coagulopathy (4, 5, 7, 8, 20). Also different from previous reports, respiratory symptoms were more common initial symptoms than gastrointestinal symptoms, and aseptic meningitis was not commonly identified in our study (3). Although generally mild, acute myocardial injury was the most common complication in our cohort. These differences may be related to difference in the age groups of the study population, and subgroups of enterovirus. In addition, a previous study from Li et al. has reported that there have been multiple genotypes of E-11, with different pathogenicity and clinical features, circulated in mainland China (21). Although the E-11genotype identified in our study is most closely related to E-11 strain D207, whether these clinical features found in our cohort are characteristic to this E-11 genotype need to be further studied.
Mortality was high (20%) in the severe group, who had variable degrees of hepatic dysfunction in our study. Early identification of neonates with the trend of haemorrhage-hepatitis syndrome is therefore crucial for prompt treatment. Previous studies have reported that factors associated with hepatic necrosis with coagulopathy in echovirus infection include prematurity, maternal history of illness, early age of onset, higher WBC count and lower haemoglobin levels (21, 22). Similarly, we found that prematurity, early age of onset (<3 days) and lower haemoglobin levels were significantly more common in severe cases. In addition, PN at disease onset is associated with an increased risk of haemorrhage-hepatitis syndrome. Moreover, an early decrease in PLT by <140 × 10⁹/L within the first three days of illness is an early marker of severe disease. These risk factors and early markers could be helpful for early identification of haemorrhage-hepatitis syndrome. Multiple imputation may reduce bias and increase power compared with complete case analysis (23). Gestational age and other variables appeared only in multiple imputation analysis, possibly due to the inclusion of larger numbers of study subjects in multiple imputation analysis, allowing more variables to achieve statistical significance.
Some studies have indicated that passive transplacental acquisition of antibodies prevents severe, systemic echovirus disease or may be the reason for asymptomatic infection (7, 24). However, the immune characteristics of haemorrhage-hepatitis syndrome remain unclear owing to limited studies. Previous studies have demonstrated that E-11 infection affects specific cell populations in the human intestine (25). In our study, we detected elevated IP-10 and decreased IFN-α in critically ill neonates. Meanwhile we also found elevated IP-10 level in the livers of patients with haemorrhage-hepatitis syndrome (data not shown). IP-10 has been shown to correlate with hepatic injury (26–30). However, whether this can be used as a marker of disease progression associated with haemorrhage-hepatitis syndrome in neonates needs to be further studied.
IP-10 could have been induced to recruit more lymphocytes as a feedback to low levels of IFN-α in cases with haemorrhage-hepatitis syndrome. Lin GL reported decreased production of type 1 IFNs as a feature of the neonatal immune system (31). We identified reduced IFN-α levels in neonates with severe disease as compared with the other neonatal patients. IFN-α is secreted by immune cells and epithelial cells and has an anti-enterovirus effect, which may restrict enterovirus replication in the human intestine (32). It has been shown that the cellular sources of IFN-α may vary during different viral infections and epithelial cells in the gut produce IFN-I in response to mucosal infections caused by different viruses (33, 34). We speculate that incomplete colonisation of intestinal flora resulting from inadequate enteral nutrition may have contributed to either immature immune function or dysfunction of intestinal epithelial cells, and thus resulting in the downregulation of IFN-α in infants with severe disease in our cohort. A study showed that some elemental diet can regulate the immunological response of intestinal tract (35). Given that PN is a risk factor for haemorrhage-hepatitis syndrome in our study and previous studies (36), we speculate that lack of enteral nutrition may aggravate deficiency of gut immune function, which plays an important role in adaptive immune insufficiency and further lead to haemorrhage-hepatitis syndrome in some neonatal patients.
Our study has some notable limitations. First, there were variations in medical treatment among different centres, and laboratory testing were not completely performed in a few centres. Second, given the retrospective nature of the study, maternal E-11 testing was not performed. Third, the patients were followed up until 4 months of age; therefore, we could not report long-term outcome data, especially long-term neurodevelopmental outcomes (37). Lastly, only one center conducted immunological testing in patients; therefore, the immunological changes found in our study needs to be confirmed by further studies. However, the new risk factors and early markers found in our study may aid the early identification of infants with haemorrhage-hepatitis syndrome that enable early intervention.
In conclusion, we found that PN at onset, thrombocytopenia and decreased haemoglobin levels in the early stage of illness are associated with haemorrhage-hepatitis syndrome in neonates with E-11 infection. We speculate that intestinal immune insufficiency may play a key role in the development of haemorrhage-hepatitis syndrome in neonates, which should be verified by future studies.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Institutional Review Board of Guangzhou Women and Children's Medical Centre. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
SG, HZ and WZ: conceived, designed and supervised the study. PW, YX and ML: designed the study, analyzed data and wrote the daft. HL, HW, YL, BW and SX: assisted in data collection and analysis. ML and BL: assisted in interpreting the findings of the lab. HL, and XG: interpreted the findings, commented on and helped revise drafts of the manuscript. HS, MW, LT and XC: participated in collection and management of data. All authors contributed to the article and approved the submitted version.
Guangzhou Science and Technology Plan Project (No. 202102080247), Basic and Applied Basic Research Foundation of Guangdong Province (No. 2022A1515012354) and the Foundation of Guangzhou Women and Children's Medical Center (No. CWCMC2020-6-011). Funds of Guangzhou Women and Children's Medical Center received for open access publication fees.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2023.1063558/full#supplementary-material.
1. Oberste MS, Nix WA, Kilpatrick DR, Flemister MR, Pallansch MA. Molecular epidemiology and type-specific detection of echovirus 11 isolates from the americas, Europe, Africa, Australia, southern Asia and the Middle East. Virus Res. (2003) 91:241–8. doi: 10.1016/S0168-1702(02)00291-5
2. Chuang YY, Huang YC. Enteroviral infection in neonates. J Microbiol Immunol Infect. (2019) 52:851–7. doi: 10.1016/j.jmii.2019.08.018
3. Rodà D, Pérez-Martínez E, Cabrerizo M, Trallero G, Martínez-Planas A, Luaces C, et al. Clinical characteristics and molecular epidemiology of enterovirus infection in infants <3 months in a referral paediatric hospital of Barcelona. Eur J Pediatr. (2015) 174:1549–53. doi: 10.1007/s00431-015-2571-z
4. Berry PJ, Nagington J. Fatal infection with echovirus 11. Arch Dis Child. (1982) 57:22–9. PMID: 7199896; PMCID: 2863273
5. Modlin JF. Fatal echovirus 11 disease in premature neonates. Pediatrics. (1980) 66:775–80. doi: 10.1542/peds.66.5.775
6. Ho SY, Chiu CH, Huang YC, Chen CJ, Lien R, Chu SM, et al. Investigation and successful control of an echovirus 11 outbreak in neonatal intensive care units. Pediatr Neonatol. (2020) 61:180–7. doi: 10.1016/j.pedneo.2019.09.012
7. Nagington J, Wreghittt TG, Gandy G, Roberton NR, Berry PJ. Fatal echovirus 11 infections in outbreak in special-care baby unit. Lancet. (1978) 2:725–8. doi: 10.1016/S0140-6736(78)92714-9
8. Modlin JF. Perinatal echovirus infection: insights from a literature review of 61 cases of serious infection and 16 outbreaks in nurseries. Rev Infect Dis. (1986) 8:918–26. doi: 10.1093/clinids/8.6.918
9. Messacar K, Spence-Davizon E, Osborne C, Press C, Schreiner TL, Martin J, et al. Clinical characteristics of enterovirus A71 neurological disease during an outbreak in children in Colorado, USA, in 2018: an observational cohort study. Lancet Infect Dis. (2020) 20:230–9. doi: 10.1016/S1473-3099(19)30632-2
10. Bubba L, Broberg EK, Jasir A, Simmonds P, Harvala H. Enterovirus study collaborators. Circulation of non-polio enteroviruses in 24 EU and EEA countries between 2015 and 2017: a retrospective surveillance study. Lancet Infect Dis. (2020) 20:350–61. doi: 10.1016/S1473-3099(19)30566-3
11. Bersani I, Auriti C, Piersigilli F, Dotta A, Diomedi-Camassei F, Di Pede A, et al. Neonatal acute liver failure due to enteroviruses: a 14 years single NICU experience. J Matern Fetal Neonatal Med. (2020) 33:2576–80. doi: 10.1080/14767058.2018.1555806
12. Khetsuriani N, Lamonte A, Oberste MS, Pallansch M. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the United States, 1983–2003. Pediatr Infect Dis J. (2006) 25:889–93. doi: 10.1097/01.inf.0000237798.07462.32
13. Newland CD. Acute liver failure. Pediatr Ann. (2016) 45:e433–8. doi: 10.3928/19382359-20161128-01
14. Aronson PL, Lyons TW, Cruz AT, Freedman SB, Okada PJ, Fleming AH, et al. Pediatric emergency medicine clinical research network (PEM CRC) herpes Simplex virus (HSV) study group. Impact of enteroviral polymerase chain reaction testing on length of stay for infants 60 days old or younger. J Pediatr. (2017) 189:169–74. doi: 10.1016/j.jpeds.2017.06.021
15. Gill PJ, Richardson SE, Ostrow O, Friedman JN. Testing for respiratory viruses in children: to swab or not to swab. JAMA Pediatr. (2017) 171:798–804. doi: 10.1001/jamapediatrics.2017.0786
16. Tomatis Souverbielle C, Feister J, Leber A, Salamon D, Mejias A, Ramilo O, et al. Multiple sites PCR testing for enteroviruses in young febrile infants. Lancet Infect Dis. (2019) 19:239–40. doi: 10.1016/S1473-3099(19)30042-8
18. Bessaud M, Delpeyroux F. Enteroviruses-the famous unknowns. Lancet Infect Dis. (2020) 20:268–9. doi: 10.1016/S1473-3099(19)30636-X
19. Li J, Yan D, Chen L, Zhang Y, Song Y, Zhu S, et al. Multiple genotypes of echovirus 11 circulated in mainland China between 1994 and 2017. Sci Rep. (2019) 9:10583. doi: 10.1038/s41598-019-46870-w
20. Modlin JF. Echovirus infections of newborn infants. Pediatr Infect Dis J. (1988) 7:311–2. doi: 10.1097/00006454-198805000-00002
21. Bouwman JJ, Visseren FL, Bosch MC, Bouter KP, Diepersloot RJ. Procoagulant and inflammatory response of virus-infected monocytes. Eur J Clin Invest. (2002) 32:759–66. doi: 10.1046/j.1365-2362.2002.01041.x
22. Lin TY, Kao HT, Hsieh SH, Huang YC, Chiu CH, Chou YH, et al. Neonatal enterovirus infections: emphasis on risk factors of severe and fatal infections. Pediatr Infect Dis J. (2003) 22:889–94. doi: 10.1097/01.inf.0000091294.63706.f3
23. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. (2011) 30:377–99. doi: 10.1002/sim.4067
24. Modlin JF, Polk BF, Horton P, Etkind P, Crane E, Spiliotes A. Perinatal echovirus infection: risk of transmission during a community outbreak. N Engl J Med. (1981) 305:368–71. doi: 10.1056/NEJM198108133050703
25. Drummond CG, Bolock AM, Ma C, Luke CJ, Good M, Coyne CB. Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner. Proc Natl Acad Sci U S A. (2017) 114:1672–7. doi: 10.1073/pnas.1617363114
26. Wada N, Takaki A, Ikeda F, Yasunaka T, Onji M, Nouso K, et al. Serum-inducible protein (IP)-10 is a disease progression-related marker for non-alcoholic fatty liver disease. Hepatol Int. (2017) 11:115–24. doi: 10.1007/s12072-016-9773-y
27. Zhao K, Yang T, Sun M, Zhang W, An Y, Chen G, et al. IP-10 Expression in patients with chronic HBV infection and its ability to predict the decrease in HBsAg levels after treatment with entecavir. Mol Cells. (2017) 40:418–25. doi: 10.14348/molcells.2017.0051
28. Wang C, Ji D, Chen J, Shao Q, Li B, Liu J, et al. Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Clin Gastroenterol Hepatol. (2017) 15:132–6. doi: 10.1016/j.cgh.2016.06.023
29. Harvey CE, Post JJ, Palladinetti P, Freeman AJ, Ffrench RA, Kumar RK, et al. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J Leukoc Biol. (2003) 3:360–9. doi: 10.1189/jlb.0303093
30. Wells AI, Grimes KA, Kim K, Branche E, Bakkenist CJ, DePas WH, et al. Human FcRn expression and type I interferon signaling control echovirus 11 pathogenesis in mice. Plos Pathog. (2021) 1:e1009252. doi: 10.1371/journal.ppat.1009252
31. Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol. (2018) 9:2147. doi: 10.3389/fimmu.2018.02147
32. Saxena K, Simon LM, Zeng XL, Blutt SE, Crawford SE, Sastri NP, et al. A paradox of transcriptional and functional innate interferon responses of human intestinal enteroids to enteric virus infection. Proc Natl Acad Sci U S A. (2017) 114:E570–9. doi: 10.1073/pnas.1615422114
33. Swiecki M, Colonna M. Type I interferons: diversity of sources, production pathways and effects on immune responses. Curr Opin Virol. (2011) 1:463–75. doi: 10.1016/j.coviro.2011.10.026
34. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. (2012) 489:231–41. doi: 10.1038/nature11551
35. Olaussen RW, Løvik A, Tollefsen S, Andresen PA, Vatn MH, De Lange T, et al. Effect of elemental diet on mucosal immunopathology and clinical symptoms in type 1 refractory celiac disease. Clin Gastroenterol Hepatol. (2005) 3:875–85. doi: 10.1016/S1542-3565(05)00295-8
36. Van Puffelen E, Vanhorebeek I, Joosten KFM, Wouters PJ, Van den Berghe G, Verbruggen SCAT. Early versus late parenteral nutrition in critically ill, term neonates: a preplanned secondary subgroup analysis of the PEPaNIC multicentre, randomised controlled trial. Lancet Child Adolesc Health. (2018) 2:505–15. doi: 10.1016/S2352-4642(18)30131-7
37. Van Hinsbergh TMT, Elbers RG, Hans Ket JCF, van Furth AM, Obihara CC. Neurological and neurodevelopmental outcomes after human parechovirus CNS infection in neonates and young children: a systematic review and meta-analysis. Lancet Child Adolesc Health. (2020) 4:592–605. doi: 10.1016/S2352-4642(20)30181-4
Keywords: newborn, echovirus 11, intensive care medicine, hepatitis, critical infection
Citation: Wang P, Xu Y, Liu M, Li H, Wang H, Liu Y, Wang B, Xia S, Su H, Wei M, Tao L, Chen X, Lu B, Gu X, Lyu H, Zhou W, Zhang H and Gong S (2023) Risk factors and early markers for echovirus type 11 associated haemorrhage-hepatitis syndrome in neonates, a retrospective cohort study. Front. Pediatr. 11:1063558. doi: 10.3389/fped.2023.1063558
Received: 7 October 2022; Accepted: 14 March 2023;
Published: 6 April 2023.
Edited by:
Maria Giulia Conti, Sapienza University of Rome, ItalyReviewed by:
Laura Pellegrinelli, University of Milan, Italy© 2023 Wang, Xu, Liu, Li, Wang, Liu, Wang, Xia, Su, Wei, Tao, Chen, Lu, Gu, Lyu, Zhou, Zhang and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhou emhvdXdlaV9wdTAwMkAxMjYuY29t Huayan Zhang emhhbmdoQGVtYWlsLmNob3AuZWR1 Sitang Gong c2l0YW5nZ0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.