
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 21 February 2023
Sec. Pediatric Surgery
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1062144
This article is part of the Research Topic Advances on Clinical and Basic Translational Research of Neonatal Babies with Surgical Issues View all 27 articles
Aim: To observe the change of capsulotomy opening diameter (COD) in aphakic eyes after primary congenital cataract removal and investigate its influencing factors.
Methods: Ocular parameters, including corneal diameter (CD), axial length (AL), anterior and posterior COD (ACOD, PCOD), and age at surgery were recorded at primary congenital cataract removal and secondary intraocular lens implantation. The concentrations of 15 kinds of cytokines in aqueous humor samples collected at the primary surgery were detected. The change (Δ) of COD between two surgeries were described, and its association was analyzed.
Results: Fifty eyes from 33 patients with congenital cataract who underwent primary and secondary surgery were enrolled. The changes in ACOD and PCOD were not statistically significant on the whole. ΔACOD was positively correlated with ΔCD and the concentrations of PDGF-AA, VEGF and TGF-β1. The concentration of FGF-2 and the interval between two surgeries showed negative correlations with ΔACOD and ΔPCOD.
Conclusion: COD in aphakic eyes kept changing after primary surgery. The positive correlation between ΔACOD and ΔCD manifested the enlargement of ACOD was influenced by lateral eye growth. Meanwhile, ΔACOD was also associated with cytokines, indicating postoperative inflammation promoted the ACOD constriction.
Congenital cataract, with an overall prevalence of 0.63–9.7 per 10,000 people, is the main cause of childhood blindness worldwide (1, 2). As a precondition for visual rehabilitation, surgery is now a safe and effective intervention for infant patients (3). Due to their undeveloped anatomical structure, majority of children <2-year-old routinely undergo secondary intraocular lens (IOL) implantation at an older age (4).
Sulcus and capsular bag are the most common positions to house an IOL during secondary surgery. The procedure of sulcus IOL implantation is relatively easier, but with higher rates of postoperative complications, such as pigment dispersion, elevated intraocular pressure and IOL decentration (5, 6). In-the-bag IOL implantation, with the advantage of conforming to physiological structure, better IOL centration and lower rates of postoperative complications, may be a better long-term choice for patients (6, 7).
Capsulotomy opening diameter (COD) is a key factor in IOL stability. Published studies had recommended an anterior COD (ACOD) of 4.0–5.0 mm and a posterior COD (PCOD) of 3.0–4.0 mm for optimal capsular outcome for secondary IOL implantation (8, 9). Along with the eye ball growth and inflammatory response, COD seemed to go through uncertain changes postoperatively such as capsulotomy opening area opacity and capsular constriction (10). Cases whose ACOD and PCOD changed similarly or inversely were occasionally seen, some of which even lost the chance to undergo in-the-bad IOL implantation due to severe COD change. Thus, it was necessary to figure out the change of COD. However, previously studies rarely focused on COD itself.
This study aimed to observe the change of COD in aphakic eyes after primary congenital cataract removal, and to investigate its influencing factors, in order to discover the change rule of COD.
Patients who were diagnosed with congenital cataract by experienced ophthalmologists and underwent primary cataract removal and secondary IOL implantation at the Eye Hospital of Wenzhou Medical University (Hangzhou branch) were preliminarily enrolled. Complete follow-up data and clear operation videos that showed COD were requisite. Exclusion criteria included ophthalmic diseases (microcornea, microphthalmia, posterior capsule defect and persistent fetal vasculature), and systemic disorders (Marfan syndrome). Patients with severe capsular fibrosis, who needed scissors or a vitrector to remove part of capsule, and whose pupils could not be dilated during surgery, were also excluded.
This study was conducted following the tenets of the Declaration of Helsinki, adhered to the ARVO statement on human subjects. It was approved by the Ethics Review Committee of Wenzhou Medical University. Informed consent forms were obtained from the legal guardians of the patients.
All surgeries were performed by the same experienced pediatric cataract surgeon (Yune Zhao) under general anesthesia using a 23-gauge microincision vitrectomy system (Accurus, Alcon Laboratories, Fort Worth, TX, United States).
Primary surgery included lensectomy, posterior capsulotomy and limited anterior vitrectomy. Two 1.0-mm clear corneal paracenteses were created at 3 and 9 o'clock using a diamond knife. A 23-gauge irrigating cannula was inserted via one port to maintain the anterior chamber, while the other 23-gauge vitrector was inserted via the other port to create a stab opening in the anterior capsule. The vitrector hole was then extended until an anterior capsulotomy opening of approximately 4.0–5.0 mm diameter was created. The cortex was aspirated in irrigation/aspiration mode. A posterior capsulotomy opening of approximately 3.0–4.0 mm diameter was performed with the vitrector. About one third of the anterior vitreous volume was removed using the same vitrectomy setting.
Compared to primary surgery, an additional scleral tunnel at 12 o'clock was made for IOL insertion at secondary surgery. After reopening the residual capsule with an iris spatula or capsulorhexis forceps and aspirating the cortex in the peripheral bag with a vitrector, the viscoelastic material was filled into the rebuilt capsular bag and a suitable IOL was placed in the bag.
The age at surgery was recorded. Axial length (AL) was measured via an A-scan (Axis nano, Quantel Medical, Cournon, France) under sedation before surgery. Corneal diameter (CD) was measured after general anethesia at the beginning of surgery using calipers from the nasal to the temporal inner side of the limbus transition zone.
The measurements of ACOD and posterior OCD (PCOD) were performed using Adobe Photoshop CC 2018 (Adobe Systems Inc., CA, United States) (Figure 1). First, we captured a legible image that clearly showed ACOD and PCOD from the operation video. In the video of the primary surgery, an image was captured under a stable anterior chamber after suturing two corneal incisions. As for the secondary surgery, images were captured after separating the fused anterior and posterior capsule and when the anterior chamber was well maintained by a 23-gauge infusion cannula with the pressure of 30 cmH2O. Second, we measured the length of CD, ACOD and PCOD from the nasal edge to the temporal edge horizontally via Adobe Photoshop CC 2018 in units of pixels. Finally, since the value of CD in units of millimeter had already been measured before, the values of ACOD and PCOD in units of millimeter were calculated by equal-scale conversion.
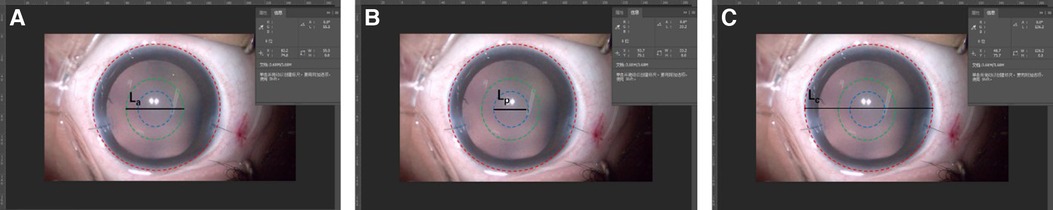
Figure 1. Measurements of anterior capsulotomy opening diameter (ACOD) and posterior capsulotomy opening diameter (PCOD) using adobe photoshop CC 2018. The red, green, and blue circles represented the cornea and, anterior and posterior capsulorhexis opening, while La, Lp, and Lc represent ACOD, PCOD and corneal diameter (CD), respectively, in units of pixels. As the value of CD (mm) was measured, the values of ACOD and PCOD (mm) could be calculated by equal-scale conversion: .
As all parameters used for the equal-scale conversion were measured manually, we used a fixed optical surface diameter of IOL (6 mm) to test the accuracy of this method (Figure 2). First, we captured a legible image that clearly showed ACOD and PCOD from the operation video of primary IOL implantation. Second, we measured the length of CD, ACOD, PCOD and IOL in units of pixels from the nasal edge to the temporal edge horizontally using Adobe Photoshop CC 2018. Third, as the value of CD (mm) had already been measured before and the optical surface diameter of IOL was fixed, the values of ACOD and PCOD (mm) could be calculated by equal-scale conversion through CD and IOL. Finally, the values of ACOD and PCOD calculated using CD and IOL were compared.
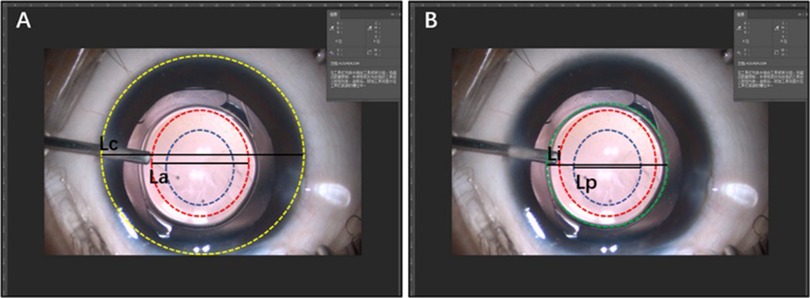
Figure 2. Verification of the accuracy of the measurement method of capsulotomy opening diameter (COD). (A) The method converted the values of ACOD and PCOD (mm) through the value of CD (mm). (B) The method converted the values of ACOD and PCOD (mm) through the value of the optical surface diameter of the intraocular lens (6 mm).
The change (Δ) of the parameters between two surgeries was defined as the parameter of secondary surgery minus that of primary surgery.
Aqueous humor samples were collected at the beginning of primary surgery using a 29-gauge needle via limbal paracentesis. All samples were collected before any intraocular operation to avoid the influence of surgical stimulation. Considering that the anterior chambers in infant patients were narrow, only 100 μl of aqueous humor was collected. After being transferred to aseptic EP tubes marked with the information of the patients, the samples were immediately stored in −80°C refrigerator until analysis.
By referring to the published studies focusing on the association between cytokines and capsular structure, a total of 15 kinds of cytokines were selected for detection, including IL-6, IL-10, IL-15, TNF-α, IFN-γ, MCP-1, IP-10, G-CSF, FGF-2, PDGF-AA, VEGF, EGF, TGF-β1, TGF-β2, and TGF-β3. The concentrations of these cytokines were measured using Luminex xMAP technology with multi-analyte profiling beads (Lincoplex cytokine/chemokine multiplex kit, HCYTO-60K; Millipore Corporation, Billerica, MA).
Data were analyzed using SPSS 22.0 (SPSS, Armonk, NY, United States). Normally distributed data were expressed as mean ± standard deviation. Two-tailed paired t test was used to compare the means between the primary and secondary parameters. The correlations between normally distributed parameters were analyzed using Pearson's correlation analysis. For data that did not fit the normal distribution, values were recorded as median and 25th to 75th interquartile range. Wilcoxon signed-rank test was used to compare the primary and secondary parameters. Spearman correlation analysis was used to analyze the correlations. A two-tailed P value less than 0.05 was considered statistically significant for all tests.
Sixty eyes from 41 congenital cataract patients were preliminarily enrolled. One eye from 1 patient, whose pupil failed to be dilated, was excluded. Six eyes from 5 patients were excluded due to concurrent ophthalmic diseases. Three eyes from 2 patients were excluded for incomplete data. Finally, 50 eyes from 33 patients who underwent primary and secondary surgery between February 2016 and April 2020 were enrolled (Figure 3). Demographic data for the enrolled patients are summarized in Table 1.
Twenty eyes that underwent primary IOL implantation were selected to test the accuracy of the measurement method. The values of ACOD and PCOD converted by CD and IOL were compared in Table 2. The result indicated that our method to measure COD in aphakic eyes was reliable.
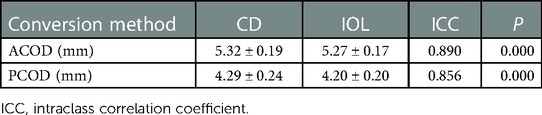
Table 2. Intraclass correlation coefficient between the values of ACOD and PCOD converted by CD and IOL.
The values of the ocular parameters were presented as mean ± standard deviation and were summarized in Table 3. No significant difference in ACOD and PCOD between two surgeries was found.
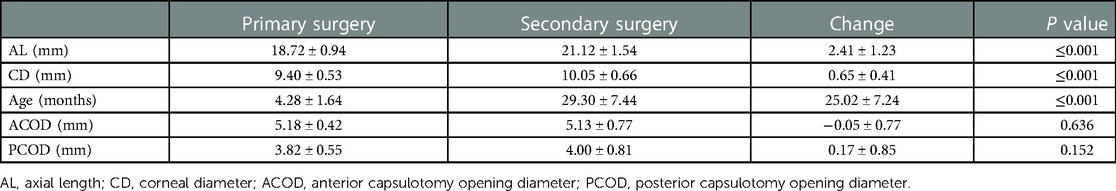
Table 3. Mean ± standard deviation of ocular parameters collected at the primary and secondary surgery.
ΔACOD showed a positive correlation with ΔCD (r2 = 0.113, y = 0.63*x + 0.46, P = 0.017) (Figure 4), but no similar correlation was observed between ΔPCOD and ΔCD. Negative correlations were shown between the interval of two surgeries with ΔACOD (r2 = 0.131, y = −0.04*x + 0.91, P = 0.010) and ΔPCOD (r2 = 0.104, y = −0.04*x + 1.12, P = 0.022) (Figure 4). No significant correlation was observed between ΔCOD and ΔAL, age at surgery, sex and eye type.
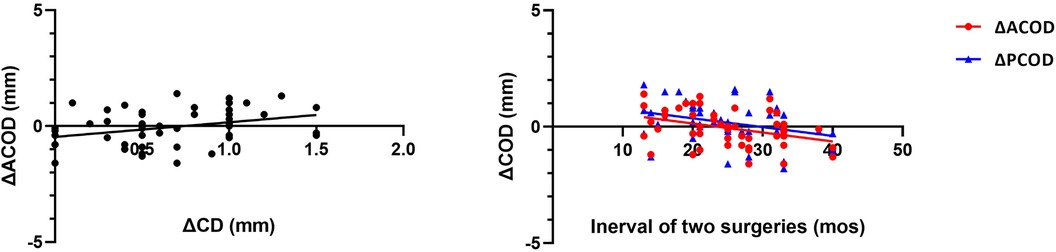
Figure 4. Comparisons of the concentrations of IL-15, TNF-α, MCP-1, G-CSF, and FGF-2 in the aqueous humor samples collected at the primary and secondary surgery. PAQ, aqueous humor sample collected at primary surgery; SAQ, aqueous humor sample collected at secondary surgery.
A total of 50 samples from 50 eyes of 33 patients were collected. The concentrations of cytokines in the aqueous humor samples are presented as the median and 25th to 75th interquartile range (Table 4).
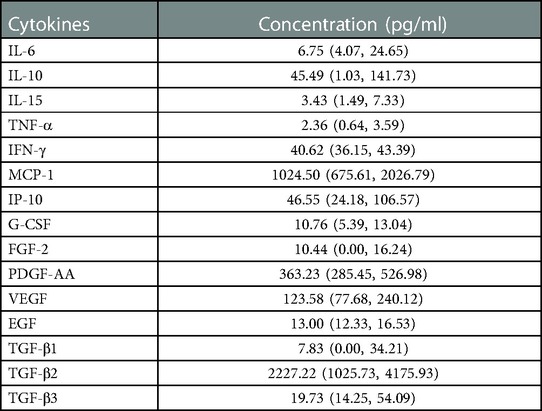
Table 4. Median and 25th to 75th interquartile range of the concentrations of cytokines in the aqueous humor samples collected at the primary surgery.
Negative correlations were observed between the concentrations of FGF-2 with ΔACOD (r2 = 0.216, y = −0.04*x + 0.52, P = 0.037) and ΔPCOD (r2 = 0.319, y = −0.06*x + 0.77, P = 0.003) (Figure 5). Positive correlations were observed between ΔACOD with the concentrations of PDGF-AA (r2 = 0.108, y = 1.56 × 10−3*x − 0.58, P = 0.016), VEGF (r2 = 0.080, y = 1.33 × 10−3*x − 0.29, P = 0.033), and TGF-β1 (r2 = 0.225, y = 0.001*x − 0.18, P = 0.013) (Figure 5).
In consideration of the postoperative complications and the complexity of IOL power calculation, for infants younger than 2 years old, especially under 6 months, lens removal with posterior capsulotomy and limited anterior vitrectomy was the more common surgery choice (4, 11). At secondary IOL implantation, only those whose residual peripheral capsule was intact with suitable COD could accept in-the-bag IOL implantation.
In a previous study, COD was measured with a self-made measuring needle with a laser marker (1 mm scale) (12). This measurement method ignored the indication error caused by the angel between the capsulotomy opening plane and the measuring needle. In this study, COD was actually calculated by equal-scale conversion. Besides, we verified the accuracy of this method with a fixed optical surface diameter of IOL (6 mm).
In the enrolled aphakic eyes of the current study, both ACOD and PCOD changed, along with age, axial length and corneal diameter. Although on the whole, the changes of AOCD and PCOD were minor and not statistically significant, the range of changes was very wide. Serious changes in COD increase the difficulty of secondary in-the-bag IOL implantation, and related factors need to be furtherly studied. In our study, ΔCD represents lateral eye growth while ΔAL represents axial growth. The positive correlation between ΔACOD and ΔCD indicated that the change of ACOD was influenced by lateral eye growth rather than axial growth, since no correlation was shown between ΔACOD and ΔAL. While ΔPCOD did not show similar positive correlation with ΔCD, it changed strongly in agreement with ΔACOD. Meanwhile, ΔACOD and ΔPCOD both showed negative correlations with the interval between two surgeries, indicating a constriction trend of COD after primary surgery.
The strength to contract the capsule was more likely due to the proliferation of lens epithelial cells (LECs). Anterior and posterior capsule opacification were the classical fibrotic disease exhibiting LECs hyperproliferation and outgrow after cataract surgery in response to surgical trauma, myofibroblast formation, matrix deposition and constriction, which were considered to be associated with wrinkling/constriction of the residual capsule (13, 14). This surgery-induced complication was commonly seen in infants after congenital cataract surgery. In this fibrotic progression, the inflammation response had long been considered to be the inducement (15).
In our study, the correlations between ΔACOD and the concentrations of FGF-2 (r2 = 0.216, y = −0.04*x + 0.52, P = 0.037) and TGF-β1 (r2 = 0.225, y = 0.001*×−0.18, P = 0.013) prompted the roles of FGF-2 and TGF-β playing in LECs proliferation and differentiation. It had been shown that FGF-2 plays an important role in the proliferation and differentiation of LECs (16, 17). Low concentrations of FGF-2 could maintain LEC proliferation while high concentrations could promote differentiation and lentoid formation in vitro (17). Meanwhile, in the human lens, LECs transdifferentiated and proliferated into myofibroblasts through an epithelial-mesenchymal transition induced by TGF-β1, and as a result, these myofibroblasts promoted lens capsule wrinkling (18–20). Studies mentioned above proved that FGF-2 and TGF-β played roles in the progression of LECs proliferation and differentiation.
PDGF-AA (r2 = 0.108, y = 1.56 × 10−3*x − 0.58, P = 0.016) and VEGF (r2 = 0.080, y = 1.33 × 10−3*x − 0.29, P = 0.033) were the other two cytokines that showed correlations with ΔACOD, which indicated the participation of them in the change of residual capsule. Previously, Wu et al. found that the preoperative concentration of PDGF-AA exhibited positive correlations with the PCO scores at 1 month and 3 months after pediatric cataract surgery (r = 0.497, P = 0.030; r = 0.647, P = 0.009) (21). As for VEGF, which was considered strongly implicated in neovascularization, although there was no published study describing its association with the capsule, its existence in some nonvascular tissues, such as the cornea, was confirmed by Gogat et al. (22). To date, the concrete mechanism of the two cytokines in the change of residual capsule has been unknown, but our findings might provide new scope for further basic research.
The main strengths of our study are as follows. First, this study investigated the postoperative capsular outcome from the perspective of COD, and furtherly came up with a hypothesis that the change of ACOD after primary surgery was influenced by the lateral eye growth and postoperative inflammation. After primary surgery, the strength of the lateral eye growth gradually decreased as the patients passing the developmental phase after birth, but the strength to contract the capsule existed persistently. As a result, ACOD showed contraction trend after primary surgery. Second, more patients with congenital cataract were included in this study compared to those in previous studies. The limitations of our study must be mentioned. First, only 100 μl of aqueous humor was collected from a congenital cataract eye per surgery for security, which limited the number of cytokines that could be detected. Second, the results only presented the change in COD in interval between primary and secondary surgery, longer follow-up was needed.
In conclusion, the results showed that the COD remained changing after primary congenital cataract removal. The correlations between ΔACOD and ΔCD, the interval between two surgeries, as well as the correlations between ΔACOD and the concentrations of FGF-2, PDGF-AA, and TGF-β1, prompted us to first put forward the hypothesis that the change in ACOD was influenced by lateral eye growth and LECs proliferation regulated by cytokines. However, it is hard to summarize the changing rule of COD simply according to the result of this study, and thus further research is needed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Review Committee of Wenzhou Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
QHZ designed and conducted the research, and wrote this article. PJC designed the research. YYZ analyzed the data. DDW helped to polish this article. YEZ designed the research and was the corresponding author. All authors contributed to the article and approved the submitted version.
National Natural Science Foundation of China (grant no. 81870680).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Foster A, Gilbert C, Rahi J. Epidemiology of cataract in childhood: a global perspective. J Cataract Refract Surg. (1997) 23(Suppl 1):601–4. doi: 10.1016/S0886-3350(97)80040-5
2. Sheeladevi S, Lawrenson JG, Fielder AR, Suttle CM. Global prevalence of childhood cataract: a systematic review. Eye (London, England). (2016) 30:1160–9. doi: 10.1038/eye.2016.156
3. Kumar P, Lambert SR. Evaluating the evidence for and against the use of IOLs in infants and young children. Expert Rev Med Devices. (2016) 13:381–9. doi: 10.1586/17434440.2016.1153967
4. Solebo AL, Cumberland P, Rahi JS. 5-year Outcomes after primary intraocular lens implantation in children aged 2 years or younger with congenital or infantile cataract: findings from the IoLunder2 prospective inception cohort study. Lancet Child Adolesc Health. (2018) 2:863–71. doi: 10.1016/S2352-4642(18)30317-1
5. Chang DF, Masket S, Miller KM, Braga-Mele R, Little BC, Mamalis N, et al. Complications of sulcus placement of single-piece acrylic intraocular lenses: recommendations for backup IOL implantation following posterior capsule rupture. J Cataract Refract Surg. (2009) 35:1445–58. doi: 10.1016/j.jcrs.2009.04.027
6. Mehta R, Aref AA. Intraocular Lens implantation in the ciliary sulcus: challenges and risks. Clin Ophthalmol. (2019) 13:2317–23. doi: 10.2147/OPTH.S205148
7. Trivedi RH, Wilson ME Jr., Facciani J. Secondary intraocular lens implantation for pediatric aphakia. J AAPOS. (2005) 9:346–52. doi: 10.1016/j.jaapos.2005.02.010
8. Wilson ME Jr., Englert JA, Greenwald MJ. In-the-bag secondary intraocular lens implantation in children. J AAPOS. (1999) 3:350–5. doi: 10.1016/s1091-8531(99)70044-3
9. Lin H, Tan X, Lin Z, Chen J, Luo L, Wu X, et al. Capsular outcomes differ with capsulorhexis sizes after pediatric cataract surgery: a randomized controlled trial. Sci Rep. (2015) 5:16227. doi: 10.1038/srep16227
10. Petric I, Lacmanović Loncar V. Surgical technique and postoperative complications in pediatric cataract surgery: retrospective analysis of 21 cases. Croat Med J. (2004) 45:287–91. PMID: 15185419.15185419
11. Lambert SR, Aakalu VK, Hutchinson AK, Pineles SL, Galvin JA, Heidary G, et al. Intraocular lens implantation during early childhood: a report by the American academy of ophthalmology. Ophthalmology. (2019) 126:1454–61. doi: 10.1016/j.ophtha.2019.05.009
12. Tan X, Lin H, Lin Z, Chen J, Tang X, Luo L, et al. Capsular outcomes after pediatric cataract surgery without intraocular Lens implantation: qualitative classification and quantitative measurement. Medicine (Baltimore). (2016) 95:e2993. doi: 10.1097/MD.0000000000002993
13. Bertelmann E, Kojetinsky C. Posterior capsule opacification and anterior capsule opacification. Curr Opin Ophthalmol. (2001) 12:35–40. doi: 10.1097/00055735-200102000-00007
14. Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res. (2009) 88:257–69. doi: 10.1016/j.exer.2008.10.016
15. Eldred JA, Dawes LJ, Wormstone IM. The lens as a model for fibrotic disease. Philos Trans R Soc Lond B Biol Sci. (2011) 366:1301–19. doi: 10.1098/rstb.2010.0341
16. Lovicu FJ, McAvoy JW. Structural analysis of lens epithelial explants induced to differentiate into fibres by fibroblast growth factor (FGF). Exp Eye Res. (1989) 49:479–94. doi: 10.1016/0014-4835(89)90056-0
17. Wang D, Wang E, Liu K, Xia CH, Li S, Gong X. Roles of TGFβ and FGF signals during growth and differentiation of mouse lens epithelial cell in vitro. Sci Rep. (2017) 7:7274. doi: 10.1038/s41598-017-07619-5
18. Novotny GE, Pau H. Myofibroblast-like cells in human anterior capsular cataract. Virchows Arch A Pathol Anat Histopathol. (1984) 404:393–401. doi: 10.1007/BF00695223
19. Hales AM, Chamberlain CG, McAvoy JW. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Visual Sci. (1995) 36:1709–13. PMID: 7601651.
20. Lovicu FJ, Schulz MW, Hales AM, Vincent LN, Overbeek PA, Chamberlain CG, et al. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. (2002) 86:220–6. doi: 10.1136/bjo.86.2.220
21. Wu X, Liu Z, Wang D, Lin D, Long E, Lin Z, et al. Preoperative profile of inflammatory factors in aqueous humor correlates with postoperative inflammatory response in patients with congenital cataract. Mol Vision. (2018) 24:414–24. PMID: 29930475.
Keywords: change, congenital cataract, capsulotomy opening diameter, association, aphakic eye
Citation: Zhao Q, Chang P, Zhao Y, Wang D and Zhao Y (2023) Capsulotomy opening diameter outcomes in aphakic eyes after primary congenital cataract removal and its association. Front. Pediatr. 11:1062144. doi: 10.3389/fped.2023.1062144
Received: 5 October 2022; Accepted: 3 February 2023;
Published: 21 February 2023.
Edited by:
Antonino Morabito, University of Florence, ItalyReviewed by:
Irina Trifanenkova, S. Fyodorov Eye Microsurgery Federal State Institution, Russia© 2023 Zhao, Chang, Zhao, Wang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yune Zhao enllaHpleWVAMTI2LmNvbQ==
Specialty Section: This article was submitted to Pediatric Surgery, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.