- Department of Pediatrics, University of Chieti, Chieti, Italy
Introduction: Increase in cardiac biomarkers during Coronavirus disease 2019 (COVID-19) was frequent regardless of the presence of myocarditis and multisystem inflammatory syndrome in children (MIS-C). Several studies described MIS-C, but few papers evaluated cardiac manifestations in children with SARS-CoV-2 infection without MIS-C and investigated the role of troponin in absence of electrocardiogram (ECG) and echocardiographic alterations. The aim of this case series is to describe the cardiac manifestations during COVID-19 in children, trying to explain the meaning of laboratory findings during COVID-19, especially of increased troponin.
Materials and methods: We conducted a retrospective case series of children aged <18 years admitted at the Department of Pediatrics, University of Chieti, for SARS-CoV-2 infection between 1st March 2020 and 31th July 2022. All patients with documented SARS-CoV-2 infection underwent a laboratory evaluation at admission. Children with increased troponin I and/or BNP underwent electrocardiographic and echocardiographic exams.
Results: 125 children were admitted for SARS-CoV-2 infection to our Department of Pediatrics, of whom 17 (13.6% of cases) with different patterns of cardiac involvement. Specifically, 5 subjects (4.0% of admitted children) were diagnosed as MIS-C and 12 children (9.6%) manifested a cardiac involvement in terms of increased troponin with or without ECG and echocardiography anomalies. Troponin, C-reactive protein, procalcitonin and BNP values resulted higher in patients with MIS-C compared to patients without MIS-C. Furthermore, patients with MIS-C had higher neutrophils and lower lymphocytes compared to patients without MIS-C. ECG abnormalities were found in 4/5 patients with MIS-C and in 2/12 patients without MIS-C. Echocardiographic anomalies were found in all patients with MIS-C, especially in terms of valve regurgitation and ejection fraction reduction and in 2/12 patients without MIS-C, especially in terms of pericardial effusion. Despite high troponin levels, children presented a favorable clinical evolution.
Conclusion: The increase in troponin level in children with COVID-19 could also be due to respiratory causes or a massive inflammatory state. In our case series, patients with increased troponin associated to COVID-19 presented a favorable clinical course with clinical and laboratory remission almost always within 7 days.
1. Introduction
At the onset of the Coronavirus disease 2019 (COVID-19) outbreak, children were marginally involved, accounting for 1.7% of cases, and considered mostly asymptomatic carrier cases (1). During the following epidemic waves, an increasing number of children exposed to COVID-19 developed a multisystem inflammatory syndrome in children (MIS-C), defined on May 14, 2020, by the Centers for Disease Control (CDC) with a Health Alert Network (1, 2). Although COVID-19 mostly showed a favorable prognosis in children, MIS-C was characterized by an overwhelming inflammatory activation, with clinical similarities with Kawasaki disease (KD) including cardiac involvement (3).
SARS-CoV-2 mainly affects the upper respiratory tract. Nevertheless, the virus can damage other tissue than lung through a direct injury or an indirect one caused by the release of proinflammatory cytokines (4). An exaggerated inflammatory response triggered by the cytokine storm could cause a multi-organ involvement (5). A higher risk of severity and mortality was described in patients with underlying cardiovascular morbidity (6).
The incidence of MIS-C is not clear, but some estimates showed that MIS-C occurs in 5.1 out of 1,000,000 person-months in individuals under the age of 21 years (7). Cardiac involvement was frequently described in children with MIS-C, with myo-pericardial inflammation, coronary dilatation or aneurysm and arrhythmias (8). The mechanism of myocardial dysfunction in MIS-C is still unclear, but possible causes include acute myocarditis, post-viral immunological reaction, and systemic inflammatory response syndrome (2). The pathophysiology of MIS-C includes a sequence of events. Firstly, neutrophils infiltrate vessel walls causing acute necrotizing arteritis and an aneurysm within the coronary artery. Macrophages and T-cell lymphocytes accumulate in the damaged vessel wall, initiating a chronic form of vasculitis, with proliferation of myofibroblasts and risk for coronary artery stenosis. In the early acute phase, myocardial edema characterizes myocarditis before evidence of an aneurysm, with possibly transient left ventricular dysfunction that can lead to cardiovascular shock (4).
In literature, clinical and laboratory data mostly derived from clinical cases. In a case series of 20 critically ill children admitted for shock, fever and SARS-CoV-2 infection between 15th and 27th April 2020, the authors found an acute myocarditis with a mean left ventricular ejection fraction of 35% and high troponin level (269 ng/ml). The first symptoms before admission were intense abdominal pain and fever for 6 days and all children showed increased inflammation indexes (9).
In a French prospective study including 21 children and adolescents with features of KD admitted between 27 April and 11 May 2020, Toubiana et al. (10) found evidence of recent SARS-CoV-2 infection in 19 (90%) subjects. The authors observed myocarditis in 16 (76%) patients, with a median left ventricular ejection fraction rate of 42%, increased troponin I and B-type natriuretic peptide in 81% and 78% patients respectively, and pericardial effusion in 48% of enrolled children. Interestingly, all 21 patients presented noticeable gastrointestinal symptoms and high levels of inflammatory markers during the early stage of disease.
Furthermore, in a multicenter case series of 183 children with MIS-C, a wide clinical spectrum was found. All patients presented with fever, 63.9% gastrointestinal symptoms, and 43.2% presented with shock. Inotropic support, mechanical ventilation, and extracorporeal membrane oxygenation were indicated in 39.3%, 23.5%, and 2.2% patients, respectively (11).
An increased volume, a diastolic dysfunction and a reduced ejection fraction of the left ventricle were frequently found during echocardiographic exam. On imaging examinations, Magnetic Resonance Imaging (MRI) findings often showed late gadolinium enhancement, native T1 and T2 enhancement, and pericardial enhancement (9). These data suggest that SARS-CoV-2 can cause myocarditis, myocardial ischemia, and heart failure in a significant percentage of infected patients (12).
Children with COVID-19 myocarditis showed higher C-reactive protein levels, variable clinical features, need for shorter inotropic therapy and faster recovery of the left ventricular systolic function compared to patients with non-COVID-19 myocarditis (13).
The management of acute MIS-C patient includes cardiac support, immunomodulation, and antiplatelet/anticoagulant treatments (14). Whittaker et al. (15) documented cardiac involvement with left ventricular dysfunction on echocardiography and troponin elevation in 62% and 66% of children with MIS-C, respectively.
The aim of this case series is to describe the cardiac manifestations during COVID-19 in children, trying to explain the meaning of laboratory findings during COVID-19, especially of increased troponin.
2. Materials and methods
We conducted a retrospective case series of all children aged <18 years admitted at the Department of Pediatrics, University of Chieti, for SARS-CoV-2 infection between 1st March 2020 and 31th July 2022. Written informed consent was obtained from the minor's legal guardian for the publication of any potentially identifiable images or data included in this article. We reviewed the medical records of children who needed hospitalization to collect clinical, laboratory, imaging and echocardiographic findings and data about COVID-19 vaccination history. The time between onset of symptoms and admission and days of hospitalization were also recorded.
SARS-CoV-2 was diagnosed at the admission using reverse-transcriptase polymerase chain reaction (PCR) on nasopharyngeal and/or oropharyngeal swab samples. Serological test for SARS-CoV-2 antibodies detection was performed in children without active infection.
Clinical evaluation included physical examination and vital signs. Main clinical symptoms at onset, including fever, mucocutaneous involvement, presence of nonsuppurative laterocervical lymphadenopathy, conjunctivitis, and gastrointestinal, respiratory, cardiovascular and neurologic symptoms were also collected.
All patients with documented SARS-CoV-2 infection underwent a laboratory evaluation at admission, including blood cell count with white blood cells (WBC, 103/mmc), platelet count (PLT, 103/mmc) and hemoglobin (Hb, g/dl); C-reactive protein (CRP, mg/L) and procalcitonin (PCT, mg/ml); troponin I (pg/ml) and brain natriuretic peptid (BNP, pg/ml), transaminases (U/L), ferritin (ng/ml), D-dimer (ng/ml). According to our Laboratory Unit, the upper limit of serum troponin level is 15.2 pg/ml and of BNP is 100 pg/ml.
Children with increased troponin I and/or BNP underwent electrocardiographic and echocardiographic exams. Electrocardiogram (ECG) data, including abnormal PR and QT intervals and ST- and T-wave changes, were recorded. Echocardiography findings were considered, including left ventricular ejection fraction, assessment of coronary arteries, and pericardial effusion.
Cardiac involvement is defined according to World Health Organization (WHO) definition as the presence of myocardial dysfunction, pericarditis, valvulitis, or coronary abnormalities (including findings on echocardiogram or elevated levels of troponin/BNP) (16). Patients with cardiac involvement were included in the analysis and divided into two groups: SARS-CoV-2 patients with and without MIS-C. According to WHO criteria, MIS-C was diagnosed in children and adolescents 0–19 years of age with fever >3 days with evidence of SARS-CoV-2 infection and exclusion of other obvious microbial cause and increased inflammation markers and two the following criteria: (a) Rash or bilateral non-purulent conjunctivitis or muco-cutaneous inflammation signs (oral, hands or feet); (b) Hypotension or shock; (c) cardiac involvement; (d) Evidence of coagulopathy (by prothrombin time, partial thromboplastin time, elevated d-Dimers); (e) Acute gastrointestinal problems (diarrhea, vomiting, or abdominal pain) (16).
In children with MIS-C, usual etiologic causes of acute myocarditis were screened, including testing of a large panel of non-SARS-CoV-2 viruses in blood, as Epstein–Barr virus, Cytomegalovirus, Parvovirus B19, Coxsackievirus, Echovirus. Other causes of infection were also excluded testing culture of urine, stool, and nasopharyngeal swabs and detection of Mycoplasma, Adenovirus, Influenza and Parainfluenza virus antibodies.
Information about need for oxygen, intravenous immunoglobulins, antiplatelet/anticoagulant treatments and inotropic support was collected. The clinical evolution including admission to Intensive Care Unit or death was also considered.
Continuous data was expressed as median and range 5%–95%. Categorical data was presented as numbers and percentages. Mann-Whitney U test was performed to compare characteristics between two groups. The statistical significance level was p < 0.05. SPSS version 25.0 for Windows (IBM, Armonk, NY, USA) and STATA/IC 15.1 (StataCorp. 2017. Stata Statistical Software: Release 15. StataCorp LLC. College Station, TX, USA) were used to perform statistical analysis.
3. Results
One hundred twenty five children were admitted for SARS-CoV-2 infection to our Department of Pediatrics. Seventeen children (13.6% of cases) showed different patterns of cardiac involvement. Specifically, 5 subjects (4.0% of admitted children) were diagnosed as MIS-C with a median age of 8.8 years (5%–95% CI 1.8–16.0); three were male (60.0%). Twelve children (9.6% of total cases) with a median age of 0.2 years (5%–95% CI 0.1–5.4, male 50%) manifested a cardiac involvement in terms of increased troponin with or without ECG and echocardiography anomalies, and showing no diagnostic criteria for MIS-C (Figure 1). Other etiologic causes of acute myocarditis (Epstein–Barr virus, Cytomegalovirus, Parvovirus B19, Coxsackievirus, Echovirus) resulted negative in children with MIS-C.

Figure 1. Flow-chart of children enrollment with main laboratory, electrocardiographic and echocardiographic findings in the two groups.
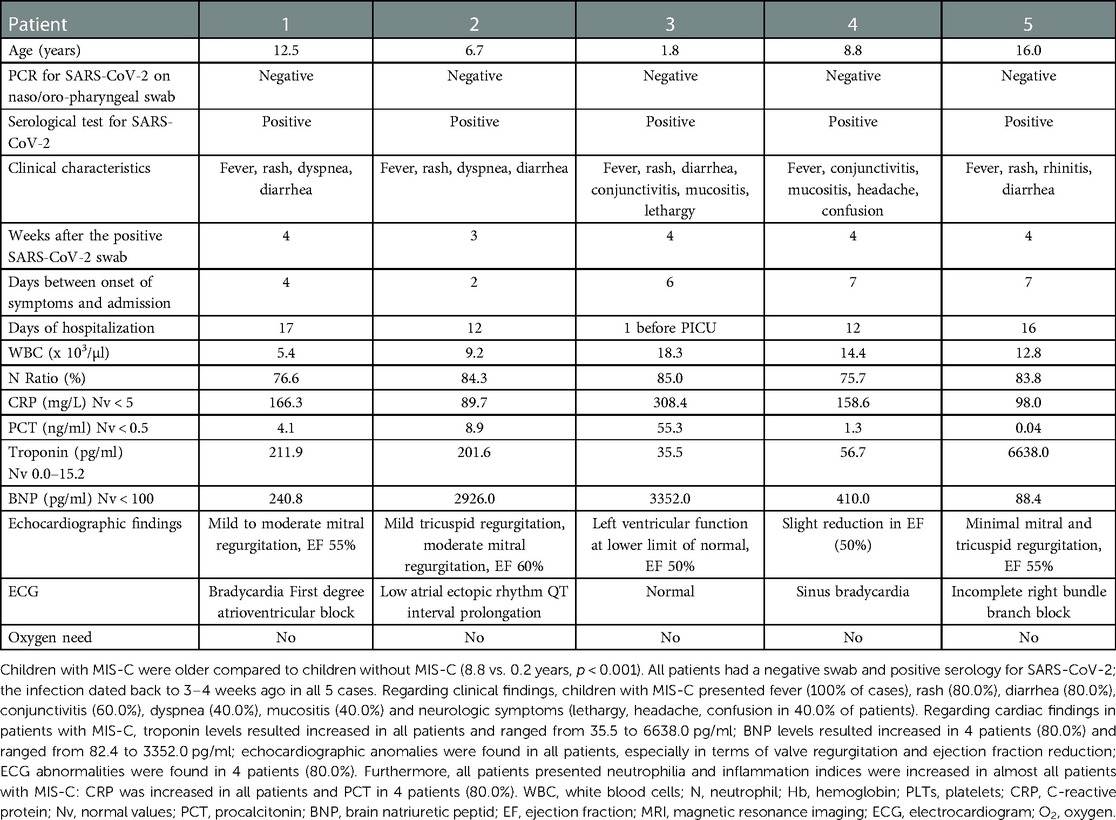
Children with MIS-C were older compared to children without MIS-C (8.8 vs. 0.2 years, p < 0.001). Patients without MIS-C were mainly infants (11/12 patients, 91.6% of cases), mostly within the first 3 months of life. The child with the highest troponin level (710 picogram/ml) was a 2-month-old infant with a minimal pericardial effusion detected by echocardiography that did not require any intervention. In this case, troponin level gradually decreased fluctuating after approximately 70 days, while increased troponin values in other patients became normal after about a week.
Nine patients (9/108, 8.3%) among children without cardiac involvement and one patient (1/17, 5.9%) with increased troponin levels were previously vaccinated for SARS-CoV-2.
Children with MIS-C presented fever (100% of cases), rash (80.0%), diarrhea (80.0%), conjunctivitis (60.0%), dyspnea (40.0%), mucositis (40.0%) and neurologic symptoms (lethargy, headache, confusion in 40.0% of patients). In patients without MIS-C, clinical characteristics ranged from fever (66.7% of patients), tachypnea (50.0%), cough (41.7%), diarrhea and weight loss (8.3%), headache (8.3%) and tachycardia (8.3%). Patients with MIS-C showed higher troponin levels compared to patients without MIS-C [201.6 (35.5–6638.0) vs. 65.5 (18.3–710.0)] but the difference was not statistically significant (p = 0.328). C-reactive protein [158.6 (89.7–109.4) vs. 3.5 (0.2–38.5); p < 0.0001], procalcitonin [4.1 (0.04–55.3) vs. 0.2 (0.01–1.4); p = 0.048], and BNP [410.0 (82.4–3352.0) vs. 24.7 (0–564.2); p = 0.008] values were higher in patients with MIS-C compared to patients without MIS-C. Values of WBS are similar between the two groups [12.8 (5.4–18.3) vs. 9.7 (2.9–19.5); p = 0.442], but patients with MIS-C had higher neutrophils [10.7 (CI 4.1–15.5) vs. 2.1 (0.6–7.1); p = 0.002] and lower lymphocytes [1.4 (0.7–2.2) vs. 6.7 (1.7–10.9); p = 0.002] compared to patients without MIS-C.
Aspartate aminotransferase [28.0 (20.0–156.0) vs. 49.0 (25.0–574.0); p = 0.377], alanine aminotransferase [21.0 (19.0–350.0) vs. 43.0 (21.0–406.0); p = 0.377], ferritin [378.0 (283.8–524.8) vs. 784.5 (186.4–1755.0); p = 0.247] and D-dimer [1.2 (0.5–10.2) vs. 0.9 (0.6–1.5); p = 0.524] were not significantly different between the two groups.
ECG abnormalities were found in 4/5 patients (80.0%) with MIS-C (Table 1), while in patients with increased troponin and without MIS-C 2/12 patients (16.7%) showed ECG abnormalities: a patient with ventricular repolarization (VR) abnormalities and one with sinus tachycardia (Table 2).
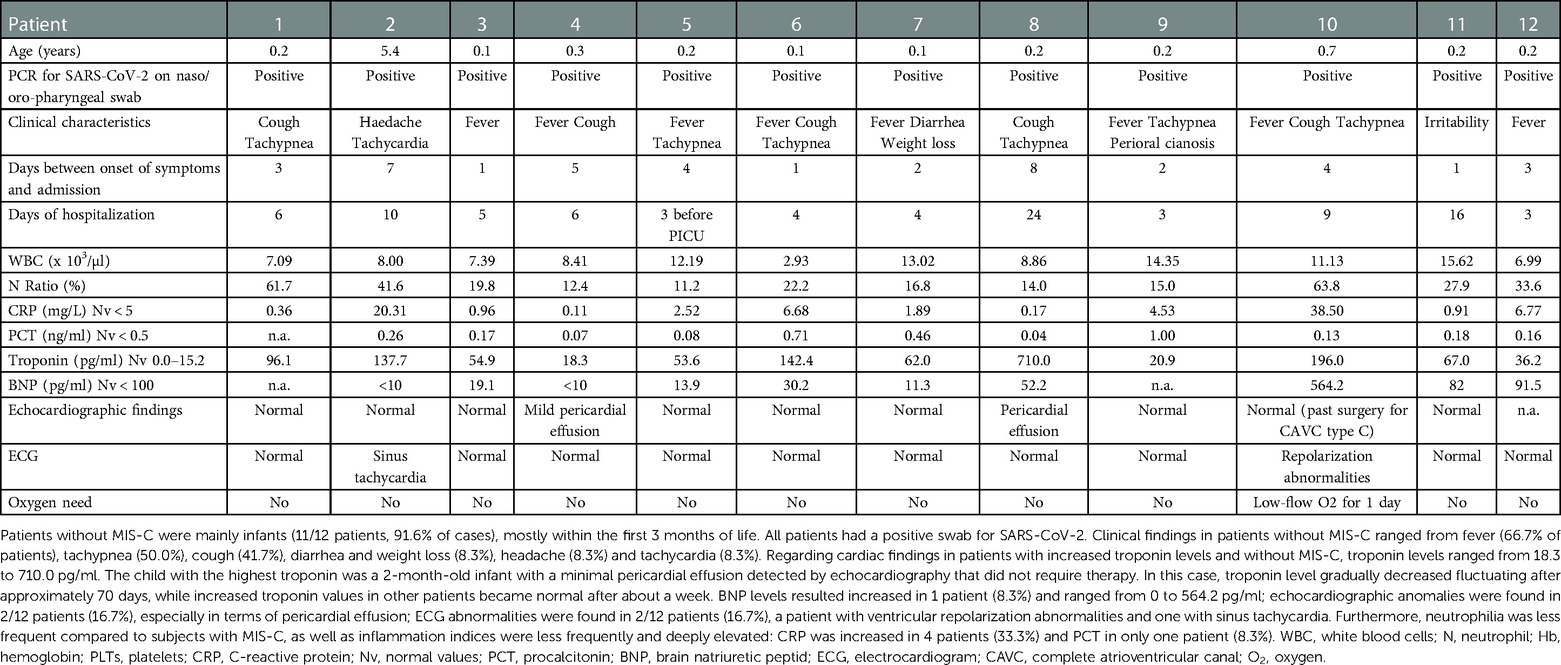
Table 2. Clinical, laboratory and cardiac findings and oxygen need of 12 patients with increased troponin levels without MIS-C.
Echocardiographic anomalies were found in all patients with MIS-C, especially in terms of valve regurgitation and ejection fraction reduction (Table 1). Conversely, echocardiographic anomalies were found in 2/12 patients (16.7%), especially in terms of pericardial effusion (Table 2).
One patient (20.0%) in the MIS-C group and one patient (8.3%) in the group without MIS-C were transferred to the Pediatric Intensive Care Unit. None of the 17 patients presented sequelae. None of the 17 patients died, but a patient in the whole group of infected children (1/125, 0.8%) died because of respiratory failure. The patient had Down syndrome and congenital heart disease (subaortic ventricular defect and patent foramen ovale with pulmonary hypertension, previously surgically corrected).
4. Discussion
In this case series, cardiac involvement was detected in 13.6% (17/125) and MIS-C in 4.0% (5/125) of hospitalized children with a recent history of COVID-19 or acute COVID-19. All the patients with MIS-C showed increased troponin level, while 12 children (9.6% patients) with current SARS-CoV-2 infection presented increased troponin levels. Despite high troponin level, only 2/12 patients had ECG anomalies and 3/12 echocardiographic ones.
In literature, several studies described MIS-C and the role of troponin increase as a marker of myocardial damage was widely reported (17–19). Otherwise, few papers evaluated cardiac manifestations in children with SARS-CoV-2 infection without MIS-C and investigated the role of troponin in absence of ECG and echocardiographic alterations. Noteworthy, increase in cardiac biomarkers during COVID-19 was frequent regardless of the presence of myocarditis, especially in the phase of severe systemic inflammation and acute respiratory distress syndrome and quantitatively associated with poor outcome (20). Indeed, an increase in cardiac biomarkers was found in up to 27% of COVID-19 patients (21).
In a single-center retrospective observational study of 759 children with increased troponin levels over an 11-year period, the authors found that this laboratory finding was associated mostly to cardiac diseases. Nevertheless, increased troponin resulted also in drug or carbon monoxide intoxication, bronchopneumonia, asthma, sepsis, septic shock, and systemic inflammatory response syndrome and hypotension or hypovolemia (22). Therefore, Yoldas et al. (22) showed that troponin elevation could be caused also by non-cardiac disease.
We speculated that the frequent increased troponin levels in children with COVID-19 could not be due to viral infection of the heart. Indeed, in a study including COVID-19 patients increased troponin levels were not correlated with left ventricular dysfunction, but with right ventricular one. These findings suggested that increased troponin was due to acute right ventricular overload secondary to parenchymal or vascular lung disease resulting in subendocardial damage of the right ventricular myocardium (23). However, respiratory distress was not significant in our 12 patients with troponin increase, such that only one required oxygen. Therefore, in children infected and without myocarditis, the increase in troponin could be mostly related to the intense inflammatory state (22).
Otherwise, another hypothesis could be that very mild myocarditis, not evident on ECG and echocardiogram findings, resulted only in troponin increase. Tunçer et al. (24) reported that a mild myocarditis evolved with complete recovery without complications, albeit elevated troponin levels. ECG anomalies were detected in 93%–100% of children with myocarditis in retrospective studies; therefore, a myocarditis without ECG alterations was described rarely (25–27). We speculated that mild cardiac involvement with no clinical significance could be detected only by laboratory tests.
Comparing patients with and without MIS-C, we found higher troponin level in patients with MIS-C, although not significantly. Furthermore, we found significant higher levels of neutrophils, C-reactive protein, procalcitonin and BNP and significant lower values of lymphocytes in patients with MIS-C compared to patients without MIS-C. Similarly, in a recent study with 233 children with MIS-C and 102 with COVID-19, patients with MIS-C had significant higher levels of troponin, BNP and C-reactive protein and lower lymphocytes compared with COVID-19 children (28). We suggested that these laboratory findings could help the physician to differentiate patients with MIS-C. Particularly, BNP seems to be a promising severity index. In a recent metanalysis including 24 studies comprised of 2,583 COVID-19 patients and 1,613 MIS-C patients, the authors suggested that BNP was the key cardiac marker that showed differences between patients with MIS-C/non-severe COVID-19 and between patients with severe/non-severe MIS-C. The authors found that other markers, such as troponin and transaminases, did not exhibit notable differences in indicating cardiac injury between patients with MIS-C and COVID-19 (29).
In our study population, ECG abnormalities were found in 80% of patients with MIS-C and 16.7% of patients without MIS-C. In literature, arrhythmic manifestations were described in a wide range of patients (from 7% to 60%) with MIS-C (14). The most frequently described ECG anomalies were non-specific anomalies of ventricular repolarization, prolongation of the QT interval and premature atrial or ventricular beats (14). First and second degree atrioventricular (AV) blocks and atrial fibrillation were also reported (8, 30). In literature, less information were reported regarding ECG changes in children with troponin elevation without MIS-C. In the 12 children without MIS-C, we found no ECG changes that were clinically significant: one reported only sinus tachycardia and the other ventricular repolarization. Furthermore, the latter was a child with a history of previous surgery for complete atrioventricular canal.
Echocardiography has a key role for the diagnosis and monitoring of myocarditis (31). In our study population, echocardiographic anomalies were found in all patients with MIS-C (especially in terms of valve regurgitation and ejection fraction reduction), confirming the key role of this tool in the diagnosis of MIS-C. Echocardiographic anomalies were also found in 16.7% of patients without MIS-C, especially in terms of pericardial effusion, probably indicating very mild pericarditis. In literature, cardiac complications occurred in approximately 30% of infected children and in nearly all those with MIS-C (2). In a study including 294 children with active or previous SARS-CoV-2 infection, of which 46 with MIS-C, the most frequent echocardiographic change was pericardial effusion, followed by left ventricular systolic dysfunction, while coronary abnormalities occurred in about one third of patients with MIS-C (2).
The lack of MRI data in patients with cardiac involvement is a limitation in this case series. Indeed, cardiac MRI is the gold standard for the quantitative evaluation of ventricular systolic function and it could detect myocardial edema. Verification of myocarditis in patients with acute cardiac syndromes but normal coronary arteries or with atypical symptoms is one of its greatest challenges in clinical practice (32).
Regarding clinical evolution, in our population one patient in the MIS-C group and one patient in the group without MIS-C were transferred to a Pediatric Intensive Care Unit. However, the clinical evolution was favorable for all patients with complete clinical remission; none of the 17 patients presented sequelae or died. In literature, a mortality rate of 1% in children with MIS-C after adequate treatment was reported (33). In a large meta-analysis of 42 studies including 275,661 children without comorbidities and 9,353 children with comorbidities, a mortality rate of 0.03% in children without comorbidities and of 1.5% in children with pre-existing comorbidities was found (34). In our study population, a patient in the whole group of infected children (1/125, 0.8%) died because of respiratory failure. The patient had Down syndrome and congenital heart disease (subaortic ventricular defect and patent foramen ovale with pulmonary hypertension, previously surgically corrected).
5. Conclusion
We found a 4% prevalence of MIS-C in subjects hospitalized for COVID-19 and a 9.6% prevalence of troponin elevation in patients infected without MIS-C. Additionally, children with MIS-C were older, had higher neutrophils, inflammation indexes, troponin and BNP levels and lower lymphocytes compared to children without MIS-C.
This case series is one of the few investigating the role of troponin in SARS-CoV-2 infected children without MIS-C. The significance of troponin increase not associated to ECG and echocardial anomalies is not yet clear. Surely, it must be kept in mind that the increase in troponin level can also occur in case of right ventricle overload due to respiratory causes or in case of a massive inflammatory state. However, this increase could also be caused by a very slight myocardial damage, not detectable on ECG and echocardiogram. In our case series, patients with increased troponin associated to SARS-CoV-2 infection presented a favorable clinical course with clinical and laboratory remission almost always within 7 days.
Nevertheless, follow-up of children with cardiac involvement associated to SARS-CoV-2 infection remains to be clarified. It is not clear whether a control echocardiogram is necessary in all patients with increased troponin. Larger multicenter studies to better define the incidence and characteristics of cardiac involvement associated to SARS-CoV-2 infection in children are needed. Finally, being a novel entity, long-term studies are needed to better define evolution and prognosis of this disease in children.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor's legal guardian for the publication of any potentially identifiable images or data included in this article.
Disclosure
All authors disclose no personal or financial support or author involvement with organizations with financial interest in the subject. All authors contributed equally to this manuscript and approved the final version and its submission to the journal. The author reports no conflicts of interest in this work. All authors disclose no prior presentation of study data as an abstract or poster.
Author contributions
PDF wrote sections of the manuscript, reviewed the manuscript and created figure and tables. DD collected data and wrote the first draft of the manuscript. MA performed statistical analysis. NR conceived the study and collected participants. FR reviewed the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
COVID-19, coronavirus disease 2019; MIS-C, multisystem inflammatory syndrome in children; IL, interleukin; CDC, centers for disease control; KD, Kawasaki disease; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; WBC, white blood cells; PLT, platelet count; Hb, hemoglobin; CRP, C-reactive protein; PCT, procalcitonin; BNP, brain natriuretic peptid; WHO, World Health Organization; ECG, electrocardiogram; AV, atrioventricular; CAVC, complete atrioventricular canal.
References
1. CDC COVID-19 Response Team. Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. Morb Mortal Wkly Rep. (2020) 69(14):422–6. doi: 10.15585/mmwr.mm6914e4
2. Cantarutti N, Battista V, Adorisio R, Cicenia M, Campanello C, Listo E, et al. Cardiac manifestations in children with SARS-COV-2 infection: 1-year pediatric multicenter experience. Children. (2021) 8(8):717. doi: 10.3390/children8080717
3. Soumya RS, Unni TG, Raghu KG. Impact of COVID-19 on the cardiovascular system: a review of available reports. Cardiovasc Drugs Ther. (2021) 35(3):411–25. doi: 10.1007/s10557-020-07073-y
4. Abi Nassif T, Fakhri G, Younis NK, Zareef R, Al Amin F, Bitar F, et al. Cardiac manifestations in COVID-19 patients: a focus on the pediatric population. Can J Infect Dis Med Microbiol. (2021) 2021:5518979. doi: 10.1155/2021/5518979
5. van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. (2020) 382(16):1564–7. doi: 10.1056/NEJMc2004973
6. Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. (2020) 309:70–7. doi: 10.1016/j.ijcard.2020.03.063
7. Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. (2020) 383:347–58. doi: 10.1056/NEJMoa2021756
8. Di Filippo P, Raso M, Cacciatore M, Patacchiola R, Renda G, Rossi N, et al. Case report: mitral valve involvement and first-degree atrial-ventricular block in two patients with multisystem inflammatory syndrome in children. Front Pediatr. (2021) 9:676934. doi: 10.3389/fped.2021.676934
9. Grimaud M, Starck J, Levy M, Marais C, Chareyre J, Khraiche D, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. (2020) 10(1):69. doi: 10.1186/s13613-020-00690-8
10. Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, et al. Kawasaki-like multisystem inflammatory syndrome in children during the COVID-19 pandemic in Paris, France: prospective observational study. Br Med J. (2020) 369:m2094. doi: 10.1136/bmj.m2094
11. Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, Herberg J, Bajolle F, Randanne PC, et al. Multisystem inflammatory syndrome in children: an international survey. Pediatrics. (2021) 147(2):e2020024554. doi: 10.1542/peds.2020-024554
12. Fried JA, Ramasubbu K, Bhatt R, Topkara VK, Clerkin KJ, Horn E, et al. The variety of cardiovascular presentations of COVID-19. Circulation. (2020) 141(23):1930–6. doi: 10.1161/CIRCULATIONAHA.120.047164
13. Vukomanovic VA, Krasic S, Prijic S, Ninic S, Minic P, Petrovic G, et al. Differences between pediatric acute myocarditis related and unrelated to SARS-CoV-2. Pediatr Infect Dis J. (2021) 40(5):e173–8. doi: 10.1097/INF.0000000000003094
14. Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. (2021) 180(2):307–22. doi: 10.1007/s00431-020-03766-6
15. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. J Am Med Assoc. (2020) 324(3):259–69. doi: 10.1001/jama.2020.10369
16. World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19: Scientific brief (2020). Available at: https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (Accessed July 25, 2022).
17. Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. (2020) 221(11):1775–81. doi: 10.1093/infdis/jiaa113; Erratum in: J Infect Dis. 2020;222(3):519.32179908
18. Li Y, Guo F, Cao Y, Li L, Guo Y. Insight into COVID-2019 for pediatricians. Pediatr Pulmonol. (2020) 55(5):E1–4. doi: 10.1002/ppul.24734
19. Su L, Ma X, Yu H, Zhang Z, Bian P, Han Y, et al. The different clinical characteristics of corona virus disease cases between children and their families in China - the character of children with COVID-19. Emerg Microbes Infect. (2020) 9(1):707–13. doi: 10.1080/22221751.2020.1744483
20. Kurz DJ, Eberli FR. Cardiovascular aspects of COVID-19. Swiss Med Wkly. (2020) 150:w20417. doi: 10.4414/smw.2020.20417
21. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5(7):811–8. doi: 10.1001/jamacardio.2020.1017
22. Yoldaş T, Örün UA. What is the significance of elevated troponin I in children and adolescents? A diagnostic approach. Pediatr Cardiol. (2019) 40(8):1638–44. doi: 10.1007/s00246-019-02198-w
23. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. (2020) 142(4):342–53. doi: 10.1161/CIRCULATIONAHA.120.047971
24. Tunçer T, Varol F, Coşkun Ş, Güzel B, Güven Ş, Çam H. Patterns of myocardial involvement during COVID-19 pandemic; from newborn to adolescents. J Curr Pediatr. (2021) 19:319–27. doi: 10.4274/jcp.2021.83798
25. Durani Y, Egan M, Baffa J, Selbst SM, Nager AL. Pediatric myocarditis: presenting clinical characteristics. Am J Emerg Med. (2009) 27(8):942–7. doi: 10.1016/j.ajem.2008.07.032
26. Freedman SB, Haladyn JK, Floh A, Kirsh JA, Taylor G, Thull-Freedman J. Pediatric myocarditis: emergency department clinical findings and diagnostic evaluation. Pediatrics. (2007) 120(6):1278–85. doi: 10.1542/peds.2007-1073
27. Chang YJ, Chao HC, Hsia SH, Yan DC. Myocarditis presenting as gastritis in children. Pediatr Emerg Care. (2006) 22(6):439–40. doi: 10.1097/01.pec.0000221346.64991.e7
28. Godfred-Cato S, Abrams JY, Balachandran N, Jaggi P, Jones K, Rostad CA, et al. Distinguishing multisystem inflammatory syndrome in children from COVID-19, kawasaki disease and toxic shock syndrome. Pediatr Infect Dis J. (2022) 41(4):315–23. doi: 10.1097/INF.0000000000003449
29. Zhao Y, Patel J, Huang Y, Yin L, Tang L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: a meta-analysis. Am J Emerg Med. (2021) 49:62–70. doi: 10.1016/j.ajem.2021.05.044
30. Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, et al. COVID-19-associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc. (2020) 9(3):407–8. doi: 10.1093/jpids/piaa061
31. Dancea AB. Myocarditis in infants and children: a review for the paediatrician. Paediatr Child Health. (2001) 6(8):543–5. doi: 10.1093/pch/6.8.543
32. Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. (2013) 6(5):833–9. doi: 10.1161/CIRCIMAGING.113.000416
33. Acevedo L, Piñeres-Olave BE, Niño-Serna LF, Vega LM, Gomez IJA, Chacón S, et al. Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 in critically ill patients: an observational multicenter study (MISCO study). BMC Pediatr. (2021) 21(1):516. doi: 10.1186/s12887-021-02974-9
Keywords: MIS-C, SARS-CoV-2, cardiac involvement, children, troponin
Citation: Di Filippo P, David D, Attanasi M, Rossi N and Chiarelli F (2023) Case report: Increased troponin level in 125 children during COVID-19. Front. Pediatr. 11:1059685. doi: 10.3389/fped.2023.1059685
Received: 1 October 2022; Accepted: 6 February 2023;
Published: 1 March 2023.
Edited by:
Estela Azeka, University of São Paulo, BrazilReviewed by:
Francesco Bianco, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyKei Yamamoto, National Center For Global Health and Medicine, Japan
© 2023 Di Filippo, David, Attanasi, Rossi and Chiarelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paola Di Filippo ZGlmaWxpcHBvcGFvbGFAbGliZXJvLml0
†These authors have contributed equally to this work
‡ORCID Paola Di Filippo orcid.org/0000-0001-7336-4292
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Paola Di Filippo
Paola Di Filippo Daniela David†
Daniela David† Marina Attanasi
Marina Attanasi