
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 23 February 2023
Sec. Pediatric Cardiology
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1051041
This article is part of the Research TopicCase Reports in Pediatric Cardiology: 2022View all 31 articles
This case report describes a 15-year-old patient with a known congenital malformation syndrome and immune deficiency, presenting with new-onset atrial fibrillation (AF) after a recent diagnosis of an intrathoracic mass. Transthoracic echocardiography showed a structurally and functionally normal heart and workup confirmed a primary diffuse large B-cell lymphoma, with pericardial and left atrial involvement on cardiac magnetic resonance imaging. Electrical cardioversion was successfully performed to convert the AF and chemotherapy was promptly started. Antiarrhythmic treatment was continued for 6 weeks, without recurrent AF. We discuss the pathogenesis of AF in the setting of malignancies as well as the management strategies of AF, mainly based on adult guidelines.
Atrial fibrillation (AF) is uncommon in children in the absence of congenital heart disease (1, 2). Epidemiological pediatric data are scarce and management is guided by studies performed in the adult population (3). The presence of AF in a young patient with a structurally normal heart requires a careful etiological workup. This case illustrates a rare malignant etiology of AF, an intrathoracic non-Hodgkin lymphoma with cardiac involvement, demonstrated on cardiac magnetic resonance imaging (MRI). To our knowledge, this is the first pediatric case described with new-onset AF caused by a neoplastic invasion of the left atrial wall.
A 15-year-old girl, diagnosed with Hay–Wells syndrome–like phenotype at birth, was hospitalized for persistent cough, fatigue, and a deteriorating general condition. Her syndromic clinical features at birth and in infancy included cleft lip, cleft palate, maxillary hypoplasia, patchy alopecia, ankyloblepharon filiforme adnatum, absent eyelashes, dystrophic nails, bilateral syndactyly of the second to the fourth toe, ectrodactyly of both thumbs, hyperkeratosis, and hypoplasia of external genitalia. Furthermore, her medical history was marked by severe growth failure, celiac disease, gastroesophageal reflux, and hypogammaglobulinemia. She had no history of cardiac symptoms nor signs prior to this admission and there was no known family history of AF.
A persistent pleural effusion, found and monitored on chest x-ray and positron emission tomography computed tomography (PET-CT) scan, was suggestive of a malignant intrathoracic process. Primary mediastinal non-Hodgkin lymphoma was confirmed at anatomopathological analysis, with the definitive diagnosis of a stage III diffuse large B-cell lymphoma. Shortly after diagnosis and before starting chemotherapy and corticosteroids, the patient presented with acute onset chest discomfort and palpitations. A cardiac monitoring showed a heart rate varying between 200 and 220 beats per minute (bpm) with normal blood pressure for age. On electrocardiogram (ECG), AF was diagnosed, with a rapid ventricular response and incomplete right bundle branch block secondary to the fast heart rate (Figure 1). On previously performed transthoracic echocardiography (TTE), a small atrial septal defect–type secundum and normal biventricular dimensions and function were seen. Previous ECG was also normal with no signs of pre-excitation or early repolarization.
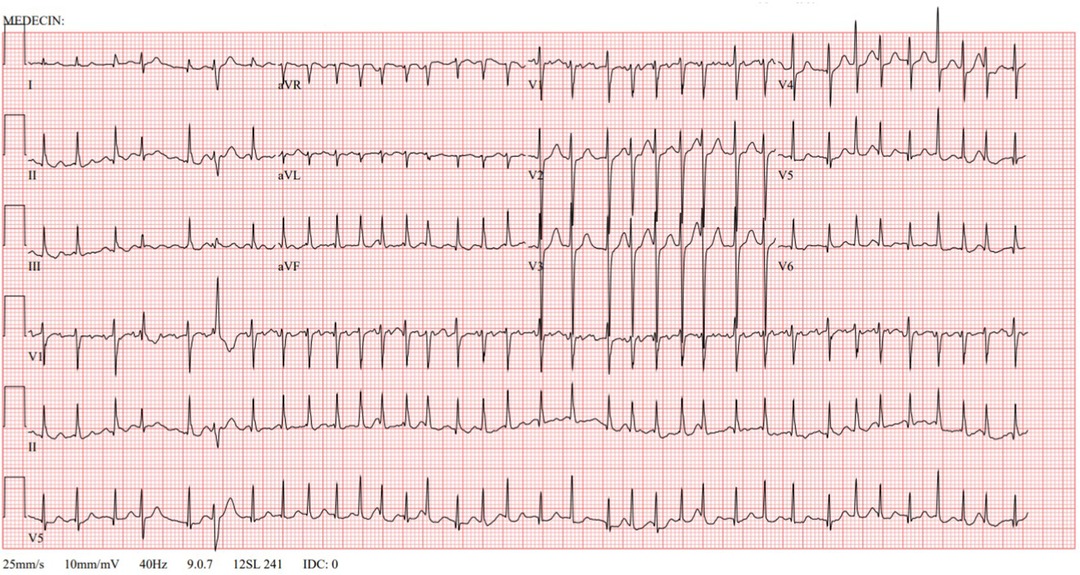
Figure 1. Electrocardiogram at onset. Atrial fibrillation with a rapid ventricular response. Incomplete right bundle branch block secondary to fast heart rate.
At the time of the acute AF, blood gas excluded electrolyte abnormalities. Hematologic analysis showed a hemoglobin level of 9 g/dl [Normal (N): 11–14.5 g/dL], hematocrit of 29% (N: 35%–47%), 3,000/mm3, neutrophils (N: 1,700–5,700/mm3) among 4,200/mm3 of white blood cells (N 4,000–10,000/mm3), and 489,000/mm³ of platelets (N: 1,75,000–3,45,000/mm3). Renal function and thyroid hormone levels were normal. Given her clinical state and hemodynamic tolerance, she was given an oral loading dose of amiodarone 800 mg/m2. Within 6 h, her ventricular response rate had dropped to 180 bpm, but she remained in AF and not in sinus rhythm. Over the next few hours, the clinical signs of increasing pallor and hepatomegaly developed and her blood pressure decreased to 79/43 mmHg. TTE showed normal cardiac function [with an ejection fraction (EF) of 70% and a shortening fraction (SF) of 39%], without enlargement of the left heart structures and no mitral valve regurgitation.
TTE was completed with a transesophageal echocardiography in order to rule out the presence of an intracardiac thrombus. Due to clinical deterioration, she was sedated (with propofol), intubated, and ventilated prior to electrical cardioversion. She returned to sinus rhythm after one shock of 1 J/kg. Anticoagulation by low-molecular-weight heparin (tinzaparin, 4,500 UI/day subcutaneously) was started and discontinued after 24 h of persistent sinus rhythm, without continuation of oral anticoagulation.
Etiological investigation was completed by performing cardiac MRI, which showed tumoral invasion into the pericardium and the lateral wall of the left atrium (Figure 2). Repeat TTE could not reproduce this finding.
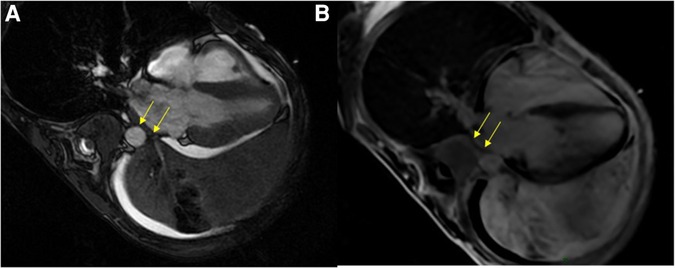
Figure 2. Cardiac MRI. SSFP four-chambers view showing the extensive tumoral process in the left pulmonary area coming into close contact with the left atrium, with paraneoplastic pleural and pericardial effusions (A); and likely tumoral invasion of the left atrial wall on the late-gadolinium-enhanced acquisition (B). MRI, magnetic resonance imaging; SSFP, steady-state free precession.
Neurological examination at all times (at presentation and following treatment) remained normal. Cerebral MRI 24 h post-AF onset ruled out both intracranial tumoral invasion and signs of ischemic stroke.
Chemotherapy was started 24 h after AF onset according to the Inter-B-NHL 2010 treatment protocol. Amiodarone in maintenance dosage (200 mg/m2 1×/day orally) was continued for 6 weeks without any use of antiarrhythmic agents thereafter. There was no recurrence of AF in this patient.
The incidence of AF in pediatrics is rare (prevalence <0.05% prior to the age of 30) and mainly documented in children with congenital heart disease, cardiomyopathy, inherited arrhythmias, hyperthyroidism, or post-open-heart surgery (2–4). Isolated AF, in the absence of underlying cardiovascular disease, represents less than 5% of all cases of AF, across all ages (1). Several risk factors for AF are described, such as hypertension, diabetes mellitus, obstructive sleep disorder, obesity, smoking, alcohol and drug use, excessive exercise, hyperthyroidism, or positive family history. The patient in this case report had none of these known risk factors (3, 5).
The pathogenesis of AF is generally multifactorial, with electrical remodeling, structural remodeling, and inflammation (6, 7). Once initiated, AF alters the electrophysiologic properties of the atrial myocardium, responsible for the maintenance of the arrhythmia. Furthermore, the role of a susceptible atrial anatomical substrate, such as myocyte degeneration, activation of fibroblasts, and enhanced connective tissue deposition leading to interstitial fibroses, has been implicated in the physiology of the onset and maintenance of AF. The presence of AF has also been associated with an increased inflammatory burden.
Invasion of the malignant tumor into the atrial wall myocytes in this case, is considered an inflammatory burden, creating a susceptible atrial anatomical structure, with the underlying milieu of the systemic proinflammatory state of cancer. In the absence of other underlying extracardiac triggers such as hypertension, hyperthyroidism, pulmonary embolism, viral infection, sepsis, or drug overdose, we considered the non-Hodgkin lymphoma with neoplastic invasion of the pericardium and the left atrial wall as the etiology AF in our patient.
The association between various cancers and AF has been described, justified by the abovementioned pathophysiological mechanisms of proinflammatory markers in both AF and cancer (8, 9). A large cohort study in a Danish population found that patients with new-onset AF had a markedly increased probability of neoplasia within 3 months following AF diagnosis, and that moreover, AF was strongly associated with metastatic cancer (10). Conversely, a recent meta-analysis suggests that patients with newly diagnosed cancer have a significantly increased risk of AF during the first 3-months of follow-up (9). Hospitalization costs, length of stay, and mortality rates are higher in cancer patients with AF than in those without AF. With regard to different cancer subtypes, in patients under the age of 65 years, AF has the highest association with lung cancer, followed by multiple myeloma and non-Hodgkin lymphoma (such as in our patient) (8).
Primary cardiac lymphoma is rare, and secondary cardiac involvement is even rarely reported. Usually, cardiac involvement is a late manifestation of lymphoma with median onset at 20 months after initial diagnosis, and often diagnosed at autopsy (11). The right side of the heart seems to be more often involved than the left and typically associated features include superior vena cava syndrome, pericardial effusion, and lymphatic return obstruction. Various arrhythmias can occur, including AF, atrioventricular block, and ventricular tachycardia (12).
Diagnostic modalities include CT and MRI, with contrast-enhanced MRI resulting in superior quality images to identify the morphology, location, and extent of intrathoracic masses (13).
Pediatric cases are very poorly described in the literature, and this patient reported is most likely the youngest to date reported with AF caused by pericardial and left atrial wall invasion of a non-Hodgkin lymphoma. Very few similar adult cases have been described (12, 14).
An added feature of note in this case is that during infancy, she was diagnosed with a Hay–Wells syndrome–like phenotype (15). The ankyloblepharon-ectodermal defects-cleft lip/palate (AEC) syndrome or Hay–Wells syndrome (MIM #106260) was first reported in 1976 (16). Hay–Wells syndrome belongs to a large, heterogeneous group of ectodermal dysplasia that affects the embryonic development of ectodermal tissues: hair, nails, teeth, sweat glands, and skin (17, 18). This very rare genetic condition is caused by a heterozygous mutation of the tumor protein p63 (TP63) gene, located on chromosome 3q28. Exome sequencing in our patient revealed de novo heterozygous variants in CHUK, PTGER4, and IFIT2. The variant in CHUK appeared to be most relevant for the AEC-like phenotype. CHUK is a direct target gene of p63 and encodes a component of the IKK complex that plays a key role in NF-κB pathway activation (15).
As in most of the classic Hay–Wells patients, dermatological features were predominant in our patient. Cardiac features are extremely rare in these patients, with only ventricular septum defects and persistent ductus arteriosus described (19). No cardiac arrhythmias nor tumor development (such as lymphoma) have been reported in the literature linked to the syndrome. We did not consider her underlying genetic condition as the cause for her malignancy or AF.
With regard to the early workup in AF, early-stage cardiomyopathy and inherited ventricular arrhythmias should be ruled out/excluded. Rare channelopathies associated with AF in children include Brugada syndrome, long QT syndrome, and short QT syndrome. Baseline 12-lead ECG is a basic yet essential screening tool in the diagnosis and has high value in establishing prognosis and orienting further therapy (3). In our patient, a 12-lead ECG taken earlier was carefully examined using the Bazett formula, and no case for channelopathy was made out.
AF can also be associated with organized supraventricular tachycardia such as atrioventricular reentrant tachycardia degenerating into AF. Patients with accessory pathways have a much higher incidence of AF than the general population, especially with manifest accessory pathways (Wolff–Parkinson–White syndrome), but it has also been reported in patients with concealed accessory pathways (20).
AF is strongly associated with heart failure, even in the early stages of cardiomyopathy, and therefore (21), diastolic function on TTE should be carefully examined. Structural changes, however, may be delayed and develop later depending on the management, control, and re-occurrence of arrhythmias (3). In this case, TTE remained normal during follow-up.
Importantly, our patient did not receive any treatment in the form of anthracyclines, monoclonal antibodies such as ibrutinib, or other oncological treatments at the onset of AF. These medications are all considered risk factors for cardiovascular events (22–24).
In the current literature, treatment strategies for AF in children are not well defined. In general, rate control is often sufficient to resolve AF-related symptoms; however, rhythm-control strategies should always be considered in children. There is no robust evidence for the best choice of pharmacological agents offered for rate control, and the current practice includes the use of beta-blockers, calcium channel blockers, other antiarrhythmic drugs such as amiodarone or combination therapies.
Conversion to sinus rhythm by antiarrhythmic drugs is observed in approximately 50% in adults (7), and due to our patient's initial hemodynamic stability, we initially chose this strategy, in the knowledge that amiodarone appears to be more effective than sotalol in restoring sinus rhythm (25, 26). Pharmacological cardioversion also has the benefit of not requiring a fasting state or sedation.
In our patient, due to the inefficacy of pharmacological cardioversion and her worsening clinical state with hypotension, synchronized electrical cardioversion was subsequently the most appropriate procedure to rapidly establish sinus rhythm and avoid further clinical deterioration. Electrical cardioversion was performed despite the risk of stroke in non-anticoagulated patients (7) because it is indeed a quicker procedure and a more effective treatment of choice in hemodynamically compromised patients. It can be performed safely under sedation with intravenous midazolam and/or propofol. Continuous cardiorespiratory monitoring is essential.
Thromboembolism is a known complication of AF. The structural and functional changes of the atrial myocardium and stasis of blood generate a prothrombotic milieu. Anticoagulation is usually indicated in order to prevent stroke, although it remains controversial as there is a lack of pediatric recommendations. In adults, various risk scores exist to guide long-term anticoagulation decisions, such as the CHA2DS2-VASc score [congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65–74, and sex (female)] recommended by the European Society of Cardiology (ESC) (7). Our patient was covered with low-molecular-weight heparin in view of electrical cardioversion, from the time of the procedure until 24 h thereafter. Continuous monitoring showed no recurrence of AF, and additional oral anticoagulation was not proposed in our patient, in accordance with adult guidelines (1, 7, 27).
The recurrent risk of AF is described between 15% and 39% after a first episode of isolated AF (4, 28). According to different case cohorts, age, male sex, obesity, and duration of the initial episode of AF were associated with a higher risk of recurrence. Throughout the treatment and follow-up of the patient's oncological condition, no recurrence was seen on monitoring. The indication for long-term antiarrhythmic therapy for AF has to be carefully balanced and weighed up, with negative AF-related symptoms on the one hand vs. the possible adverse effects on the other. Informed patient preference also plays a role (7). In cases of non-cardiac conditions associated with AF, the treatment of the underlying condition is crucial, as first-line therapy and (29) prognosis rely strongly on the underlying condition, but the lymphoma stage in our patient was unfortunately unfavorable.
In case of the recurrence of AF our patient, an electrophysiology study should be considered because other forms of supraventricular tachycardia that may trigger AF should be excluded (2, 29). Further future pediatric studies on this topic are needed in order to establish more specific guidelines on the management of AF in young patients. This study highlights the importance of clinical workup, examinations, and investigations to search for the cause of every new onset of AF in a pediatric patient.
In young patients presenting with AF, with no prior history or findings of cardiac abnormalities, the investigative workup for determining etiology is vital. Oncological conditions, and especially intrathoracic or cardiac malignant lesions, are rare but a part of the differential diagnosis of new-onset AF, as demonstrated by this case report of a 15-year-old girl with non-Hodgkin lymphoma. Cardiac MRI is a helpful diagnostic tool and electrical cardioversion is a rapid and effective treatment option. Further pediatric studies are needed to establish clear management guidelines for isolated AF in pediatric populations.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JH collected data, conceived and designed the analysis, and drafted the initial manuscript. CV conceived and designed the analysis. CB referred the patient, provided follow-up, and collected data. KC drafted the manuscript. SM provided the figures and performed the analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR Jr., Ilstrup DM, et al. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. (1987) 317(11):669–74. doi: 10.1056/NEJM198709103171104
2. Ceresnak SR, Liberman L, Silver ES, Fishberger SB, Gates GJ, Nappo L, et al. Lone atrial fibrillation in the young—perhaps not so “lone”? J Pediatr. (2013) 162(4):827–31. doi: 10.1016/j.jpeds.2012.09.016
3. Gourraud JB, Khairy P, Abadir S, Tadros R, Cadrin-Tourigny J, Macle L, et al. Atrial fibrillation in young patients. Expert Rev Cardiovasc Ther. (2018) 16(7):489–500. doi: 10.1080/14779072.2018.1490644
4. El-Assaad I, Al-Kindi SG, Saarel EV, Aziz PF. Lone pediatric atrial fibrillation in the United States: analysis of over 1500 cases. Pediatr Cardiol. (2017) 38(5):1004–9. doi: 10.1007/s00246-017-1608-7
5. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. (1998) 82(8A):2N–9N. doi: 10.1016/s0002-9149(98)00583-9
6. Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. (2009) 157(2):243–52. doi: 10.1016/j.ahj.2008.10.009
7. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612 Erratum in: Eur Heart J. (2021) 42(5):507; Eur Heart J. (2021) 42(5):546–7; and Eur Heart J. (2021) 42(40):4194.32860505
8. Zubair Khan M, Gupta A, Patel K, Abraham A, Franklin S, Kim DY, et al. Association of atrial fibrillation and various cancer subtypes. J Arrhythm. (2021) 37(5):1205–14. doi: 10.1002/joa3.12589
9. Yuan M, Zhang Z, Tse G, Feng X, Korantzopoulos P, Letsas KP, et al. Association of cancer and the risk of developing atrial fibrillation: a systematic review and meta-analysis. Cardiol Res Pract. (2019) 2019:8985273. doi: 10.1155/2019/8985273
10. Ostenfeld EB, Erichsen R, Pedersen L, Farkas DK, Weiss NS, Sørensen HT. Atrial fibrillation as a marker of occult cancer. PLoS One. (2014) 9(8):e102861. doi: 10.1371/journal.pone.0102861
11. Petersen CD, Robinson WA, Kurnick JE. Involvement of the heart and pericardium in the malignant lymphomas. Am J Med Sci. (1976) 272(2):161–5. doi: 10.1097/00000441-197609000-00005
12. Yang HC, Liao JN, Hsiao LT, Yu WC, Chen SA. An unusual etiology for a 37-year-old man with paroxysmal atrial fibrillation and termination pause. JACC Case Rep. (2021) 3(1):165–8. doi: 10.1016/j.jaccas.2020.10.031
13. O’Mahony D, Peikarz RL, Bandettini WP, Arai AE, Wilson WH, Bates SE. Cardiac involvement with lymphoma: a review of the literature. Clin Lymphoma Myeloma. (2008) 8(4):249–52. doi: 10.3816/CLM.2008.n.034
14. Hirabayashi T, Tanabe M, Onishi K, Tanigawa T, Kitamura T, Yamada N, et al. Cardiac malignant lymphoma with atrial arrhythmias. Int J Cardiol. (2007) 114(2):E42–4. doi: 10.1016/j.ijcard.2006.07.214
15. Khandelwal KD, Ockeloen CW, Venselaar H, Boulanger C, Brichard B, Sokal E, et al. Identification of a de novo variant in CHUK in a patient with an EEC/AEC syndrome-like phenotype and hypogammaglobulinemia. Am J Med Genet A. (2017) 173(7):1813–20. doi: 10.1002/ajmg.a.38274
16. Hay RJ, Wells RS. The syndrome of ankyloblepharon, ectodermal defects, and cleft lip and palate: an autosomal dominant condition. Br J Dermatol. (1976) 94(3):277–89. doi: 10.1111/j.1365-2133.1976.tb04384.x
17. Fete M, van Bokhoven H, Clements SE, McKeon F, Roop DR, Koster MI, et al. International research symposium on ankyloblepharon-ectodermal defectscleft lip/palate (AEC) syndrome. Am J Med Genet A. (2009) 149A(9):1885–93. doi: 10.1002/ajmg.a.32761
18. Rinne T, Brunner HG, van Bokhoven H. p63-associated disorders. Cell Cycle. (2007) 6(3):262–8. doi: 10.4161/cc.6.3.3796
19. Online Mendelian inheritance in man, OMIM®. Baltimore, MD: Johns Hopkins University (2022). MIM Number: 106260. Available at: https://omim.org/ (Accessed December 2, 2009).
20. Chen M, Feng X, Sun J, Wang Q, Zhang P, Wang J, et al. Risk factors responsible for atrial fibrillation development between symptomatic patients with concealed or manifest atrioventricular accessory pathways. Int J Cardiol Heart Vasc. (2015) 7:69–75. doi: 10.1016/j.ijcha.2015.02.010
21. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol. (2003) 91(6A):2D–8D. doi: 10.1016/s0002-9149(02)03373-8
22. Diamond A, Ayyappan S, Cao S, Tashtish N, Boughan K, Cooper B, et al. Risk factors for cardiovascular events and mortality in patients diagnosed with diffuse large B-cell lymphoma and treated with anthracyclines. Hematol Oncol. (2022) 40:626–36. doi: 10.1002/hon.3034
23. Palazzo AG, Zizza A, Nuzzo M, Urciuoli C, Scardia S, Romano A, et al. Atrial fibrillation with aberrant ventricular conduction after receiving bamlanivimab/etesevimab: a case report. Curr Med Res Opin. (2022) 38(7):1055–7. doi: 10.1080/03007995.2022.2081450
24. Caldeira D, Alves D, Costa J, Ferreira JJ, Pinto FJ. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. (2019) 14(2):e0211228. doi: 10.1371/journal.pone.0211228
25. Vijayalakshmi K, Whittaker VJ, Sutton A, Campbell P, Wright RA, Hall JA, et al. A randomized trial of prophylactic antiarrhythmic agents (amiodarone and sotalol) in patients with atrial fibrillation for whom direct current cardioversion is planned. Am Heart J. (2006) 151(4):863.e1–6. doi: 10.1016/j.ahj.2005.09.009
26. Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, et al. Sotalol amiodarone atrial fibrillation efficacy trial (SAFE-T) investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. (2005) 352(18):1861–72. doi: 10.1056/NEJMoa041705
27. You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, et al. Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141(2 Suppl):e531S–75S. doi: 10.1378/chest.11-2304
28. Mills LC, Gow RM, Myers K, Kantoch MJ, Gross GJ, Fournier A, et al. Lone atrial fibrillation in the pediatric population. Can J Cardiol. (2013) 29(10):1227–33. doi: 10.1016/j.cjca.2013.06.014
29. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr., et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022
Keywords: atrial fibrillation, child, lymphoma, cardiac involvement, pericardial invasion, cardioversion
Citation: Hubrechts J, Vô C, Boulanger C, Carkeek K and Moniotte S (2023) Atrial fibrillation in a pediatric patient caused by an unusual malignant etiology: A case report. Front. Pediatr. 11:1051041. doi: 10.3389/fped.2023.1051041
Received: 22 September 2022; Accepted: 25 January 2023;
Published: 23 February 2023.
Edited by:
Cecile Tissot, Clinique des Grangettes, SwitzerlandReviewed by:
Emanuele Micaglio, IRCCS San Donato Polyclinic, Italy© 2023 Hubrechts, Vô, Boulanger, Carkeek and Moniotte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jelena Hubrechts amVsZW5hLmh1YnJlY2h0c0BzYWludGx1Yy51Y2xvdXZhaW4uYmU=
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.