
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 23 February 2023
Sec. Children and Health
Volume 11 - 2023 | https://doi.org/10.3389/fped.2023.1003037
Background: Inconsistent relationships have been shown between cigarette smoking and hypospadias in offspring. The purpose of this study was to summarize epidemiological evidence to evaluate the relationship between parental smoking and the risk of hypospadias.
Methods: Up until October 2022, PubMed, EMBASE, Web of Science, and the Cochrane Library were systematically searched for qualified research. The summary RRs and 95% CIs were calculated using either a fixed-effects or a random-effects model. There were subgroup analyses undertaken to identify potential sources of heterogeneity.
Results: 44 studies with 16,637,830 participants were included in our meta-analysis. Overall, maternal active smoking [risk ratio (RR) = 0.94; 95% confidence interval (CI): 0.90–0.99; P < 0.01] was significantly associated with the risk of hypospadias. And neither paternal smoking (RR = 1.00; 95% CI: 0.86–1.15) nor maternal passive smoking (RR = 0.91; 95% CI: 0.60–1.23) was associated with the risk of hypospadias.
Conclusion: Our study discovered an association between maternal active smoking and a decreased risk of hypospadias, which may be due to the effect of smoking on androgen. However, as numerous studies have proved that cigarette smoking during pregnancy increases the risk of overall birth abnormalities in offspring, quitting cigarettes before pregnancy positively influences the health of offspring and should be advocated worldwide.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42022319378].
Hypospadias is a male urogenital congenital defect characterized by a disturbed urethral fusion between gestational weeks 8 and 14, which locates the urethral meatus ventrally from the penile glans to the scrotum or perineum (1). It occurs in roughly 0.5 percent of live newborns (2). However, the risk factors and the etiology of hypospadias remain controversial.
Extensive clinical and laboratory studies on the biological mechanisms by which tobacco smoke affects fetal development have revealed that many of the 7,000 compounds can cross the placental barrier and have adverse effects on the developing fetus (3–5). Moreover, smoking during pregnancy seems to be associated with various congenital abnormalities, such as gastrointestinal abnormalities, eye defects, digit anomalies, oral clefts, limb abnormalities, musculoskeletal deformities, heart defects, and cryptorchidism (6). Even though smoking during pregnancy increases the risk of most congenital defects, many pregnant women continue to smoke (7).
A previous meta-analysis of 15 studies on this topic revealed that pregnant women who smoke have a decreased risk of hypospadias (6), while a recent meta-analysis of 12 studies indicates that pregnant women who smoke have an increased risk of hypospadias (8). However, the number of studies included in these two meta-analyses is quite limited. Moreover, despite the fact that paternal smoking and maternal passive smoking are more common, these two meta-analyses did not conduct a meta-analysis of the association between maternal passive smoking or paternal smoking and the risk of hypospadias. Numerous studies evaluating the risk of hypospadias associated with paternal active smoking and maternal passive smoking produced equivocal findings.
Notably, a number of studies, including a large number of cohort studies with adequate sample sizes, have been published, which will help provide an adequate number of studies to assess the risk of hypospadias with greater confidence and to investigate plausible causes for heterogeneity. Prior meta-analyses did not consider the duration of smoking exposure. Early in pregnancy, the important phase of fetal urethral development occurs. Examining the relationship between parental smoking in early pregnancy and hypospadias is crucial, as this may help explain causation.
Therefore, with newly accumulating evidence, we aimed to conduct a meta-analysis with two aims: (i) to determine the risk of hypospadias associated with parental smoking, including maternal active smoking, paternal active smoking, and maternal passive smoking; and (ii) to identify potential moderators of heterogeneity using subgroup, including subgroups based on smoking exposure time, and other potential factors.
This research followed PRISMA standards and was pre-registered with PROSPERO: CRD42022319378, outlining our investigation's objectives and procedures.
This meta-analysis included articles published before October 23, 2022, about maternal or paternal smoking and the risk of hypospadias. Using the Boolean approach, relevant papers were retrieved from PubMed, EMBASE, Web of Science, and the Cochrane Library. The following search terms were utilized in the systemic search: (smoking OR tobacco OR cigarette OR gestational cigarette exposure OR gestational smoking OR gestational tobacco exposure OR factor) AND (hypospadias OR congenital anomalies OR genitourinary anomalies). The reference lists of included papers were also searched to find any additional research that may have been overlooked.
In accordance with the research question (PICO), the following inclusion criteria were established for the selection of articles: studies on women who gave birth to a live-born son (patients, P); studies that examined maternal active smoking/maternal passive smoking/paternal smoking during pregnancy for risk of hypospadias (intervention, I); studies that used no cigarette exposure during pregnancy as a comparison (comparison, C); and studies that described the risk of hypospadias in their offspring (outcome, O).
Studies were excluded if the following criteria were met: (1) statistical data for case and control groups or effect estimates were unavailable. (2) the absence of a non-smoking control group.
Two independent reviewers screened the abstracts and titles of the studies retrieved during the search procedure to identify those studies that potentially meet the inclusion criteria. Then, reviewers evaluated the entire text of the identified studies to determine which ones should be included in the study. Disputes were resolved through discussion or consultation with a third author.
The fundamental data from selected studies would be extracted, including the first author, the year of publication, the participants’ country, the study period, the number of hypospadias cases and sample size of cohort studies, or the total sample size of case and control group for case-control studies, the exposure of interest, the assessment method of smoking status, the reported exposure time, the assessment method of hypospadias, whether the confounding factors were controlled, and quality score (Table 1).
The Newcastle-Ottawa Scale (NOS), the Agency Guidelines for Healthcare Research and Quality (AHRQ), was used to assess study quality and the potential for bias. A star system (range, 0–9 stars) was developed to rate the quality of research in the three categories of participant selection, group comparability, and exposure and result of interest for investigations, respectively. A study with seven or more stars as being low risk of bias was defined. The details are listed in Table 2.
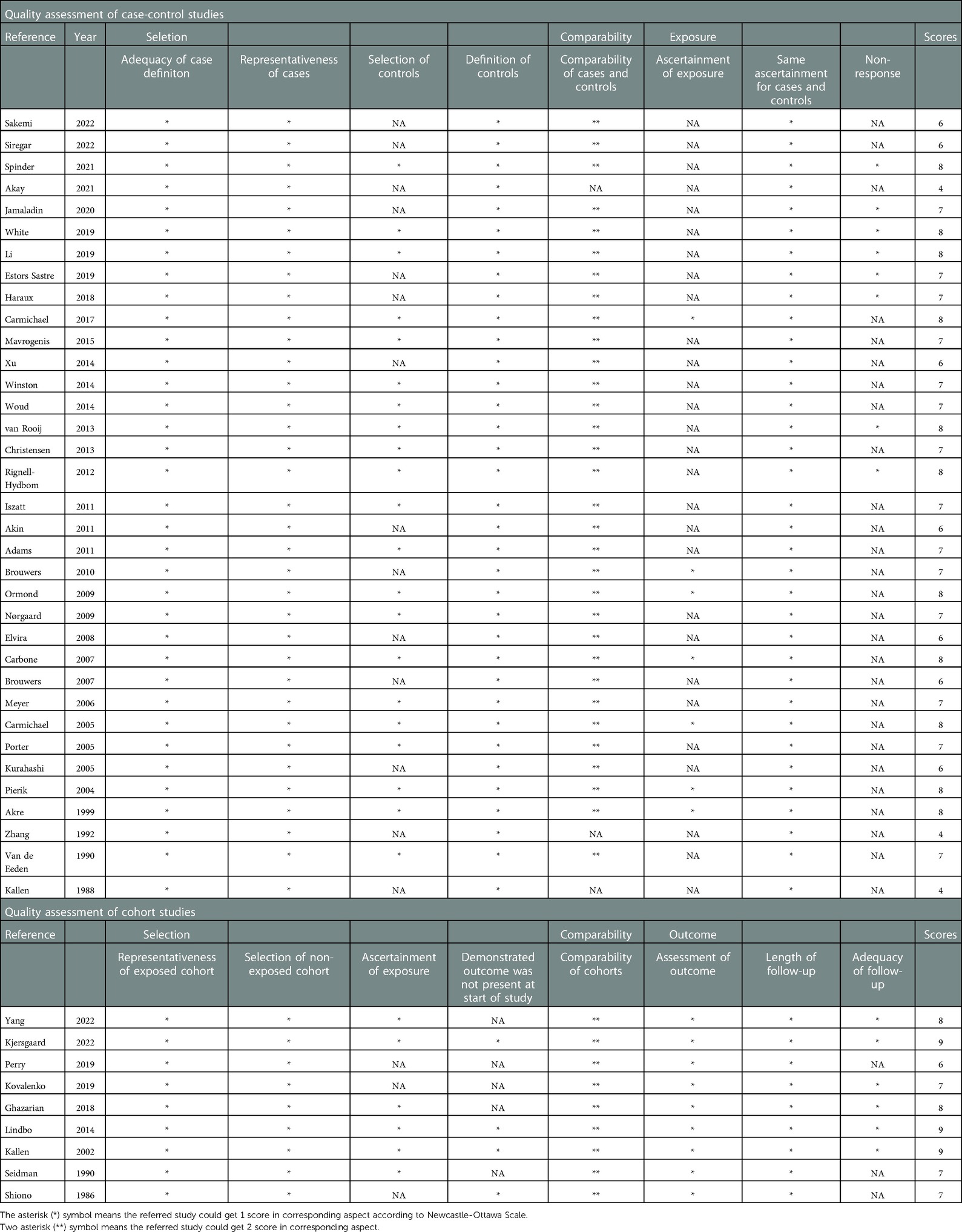
Table 2. Evaluation of the quality of included studies (case-control or cohort studies) using the Newcastle-Ottawa Scale (NOS).
The association between parental smoking and hypospadias in offspring was evaluated using RRs. Due to the low incidence of hypospadias, ORs were regarded as RRs directly. First, we estimated the summary RRs and 95% CIs for the risk of hypospadias associated with parental smoking using the RRs and 95% CIs from each study. Then, using the chi-square test (with a p-value of <0.1 and ≥0.1indicating high and low heterogeneity, respectively) and I2 index statistics (varied from 0% to 100%), we determined the heterogeneity of these studies, which was described as no (0%–25%), low (26%–50%), moderate (51%–74%), and high (75%–100%). When there was evidence of heterogeneity (I2 > 50%) across studies, random-effects models were used to generate the combined RRs and the corresponding 95% CIs, whereas fixed-effects models were employed otherwise. Subgroup analyses were undertaken based on the study's primary outcomes to investigate the potential causes of heterogeneity: study design, participants’ region by continent, assessment methods of smoking, whether the confounding factors were controlled, smoking exposure time, and quality score.
Furthermore, publication bias was to be examined visually using funnel plots if the number of studies in either cohort equalled or exceeded 10. Stata version 15.1 and the statistical software R 4.1.1 were used to conduct all analyses.
Figure 1 is a diagrammatic illustration of the study identification and inclusion process. 126,938 articles were identified using our systematic search approach in PubMed, the Cochrane Library, EMBASE, and Web of Science. We retained 107,865 articles after removing duplicate studies. Following a review of the titles and abstracts, it was determined that 107,761 papers did not meet the research objective. After comprehensively screening 204 full texts, 58 papers were found to meet our inclusion criteria. Additionally, we searched the reference lists of chosen papers for relevant studies. We found one additional article (9). After reviewing the entire texts of fifty-nine studies, we found that several studies used similar initial data, running the risk of the same patients being included in the meta-analysis twice [Kallen et al. (10) and Kallen et al. (11); Giordano et al. (12) and Carbone et al. (13); Carmichael et al. (14), Carmichael et al. (15), Van Zutphen et al. (16) and Hoyt et al. (17); de Kort et al. (18), Rocheleau et al. (19), and Ormond et al. (20); Rappazzo et al. (21) and Winston et al. (22); Caton et al. (23) and Carmichael et al. (1); Leite et al. (24) and Lindbo et al. (25); Agopian et al. (26), White et al. (27) and Sheth et al. (28); Haraux et al. (29) and Haraux et al. (30); Trabert et al. (31) and Ghazarian et al. (32); Akre et al. (33) and Kjersgaard et al. (34)]. After comparing the quality and methods of these articles, we selected Kallen et al. 1988, Carbone et al. Carmichael et al. 2017a, Ormond et al. Winston et al. Carmichael et al. 2005, Lindbo et al. Agopian et al. Haraux et al. 2018, Ghazarian et al. and Kjersgaard et al. for data extraction, as they more closely met our inclusion criteria and had the lower risk of bias.
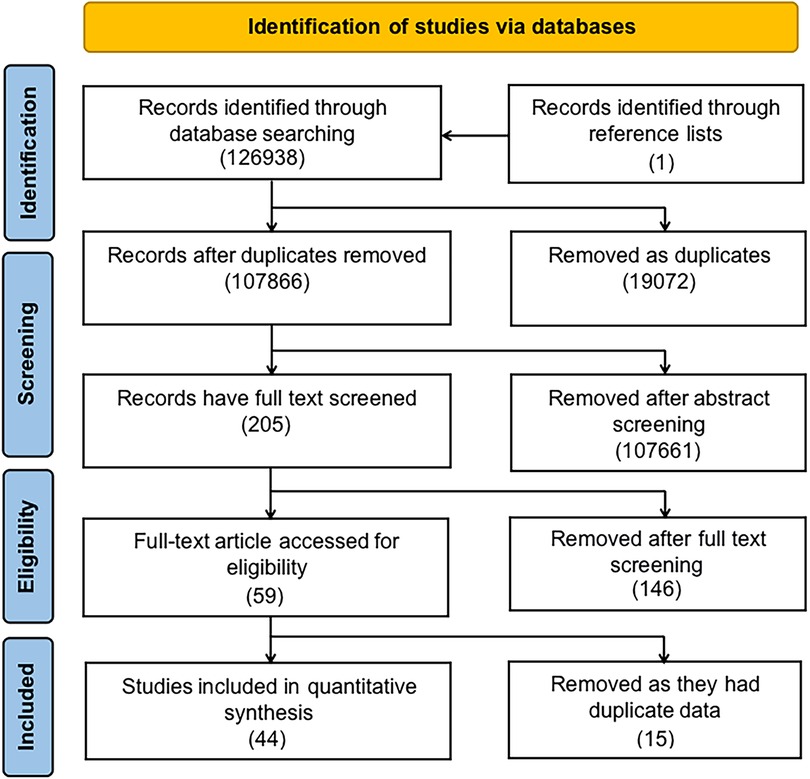
Figure 1. Flow diagram illustrates the identification of studies from the literature and their subsequent inclusion or exclusion from the meta-analysis.
Our meta-analysis includes thirteen studies from Hackshaw et al. (6) and nine studies from Zhang et al. (8). Two of the studies included by Hackshaw et al. were excluded. One was excluded due to data overlap (23), while another study was a book, for which data were unavailable (35). Three of the studies included by Zhang et al. were excluded due to data overlap (11, 12, 33).
Table 1 summarizes the included studies’ features, which comprised 16,637,830 participants and were published between 1986 and 2022. 42 studies, 9 studies, and 3 studies reported maternal active smoking, paternal smoking, and maternal passive smoking, respectively. 35 (79.5%) of the 44 papers included in this study employed a case-control design (1, 9, 10, 13, 14, 20, 22, 27, 30, 36–61), whereas 9 (20.5%) employed a cohort study design (25, 32, 34, 62–67).
The quality assessment scores of included studies are summarized in Table 2. Overall, 75.0% (33/44) of these studies received a score higher than 6. The mean value was 6.86 stars for 35 case-control studies and 7.78 stars for 9 cohort studies. The evaluation of the study's quality revealed little evidence of considerable bias that could have significantly impacted the study's outcomes.
The meta-analysis of the combined RR (Figure 2) indicated that maternal active smoking was associated with a lower risk of offspring hypospadias (RR = 0.94, 95% CI: 0.90–0.99). Moderate heterogeneity between studies led to the use of a random-effects model (I2 = 56.1%; P < 0.001).
Figure 3 summarizes risk estimates between paternal smoking and hypospadias in offspring. There was no significant association between paternal smoking and the risk of hypospadias (RR = 1.00; 95% CI: 0.86–1.15). Moreover, low evidence of heterogeneity was found, and a fixed-effects model was employed (I2 = 31.1%; P = 0.170).
Figure 4 summarizes risk estimates between maternal passive smoking and hypospadias in offspring. Overall, there was no association between maternal passive smoking and the risk of hypospadias (RR = 0.91, 95% CI: 0.60–1.23). In addition, moderate heterogeneity was detected, and the random-effects model was utilized (I2 = 69.3%; P = 0.039).
We performed a subgroup analysis based on the risk of hypospadias and smoking exposure time to determine whether smoking exposure time was the source of heterogeneity (Figure 6). However, no differences were identified (P = 0.804; Figure 6).
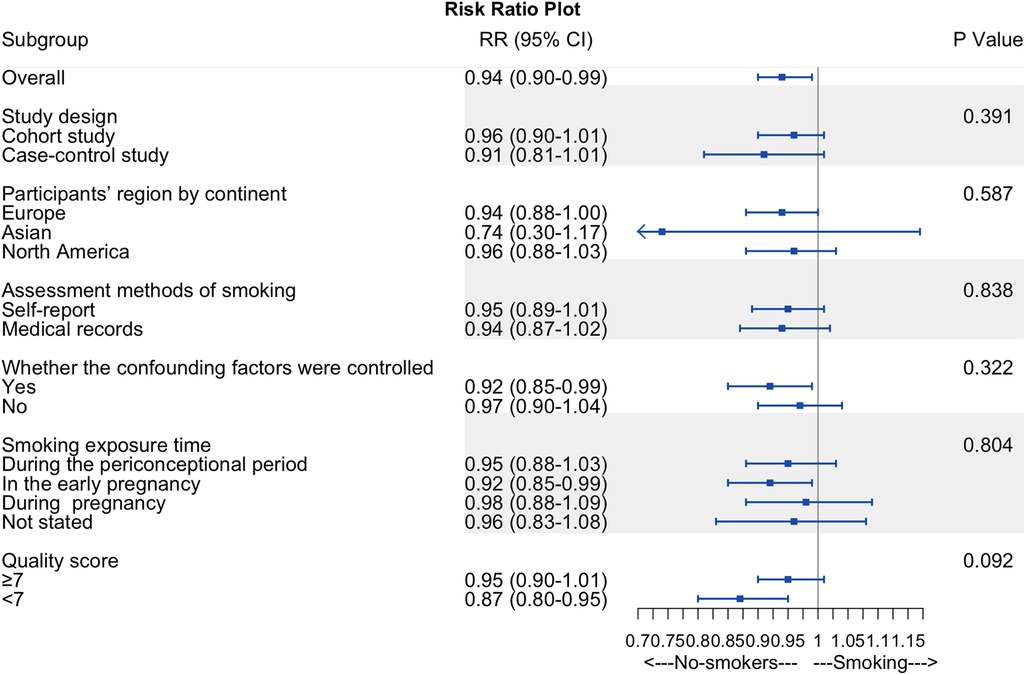
Figure 6. Summary forest plot of subgroup analyses. Summary forest plot of subgroup analyses, including study design, participants’ region by continent, assessment methods of smoking, whether the confounding factors were controlled, smoking exposure time, and quality score.
Other subgroup analyses of maternal active smoking failed to explain the overall analysis's heterogeneity and revealed consistent findings within subgroups (Figure 6). We found no difference in RR between cohort and case-control studies, participants’ region by continent, the assessment methods of smoking, quality scores, and whether the confounding factors were controlled.
The funnel plot (Figure 5) indicated no evidence of publication bias in risk estimates for maternal active smoking and offspring hypospadias.
We did not analyze publication bias in risk estimates for paternal smoking or maternal passive smoking using a funnel plot because the number of included studies was less than 10.
There is considerable debate over the association between paternal smoking and offspring hypospadias. Our study demonstrated an association between maternal active smoking and a decreased risk of hypospadias in offspring. However, no association was found between maternal passive smoking or paternal smoking and the risk of offspring hypospadias.
Our subgroup analysis showed that the risk of offspring hypospadias was consistent regardless of whether the mother smoked in early pregnancy or during the entire pregnancy, which accorded with the results of a prior Danish study. Eighty-eight percent of pregnant women who smoked at the 16th week continued to do so throughout their pregnancy (68). Consequently, even if some studies collected data on smoking during early pregnancy, it could be a good approximation of exposure status throughout pregnancy.
There are two meta-analyses examining the association between maternal smoking and the risk of hypospadias in offspring (6, 8). However, no meta-analysis has evaluated the risk of hypospadias related to maternal passive smoking or paternal smoking. The 15-study meta-analysis by Hackshaw et al. reported an odds ratio (OR) of 0.90 (95% CI: 0.85; 0.95) for having offspring with hypospadias (6), whereas 12-study meta-analysis by Zhang et al. reported an odds ratio (OR) of 1.16 (95% CI: 1.01; 1.33) for having offspring with hypospadias. Our study has some significant advantages over the previous meta-analysis; for example, we included more existing articles with a total of 16,637,830 participants to more precisely estimate the association between parental smoking and the risk of hypospadias in offspring. We also performed the first meta-analysis on the association between maternal passive smoking and paternal smoking and the risk of offspring hypospadias. Additionally, we performed subgroup analyses to explore potential sources of heterogeneity.
The specific mechanisms underlying the association between maternal smoking and hypospadias in offspring remain unknown and require further study, but various hypotheses based on epidemiological and animal data have been offered. One hypothesis is that a higher androgenic steroid level in the fetuses of pregnant smokers promotes normal urethral closure. According to a prior study, estrogens may inhibit the activity of fetal androgens (69). Smoking may have anti-estrogenic effects by increasing estradiol metabolism to metabolites with little estrogenic activity via increased 2-hydroxylation (70), which is consistent with the finding that smoking women have a higher androgenic steroid level (71, 72). In addition, smoking reduces the activity of 21- or 11-hydroxylase in the adrenal cortex, increasing adrenal androgen secretion (73, 74). A higher androgenic steroid level could compensate for intrinsic fetal endocrine testicular insufficiency, fetal androgen receptor deficiency, or decreased fetal 5-reductase activity (65), which promotes normal urethral closure, in fetuses of pregnant smokers.
Our study should also be viewed with certain limitations. First, only one study indicated the exclusion of passive smokers from the comparison of active smokers and no-smokers, as well as the exclusion of active smokers from the comparison of passive smokers and no-smokers (14). The imprecision of the categorization of included studies may introduce bias into the study's findings. Second, there were a limited number of studies on maternal passive smoking, which may have led to biased results. The relationship between maternal passive smoking and the risk of hypospadias requires additional research. Third, cigarette smoking is associated with an increased risk of the majority of congenital defects (6). Our study did not assess the association between smoking and other congenital defects, which may have affected the study's credibility.
In conclusion, the purpose of our study, which included a significant number of participants to ensure statistical power, was to examine the association between parental smoking and hypospadias. Our study demonstrated an association between maternal active smoking and a decreased risk of hypospadias. No association was identified between parental smoking or passive smoking and the risk of hypospadias in offspring. However, quitting cigarettes before pregnancy indeed has a good effect on the health of the offspring and should be advocated worldwide.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The meta-analysis was conceived and designed by Z-hY and XL. Z-hY and H-sC independently extracted the data from PubMed, the Cochrane Library, EMBASE, and Web of Science. Z-hY led the data analysis and interpretation, drafted the manuscript, and revised the content in response to feedback. Z-CZ played the role of the second reviewer. XW assisted with database retrieval and data collecting. H-sC assisted with data interpretation and gave crucial draft revisions. G-hW contributed to the conception and design, data interpretation, and revision of drafts. XL acted as the corresponding author, provided funding support, assisted with data interpretation, provided critical revision of drafts, and served as the third (mediating) reviewer. All authors contributed to the article and approved the submitted version.
I would like to appreciate the Department of Urology at Children's Hospital of Chongqing Medical University and the National Natural Science Foundation of China for their financial support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Suzan LC, Gary MS, Cecile L, Edward JL, Richard SO. Hypospadias and maternal exposures to cigarette smoke. Paediatr Perinat Epidemiol. (2005) 19:406–12. doi: 10.1111/j.1365-3016.2005.00680.x
2. Lund L, Engebjerg MC, Pedersen L, Ehrenstein V, Norgaard M, Sorensen HT. Prevalence of hypospadias in danish boys: a longitudinal study, 1977-2005. Eur Urol. (2009) 55:1022–6. doi: 10.1016/j.eururo.2009.01.005
3. Quinton AE, Cook CM, Peek MJ. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG. (2008) 115:780–4. doi: 10.1111/j.1471-0528.2008.01691.x
4. Talbot P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res C Embryo Today. (2008) 84:61–72. doi: 10.1002/bdrc.20120
5. Rogers JM. Tobacco and pregnancy. Reprod Toxicol. (2009) 28:152–60. doi: 10.1016/j.reprotox.2009.03.012
6. Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. (2011) 17:589–604. doi: 10.1093/humupd/dmr022
7. Tong VT, Dietz PM, Morrow B, D’Angelo DV, Farr SL, Rockhill KM, et al. Trends in smoking before, during, and after pregnancy – pregnancy risk assessment monitoring system. MMWR Surveill Summ. (2013) 62:1–19.24196750
8. Zhang Q, Zhang ZC, He XY, Liu ZM, Wei GH, Liu X. Maternal smoking during pregnancy and the risk of congenital urogenital malformations: a systematic review and meta-analysis. Front Pediatr. (2022) 10:973016. doi: 10.3389/fped.2022.973016
9. Rodriguez-Pinilla E, Mejias C, Prieto-Merino D, Fernandez P, Martinez-Frias ML, Group EW. Risk of hypospadias in newborn infants exposed to valproic acid during the first trimester of pregnancy: a case-control study in Spain. Drug Saf. (2008) 31:537–43. doi: 10.2165/00002018-200831060-00008
10. Kallen B. Case-control study of hypospadias, based on registry information. Teratology. (1988) 38:45–50. doi: 10.1002/tera.1420380107
11. Kallen B, Winberg J. An epidemiologic study of hypospadias in Sweden. Acta Pediatrica Scandinavica. (1982) 293:1–21. doi: 10.1111/j.1651-2227.1982.tb09577.x
12. Giordano F, Carbone P, Nori F, Mantovani A, Taruscio D, Figa-Talamanca I. Maternal diet and the risk of hypospadias and cryptorchidism in the offspring. Paediatr Perinat Epidemiol. (2008) 22:249–60. doi: 10.1111/j.1365-3016.2007.00918.x
13. Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, et al. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: a population-based case-control study in rural sicily. Int J Androl. (2007) 30:3–13. doi: 10.1111/j.1365-2605.2006.00703.x
14. Carmichael SL, Ma C, Shaw GM, National Birth Defects Prevention Study. Maternal smoking, alcohol, and caffeine exposures and risk of hypospadias. Birth Defects Res. (2017) 109:1127–33. doi: 10.1002/bdr2.1044
15. Carmichael SL, Ma C, Tinker S, Shaw GM, National Birth Defects Prevention Study. Maternal stressors and social support and risks of delivering babies with gastroschisis or hypospadias. Am J Epidemiol. (2017) 185:1240–6. doi: 10.1093/aje/kww121
16. Van Zutphen AR, Werler MM, Browne MM, Romitti PA, Bell EM, McNutt LA, et al. Maternal hypertension, medication use, and hypospadias in the national birth defects prevention study. Obstet Gynecol. (2014) 123:309–17. doi: 10.1097/AOG.0000000000000103
17. Hoyt AT, Canfield MA, Romitti PA, Botto LD, Anderka MT, Krikov SV, et al. Associations between maternal periconceptional exposure to secondhand tobacco smoke and major birth defects. Am J Obstet Gynecol. (2016) 215:613.e1–e11. doi: 10.1016/j.ajog.2016.07.022
18. de Kort CA, Nieuwenhuijsen MJ, Mendez MA. Relationship between maternal dietary patterns and hypospadias. Paediatr Perinat Epidemiol. (2011) 25:255–64. doi: 10.1111/j.1365-3016.2011.01194.x
19. Rocheleau CM, Romitti PA, Sanderson WT, Sun L, Lawson CC, Waters MA, et al. Maternal occupational pesticide exposure and risk of hypospadias in the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. (2011) 91:927–36. doi: 10.1002/bdra.22860
20. Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, et al. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect. (2009) 117:303–7. doi: 10.1289/ehp.11933
21. Rappazzo KM, Warren JL, Davalos AD, Meyer RE, Sanders AP, Brownstein NC, et al. Maternal residential exposure to specific agricultural pesticide active ingredients and birth defects in a 2003-2005 North Carolina birth cohort. Birth Defects Res. (2019) 111:312–23. doi: 10.1002/bdr2.1448
22. Winston JJ, Meyer RE, Emch ME. Geographic analysis of individual and environmental risk factors for hypospadias births. Birth Defects Res A Clin Mol Teratol. (2014) 100:887–94. doi: 10.1002/bdra.23306
23. Caton AR, Bell EM, Druschel CM, Werler MM, Mitchell AA, Browne ML, et al. Maternal hypertension, antihypertensive medication use, and the risk of severe hypospadias. Birth Defects Res A Clin Mol Teratol. (2008) 82:34–40. doi: 10.1002/bdra.20415
24. Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking in pregnancy and risk for congenital malformations: results of a danish register-based cohort study. Acta Obstet Gynecol Scand. (2014) 93:825–34. doi: 10.1111/aogs.12433
25. Lindbo D, Arendt LH, Ernst A, Lunddorf LLH, Brix N, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and genital anomalies in boys: a register-based cohort and sibling-matched design study. Clin Epidemiol. (2022) 14:901–10. doi: 10.2147/CLEP.S368826
26. Agopian AJ, Hoang TT, Mitchell LE, Morrison AC, Tu D, Nassar N, et al. Maternal hypertension and risk for hypospadias in offspring. Am J Med Genet A. (2016) 170:3125–32. doi: 10.1002/ajmg.a.37947
27. White JT, Kovar E, Chambers TM, Sheth KR, Peckham-Gregory EC, O'Neill M, et al. Hypospadias risk from maternal residential exposure to heavy metal hazardous air pollutants. Int J Environ Res Public Health. (2019) 16:930. doi: 10.3390/ijerph16060930
28. Sheth KR, Kovar E, White JT, Chambers TM, Peckham-Gregory EC, O'Neill M, et al. Hypospadias risk is increased with maternal residential exposure to hormonally active hazardous air pollutants. Birth Defects Res. (2019) 111:345–52. doi: 10.1002/bdr2.1461
29. Haraux E, Braun K, Buisson P, Stephan-Blanchard E, Devauchelle C, Ricard J, et al. Maternal exposure to domestic hair cosmetics and occupational endocrine disruptors is associated with a higher risk of hypospadias in the offspring. Int J Environ Res Public Health. (2016) 14:27. doi: 10.3390/ijerph14010027
30. Haraux E, Tourneux P, Kouakam C, Stephan-Blanchard E, Boudailliez B, Leke A, et al. Isolated hypospadias: the impact of prenatal exposure to pesticides, as determined by meconium analysis. Environ Int. (2018) 119:20–5. doi: 10.1016/j.envint.2018.06.002
31. Trabert B, Longnecker MP, Brock JW, Klebanoff MA, McGlynn KA. Maternal pregnancy levels of trans-nonachlor and oxychlordane and prevalence of cryptorchidism and hypospadias in boys. Environ Health Perspect. (2012) 120:478–82. doi: 10.1289/ehp.1103936
32. Ghazarian AA, Trabert B, Graubard BI, Longnecker MP, Klebanoff MA, McGlynn KA. Placental weight and risk of cryptorchidism and hypospadias in the collaborative perinatal project. Am J Epidemiol. (2018) 187:1354–61. doi: 10.1093/aje/kwy005
33. Akre O, Boyd HA, Ahlgren M, Wilbrand K, Westergaard T, Hjalgrim H, et al. Maternal and gestational risk factors for hypospadias. Environ Health Perspect. (2008) 116:1071–6. doi: 10.1289/ehp.10791
34. Kjersgaard CL, Arendt LH, Ernst A, Sondergaard Lindhard M, Olsen J, Henriksen TB, et al. Lifestyle in pregnancy and hypospadias in sons: a study of 85,923 mother-son pairs from two danish pregnancy cohorts. Clin Epidemiol. (2022) 14:149–57. doi: 10.2147/CLEP.S335877
35. Heinonen OP. Birth defects and drugs in pregnancy. Littleton: Massachusetts Publishing Sciences Group, Inc (1977).
36. Sakemi Y, Shono T, Nakashima T, Yamashita H, Sugino N, Bonno M. Abnormal placental cord insertion, hypertensive disorders of pregnancy and birth length may be involved in development of hypospadias in male fetuses. Birth Defects Res. (2022) 114:271–6. doi: 10.1002/bdr2.1995
37. Siregar S, Sibarani J, Saputra D. The role of maternal and environmental factors during pregnancy on the risk of hypospadias occurrence. Glob Pediatr Health. (2022) 9:2333794X221105254. doi: 10.1177/2333794X221105254
38. Spinder N, Bergman JEH, van Tongeren M, Boezen HM, Kromhout H, de Walle HEK. Maternal occupational exposure to endocrine-disrupting chemicals and urogenital anomalies in the offspring. Hum Reprod. (2021) 37:142–51. doi: 10.1093/humrep/deab205
39. Akay MA, Yildiz GE. Impact of gestational and parental factors and maternal intake of progesterone on the development of hypospadias: a retrospective case-control study. Taiwan J Obstet Gynecol. (2021) 60:894–8. doi: 10.1016/j.tjog.2021.08.001
40. Jamaladin H, van Rooij I, van der Zanden LFM, van Gelder M, Roeleveld N. Maternal hypertensive disorders and subtypes of hypospadias: a Dutch case-control study. Paediatr Perinat Epidemiol. (2020) 34:687–95. doi: 10.1111/ppe.12683
41. Li X, Sundquist J, Hamano T, Sundquist K. Family and neighborhood socioeconomic inequality in cryptorchidism and hypospadias: a nationwide study from Sweden. Birth Defects Res. (2019) 111:78–87. doi: 10.1002/bdr2.1444
42. Sastre E, C B, Artero C, Gonzalez Ruiz Y, Fernandez Atuan RL, Bragagnini Rodriguez P, et al. Occupational exposure to endocrine-disrupting chemicals and other parental risk factors in hypospadias and cryptorchidism development: a case-control study. J Pediatr Urol. (2019) 15:520.e1–e8. doi: 10.1016/j.jpurol.2019.07.001
43. Mavrogenis S, Urban R, Czeizel AE. Pregnancy complications in the mothers who delivered boys with isolated hypospadias - a population-based case-control study. J Matern Fetal Neonatal Med. (2015) 28:489–93. doi: 10.3109/14767058.2014.921902
44. Xu LF, Liang CZ, Lipianskaya J, Chen XG, Fan S, Zhang L, et al. Risk factors for hypospadias in China. Asian J Androl. (2014) 16:778–81. doi: 10.4103/1008-682X.131704
45. Woud SG, van Rooij IA, van Gelder MM, Olney RS, Carmichael SL, Roeleveld N, et al. Differences in risk factors for second and third degree hypospadias in the national birth defects prevention study. Birth Defects Res A Clin Mol Teratol. (2014) 100:703–11. doi: 10.1002/bdra.23296
46. van Rooij IA, van der Zanden LF, Brouwers MM, Knoers NV, Feitz WF, Roeleveld N. Risk factors for different phenotypes of hypospadias: results from a Dutch case-control study. BJU Int. (2013) 112:121–8. doi: 10.1111/j.1464-410X.2012.11745.x
47. Christensen JS, Asklund C, Skakkebaek NE, Jorgensen N, Andersen HR, Jorgensen TM, et al. Association between organic dietary choice during pregnancy and hypospadias in offspring: a study of mothers of 306 boys operated on for hypospadias. J Urol. (2013) 189:1077–82. doi: 10.1016/j.juro.2012.09.116
48. Rignell-Hydbom A, Lindh CH, Dillner J, Jonsson BA, Rylander L. A nested case-control study of intrauterine exposure to persistent organochlorine pollutants and the risk of hypospadias. PLoS One. (2012) 7:e44767. doi: 10.1371/journal.pone.0044767
49. Adams SV, Hastert TA, Huang Y, Starr JR. No association between maternal pre-pregnancy obesity and risk of hypospadias or cryptorchidism in male newborns. Birth Defects Res A Clin Mol Teratol. (2011) 91:241–8. doi: 10.1002/bdra.20805
50. Iszatt N, Nieuwenhuijsen MJ, Nelson P, Elliott P, Toledano MB. Water consumption and use, trihalomethane exposure, and the risk of hypospadias. Pediatrics. (2011) 127:e389–97. doi: 10.1542/peds.2009-3356
51. Akin Y, Ercan O, Telatar B, Tarhan F, Comert S. Hypospadias in Istanbul: incidence and risk factors. Pediatr Int. (2011) 53:754–60. doi: 10.1111/j.1442-200X.2011.03340.x
52. Brouwers MM, van der Zanden LF, de Gier RP, Barten EJ, Zielhuis GA, Feitz WF, et al. Hypospadias: risk factor patterns and different phenotypes. BJU Int. (2010) 105:254–62. doi: 10.1111/j.1464-410X.2009.08772.x
53. Nørgaard M, Wogelius P, Pedersen L, Rothman KJ, Sørensen HT. Maternal use of oral contraceptives during early pregnancy and risk of hypospadias in male offspring. Urology. (2009) 74:583–7. doi: 10.1016/j.urology.2009.04.034
54. Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr. (2007) 166:671–8. doi: 10.1007/s00431-006-0304-z
55. Meyer KJ, Reif JS, Veeramachaneni DN, Luben TJ, Mosley BS, Nuckols JR. Agricultural pesticide use and hypospadias in eastern Arkansas. Environ Health Perspect. (2006) 114:1589–95. doi: 10.1289/ehp.9146
56. Porter MP, Faizan MK, Grady RW, Mueller BA. Hypospadias in Washington state: maternal risk factors and prevalence trends. Pediatrics. (2005) 115:e495–9. doi: 10.1542/peds.2004-1552
57. Kurahashi N, Sata F, Kasai S, Shibata T, Moriya K, Yamada H, et al. Maternal genetic polymorphisms in CYP1A1, GSTM1 and GSTT1 and the risk of hypospadias. Mol Hum Reprod. (2005) 11:93–8. doi: 10.1093/molehr/gah134
58. Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect. (2004) 112:1570–6. doi: 10.1289/ehp.7243
59. Olof A, Loren L, Sven C, Par S, Anders E. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. (1999) 10:364–9. doi: 10.1097/00001648-199907000-00005
60. Juim Z, David AS, Pamela JS, Wenwei C. A case-control study of paternal smoking and birth defects. Int J Epidemiol. (1992) 21:273–8. doi: 10.1093/ije/21.2.273
61. Stephen KVDE, Margaret RK, Janet RD, Thomas LV. A case-control study of maternal smoking and congenital malformations. Paediatr Perinat Epidemiol. (1990) 4:147–55. doi: 10.1111/j.1365-3016.1990.tb00630.x
62. Yang L, Wang H, Yang L, Zhao M, Guo Y, Bovet P, et al. Maternal cigarette smoking before or during pregnancy increases the risk of birth congenital anomalies: a population-based retrospective cohort study of 12 million mother-infant pairs. BMC Med. (2022) 20:4. doi: 10.1186/s12916-021-02196-x
63. Perry MF, Mulcahy H, DeFranco EA. Influence of periconception smoking behavior on birth defect risk. Am J Obstet Gynecol. (2019) 220:588.e1–e7. doi: 10.1016/j.ajog.2019.02.029
64. Kovalenko AA, Brenn T, Odland JO, Nieboer E, Krettek A, Anda EE. Risk factors for hypospadias in northwest Russia: a murmansk county birth registry study. PLoS One. (2019) 14:e0214213. doi: 10.1371/journal.pone.0214213
65. Kallen K. Role of maternal smoking and maternal reproductive history in the etiology of hypospadias in the offspring. Teratology. (2002) 66:185–91. doi: 10.1002/tera.10092
66. Daniel SS, Pnina E-H, Rena G. Effect of maternal smoking and age on congenital anomalies. Obstet Gynecol. (1990) 76:1046–50.2234712
67. Patricia HS, Mark AK, Heinz WB. Congenital malformations and maternal smoking during pregnancy. Teratology. (1986) 34:65–71. doi: 10.1002/tera.1420340109
68. Kirsten W, Tine Brink H, Morten H, Niels Jørgen S. Smoking habits among Danish pregnant women from 1989 to 1996 in relation to sociodemographic and lifestyle factors. Acta Obstet Gynecol Scand. (1998) 77:836–40.9776597
69. Sharpe R, Skakkebaek N. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet. (1993) 341:1392–5. doi: 10.1016/0140-6736(93)90953-E
70. Michnovicz J, Naganuma H, Hershcopf R, Bradlow H, Fishman J. Increased urinary catechol estrogen excretion in female smokers. Steroids. (1988) 52:69–83. doi: 10.1016/0039-128X(88)90218-8
71. Cassidenti DL, Pike MC, Vijod AG, Stanczyk FZ, Lobo RA. A reevaluation of estrogen status in postmenopausal women who smoke. Am J Obstet Gynecol. (1992) 166:1444–8. doi: 10.1016/0002-9378(92)91617-J
72. Law MR, Cheng R, Hackshaw AK, Allaway S, Hale AK. Cigarette smoking, sex hormones and bone density in women. Eur J Epidemiol. (1997) 13:553–8. doi: 10.1023/A:1007389712487
73. Robert LB, Andrew JF, Rapin O. Cotinine and nicotine inhibit human fetal adrenal 11 beta-hydroxylase. J Clin Endocrinol Metab. (1989) 69:1221–4. doi: 10.1210/jcem-69-6-1221
Keywords: hypospadias, maternal active smoking, maternal passive smoking, paternal smoking, and meta-analysis
Citation: Ye Z-H, Chen H-S, Zhang Z-C, Wang X, Liu X and Wei G-H (2023) Parental smoking and risk of hypospadias: An updated meta-analysis of observational studies. Front. Pediatr. 11:1003037. doi: 10.3389/fped.2023.1003037
Received: 26 August 2022; Accepted: 26 January 2023;
Published: 23 February 2023.
Edited by:
Wenquan Niu, China-Japan Friendship Hospital, ChinaReviewed by:
Loes Van Der Zanden, Radboud University Medical Center, Netherlands© 2023 Ye, Chen, Zhang, Wang, Liu and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing Liu ZHIubGl1eDAyMTdAZ21haWwuY29t
Specialty Section: This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
Abbreviations RR, risk ratio; CI, confidence interval.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.