- Department of Pediatrics, The First Hospital of Jilin University, Changchun, China
Background: Recently, there was an outbreak in China of the Omicron (B.1.1.529) variant, the corresponding clinical characteristics of Chinese children with the Omicron variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were then reviewed and summarized retrospectively.
Methods: From March to April 2022, a total of 134 children infected with the Omicron variant were included in the study. Data such as sex, age, clinical symptoms, laboratory examinations, and imaging features were collected for further analyses.
Results: Half of the children were male and the median age was 5.67 years. The most SARS-CoV-2 Omicron variant was identified in mild (122, 91%), and the most three frequent symptoms were as cough (108, 80.6%), fever (75, 56%), and sore throat (38, 28.4%). Among age groups, no significant difference was observed in the distribution of symptoms, and no statistical difference was found in different clinical types among sex or age groups. Laboratory examinations revealed that white blood cells, neutrophils, and hemoglobin decreased; and monocytes, C-reactive protein (CRP), and aspartate aminotransferase (AST) increased. Further analyses showed that neutrophils, hemoglobin, CRP, and AST exhibited significant differences among age groups. Radiological abnormalities were found in nine cases, with small patchy high-density shadows. Of the 76 cured cases discharged from the hospital, the median hospital stay was 13 days (mean, 12 days).
Conclusions: In China, most children with Omicron SARS-CoV-2 infection have mild presentation. The findings of this study may help other districts improve the management of children with Omicron SARS-CoV-2 infection in China.
Introduction
On November 24, 2021, a novel severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) variant, Omicron (B.1.1.529), was first identified in South Africa, which was responsible for a fourth wave of coronavirus disease 2019 (COVID-19) (1–3). In March 2022, an outbreak of Omicron variant infection was first recognized in Changchun, China.
The clinical manifestations caused by the Omicron variant strain are significantly different from those caused by the previous three viral strains and have garnered great attention (4, 5). Although many studies have revealed that the clinical characteristics caused by Omicron variant are significantly different from those caused by other variants (5–7), more investigation is needed in the Chinese population. Therefore, in this study, the clinical characteristics of Chinese children were collected and reviewed from March to April 2022, and then compared with the characteristics of children infected with Delta variants. Some novel findings were observed, which are inconsistent with those children infected with Omicron in South Africa (such as symptoms, oxygen use) (8) or with the Delta variant in this area. The findings of this study may improve the management of children with Omicron infection in other regions.
Materials and methods
Subjects
The retrospective study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Jilin University (No. 2022-290). Written informed consent was obtained from the parents. From March 2022 to April 2022, children (≤4 years) admitted to the hospital with a diagnosis of COVID-19 were included for analysis. The diagnosis and treatment of COVID-19 were according to the guidelines issued by the National Health Commission of the People's Republic of China (9th version) (9). Diagnostic criteria were: previous contact history, symptoms such as fever and cough, radiological evidence; SARS-CoV-2 PCR (+), IgM or IgG (+, unvaccinated status). Discharge criteria were: recovery (temperature, respiratory symptoms, and radiological features) and sequential PCR (-, at least two times with a 1-day interval).
Data collection
Data such as demographic characteristics, clinical type, clinical manifestations, treatment, laboratory examinations [e.g., SARS-COV-2 PCR, whole blood count, C-reactive protein (CRP), chemistry analysis], and radiological features were collected from the electronic medical records using a structured questionnaire.
Statistical analyses
Statistical analyses was performed using SPSS (version 24.0). The quantitative data with normal distribution are expressed as the mean ± standard deviation (SD) and were compared using the t-test. Otherwise the median and interquartile range (IQR) were reported and compared using the Mann-Whitney test or Kruskal-Wallis test. The categorical data were reported with frequency (percentage) and compared using the chi square test and Fisher's exact test. P < 0.05 was considered statistically significant.
Statistical analysis was performed using R language (version 4.1.2). Continuous data that follow a normal distribution are expressed as the mean ± standard deviation, and otherwise are expressed as the median and interquartile range. Comparisons between groups were analyzed using the Mann-Whitney U test. Categorical data are expressed as frequencies (percentages), and comparisons were analyzed using the chi-square and Fisher's exact test. P < 0.05 is considered statistically significant.
Results
Baseline characteristics
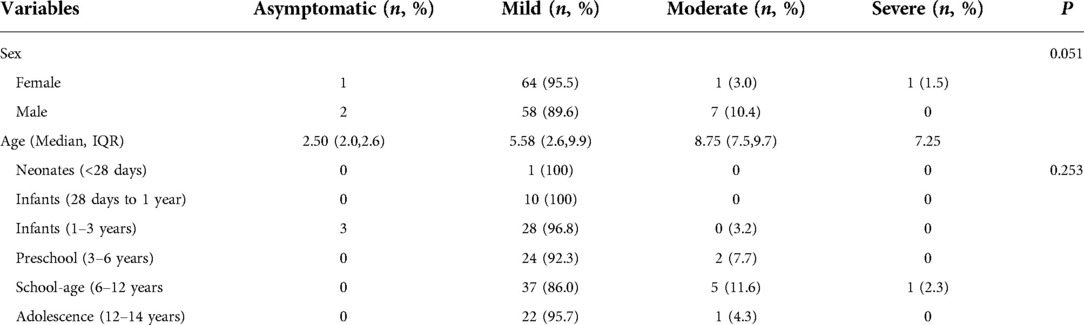
A total of 134 children with COVID-19 were included in this study, of whom 67 (50.0%) were males. The median age was 5.67 (IQR, 2.58–9.92) years. The age distributions were as follows: neonates (<28 days; n = 1), infants (28 days to 1 year; n = 10), infants (1–3 years old; n = 31), preschool (3–6 years old; n = 26), school-age (6–12 years old; n = 43), and adolescence (12–14 years old; n = 23). There were 3 asymptomatic cases (2.2%), 122 mild cases (91%), 8 moderate cases (6%), and 1 severe case (0.7%). Table 1 shows the baseline characteristics of the children with COVID-19.
Clinical types
In terms of clinical types, the median age was as follows: asymptomatic group, 2.50 (IQR, 2.0–2.6) years; mild group, 5.58 (IQR, 2.6–9.9) years, moderate group 8.75 (IQR, 7.5–9.7) years, and severe group, 7.25 years. There was no statistically significant difference in clinical classification between sex and age groups.
Symptoms
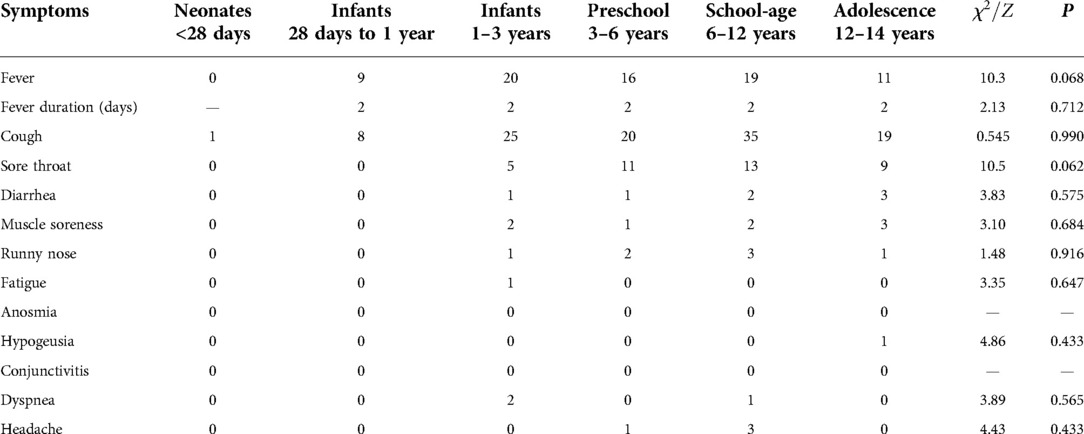
The main symptoms of children infected with Omicron variant were cough (n = 108, 80.6%), fever (n = 75, 56%), sore throat (n = 38, 28.4%), muscle soreness (n = 8, 6%), diarrhea (n = 7, 5.2%), runny nose (n = 7, 5.2%), headache (n = 4, 3%), dyspnea (n = 3, 2.2%), fatigue (n = 1, 0.7%), and hypogeusia (n = 1, 0.7%). There was no statistically significant difference in the distribution of symptoms among age groups, which may be related to the small sample size of each symptom (Table 2).
Laboratory examinations
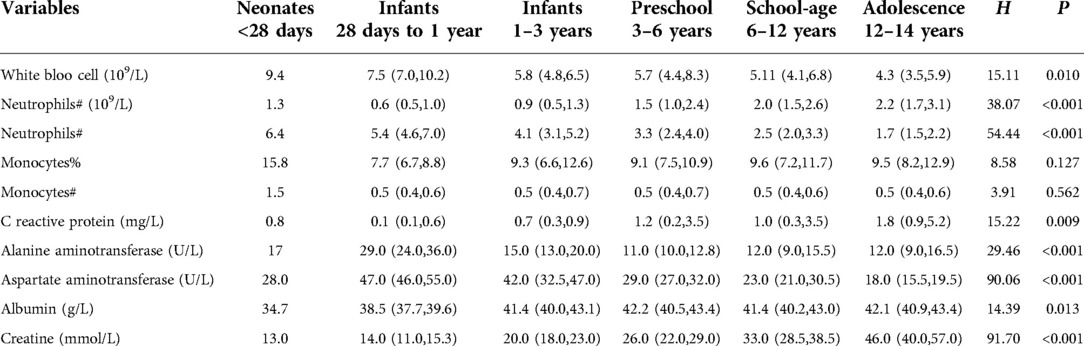
Routine blood examination showed that white blood cells (WBCs) were elevated in 8 (6.0%) patients and decreased in 27 (20.1%) patients; neutrophils were decreased in 51(38.1%) patients; lymphocytes were decreased in 4 (3.0%) patients; monocytes and its proportion were increased in 22 (16.4%) and 85 (63.4%) patients, respectively; and hemoglobin (HGB) was decreased in 7 (5.2%) patients. C-reactive protein (CRP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were elevated in 58 (43.3%) patients, 3 (2.2%) patients, and 18 (13.4%) patients, respectively. Only two (1.5%) patients a had decreased level of albumin. Further statistical analyses showed that neutrophil, hemoglobin, CRP, and AST were significantly different among age groups (Tables 3, 4).
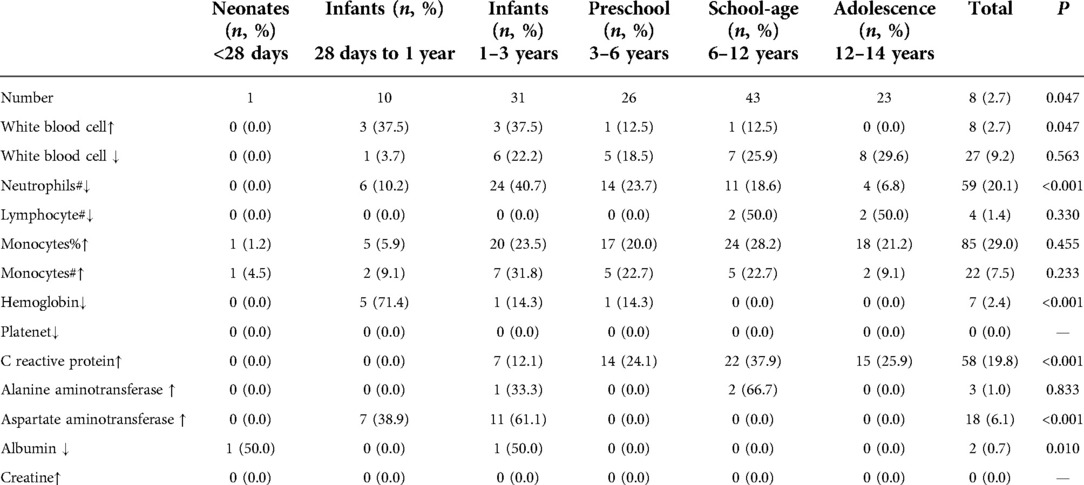
Table 3. The distribution of abnormal lab examinations between age groups among children with omicron infection.
Imaging
Nine cases had radiological abnormalities, and all patients showed small patchy high-density shadows including the right side (n = 8), left side (n = 1), right upper lobe (n = 6), right lower lobe (n = 2), and left lower lobe (n = 1). Most abnormalities recovered within 1 week, except two children (one was significantly improved after 3 days, and another showed that the primary lesion disappeared and a new lesion presented on the contralateral lung).
Hospital stay
Most children were in good condition. Due to laryngeal obstruction, one child was treated with oxygen therapy, and the dyspnea was relieved after symptomatic treatment. No death was reported. To date, 76 patients were cured and discharged, with a median stay of 13 days (mean, 12 days).
Vaccination status
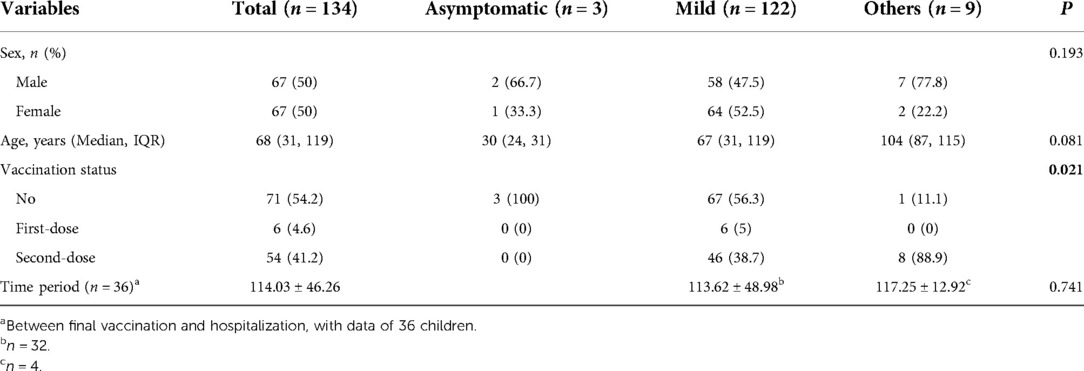
The vaccination status was available in 131 (131/134, 97.8%) children. There were three children with incomplete data. Of the 131 children, 71 had not received the COVID-19 vaccine, 6 had their first vaccine dose, and 54 had their second vaccine dose. Statistical analysis showed no significant difference between the mild form and other types regarding age, vaccination status, and period between final vaccination and hospitalization (Table 5).
Discussion
According to a report from Jilin CDC (http://wjw.jlcity.gov.cn/), the Omicron epidemic in Changchun is more severe than the previous COVID-19 epidemic. For example, the number of children with Omicron infection is larger than that caused by the Delta variant reported in other Chinese cities in 2021. Due to the zero COVID-19 strategy, little evidence is available characterizing children with Omicron infection in China. Hence, the characteristics of childhood COVID-19 were reviewed and summarized in the report.
Previously, very few COVID-19 children were studied. For example, in 2021, Huang et al. (4) studied 21 children infected with the Delta variant in southern China. Sheng et al. (10) reviewed 11 children with the Delta virus infection in central China. Moreover, during that year, a small cohort of children infected with Omicron was reported in the UK (n = 55) (11) and Spain (n = 15) (12). During an outbreak of Omicron variant in Shanghai, beginning March 7, 2022, a total of 376 children with Omicron was reviewed and reported in preprint without complete peer review (13). However, in 2021, large cohorts of childhood COVID-19 were reported. For example, in South Africa, a total of 6,287 children with confirmed COVID-19 cases were reported, including 869 cases (0–4 years), 1,231 cases (5–9 years), 2,023 cases (10–14 years), and 2,164 cases (15–19 years) (8). Similarly, a Omicron cohort of 22,772 children was reviewed in the USA (14). In general, limited evidence is available on childhood COVID-19 in China or abroad, especially for Omicron infection. Fortunately, in the March, 2022, a relatively large number (n = 134) of childhood COVID-19 cases were reported. Hence, a rapid review of their characteristics was performed, and we shared our experience with Chinese healthcare providers in improving the management of Chinese children with COVID-19.
First, the sex distribution was equal. Although previous study showed that sex could influence virus-driven T cell differentiation and maintenance in tissue sites and impact the anti-viral immune response (15), in this study, the male to female ratio was 1:1, and no statistical difference was found in the sex distribution of clinical types. Our findings were similar to previous Chinese reports in 2021. For example, the sex ratio of children infected with the Delta variant was 1:1.3 in Guangzhou (4) and 1:1.2 in Jingmen (10). In addition, similar findings were observed with the Omicron variant. For example, in the Qatar cohort, 54.6% of children were female (16). Other female proportion, such as 55.0% in Spain (12), 45.2% in Shanghai (13), and 47.3% in the USA (14) were also reported, displaying an equal distribution. However, this finding is not consistent with the COVID-19 in the adult. Previously, several reports point to sex differences in COVID-19 resulting from male patients having higher rates of infection, which is explained by social behaviour and human biology (17, 18). In addition, the median age of the children included was low and reported at 5.67 years old (IQR, 2.58–9.92), and approximately one-third of children were school-age, and more than half were 1–6 years old. This finding is inconsistent with previous reports. For example, the median age of children infected with Delta variant was 7 (IQR, 4–12) years (4). Jeane et al. (8) reported that children infected with the Omicron variant have a mean age of 4.2 years (SD 4.1) (8). In the USA COVID-19 cohort, the Omicron cohort was younger than the Delta cohort (14), and in most studies (12, 13, 16), the median age of Omicron children is reportedly between 6 and 7 years. In addition, our data also demonstrated that there was no significant difference in clinical types among age groups. In general, unlike other respiratory viral infections (such as respiratory syncytial virus) (19), the SARS-COV-2 infection in children has no age-dependent distribution. Interestingly, a previous study demonstrated that children aged 6 through 15 years had a longer persistence of viral genome in nasopharyngeal samples (20).
Second, most children infected with Omicron variants have mild symptoms. This means that during the epidemic in Changchun, the development of children's clinical type is different from previous epidemics, which happened in other Chinese cities. In the study, mild patients accounted for approximately 90% of all children. Nevertheless, as reported previously, children infected with Delta variant have relatively severe presentation, and the moderate type is significantly higher in previous studies (33.3% and 63.6%) than that of our study (4, 10). However, mild infection was significantly higher in children with Omicron infection than in those with the Delta variant (52.4% vs. 18.2%) (4, 10). Similar findings were observed in adulthood COVID-19 caused by the Omicron variant, which is that most patients have mild presentation (21, 22). In Shanghai, approximately one-third of Omicron-infected children were asymptomatic, and no severe disease was diagnosed (13). Wang et al. found that most children (82.9%) infected with Omicron variant had mild symptoms, mainly respiratory infection (23), which is consistent with the study by Ma et al. (24). According to the cohort in Qatar, among Omicron-infected children, 97.8% had mild, 2.2% had moderate, and none had severe/critical disease, Omicron variant infection (vs. Delta) was associated with significantly lower odds of moderate or severe/critical disease (16). Moreover, fewer comorbidities were reported in the Omicron cohort than in the Delta cohort (14).
In this study, only nine children had abnormal radiological features, with associated clinical characteristics (e.g., small patchy, high-density, unilateral). Similar radiological findings were reported by Huang et al. (4). In addition, radiological evidence also supported the mild presentation of children infected with the Omicron variant. No death was reported in the study, and only one child required oxygen therapy. Similarly, in the Spain cohort, only 2 of 94 (2.1%) patients were hospitalized, and no patient needed intensive care unit admission or died (12). Indirectly, this point is supported by that severe clinical outcomes in children infected with Omicron variant were significantly lower than those in the matched Delta cohort (14). However, in the UK cohort, 6.7% (3/45) had an oxygen requirement, 4.4% (2/45) required ventilation (1 invasive and 1 non-invasive), and 51.1% (23/45) received medication (11). In the UK cohort, more intervention were given, which may be explained by the cohort being younger. The data aremalso inconsistent with data from South Africa. According to the report from Jeané et al. (8), 20% of children required oxygen therapy, 5% were ventilated, and 3% were died during the study period. The difference in outcomes strengthens the role of geographical differences. In addition, the median hospital stay in our study was 13 days, which is shorter than that (median, 19 days) of the previous report by Huang et al. (4). In the UK cohort, the length of stay was shorter, ranging from 0 to 9 days with an average of 2 days (11). In a word, these characteristics such as clinical type, radiological evidence, treatment, and hospital period demonstrated that children with Omicron infection show a mild presentation.
Third, in the study, cough was the first symptom of children with Omicron infection, which accounted for 80.6% of all cases. Similar data were observed in the cohort conducted in UK, where fever and/or respiratory symptoms (86%) were the most common symptoms (11). However, another two reports in China (2021) showed that fever was the prominent symptom among children infected with Delta variant, with a proportion as high as 76.2% and 73% (4, 10). Moreover, 33.3% and 55% of children had cough in two studies (4, 10). Jeane et al. (8) reported that fever (61%) and cough (57%) were the most common symptoms among children with Omicron (B.1.1.529) variant infection, which is also inconsistent with our data. These results indicate that the symptoms caused by the Omicron variant are different from those caused by Delta variant and have geographical differences, due to the ethnic differences.
Fourth, laboratory examinations partly showed an abnormal status. For example, although the mean level of WBC (5.9 ± 2.2 × 109/L), neutrophils (1.9 ± 1.5 × 109/L), and lymphocytes (3.3 ± 1.7) were all within the normal range, a significant proportion of children showed an decreasing levels of WBCs (20.1%) and neutrophils (38.1%) and an increasing level of monocytes (63.4%) when compared with corresponding references. Increased percentage of monocytes was also observed in other studies (25). Although the underlying mechanism remains unclear and require further investigation, this may be explained by that (1) the SARS-CoV-2 infection could decrease the subset of neutrophils (26, 27), and increase the total of monocytes in mild group and decrease it in severe group (28, 29); (2) during the early stage, the decreased neutrophils and monocytes are known as an ongoing inflammatory status and risk factors of poor outcome among COVID-19 patients (29–31). Hence, patients may benefit from the normal or high numbers of neutrophils and monocytes. In addition, our study demonstrated that 43.3% of children infected with the Omicron variant showed elevated CRP. However, in a previous study of children infected with Delta variant, none (n = 21) showed elevated CRP (4), suggesting a different characteristic of children infected with the Omicron variant. Although our study had several interesting findings, the small sample size remains a concern, which may have led to significant selection bias. Therefore, further analysis of Chinese children with Omicron infection should be performed to improve the knowledge of the infection among Chinese children.
Fifth, vaccination status was also investigated. However, no significant difference was found between the mild form and other types regarding age, vaccination status, and period between final vaccination and hospitalization. In the study, half of the children had not received the SARS-CoV-2 vaccine. Recently, several studies have investigated the effects of COVID-19 vaccination against the Omicron variant. These findings support the efficacy of the SARS-CoV-2 vaccine. However, limitations remain for current vaccines. For example, the inactivated COVID-19 vaccine has a protective role for children, and more doses will be helpful to produce the IgG antibodies (23, 32). But, the production of IgG declines over time (32). In general, children are susceptible to infection with the Omicron variant (32). Meanwhile, children are more likely to be susceptible to vaccine breakthrough infections or reinfections due to the Omicron variant than previous variants (33).
Conclusions
In conclusion, our study showed that in Chinese children with Omicron infection, cough and fever are the most common symptoms, their presentations are usually mild, no special treatment is required, and the outcomes are usually good.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by The study protocol was approved by the Ethics Committee of The First Affiliated Hospital of Jilin University (No. 2022-290). The patients/participants provided their written informed consent to participate in this study.
Author contributions
HLY and YCL designed/performed most of the investigation, data analysis and wrote the manuscript; ZM and HYZ contributed to interpretation of the data and analyses. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang L, Cheng G. Sequence analysis of the emerging SARS-CoV-2 variant Omicron in South Africa. J Med Virol. (2022) 94(4):1728–33. doi: 10.1002/jmv.27516
2. Gowrisankar A, Priyanka TMC, Banerjee S. Omicron: a mysterious variant of concern. Eur Phys J Plus. (2022) 137(1):100. doi: 10.1140/epjp/s13360-021-02321-y
3. Mohapatra RK, Kuppili S, Kumar Suvvari T, Kandi V, Behera A, Verma S, et al. SARS-CoV-2 and its variants of concern including Omicron: a never ending pandemic. Chem Biol Drug Des. (2022) 99(5):769–88. doi: 10.1111/cbdd.14035
4. Huang CM, Hu Z, Lin J, Deng X. Clinical characteristics of infection with SARS-CoV-2 Delta variant in children and youth in Guangzhou city. Chin J Infect Control. (2021) 20:976–83. doi: 10.12138/j.issn.1671-9638.20211781
5. Maslo C, Friedland R, Toubkin M, Laubscher A, Akaloo T, Kama B. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. (2022) 327(6):583–4. doi: 10.1001/jama.2021.24868
6. Modes ME. Clinical characteristics and outcomes among adults hospitalized with laboratory-confirmed sars-cov-2 infection during periods of b. 1.617. 2 (delta) and b. 1.1. 529 (omicron) variant predominance—one hospital, California, July 15–September 23, 2021, and December 21, 2021–January 27, 2022. Morb Mortal Wkly Rep. (2022) 71:217. doi: 10.15585/mmwr.mm7106e2
7. Kim MK, Lee B, Choi YY, Um J, Lee KS, Sung HK, et al. Clinical characteristics of 40 patients infected with the SARS-CoV-2 omicron variant in Korea. J Korean Med Sci. (2022) 37(3):e31. doi: 10.3346/jkms.2022.37.e31
8. Cloete J, Kruger A, Masha M, du Plessis NM, Mawela D, Tshukudu M, et al. Paediatric hospitalisations due to COVID-19 during the first SARS-CoV-2 omicron (B.1.1.529) variant wave in South Africa: a multicentre observational study. Lancet Child Adolesc Health. (2022) 6(5):294–302. doi: 10.1016/s2352-4642(22)00027-x
9. China, N.H.C.o. New coronavirus pneumonia prevention and control program (9th edn). 2022 (cited 2022 13th, May); Available at: http://www.nhc.gov.cn/cms-search/xxgk/getManuscriptXxgk.htm?id=b74ade1ba4494583805a3d2e40093d88 (2022).
10. Sheng JF, Shao L, Wang YL. Clinical features of children with coronavirus disease 2019 caused by Delta variant infection. Zhongguo Dang Dai Er Ke Za Zhi. (2021) 23(12):1267–70. doi: 10.7499/j.issn.1008-8830.2110043
11. Clark M. Clinical characteristics of SARS-CoV-2 omicron infection in children under one year. Available SSRN. (2022):4013461.
12. Tagarro A, Coya ON, Pérez-Villena A, Iglesias B, Navas A, Aguilera-Alonso D, et al. Features of COVID-19 in children during the omicron wave compared with previous waves in Madrid, Spain. Pediatr Infect Dis J. (2022) 41(5):e249–e51. doi: 10.1097/inf.0000000000003482
13. Wang X, Chang H, Tian H, Zhu Y, Li J, Wei Z, et al. Epidemiological and clinical features of SARS-CoV-2 infection in children during the outbreak of Omicron variant in Shanghai, march 7-march 31, 2022. medRxiv. (2022) 16(6):1059–65. doi: 10.1111/irv.13044
14. Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Incidence rates and clinical outcomes of sars-cov-2 infection with the omicron and delta variants in children younger than 5 years in the us. JAMA Pediatr. (2022) 176(8):811–3. doi: 10.1001/jamapediatrics.2022.0945
15. Poon MML, Byington E, Meng W, Kubota M, Matsumoto R, Grifoni A, et al. Heterogeneity of human anti-viral immunity shaped by virus, tissue, age, and sex. Cell Rep. (2021) 37(9):110071. doi: 10.1016/j.celrep.2021.110071
16. Butt AA, Dargham SR, Loka S, Shaik RM, Chemaitelly H, Tang P, et al. COVID-19 disease severity in children infected with the omicron variant. Clin Infect Dis. (2022) 75(1):e361–e7. doi: 10.1093/cid/ciac275
17. Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. (2020) 11(1):29. doi: 10.1186/s13293-020-00304-9
18. Ramírez-Soto MC, Arroyo-Hernández H, Ortega-Cáceres G. Sex differences in the incidence, mortality, and fatality of COVID-19 in Peru. PLoS One. (2021) 16(6):e0253193. doi: 10.1371/journal.pone.0253193
19. Brenes-Chacon H, Garcia-Mauriño C, Moore-Clingenpeel M, Mertz S, Ye F, Cohen DM, et al. Age-dependent interactions among clinical characteristics, viral loads and disease severity in young children with respiratory syncytial virus infection. Pediatr Infect Dis J. (2021) 40(2):116–22. doi: 10.1097/inf.0000000000002914
20. Bahar B, Jacquot C, Mo YD, DeBiasi RL, Campos J, Delaney M. Kinetics of viral clearance and antibody production across age groups in children with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr. (2020) 227:31–7. doi: 10.1016/j.jpeds.2020.08.078
21. Bouzid D, Visseaux B, Kassasseya C, Daoud A, Fémy F, Hermand C, et al. Comparison of patients infected with delta versus omicron COVID-19 variants presenting to Paris emergency departments: a retrospective cohort study. Ann Intern Med. (2022) 175(6):831–7. doi: 10.7326/m22-0308, www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M22-030835286147
22. Zeng QL, Lv YJ, Liu XJ, Jiang ZY, Huang S, Li WZ, et al. Clinical characteristics of omicron sars-cov-2 variant infection after non-mrna-based vaccination in China. Front Microbiol. (2022) 13:901826. doi: 10.3389/fmicb.2022.901826
23. Wang XL, Zhai J, Zou YX. Clinical characteristics and vaccination status of SARS-CoV-2 omicron variant infected children. Zhonghua Er Ke Za Zhi. (2022) 60(7):671–5. doi: 10.3760/cma.j.cn112140-20220506-00417
24. Ma WJ, Wang XS, Tian H, Zhu YF, Wei ZQ, Xu J, et al. Characteristics of SARS-CoV-2 Omicron infection in children imported from Hong Kong. Zhonghua Er Ke Za Zhi. (2022) 60(6):539–44. doi: 10.3760/cma.j.cn112140-20220423-00367
25. Xu C, Ma M, Yi Y, Yi C, Dai H. Clinical features and high-resolution chest computerized tomography findings of children infected by the B.1.617.2 variant of coronavirus disease 2019. Ann Med. (2022) 54(1):2391–401. doi: 10.1080/07853890.2022.2114608
26. Volberding PJ, Xin G, Kasmani MY, Khatun A, Brown AK, Nguyen C, et al. Suppressive neutrophils require PIM1 for metabolic fitness and survival during chronic viral infection. Cell Rep. (2021) 35(8):109160. doi: 10.1016/j.celrep.2021.109160
27. Lam CW, Chan MH, Wong CK. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev. (2004) 25(2):121–32.18458712
28. Qin S, Jiang Y, Wei X, Liu X, Guan J, Chen Y, et al. Dynamic changes in monocytes subsets in COVID-19 patients. Hum Immunol. (2021) 82(3):170–6. doi: 10.1016/j.humimm.2020.12.010
29. Gatti A, Radrizzani D, Viganò P, Mazzone A, Brando B. Decrease of non-classical and intermediate monocyte subsets in severe acute SARS-CoV-2 infection. Cytometry Part A. (2020) 97(9):887–90. doi: 10.1002/cyto.a.24188
30. Belchamber KBR, Thein OS, Hazeldine J, Grudzinska FS, Faniyi AA, Hughes MJ, et al. Dysregulated neutrophil phenotype and function in hospitalised non-ICU COVID-19 pneumonia. Cells. (2022) 11(18):2901. doi: 10.3390/cells11182901
31. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. (2020) 71(15):762–8. doi: 10.1093/cid/ciaa248
32. Li X, Wu L, Qu Y, Cao M, Feng J, Huang H, et al. Clinical characteristics and vaccine effectiveness against SARS-CoV-2 omicron subvariant BA.2 in the children. Signal Transduct Targeted Ther. (2022) 7(1):203. doi: 10.1038/s41392-022-01023-w
Keywords: SARS-CoV-2, breakthrough infection, children, omicron variant, clinical characteristics 4 4
Citation: Li Y-c, Ma Z, Zhong H-y and You H-l (2022) Clinical characteristics of children with omicron SARS-CoV-2 infection in Changchun, China from march to april 2022: A retrospective study. Front. Pediatr. 10:990944. doi: 10.3389/fped.2022.990944
Received: 11 July 2022; Accepted: 31 October 2022;
Published: 15 November 2022.
Edited by:
Thomas S Murray, Yale University, United StatesReviewed by:
Qing-Lei Zeng, First Affiliated Hospital of Zhengzhou University, ChinaKamlendra Singh, University of Missouri, United States
© 2022 Li, Ma, Zhong and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai-long You eW91aGxAamx1LmVkdS5jbg==
Specialty Section: This article was submitted to Pediatric Infectious Diseases, a section of the journal Frontiers in Pediatrics
 Yan-chun Li
Yan-chun Li Hai-long You
Hai-long You