
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pediatr., 21 October 2022
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.989518
This article is part of the Research TopicOptimizing School Readiness for Children with Developmental DisabilitiesView all 11 articles
This paper discusses possibilities for early detection and early intervention in infants with or at increased risk of neurodevelopmental disorders in low- and middle-income countries (LMICs). The brain's high rate of developmental activity in the early years post-term challenges early detection. It also offers opportunities for early intervention and facilitation of school readiness. The paper proposes that in the first year post-term two early detection options are feasible for LMICs: (a) caregiver screening questionnaires that carry little costs but predict neurodevelopmental disorders only moderately well; (b) the Hammersmith Infant Neurological Examination and Standardized Infant NeuroDevelopmental Assessment (SINDA) which are easy tools that predict neurodisability well but require assessment by health professionals. The young brain's neuroplasticity offers great opportunities for early intervention. Ample evidence indicates that families play a critical role in early intervention of infants at increased risk of neurodevelopmental disorders. Other interventional key elements are responsive parenting and stimulation of infant development. The intervention's composition and delivery mode depend on the infant's risk profile. For instance, in infants with moderately increased risk (e.g., preterm infants) lay community health workers may provide major parts of intervention, whereas in children with neurodisability (e.g., cerebral palsy) health professionals play a larger role.
Global mortality in children aged under 5 years decreased by 60% between 1990 and 2020 due to the impact of the United Nations' Millennium Development Goals (1). Unfortunately, this accomplishment was not paralleled by a similar decrease in childhood disability (2). The combination of an increase in surviving children particularly in low- and middle-income countries (LMICs), a rapid population growth in LMICS, and often fragile health care systems in these countries, contributed to a high prevalence of children with neurodevelopmental disabilities (1, 2). It has been estimated that over 53 million children under 5 years had neurodevelopmental disabilities globally in 2016 (3). Over 90% of these children lived in LMICs (1, 4).
The United Nations Convention on the Rights of Persons with Disabilities (2006) and the United Nations Sustainable Development Goal 4 (2015) declared that children with disabilities have the right of inclusive education (5, 6). Nonetheless, UNICEF statistics revealed that many children with disabilities do not receive proper support and adequate education (7). UNICEF's data indicate that children with disabilities are 25% less likely to receive early stimulation and responsive care, 25% less likely to attend early childhood education and 49% more likely to have never attended primary school than children without disabilities (7). In order to improve this situation, it is mandatory that children with neurodevelopmental disorders, such as cerebral palsy (CP), intellectual disability and autism spectrum disorders (ASD), are detected at early age and receive early intervention (2, 8). Early detection and early intervention will result in improved school readiness, as they allow for optimal preparation of family and child so that the child may fully engage in learning experiences at school.
This perspective paper aims to discuss methods available for early detection and early intervention in infants with an increased biological risk of or with a neurodevelopmental disorder (hereafter: infants with R-ND). It pays special attention to those methods that are mostly geared to the health care situation in LMICs. Early detection and early intervention occur in a developmental timeframe that is characterized by abundant brain development. Therefore, the paper first summarizes the developmental changes in the young human brain and its implications for early detection and early intervention. It focuses on the first two postnatal years. The following two sections briefly review knowledge on early detection of and early intervention in infants with R-ND. The last section discusses how early detection and early intervention in infants with R-ND may be achieved best in LMICs. It stresses the importance of family involvement and the need of adaptation to local situations, including cultural habits and beliefs.
The development of the human nervous system is a long-lasting and intricate process based on ingenious interactions between genes, environmental information and experience (9). Figure 1 provides an overview of the elementary components of brain development. The majority of neurons and glial cells are generated during prenatal life. Many neurons do not stay at their origin's site but migrate during gestation to their final destination. Neuronal differentiation, synapse production and myelination start early in fetal life to become very active in gestation's last trimester and the first year post-term. Thereafter, these processes continue at a slower pace.
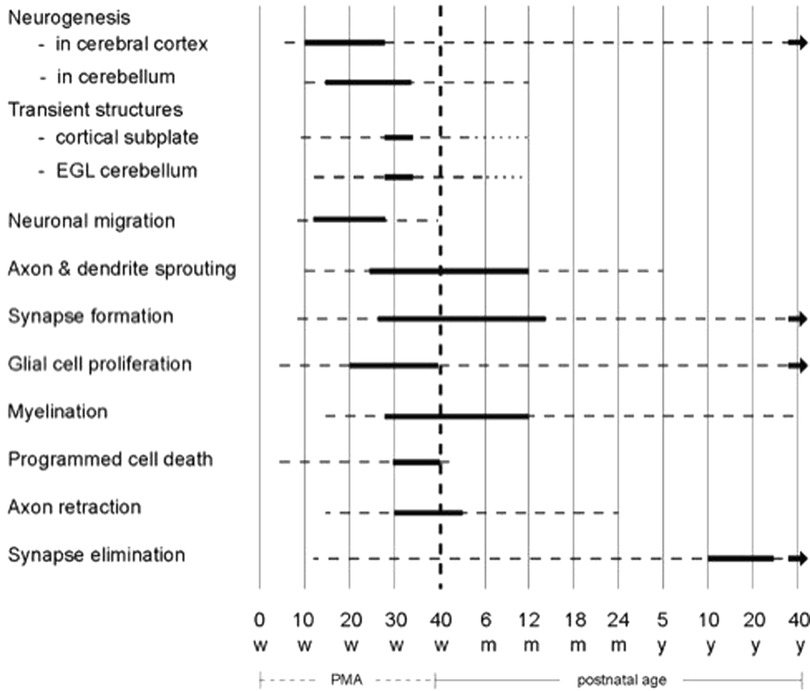
Figure 1. Schematic overview of the developmental processes occurring in the human brain. The bold lines indicate that the processes mentioned on the left side are very active, the broken lines denote that the processes still continue but less abundantly. The diagram is based on reference (9). EGL = external granular layer; m = months; PMA = postmenstrual age; w = weeks; y = years. Figure reproduced with permission from “Early Detection and Early Intervention in Developmental Motor Disorders—from neuroscience to participation” by Mijna Hadders-Algra (ed.) published by Mac Keith Press in its Clinics in Developmental Medicine Series, ISBN number 978-1-911612-43-8 (11).
Brain development is not only a matter of production of elements; it also involves massive elimination. About half of generated neurons die through programmed cell death, particularly during gestation's third trimester. Also, axons are initially produced in excess and later partially removed, especially during the end of gestation and the first 3 months post-term. Throughout life, synapses are formed and eliminated, with synapse elimination peaking between the onset of puberty and early adulthood (9).
The combination of production and regression gives rise to temporary structures and connections. Major transient structures are the cortical subplate and the cerebellar external granular layer (EGL; Table 1). The cortical subplate is a temporary structure between the developing white matter and cortical plate. It hosts the first generations of cortical neurons and plays a critical role in cortical development being the major site of neuronal differentiation, synaptogenesis and synaptic activity in the fetal cortex. It receives the first cortical afferents (10). The cortical subplate, which is most prominently present between 28- and 34-weeks postmenstrual age (PMA), mediates fetal behavior. From mid-gestation neurons in the subplate start to die and next generations of migrating cortical neurons begin to populate the cortical plate, i.e., the site of the permanent cortical networks. Around 3 months post-term, the subplate has largely disappeared in the primary motor, sensory and visual cortex, but it takes until the age of 12 months before the subplate has largely dissolved in the frontal, temporal and parietal association areas (9, 10). This means that infant behavior before subplate dissolution is based on activity in the networks in the “fetal” subplate and the cortical plate. First, after the disappearance of major parts of the cortical subplate, infant behavior is mainly mediated by the permanent cortical networks (9, 10). The other significant temporary structure is the cerebellar EGL. The EGL produces the granule cells, the most numerous cells of the brain. The EGL emerges around 15 weeks PMA and is most prominently present between 28- and 34-weeks PMA. Thereafter, it shrinks and disappears completely between 6- and 12-months post-term (9).
As mentioned above, axon development is also characterized by a combination of growth and regression. A well-known example is the axon retraction in the corticospinal tract (11). This tract begins with bilateral projections. Retraction of the ipsilateral projection starts in gestation's last trimester and is largely completed around the age of 2 years (11). This implies that, first at 2 years, the corticospinal tract has achieved its adult configuration with predominantly contralateral projections.
The brain's developmental activity in the first two years post-term results in specific windows of vulnerability for adverse events, such as inadequate nutrition, preterm birth, or hypoxic-ischemic events (12). The events' unfavorable effect often impacts development in multiple domains, including motor, cognitive, communication and socio-emotional abilities (12). The brain's high developmental activity also has important implications for early detection and early intervention in neurodevelopmental disorders. It offers opportunities and challenges. The brain's great developmental activity generates the opportunity of high neuroplasticity. Neuroplasticity may result in “growing out of dysfunction”. This means that signs of neurological dysfunction that may be present at early age in infants with prenatal, perinatal, or neonatal complications (with or without a brain lesion) may disappear with increasing age (13, 14). Moreover, the high neuroplasticity offers opportunities for early intervention. For instance, it is well known that developmental stimulation in preterm infants results in improved cognitive and motor outcome (15).
The brain's high rate of developmental activity also induces challenges, particularly for early detection of neurodevelopmental disorders. The developmental changes may not only result in resolution of neurological signs, but they may also be associated with the emergence of signs, i.e., “growing into a deficit”. The developing brain usually needs time to express signs of specific neurodevelopmental disorders. The early signs of CP manifest especially from 3 months post-term onwards, i.e., from the time that the cortical subplate in the primary motor and sensory cortex has dissolved (16). Ample evidence has demonstrated that abnormal general movements at 3 months post-term are a powerful predictor of CP (16, 17). The asymmetries of unilateral spastic CP are subtly expressed from 3 to 5 months onwards and become increasingly clear during the rest of the first year when the corticospinal tract reorganizes (18, 19). The early signs of ASD such as impaired social communication, atypical sensory responsivity and repetitive behavior, become clinically predictive from 12 months onwards, i.e., at the age that the cortical subplate has largely disappeared in the cortical association areas and the EGL has vanished (20).
The above described and other early signs of increased risk of disability generally do not allow for the diagnosis of a specific neurodevelopmental disorder. Currently the average age at the diagnosis of CP is 12 months (21), and of ASD, 43 months (22). Nonetheless, it is important to realize that a diagnosis is not needed to start early intervention. Knowing that an infant is at increased risk of neurodevelopmental disorders invokes the need of early intervention (17).
World-wide developmental screening tools are most often used to detect infants with R-ND. Commonly applied methods are caregiver questionnaires [e.g., Parents' Evaluations of Developmental Status (PEDS) (23), Ages and Stages Questionnaire (ASQ) (24)], and the Denver Developmental Screening Test (25). These methods are largely based on attainment of developmental milestones. From the age of 2 years these methods are relatively good in detecting children with developmental delay (26–28). However, their ability to detect children with neurodevelopmental disorders during the first two years is less satisfactory, with sensitivities of 40%–60% and specificities of 59%–77% (29, 30). The most frequently used caregiver questionnaire to detect ASD is the Modified Checklist for Autism in Toddlers [M-CHAT (31)]. In children aged at least 12 months M-CHAT has moderate predictive power in children at increased familial risk of ASD (32).
Five years ago, a systematic review on early prediction of CP indicated that the best methods available for young infants were magnetic resonance imaging (MRI) at term age, and the general movement assessment (GMA) around 3-month post-term (17). In term infants with hypoxic-ischemic encephalopathy, MRI-scans predict CP with sensitivities and specificities of 70%–90% (32). In preterm infants, term-MRI predicts CP with a sensitivity and specificity of 77%–79% (33). GMA is based on the evaluation of the quality of 3 min of general movements in supine. The presence of general movements with seriously reduced movement variation and lacking the age-specific fidgety movements around 3 months post-term predicts CP with a sensitivity and specificity of 91%–98% (16, 34).
The review of Novak et al. (17) also indicated that throughout infancy the Hammersmith Infant Neurological Examination (HINE) is a good instrument to detect CP. It does not only predict CP, but also intellectual disability [Table 2 (39, 40)]. More recently, the Standardized Infant Neurodevelopmental Assessment (SINDA) has been developed. SINDA consists of a neurological, developmental, and socio-emotional scale (36–38). SINDA's neurological scale predicts CP and intellectual disability well; its developmental scale also predicts intellectual disability (Table 2; 15, 32, 45).
This section focusses on early intervention in infants with R-ND during the first two years. Families play a pivotal role in early intervention (41–43). They form the infants' major environment. Also, family members are the key persons impacting child development through daily interaction during caregiving and play. Details of the intervention approach depend in part on the nature of the infant's risk profile. To this end three groups of infants may be distinguished: (a) infants with prenatal, perinatal, or neonatal complications without a significant brain lesion; (b) infants with a significant brain lesion or neurological signs suggestive of such a lesion; and (c) infants at increased familial risk of ASD.
For the first group of infants, many intervention programs are available (44). Ample evidence exists that sensitive and responsive parent-infant interaction and stimulation of infant development are associated with better family well-being and favorable infant development (11, 32, 45).
Less evidence exists on the effective elements of early intervention in infants with a significant brain lesion (32, 45, 46). Nonetheless, available information suggests that the following key elements are beneficial (32, 45, 46): (a) family involvement; (b) focus on the child's activity domain, i.e., on the child's mobility, learning and knowledge, and communication, and not on impairments such as deviant muscle tone or atypical reflexes; (c) early introduction of assistive devices to promote activities and participation and to prevent contractures and deformities; (d) emphasis on activities and participation of family and child (45). Programs that include these elements are Goals Activity Motor Enrichment (GAME) (47, 48), the Small Step Program (49), COPing with and CAring for infants with special needs (COPCA) (50–52), and - for infants at increased risk of unilateral CP - baby constraint-induced movement therapy (baby-CIMT) (53), and intensive bimanual activities (54). These programs aim to challenge children to explore by self-generated movements with trial and error their own body and the physical and social world.
Knowledge on effective intervention in infants at increased risk of ASD is limited as most intervention studies have been performed in children diagnosed with ASD, implying an age of at least 2.5 years (32). Recent systematic reviews (55–59) suggested but did not prove that in children with ASD, a developmental approach with or without behavioral components is associated with a positive effect on social communication. The evidence on the effect of intervention in infants at increased risk of ASD is very limited (55). The data available suggest that a caregiver-mediated social communication intervention may be associated with improved child attention and social communication and better caregiver responsiveness (55, 60, 61).
The rapidly developing brain during infancy imposes challenges for early detection and offers opportunities for early intervention. This is true for high income countries (HICs), but the situation in LMICs is significantly more challenging due to the large number of infants with R-ND in combination with limited resources for early detection and early intervention (62, 63).
Early detection by means of caregiver questionnaires is more cost-effective than that based on testing by professionals. This makes questionnaires (especially PEDS and ASQ) attractive for LMICs despite their less favorable detection properties than assessments by professionals. Nonetheless, barriers such as low caregiver education, illiteracy, and linguistic and cultural diversity may impede general implementation of screening questionnaires (64–67). Assistance by paraprofessional community health workers (CHWs) (68) may reduce these barriers (69) but will increase costs.
The best tools for detection of infants at high risk of neurodevelopmental disorders in the first year post-term are MRI at term, GMA, HINE and SINDA. MRI requires expensive equipment making it less feasible for LMICs. Videorecording of spontaneous movements in GMA is easy and may be performed by caregivers using mobile phones, although educational and linguistic barriers may limit successful recording (70, 71). The latter problem may be solved by videorecording by lay CHWs (68). However, the evaluation of general movement quality requires ample experience, which hampers the implementation of GMA, particularly in LMICs (72, 73). In the future, this situation may change through the application of automated GMA (74–76). Of the best detection tools, HINE and SINDA's neurological scale are the most cost-effective options. HINE and SINDA require the skills of health professionals working in infant health care. Both methods take relatively little time, they do not require an expensive toolkit and they have good predictive properties. HINE covers a larger age range than SINDA. Yet, SINDA's neurological scale has the practical advantages of having a detailed manual and being easier than HINE, as its items and cut-off for “at risk” are independent of infant age (Table 2).
Most early childhood development programs in LMICs focus on health and nutrition in children living in poverty (77). Of course, attention to health and nutrition is quintessential, as health and growth are basic requirements for children to reach their developmental potential. However, the LMIC-literature pays little attention to early intervention in infants at increased risk of neurodevelopmental disorders due to prenatal, perinatal, or neonatal complications, e.g., preterm infants. But it is conceivable that the early intervention strategies that are effective in preterm infants in HICs are also beneficial for preterm infants in LMICs. Actually, the effective strategies to promote development in socially disadvantaged infants in LMICs have large similarities to those applied in preterm infants in HICs (45, 78, 79). Key-elements of both approaches are family involvement, support of caregivers in provision of responsive caregiving, and stimulation of infant development (15, 45, 78, 80). These interventions may be provided by trained lay CHWs to groups of caregivers in the local community with or without home visits by the CHW (81). The home visits may also be replaced by tele-coaching (82). It is conceivable that similar family-community approaches may also work in young children at increased risk of or with ASD. Yet, as described above, evidence on the best intervention approaches in these children is still lacking.
Gradually it is becoming clear which early intervention strategies are beneficial for infants with R-ND due to a significant brain lesion. Essential elements are family involvement, focus on activities and participation of child and family, and prevention of contractures and deformities. Guidance of families with a child with neurodisability is more complex than guidance of families with a preterm infant. It requires more professional effort. Studies performed in LMICs indicate that a combination of caregiver group sessions ran by health professionals in combination with (a) tele-coaching by health professionals and/or (b) home visits by trained lay CHWs may be feasible means to deliver intervention services in infants at increased likelihood of or with neurodevelopmental disorders (82, 83). In the implementation of these early intervention services, it is important to recognize cultural diversity in understanding neurodisability (84). Accordingly, the first steps in early intervention consist of discussing with the family the child's condition, its significance for child, family and community, and the goals of early intervention.
In conclusion, the young brain's neuroplasticity imposes challenges and offers opportunities. It is challenging to detect in the first year infants with R-ND, as the brain needs time to get rid of its temporary structures and to express specific dysfunction. Nonetheless, our hands are not empty: the PEDS, ASQ, HINE and SINDA offer feasible early detection tools for LMICs. Early intervention needs to be geared to the characteristics of child and family. In early intervention for infants with R-ND, the family plays a critical role. In LMICs, families generally are firmly imbedded in the local community, as LMIC-societies function more collectivistic than societies in the individualistic HICs (85). The interdependent societal organization in LMICs may offer specific opportunities for early intervention (84), e.g., through the help of lay CHWs. Cultural integration is a prerequisite for successful early intervention in LMICs (86–88). Adequate early intervention in infants with R-ND will pave the way for school readiness by enhancing attitudes, awareness, knowledge and skills of families and communities, early implementation of assistive devices, and optimizing children's motor, cognitive, communication and socio-emotional skills (1, 8, 45).
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
MH-A is the only author of the paper.
Funding for the open access has been received from Stichting Ontwikkelingsneurofysiologie Groningen - writing of the paper has not been funded. The Stichting Ontwikkelingsneurofysiologie Groningen is small fund that does not have grant numbers.
I gratefully acknowledge the critical and constructive comments of Schirin Ahkbari Ziegler, Roelof Hadders, Bolajoko Olusanya on a previous draft of the manuscript.
MH-A is one of the authors of the manual of the SINDA.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ASD, Autism spectrum disorders; ASQ, Ages and Stages Questionnaire; CHW, Community health worker; CIMT, Constraint-induced movement therapy; COPCA, COPing with and CAring for infants with special needs; CP, Cerebral palsy; EGL, External granular layer; GAME, Goals Activity Motor Enrichment; GMA, General movement assessment; HICs, High-income countries; HINE, Hammersmith Infant Neurological Examination; LMICs, Low- and middle-income countries; M-CHAT, Modified Checklist for Autism in Toddlers; MRI, Magnetic resonance imaging; R-ND, increased biological Risk of or with a Neurodevelopmental Disorder; PEDS, Parents' Evaluations of Developmental Status; PMA, Postmenstrual age; SINDA, Standardized Infant Neurodevelopmental Assessment
1. The Global Research on Developmental Disabilities Collaborators. Accelerating progress on early childhood development for children under 5 years with disabilities by 2030. Lancet Glob Health. (2022) 10:e438–e44. doi: 10.1016/S2214-109X(21)00488-5
2. Olusanya BO, Boo NY, Kraus de Camargo O, Hadders-Algra M, Wertlieb D. Davis A on behalf of the Global Research on Developmental Disabilities Collaborators (GRDDC). repositioning global child health for inclusive education and development. Bull World Health Organ. (2022) 100:459–61. doi: 10.2471/BLT.22.288103
3. Global Research on Developmental Disabilities Collaborators. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet Glob Health. (2018) 6:e1100–e21. doi: 10.1016/S2214-109X(18)30309-7
4. Vawter-Lee M, McGann PT. The increasing global burden of childhood disability: a call for action. Pediatrics. (2020) 146:e20201119. doi: 10.1542/peds.2020-1119
6. Sabatello M, Layden MF. Children with disabilities: achievements, prospects, and challenges ahead. In: Todres J, King SM, editors. The Oxford handbook on children's rights law. Oxford: Oxford University Press, Oxford Handbooks Online (2022). p. 1–21. doi: 10.1093/oxfordhb/9780190097608.013.29
7. United Nations. SDG United Nations Children's Fund (UNICEF). Seen, Counted, Included: Using data to shed light on the well-being of children with disabilities, UNICEF, New York (2021).
8. Smythe T, Almasri N, Moreno Angarita M, Berman BD, Kraus De Camargo O, Hadders-Algra M, et al. The role of parenting interventions in optimizing school readiness for children with disabilities in low and middle income settings. Front Pediatr. (2022) 10:927678. doi: 10.3389/fped.2022.927678
9. Hadders-Algra M. Early human brain development: starring the subplate. Neurosci Biobehav Rev. (2018) 92:276–90. doi: 10.1016/j.neubiorev.2018.06.017
10. Kostović I. The enigmatic fetal subplate compartment forms an early tangential cortical nexus and provides the framework for construction of cortical connectivity. Prog Neurobiol. (2020) 194:101883. doi: 10.1016/j.pneurobio.2020.101883
11. Eyre JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev. (2007) 31:1136–49. doi: 10.1016/j.neubiorev.2007.05.011
12. Hadders-Algra M. Neurodevelopmental mechanisms in early life. In: Hadders-Algra M, editors. Early detection and early intervention in developmental motor disorders – from neuroscience to participation. London: Mac Keith Press (2021). p. 25–38.
13. Hadders-Algra M. Two distinct forms of minor neurological dysfunction: perspectives emerging from a review of data of the Groningen Perinatal Project. Dev Med Child Neurol. (2002) 44:561–71. doi: 10.1017/s0012162201002560
14. Straathof EJM, Hamer EG, Hensens KJ, La Bastide-van Gemert S, Heineman KR, Hadders-Algra M. Development of muscle tone impairments in high-risk infants: associations with cerebral palsy and cystic periventricular leukomalacia. Eur J Paediatr Neurol. (2022) 37:12–8. doi: 10.1016/j.ejpn.2021.12.015
15. Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst Rev. (2015) 2015:CD005495. doi: 10.1002/14651858.CD005495.pub4
16. Hadders-Algra M. Neural substrate and clinical significance of general movements: an update. Dev Med Child Neurol. (2018) 60:39–46. doi: 10.1111/dmcn.13540
17. Novak I, Morgan C, Adde L, Blackman J, Boyd RN, Brunstrom-Hernandez J, et al. Early accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. (2017) 171:897–907. doi: 10.1001/jamapediatrics.2017.1689
18. Pascal A, Govaert P, Ortibus E, Naulaers G, Lars A, Fjørtoft T, et al. Motor outcome after perinatal stroke and early prediction of unilateral spastic cerebral palsy. Eur J Paediatr Neurol. (2020) 29:54–61. doi: 10.1016/j.ejpn.2020.09.002
19. Ryll UC, Krumlinde-Sundholm L, Verhage CH, Sicola E, Sgandurra G, Bastiaenen CH, et al. Predictive validity of the Hand Assessment for Infants in infants at risk of unilateral cerebral palsy. Dev Med Child Neurol. (2021) 63:436–43. doi: 10.1111/dmcn.14739
20. Hadders-Algra M. Emerging signs of Autism Spectrum Disorder in infancy: putative neural substrate. Dev Med Child Neurol. (2022), (2022) 64:1344–50. doi: 10.1111/dmcn.15333
21. Granild-Jensen JB, Rackauskaite G, Flachs EM, Uldall P. Predictors for early diagnosis of cerebral palsy from national registry data. Dev Med Child Neurol. (2015) 57:931–5. doi: 10.1111/dmcn.12760
22. Van‘t Hof M, Tisseur C, van Berckelear-Onnes I, van Nieuwenhuyzen A, Daniels AM, Deen M, Hoek HW, et al. Age at autism spectrum disorder diagnosis: a systematic review and meta-analysis from 2012 to 2019. Autism. (2021) 25:862–73. doi: 10.1177/1362361320971107
23. Glascoe FP, Robertshaw NS. PEDS’ developmental milestones a tool for surveillance and screening professionals manual. 2nd ed. Nashville, TN: Ellsworth / Vandermeer Press, LLC (2010). 177.
24. Squires J, Bricker D. Ages / stages questionnaires®, third edition (ASQ- 3™). A parent-completed child-monitoring system. Baltimore, MD: Paul H. Brookes Publishing Co (2009). 191.
25. Frankenburg WK. Denver II: Technical manual. Denver, CO: Denver Developmental Materials (1996). 89.
26. Glascoe FP. Screening for developmental and behavioral problems. Ment Retard Dev Disabil Res Rev. (2005) 11:173–9. doi: 10.1002/mrdd.20068
27. Limbos MM, Joyce DP. Comparison of the ASQ and PEDS in screening for developmental delay in children presenting for primary care. J Dev Behav Pediatr. (2011) 32:499–511. doi: 10.1097/DBP.0b013e31822552e9
28. Fauls JR, Thompson BL, Johnston LM. Validity of the Ages and Stages Questionnaire to identify young children with gross motor difficulties who require physiotherapy assessment. Dev Med Child Neurol. (2020) 62:837–44. doi: 10.1111/dmcn.14480
29. Agarwal PK, Shi L, Daniel LM, Yang PH, Khoo PC, Quek BH, et al. Prospective evaluation of the Ages and Stages Questionnaire 3rd Edition I very low-birthweight infants. Dev Med Child Neurol. (2017) 59:484–9. doi: 10.1111/dmcn.13307
30. Rubio-Codina M, Araujo MC, Attanasio O, Muñoz P, Grantham-McGregor S. Concurrent validity and feasibility of short tests currently used to measure early childhood development in large scale studies. PLoS One. (2016) 11:e0160962. doi: 10.1371/journal.pone.0160962
31. Robins DL, Fein D, Barton ML, Green JA. The Modified Checklist for Autism in Toddlers: An initial study investigating the early detection of autism and pervasive developmental disorders. J Autism Dev Disord. (2001) 31:131–44. doi: 10.1023/a:1010738829569
32. Hadders-Algra M. Early diagnostics and early intervention in neurodevelopmental disorders – age-dependent challenges and opportunities. J Clin Med. (2021) 10:861. doi: 10.3390/jcm10040861
33. Van’t Hooft J, van der Lee JH, Opmeer BC, Ensing S, Kwee A, Mol BW. Predicting developmental outcomes in premature infants by term equivalent MRI: systematic review and meta-analysis. Syst Rev. (2015) 4:71. doi: 10.1186/s13643-015-0058-7
34. Bosanquet M, Copeland L, Ware R, Boyd R. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol. (2013) 55:418–26. doi: 10.1111/dmcn.12140
35. Majnemer A, Snider L, Hadders-Algra M. Assessment of infants and toddlers. In: Hadders-Algra M, editors. Early detection and early intervention in developmental motor disorders – from neuroscience to participation. London: Mac Keith Press (2021). p. 144–70.
36. Hadders-Algra M, Tacke U, Pietz J, Rupp A, Philippi H. Reliability and predictive validity of the standardized infant NeuroDevelopmental assessment neurological scale. Dev Med Child Neurol. (2019) 61:654–60. doi: 10.1111/dmcn.14045
37. Hadders-Algra M, Tacke U, Pietz J, Rupp A, Philippi H. Standardized infant NeuroDevelopmental assessment developmental and socio-emotional scales: reliability and predictive value in an at-risk population. Dev Med Child Neurol. (2020) 62:845–53. doi: 10.1111/dmcn.14423
38. Hadders-Algra M, Tacke U, Pietz J, Philippi H. SINDA: standardized infant NeuroDevelopmental assessment. An instrument for early detection of neurodevelopmental disorders. London: Mac Keith Press (2022). 184.
39. Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. (2016) 58:240–45. doi: 10.1111/dmcn.12876
40. Romeo DM, Cowan FM, Haataja L, Ricci D, Pede E, Gallini F, et al. Hammersmith Infant Neurological Examination for infants born preterm: predicting outcomes other than cerebral palsy. Dev Med Child Neurol. (2020) 64:871–80. doi: 10.1111/dmcn.14768
41. Rosenbaum P, King S, Law M, King G, Evans J. Family-centred services: a conceptual framework and research review. Phys Occup Ther Pediatr. (1998) 18:1–20. doi: 10.1300/J006v18n01_01
42. Law M, Teplicky R, King S, King G, Kertoy M, Moning T, et al. Family-centred service: moving ideas into practice. Child Care Health Dev. (2005) 31:633–42. doi: 10.1111/j.1365-2214.2005.00568.x
43. Akhbari Ziegler S, Dirks T, Hadders-Algra M. Coaching in early physical therapy intervention: the COPCA program as an example of translation of theory into practice. Disabil Rehabil. (2019) 41:1846–54. doi: 10.1080/09638288.2018.1448468
44. Puthussery S, Chutiyami M, Tseng PC, Kilby L, Kapadia J. Effectiveness of early intervention programs for parents of preterm infants: a meta-review of systematic reviews. BMC Pediatr. (2018) 18:223. doi: 10.1186/s12887-018-1205-9
45. Hadders-Algra M. Early intervention in the first 2 years post-term. In: Hadders-Algra M, editors. Early detection and early intervention in developmental motor disorders – from neuroscience to participation. London: Mac Keith Press. (2021). p. 198–227.
46. Morgan C, Fetters L, Adde L, Badawi N, Bancale A, Boyd RN, et al. Early intervention for children aged 0 to 2 years with or at high risk of cerebral palsy: international clinical practice guideline based on systematic reviews. JAMA Pediatr. (2021) 175:846–58. doi: 10.1001/jamapediatrics.2021.0878
47. Morgan C, Novak I, Dale RC, Badawi N. Optimising motor learning in infants at high risk of cerebral palsy: a pilot study. BMC Pediatr. (2015) 15:30. doi: 10.1186/s12887-015-0347-2
48. Morgan C, Novak I, Dale RC, Guzzetta A, Badawi N. Single blind randomised controlled trial of GAME (Goals–Activity – Motor Enrichment) in infants at high risk of cerebral palsy. Res Dev Disabil. (2016) 55:256–67. doi: 10.1016/j.ridd.2016.04.005
49. Holmström L, Eliasson AC, Almeida R, Furmark C, Weiland AL, Tedroff K, et al. Efficacy of the small step program in a randomized controlled trial for infants under 12 months old at risk of cerebral palsy (CP) and other neurological disorders. J Clin Med. (2019) 8:pii: E1016. doi: 10.3390/jcm8071016
50. Hielkema T, Blauw-Hospers CH, Dirks T, Drijver-Messelink M, Bos AF, Hadders-Algra M. Does physiotherapeutic intervention affect motor outcome in high-risk infants? An approach combining a randomized controlled trial and process evaluation. Dev Med Child Neurol. (2011) 53:e8–e15. doi: 10.1111/j.1469-8749.2010.03876.x
51. Blauw-Hospers CH, Dirks T, Hulshof LJ, Bos AF, Hadders-Algra M. Pediatric physical therapy in infancy: from nightmare to dream? A two-arm randomized trial. Phys Ther. (2011) 91:1323–38. doi: 10.2522/ptj.20100205
52. Hielkema T, Boxum AG, Hamer EG, La Bastide-Van Gemert S, Dirks T, Reinders-Messelink HA, et al. LEARN2MOVE 0-2 years, a randomized early intervention trial for infants at very high risk of cerebral palsy: family outcome and infant's Functional outcome. Disabil Rehabil. (2020) 42:3762–70. doi: 10.1080/09638288.2019.1610509
53. Eliasson AC, Nordstrand L, Ek L, Lennartsson F, Sjöstrand L, Tedroff K, et al. The effectiveness of Baby-CIMT in infants younger than 12 months with clinical signs of unilateral cerebral palsy; an explorative study with randomized design. Res Dev Disabil. (2018) 72:191–201. doi: 10.1016/j.ridd.2017.11.006
54. Chamudot R, Parush S, Rigbi A, Horovitz R, Gross-Tsur V. Effectiveness of modified constraint-induced movement therapy compared with bimanual therapy home programs for infants with hemiplegia: a randomized controlled trial. Am J Occup Ther. (2018) 72:p1–p7206205010. doi: 10.5014/ajot.2018.025981
55. French L, Kennedy EMM. Annual research review: early intervention for infants and young children with, or at-risk of, autism spectrum disorder: a systematic review. J Child Psychol Psychiatry. (2018) 59:444–56. doi: 10.1111/jcpp.12828
56. Sandbank M, Bottema-Beutel K, Crowley S, Tacke U, Gerstl L, Hilgendorff A, et al. Project AIM: autism intervention meta-analysis for studies of young children. Psychol Bull. (2020) 146:1–29. doi: 10.1037/bul0000215
57. Reichow B, Hume K, Barton EE, Boyd BA. Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD). Cochrane Database Syst Rev. (2018) 5:CD009260. doi: 10.1002/14651858.CD009260.pub3
58. Fuller EA, Kaiser AP. The effects of early intervention on social communication outcomes for children with autism spectrum disorder: a meta-analysis. J Autism Dev Disord. (2020) 50:1683–700. doi: 10.1007/s10803-019-03927-z
59. Rodgers M, Marshall D, Simmonds M. Interventions based on early intensive applied behaviour analysis for autistic children: a systematic review and cost-effectivenesss analysis. Health Technol Assess. (2020) 24:1–306. doi: 10.3310/hta24350
60. Green J, Pickles A, Pasco G, Bedford R, Wan MW, Elsabbagh M, et al. Randomised trial of a parent-mediated intervention for infants at high risk for autism: longitudinal outcomes to age 3 years. J Child Psychol Psychiatry. (2017) 58:1330–40. doi: 10.1111/jcpp.12728
61. Brian J, Drmic I, Roncadin C, Dowds E, Shaver C, Smith IM, et al. Effectiveness of a parent-mediated intervention for toddlers with autism-spectrum disorder: evidence form a large community implementation. Autism. (2022) 26:1882–97. doi: 10.1177/13623613211068934
62. Olusanya BO, Gladstone M, Wright SM, Hadders-Algra M, Boo NY, Nair MKC, et al. Cerebral palsy and developmental intellectual disability in children younger than 5 years: findings from the GBD-WHO Rehabilitation Database 2019. Front Public Health. (2022) 10:894546. doi: 10.3389/fpubh.2022.894546
63. Almasri NA, Smythe T, Hadders-Algra M, Olusanya BO, On behalf of the Global Research on Developmental Disabilities Collaborators (GRDDC). Prioritising rehabilitation in early childhood for inclusive education: a call to action. Disabil Rehabil. (2022) 1–5. doi: 10.1080/09638288.2022.2118870
64. Overs BJ, Woolfenden S, Williams K, Jalaludin B, Axelsson EL, Dissanayake C, et al. Predictors of developmental surveillance completion at six months of age in south western Sydney. Child Care Health Dev. (2017) 43:307–15. doi: 10.1111/cch.12425
65. Marlow M, Servili C, Tomlinson M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: recommendations for use in low- and middle-income countries. Autism Res. (2019) 12:176–99. doi: 10.1002/aur.2033
66. Colbert AM, Connery AK, Lamb MM, Bauer D, Olson D, Paniagua-Avila A, et al. Caregiver rating of early childhood developmental: reliability and validity of the AS!-3 in rural Guatamala. Early Hum Dev. (2021) 161:105453. doi: 10.1016/j.earlhumdev.2021.105453
67. Boggs D, Milner KM, Chandna J, Black M, Cavallera V, Dua T, et al. Rating early child development outcome measurement tools for routine health programme use. Arch Dis Child. (2019) 104(Suppl 1):S22–33. doi: 10.1136/archdischild-2018-315431
68. Olaniran A, Smith H, Unkels R, Bar-Zeev S, van den Broek N. Who is a community health worker? – A systematic review of definitions. Glob Health Action. (2017) 10:1272223. doi: 10.1080/16549716.2017.1272223
69. Sadoo S, Nalugya R, Lassman R, Kohli-Lynch M, Chariot G, Davies HG, et al. Early detection and intervention for young children with ealy developmental disabilities in Western Uganda: a mixed-methods evaluation. BMC Pediatr. (2022) 22:158. doi: 10.1186/s12887-022-03184-7
70. Adde L, Brown A, van den Broeck C, DeCoen K, Eriksen BH, Fjørtoft T, et al. In-Motion-App for remote General Movement Assessment: a multi-site observational study. BMJ Open. (2021) 11:e042147. doi: 10.1136/bmjopen-2020-042147
71. Kwong AKL, Eeles AL, Olsen JE, Zannino D, Kariotis T, Spittle AJ. The Baby Moves smartphone app for General Movement Assessment: Engagement amongst extremely preterm and term-born infants in a state-wide geographical study. J Paediatr Child Health. (2019) 55:548–54. doi: 10.1111/jpc.14240
72. Datta AN, Furrer MA, Bernhardt I, Hüppi PS, Borradori-Tolsa C, Bucher HU, et al. Fidgety movements in infants born very preterm; predictive value for cerebral palsy in a clinical multicentre setting. Dev Med Child Neurol. (2017) 59:618–24. doi: 10.1111/dmcn.13386
73. Spittle AJ, Hadders-Algra M. Assessments in the neonatal period and early infancy. In: Hadders-Algra M, editors. Early detection and early intervention in developmental motor disorders – from neuroscience to participation. London: Mac Keith Press (2021). p. 124–43.
74. Marchi V, Hakala A, Knight A, D'Acunto F, Scattoni ML, Guzzetta A, et al. Automated pose estimation captures key aspects of general movements at eight to 17 weeks from conventional videos. Acta Paediatr. (2019) 108:1817–24. doi: 10.1111/apa.14781
75. Schroeder AS, Hesse N, Weinberger R, Tacke U, Gerstl L, Hilgendorff A, et al. General Movement Assessment from videos of computed 3D infant body models is equally effective compared to conventional RGB video rating. Early Hum Dev. (2020) 144:104967. doi: 10.1016/j.earlhumdev.2020.104967
76. Raghuram K, Orlandi S, Church P, Chau T, Uleryk E, Pechlivanoglou P, et al. Automated movement recognition to predict motor impairment in high-risk infants: a systematic review of diagnostic test accuraty and meta-analysis. Dev Med Child Neurol. (2021) 63:637–48. doi: 10.1111/dmcn.14800
77. Milner KM, Bhopal S, Black M, Dua T, Gladstone M, Hamadani J, et al. Counting outcomes, coverage and quality for early child development programmes. Arch Dis Child. (2019) 104(Suppl 1):S13–21. doi: 10.1136/archdischild-2018-315430
78. Jeong J, Franchett EE, Ramos de Oliveira CV, Rehmani K, Yousafzai AK. Parenting interventions to promote early child development in the first three years of life: a global systematic review and meta-analysis. PLoS Med. (2021) 18:e1003602. doi: 10.1371/journal.pmed.1003602
79. Zhang L, Ssewanyana D, Martin MC, Lye S, Moran G, Abubakar A, et al. Supporting child development through parenting interventions in low- to middle-income countries: an updated systematic review. Front Public Health. (2021) 9:671988. doi: 10.3389/fpubh.2021.671988
80. Prime H, Andrews K, McTavish J, Harris M, Janus M, Bennett T, et al. The application of positive parenting interventions to academic school readiness: a scoping review. Child Care Health Dev. (2021) 47:1–14. doi: 10.1111/cch.12810
81. Lopez Garcia I, Saya UY, Luoto JE. Cost-effectiveness and economic returns of group-based parenting interventions to promote early childhood development: results from a randomized controlled trial in rural Kenya. PLoS Med. (2021) 18:e1003746. doi: 10.1371/journal.pmed.1003746
82. Akhbari Ziegler S, de Souza Morais RL, Magalhães L, Hadders-Algra M. The potential of COPCA's Coaching for families with infants with special needs in low- and middle-income countries. Front Pediatr. (2022).
83. Karim T, Muhit M, Jahan I, Galea C, Morgan C, Smithers-Sheedy H, et al. Outcome of community-based early intervention and rehabilitation for children with cerebral palsy in rural Bangladesh: a quasi-experimental study. Brain Sci. (2021) 11:1189. doi: 10.3390/brainsci11091189
84. Bannink Mbazzi F, Kawesa ES. ‘Impairments of the brain’: Global South perspectives on childhood neurodevelopmental disability. Dev Med Child Neurol. (2022) 64:1193–1201. doi: 10.1111/dmcn.15253
85. Wu S, Keysar B. The effect of culture on perspective taking. Psychol Sci. (2007) 18:600–06. doi: 10.1111/j.1467-9280.2007.01946.x
86. Cavallera V, Tomlinson M, Radner J, Coetzee B, Daelmans B, Hughes R, et al. Scaling early child development: what are the barriers and enablers? Arch Dis Child. (2019) 104(Suppl 1):S43–50. doi: 10.1136/archdischild-2018-315425
87. Sapiets SJ, Totsika V, Hastings RP. Factors influencing access to early intervention for families of children with developmental disabilities: a narrative review. J Appl Res Intellect Disabil. (2021) 34:695–711. doi: 10.1111/jar.12852
88. Mwangi LW, Abuga JA, Cottrell E, Kariuki SM, Kinyanjui SM, Newton CR. Barriers to access and utilization of healthcare by children with neurological impairments and disability in low-and middle-income countries: a systematic review. Wellcome Open Res. (2022) 6:61. doi: 10.12688/wellcomeopenres.16593.2
Keywords: brain development, cortical subplate, infant, early detection, early intervention, neurodevelopmental disorders, cerebral palsy, low and middle income countries
Citation: Hadders-Algra M (2022) The developing brain: Challenges and opportunities to promote school readiness in young children at risk of neurodevelopmental disorders in low- and middle-income countries. Front. Pediatr. 10:989518. doi: 10.3389/fped.2022.989518
Received: 8 July 2022; Accepted: 27 September 2022;
Published: 21 October 2022.
Edited by:
Gerry Leisman, University of Haifa, IsraelReviewed by:
Ana Elisa Toscano, Federal University of Pernambuco, Brazil© 2022 Hadders-Algra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mijna Hadders-Algra bS5oYWRkZXJzLWFsZ3JhQHVtY2cubmw=
Specialty Section: This article was submitted to Children and Health, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.