
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 16 September 2022
Sec. Pediatric Immunology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.988645
Ataxia-telangiectasia (A-T) is a syndromic inborn error of immunity (IEI) characterized by genomic instability, defective reparation of the DNA double-strand breaks, and hypersensitivity to ionizing radiation disturbing cellular homeostasis. The role of imaging diagnostics and the conscious choice of safe and advantageous imaging technique, as well as its correct interpretation, are crucial in the diagnostic process and monitoring of children with A-T. This study aimed at defining the role of a radiologist in the early diagnosis of A-T, as well as in detecting and tracking disease complications associated with infections, inflammation, lymphoproliferation, organ-specific immunopathology, and malignancy. Based on our single-center experience, retrospective analysis of investigations using ionizing radiation-free techniques, ultrasound (US), and Magnetic Resonance Imaging (MRI), was performed on regularly followed-up 11 pediatric A-T patients, 6 girls and 5 boys, aged from 2 to 18 years, with the longest period of observation coming to over 13 years. Our attention was especially drawn to the abnormalities that were observed in the US and MRI examinations of the lungs, abdominal cavity, and lymph nodes. The abdominal US showed no abnormalities in organ dimensions or echostructure in 4 out of 11 children studied, yet in the other 7, during follow-up examinations, hepato- and/or splenomegaly, mesenteric, visceral, and paraaortic lymphadenopathy were observable. In 2 patients, focal changes in the liver and spleen were shown, and in one patient progressive abdominal lymphadenopathy corresponded with the diagnosis of non-Hodgkin lymphoma (NHL). The lung US revealed multiple subpleural consolidations and B line artifacts related to the interstitial-alveolar syndrome in 5 patients, accompanied by pleural effusion in one of them. The MRI investigation of the lung enabled the detection of lymphatic nodal masses in the mediastinum, with concomitant airway lesions characteristic of bronchiectasis and focal parenchymal consolidations in one A-T patient with chronic respiratory failure. This patient also manifested organomegaly and granulomatous liver disease in abdominal MRI examination. Our study shows that the use of modern US capabilities and MRI is safe and efficient, thereby serving as a recommended advantageous imaging diagnostic tool in monitoring children with IEI and DNA instability syndromes.
Ataxia-telangiectasia (A-T) is a syndromic inborn error of immunity (IEI) characterized by genomic instability, defective response to genotoxic factors, impaired reparation of the DNA double-strand breaks, and hypersensitivity to ionizing radiation. A dysregulation of ataxia-telangiectasia mutated (ATM) protein kinase nuclear and cellular functions underpinning the pathophysiology of the disease are related to the defective generation of reactive oxygen species, mitochondrial dysfunctions, alterations in transcription and splicing, and impaired cellular protein homeostasis (1, 2). The multiplicity of ATM nuclear and cellular activities maintaining homeostasis are reflected in the constellation of phenotypic features of A-T.
A-T is a complex, multisystemic disease at the interface of immunodeficiency, infections, autoimmunity, autoinflammation, lymphoproliferation, and malignancy. The leading A-T symptomatology is characterized by neurodegeneration and progressively debilitating cerebellar ataxia with postural instability, oculomotor apraxia, dysarthria, and orolingual insufficiency, as well as extrapyramidal dysfunctions with choreoathetotic movements, dystonia, and muscle tremor. Affected children suffer from chronic rhinosinusitis, obstructive airway disease, bronchiectasis, and interstitial lung disease or pneumonia with fibrosis. The respiratory disease is exacerbated by dysfunctional swallowing, gastroesophageal reflux, aspiration episodes, and ineffective coughing (3, 4). An impaired response to oxidative stress may have a causal relationship with chronic non-alcoholic fatty liver disease in pediatric patients with A-T (5, 6). The extended A-T phenotype also includes hormonal dysfunctions, such as growth hormone deficiency, gonadal failure, and diabetes (7, 8), cutaneous and systemic laryngeal, pulmonary, and hepatosplenic granulomatosis (9–11). A-T is, therefore, a multisystemic devastating disease, burdened with a high rate of malignant transformation, significantly reducing life expectancy (12). While the monitoring of the clinical course of A-T in children relies on a multidisciplinary team of a pediatrician, neurologist, endocrinologist, pulmonologist, gastroenterologist, and hematologist under the pediatric immunologist’s supervision (13, 14), the radiologist’s role in diagnosing and following-up of affected patients cannot be overestimated. The A-T patient group is affected with a severe IEI with susceptibility to ionizing radiation, thus the choice of an adequate, safe, and providing best answer to the clinician’s requests imaging diagnostic technique is crucial for the patient’s monitoring.
According to the expert guidelines (13, 15, 16) application of ionizing radiation-free imaging techniques for the assessment of the respiratory tract should be accompanied by regular lung function monitoring aimed at early detection of lung disease progression and indicating the need for therapeutic interventions.
In this study, we aimed at defining the role of a radiologist in the early diagnosis of A-T, as well as in detecting and tracking disease complications associated with infections, inflammation, lymphoproliferation, organ-specific immunopathology, and malignancy. Based on our single-center experience, we also tried to find out whether investigations using ionizing radiation-free techniques, ultrasound (US), and Magnetic Resonance Imaging (MRI), as these techniques require no ionizing radiation and therefore, can be an elegant alternative to X-ray or CT imaging.
We retrospectively reviewed medical records of 11 children with A-T, 6 girls and 5 boys, aged from 2 to 18 years, who had been diagnosed and treated in our university pediatric tertiary care center. All the children studied were regularly monitored in the pediatric immunology unit and simultaneously, followed up in the pediatric radiology department. The mean age of the definitive A-T diagnosis, the age of the first diagnostic imaging examination, the technique that had been used, as well as the time of observation, were investigated. An in-depth analysis of findings observable in imaging of the lungs, abdominal cavity, lymph nodes, and due to special clinical indications, also in the neck with larynx, and joints was performed. The US and MRI examinations were performed during follow-up visits and additionally, during exacerbations of chronic symptoms or acute pathology.
The basic US examination protocol included the lungs (transthoracic US), peripheral lymph nodes, and abdominal cavity, with the latter supplied by analysis of vascular flow in Color Doppler (CD). Currently, superb microvascular imaging (SMI) which enables visualization of low microvascular flow, as well as elastography using low-frequency vibrations to show elasticity/stiffness of the organ, are also included in the protocol of the US examination of the abdominal cavity. The US of the abdominal cavity and peripheral lymph nodes were conducted in a supine position, while the lung US was done in a sitting patient.
The chest US examination protocol was implemented according to the following standard: the examination was performed using a linear probe of 5–12 mHz (L12-5) frequency and, depending on the patient’s age, with either a convex probe of 1–5 mHz (C5-1) frequency, a convex probe of 4–9 mHz (C9-4) frequency or a microconvex probe of 5–8 mHz (C8-5) frequency through longitudinal and transverse sections of the anterior, lateral, and posterior walls of the chest. The preliminary preset was soft tissue, excluding artifact reduction options (SonoCT, XRes). Doppler imaging was used for the evaluation of vascularization of the inflammatory changes. The chest US examination was performed by applying the probe to the anterior, lateral, and posterior surfaces of the chest. Transverse sections of the chest wall were obtained by the transverse application of the probe and scanning the whole available area in the craniocaudal direction. Longitudinal sections were obtained by applying the probe along the parasternal line, the midclavicular line, the anterior axillary line, the midaxillary line, the posterior axillary line, the scapular line, and the paravertebral line moving the probe along the intercostal spaces. In every patient the following elements were evaluated: the quality (free flowing or organized, localization) and quantity (fluid layer in millimeters) of any fluid present in the pleural space, the shape and thickness of the pleural line, the lung sliding sign, A-lines and B-lines artifacts (their number, localization, and morphology, including single ones as well as “lung rockets” complexes and “white lung” images) and alveolar consolidations (their number, dimensions, localization, morphology, presence of bronchogram and its characteristic (air or fluid) and vascularization).
The diagnostic monitoring protocol also included MRI in A-T patients to monitor the liver and spleen pathology. This technique allows to achieve high resolution and precision of focal lesions analysis due to diffusion-weighted and susceptibility-weighted imaging (DWI and SWI, respectively) or fat saturation sequences. The chest MRI protocol implemented in the patients studied has been displayed in Table 1.
Eleven pediatric patients with a genetically confirmed diagnosis of A-T, aged from 2 to 18 years, were included in the study group. The mean age of initial diagnosis of A-T was between 3 and 4 years. All the patients studied had the classical form of A-T with the early onset disease as defined by Driessen et al. (17), neurodegeneration and progressive cerebellar ataxia, severe antibody deficiency with impaired B- and T-cell homeostasis, remarkably increased alpha-fetoprotein (AFP) levels and recurrent respiratory tract infections. Besides infectious complications, immune dysregulation in the form of autoimmunity was also a frequent sequela among the A-T children studied, with autoimmune hemolytic anemia diagnosed in 4 out of 11 of them. Malignancy, in the form of non-Hodgkin lymphoma (NHL), was diagnosed in one patient. Two patients deceased due to liver failure and a chronic Epstein–Barr virus (EBV) infection. Whereas hypogammaglobulinemia, with low serum IgG and IgG2 levels in 9 out of 11 patients, immunoglobulin replacement therapy (Ig-RT) has been implemented, either as a hospital-based intravenous (IVIg) or home-based subcutaneous (SCIg) treatment. The youngest participant had his first diagnostic imaging examination 5 months before the final diagnosis was established. The longest period of observation in one patient was 13 years, from the diagnosis of A-T at the age of 5 years to his death. Other A-T patients have been monitored in our pediatric radiology department for a period from 2 to 10 years. The basic demographic data of the A-T children studied are displayed in Table 2.
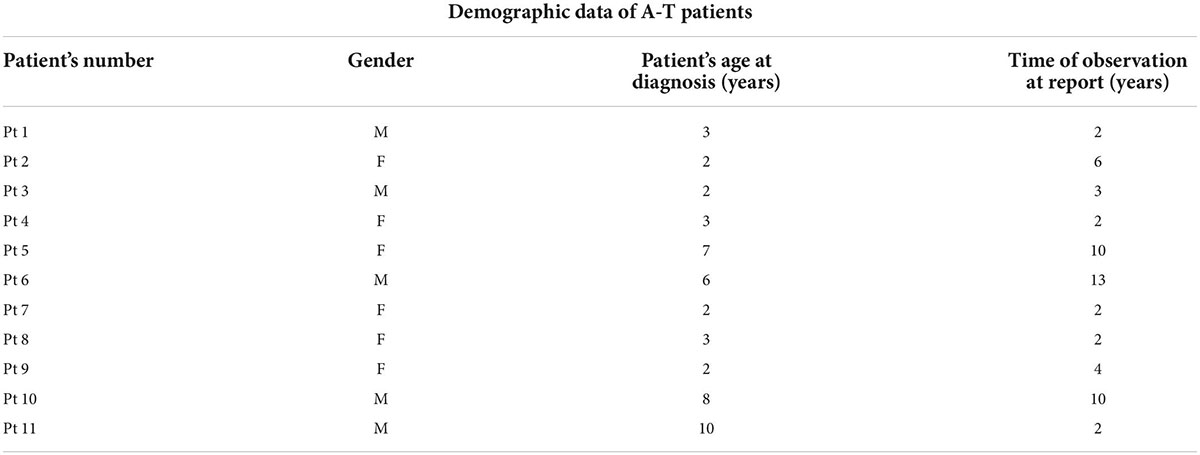
Table 2. Demographic data of ataxia-telangiectasia (A-T) patients and the time of observation by a pediatric radiologist.
All participants of the study underwent an abdominal US examination. The most common reasons for the referral were searching for the infection outbreak site, and non-infectious disease sequelae, such as lymphoproliferation, lymphadenopathy, liver disease, and granulomatosis. In only 4 patients observed over time, there were no abnormalities in terms of organ measurements and their echostructure. In the other 7 patients, the sonographic image of the abdomen was normal in the early stage of the disease. Then, during follow-up examinations, hepato- or splenomegaly as well as mesenteric, visceral, and paraaortic lymph node enlargement was diagnosed. Besides, in two patients, focal changes in the liver and spleen were observed.
Cervical, submandibular, and supraclavicular lymph nodes size and echostructure were also investigated and abnormalities were detected in 5 patients. In one female patient, in whom lymphadenopathy in the form of pathological lymph node echostructure with dense hilum, decreased echogenicity and change of shape were described, and in the follow-up examination enlargement of mediastinal and supraclavicular lymph nodes was revealed, the diagnosis of NHL was established. Other abnormalities in A-T patients included a change of lymph node shape from oval to round and decreased echogenicity. The pathological transformation of a cervical lymph node which requires a pediatrician’s awareness due to its possible malignant, infectious, or immunological etiology and thus needs further diagnostic procedures, is shown in Figure 1. No pathological vascular flow was diagnosed in the CD examination, apart from one case, in which a hilum overlay was accompanied by abnormal peripheral flow in the lymph nodes.
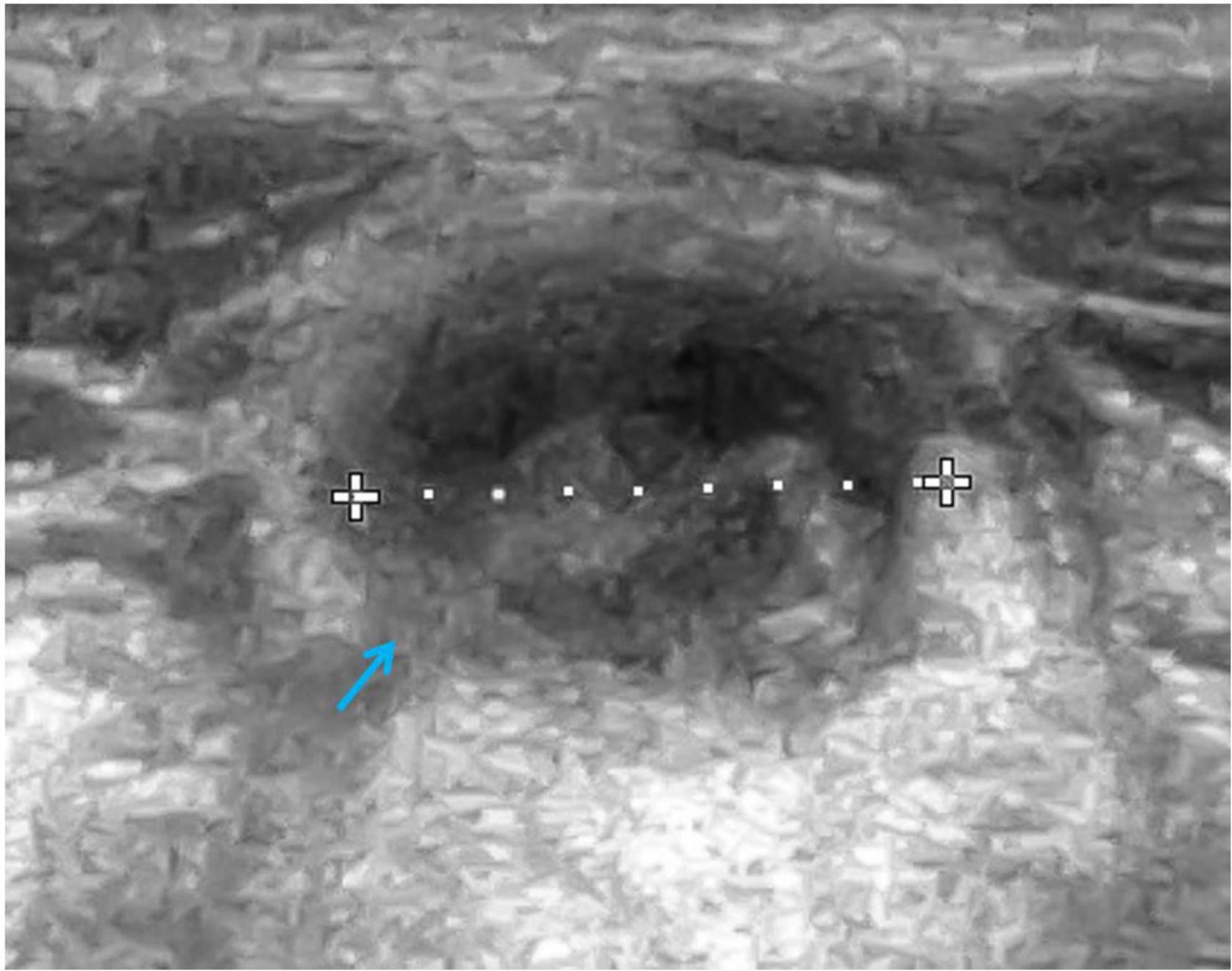
Figure 1. Ultrasound (US) examination of the lymph node, linear probe. A cervical lymph node with an abnormal echostructure, heterogeneous hypoechogenicity, and change of a shape from oval to round (marked with an arrow).
In 5 out of our 11 A-T patients who underwent lung US examination, multiple, partially merging subpleural consolidations and numerous B line artifacts were detected, corresponding with the interstitial-alveolar syndrome. In two cases, pleural effusion was found. The lung US images of consolidations and pleural effusion are shown in Figures 2A,B, respectively. The summary of the abdominal, lymph node, and lung US findings detected and monitored in the A-T children studied, has been displayed in Table 3.
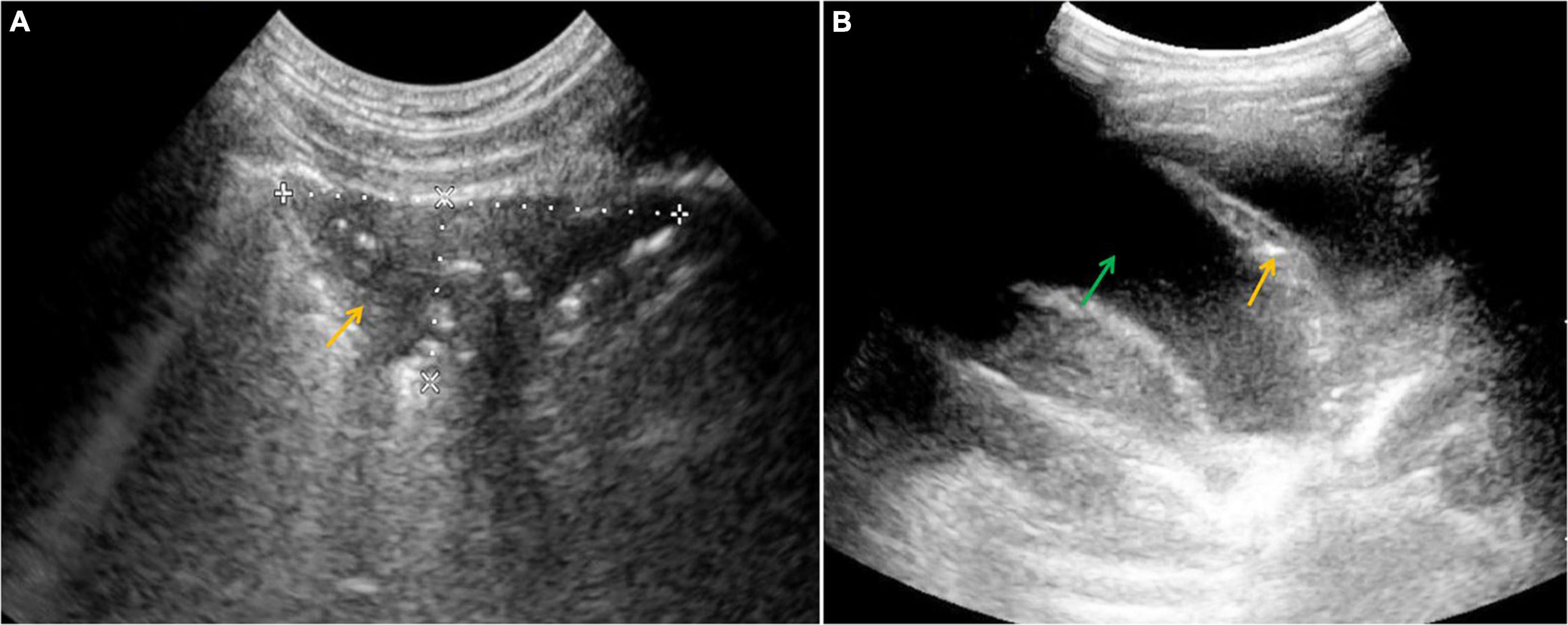
Figure 2. Ultrasound (US) examination of the lung, convex probe, (A) an area of subpleural consolidation (marked with an arrow), (B) pleural effusion (a green arrow shows the fluid and an orange one shows atelectasis).

Table 3. Findings detected in the abdominal cavity, peripheral lymph node, and lung ultrasound (US) in ataxia-telangiectasia (A-T) children.
In 6 children, before establishing the definitive A-T diagnosis, chest X-rays (CXR) were performed due to the suspicion of pneumonia. In 5 of them, consolidations characteristic of respiratory tract infection were detected. Either sign of atelectasis or emphysema were not observable, yet in one patient CXR image suggested bronchiectasis. No chest CT examinations were conducted due to the need for extended diagnostics and instead, lung MRI was proposed.
The efficacy of lung MRI has been increasingly highlighted due to the need for special ionizing radiation-free care that patients with IEI and defective DNA reparation should receive. In the study group, MRI revealed lymphatic nodal masses in the mediastinum, airway lesions characteristic of bronchiectasis, as well as parenchymal consolidations. Moreover, in the female patient with abnormal lymph node image of the neck, MRI enabled the assessment of their enhancement after contrast dose administration. MR images of lymphadenopathy and bronchiectasis are shown in Figures 3A–C. The diagnostic monitoring protocol also included MRI in A-T patients to monitor the liver and spleen pathology. The findings detected in MRI examinations in our A-T patients studied, are summarized in Table 4. The MRI examination of the brain was performed on one A-T patient with NHL and it did not reveal any abnormal imaging features. The majority of the examinations in children affected with A-T were performed without sedation on free or held breath. One A-T patient with the end-stage of the disease, chronic respiratory failure, and abdominal organomegaly, in whom MRI was made because of granulomatous lesions in the abdomen, which is displayed in Figure 4, required an anesthesiologist’s assistance.
Figure 3. Magnetic resonance imaging (MRI) examination of the chest. (A) T2 -weighted Short-TI Inversion Recovery (STIR) axial image showing enlarged perivascular lymph nodes (marked with arrows), (B) coronal image—enlarged cervical lymph nodes (marked with an arrow). (C) T2-weighted blade axial image—bronchiectasis (marked with arrows) in upper lobes of both lungs, more severe on the right side.
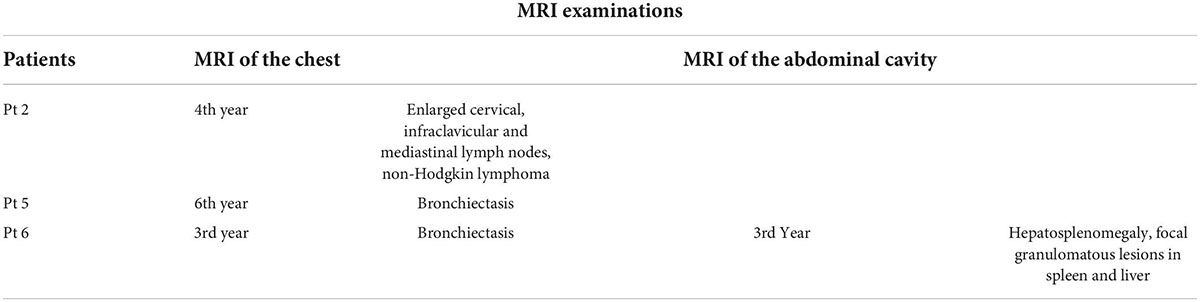
Table 4. Findings detected in magnetic resonance imaging (MRI) examinations of the abdominal cavity and the chest in ataxia-telangiectasia (A-T) patients and the time of observation.
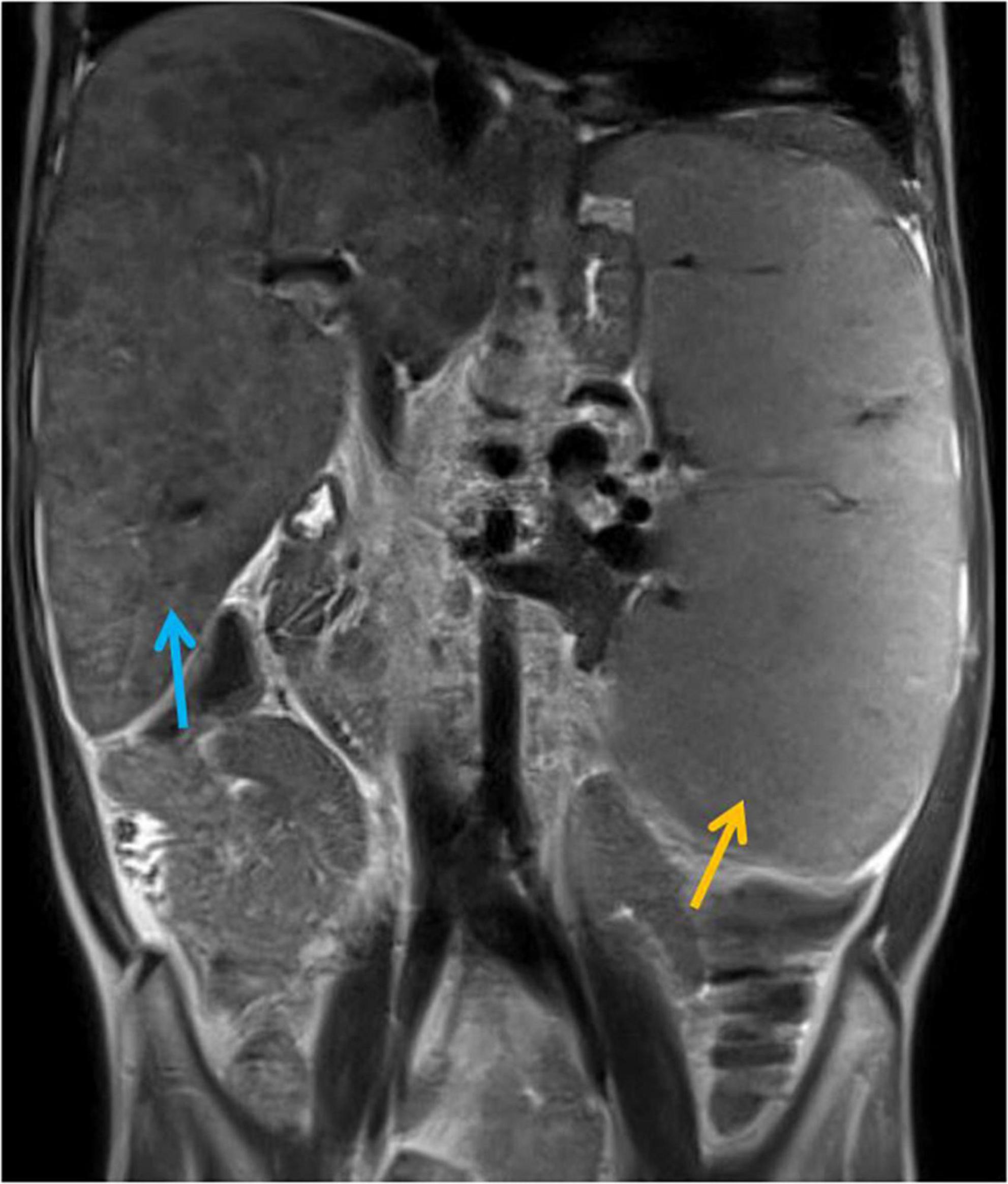
Figure 4. Magnetic Resonance Imaging (MRI) examination of the abdominal cavity, T2-weighted coronal image. Massive hepatosplenomegaly (the liver and spleen are marked with blue and orange arrows, respectively) and granulomatous lesions in the liver and spleen resulting in severe portal hypertension, hypersplenism, and ultimately, hepato-renal syndrome.
While protection against ionizing radiation and the use of lung US has been underscored in children with DNA reparation defects, the use of this diagnostic technique is increasingly proposed in the analysis of respiratory tract pathologies in pediatric patients (18–20). Its usefulness in detecting and monitoring pneumonic consolidations and atelectasis, an alveolar-interstitial syndrome that may correlate with interstitial lung disease in IEI or pulmonary fibrosis, pleural effusion, as well as diaphragmatic excursion has been documented (21–24). The lung US findings correlated with pulmonary symptoms supporting the role of US imaging in monitoring lung disease in A-T and supporting the significance and predictive role of this imaging technique in clinical pathology. The lung US is therefore a promising imaging technique in A-T patients requiring regular follow-up examinations, being a convenient, inexpensive, available at the bedside, and safe ionizing radiation-free choice. However, like all US examinations, its outcome depends on the skills and experience of the physician as well as the quality of the device.
Consistently with results of other reports (5, 6, 25), indicating that A-T patients are affected with chronic inflammatory non-alcoholic fatty liver disease, a high rate of hepatomegaly, with concomitant focal lesions in the liver and/or spleen was noted in our pediatric study group. The impaired nuclear and cellular ATM kinase activity, with defective DNA double-strand break reparation, oxygen species production, and disturbed protein homeostasis are hypothesized to contribute to the organ-specific immunopathology in the liver. Systemic granulomatosis involving the liver and the spleen as a unique form of A-T-related granulomatous disease has also been reported in one of our patients (9, 11). US evaluation of peripheral lymph nodes using a basic examination technique (26) and sonoelastography and CD (27) is helpful in distinguishing between benign and malignant lymphadenopathy. The latter options have been reported to show very good sensitivity and specificity in detecting and monitoring lymph node malignancies and thereby their eligibility in routine monitoring of cervical lymphadenopathy in children has been defined (27, 28). Concerning US features for malignancy may include heterogeneity of the node, round shape as opposed to a normal oval shape, narrow or absent hilum, irregular borders, cystic necrosis, or irregular blood flow to the capsule (29, 30). Radiologists could also guide pediatricians in the differential diagnosis of peripheral lymphadenopathy, discrimination between its infectious, such as EBV, Cytomegalovirus, Parvovirus B19, Toxoplasma gondii, Bartonella henselae, Mycobacterium tuberculosis, and non-infectious causes, for example, Kawasaki disease, autoimmune lymphoproliferative syndrome, Kikuchi-Fujimoto, and Castleman disease, as well as malignant disorders, such as NHL (31–33). Whereas in most cases, lymphadenopathy is benign in its nature, watchful observation and a clinical examination with an US evaluation are required, while MRI imaging is advocated primarily in preparation for possible surgical interventions (29, 34).
The lung US examination has emerged as a complementary imaging technique to high-resolution computer tomography (HRCT), being of utmost importance in DNA reparation defects due to the increased risk of malignancy, and proved to be a valuable and applicable method for the assessment of ILD (35, 36). Multiple, diffuse B-line artifacts representing the sonographic hallmarks of the pulmonary interstitial-alveolar syndrome were found in as many as 5 out of 11 A-T children studied. Whereas ILD in IEI may occur in diverse clinical entities (37) and these conditions may share a similar B-line distribution pattern, lung US may play an adjuvant role when combined with clinical patient profile and aid in differentiating between cardiogenic and non-cardiogenic pulmonary edema, interstitial pneumonia, and pulmonary fibrosis (35, 36, 38). ILD is an inflammatory organ-specific immunopathology, characterized by multifactorial etiology, predominately related to immune dysregulation and hyperinflammation. The diagnosis of ILD in A-T patients is complex and includes clinical manifestations, such as cough and dyspnea, thoracic imaging findings, and/or surgical lung biopsy to indicate precisely the nature of possible etiologies of this condition. The pathogenesis of ILD in A-T patients is unknown, yet the role of respiratory viruses, Mycoplasma pneumoniae, and herpes viruses, for example, EBV, cytomegalovirus, and human herpes virus 6 (HHV6) infection in initiating and triggering the progression of chronic interstitial infiltrates and pulmonary fibrosis needs to be clarified (39–41). Among our 5 A-T children studied, who presented features of ILD in lung US examination, we have found EBV-DNA in peripheral blood and CMV-DNA was persistently present in 3, and HHV6 in one of them, reflecting a combined immunodeficiency (37, 40). It has been hypothesized that the immunodeficiency phenotype and lymphocyte dysregulation-driven hyperinflammation due to the lack of ATM activity in A-T are contributory factors to interstitial pneumopathy and lung fibrosis (42, 43).
Due to increasing concerns about potentially harmful effects on imaging techniques based on exposure to ionizing radiation in children, in particular in those affected with syndromic DNA reparation defects, MRI of the respiratory system plays an important role in diagnosing and monitoring a spectrum of thoracic disorders. MRI has been adopted for evaluation of the diseases in the lung parenchyma, interstitial lung disease, disorders of large as well as medium and small airways, abnormalities of thoracic vasculature, pleural diseases, masses, and chest wall pathologies (44–50). The role of MRI in neuroimaging and investigating neurodegeneration in A-T has also been recently defined in several reports (51, 52). With the ever-increasing technical improvements and number of indications in pediatric patients, the cooperation of pediatric radiologists and pediatric clinicians is required to define and delineate guidelines for MRI imaging feasibility and application in A-T (48, 51, 52).
Despite the relatively small number of participants due to the rarity of A-T which is a major limitation of this study, the use of modern US capabilities and MRI is efficient and recommended imaging diagnostic tool in monitoring children with IEI and DNA instability syndromes.
The radiologist’s approach provides an integral contribution to the multidisciplinary care of respiratory tract disorders in A-T patients. This also includes regular lung function monitoring aimed at early detection of lung disease progression and indicating the need for therapeutic interventions. Progressive neurodegeneration and wheelchair bounding lead to an inability to perform the required respiratory maneuvers during spirometry thereby making it difficult to obtain reliable reproducible lung function tests. For these A-T children who cannot manage to perform spirometry reliably, impulse oscillometry, measuring the airway resistance, is an alternative. Chronic lower airway infections and the hyperinflammatory state lead to bronchial wall damage and the development of bronchiectasis observable in MRI and manifesting as obstructive ventilation impairment. Difficulties in expiration due to muscle fatigue and atrophy, and chest wall deformity resulting in reduced tidal volume and restrictive ventilation disorders which may accompany imaging features of ILD and lung fibrosis (13, 15).
In conclusion, while the analyzed patient group is affected with a severe IEI with susceptibility to ionizing radiation, it is characterized by the need for constant clinical supervision of the multidisciplinary team including an experienced pediatric radiologist and immunologist. The choice of an adequate, safe imaging diagnostic technique is crucial for the patient’s monitoring and, at the same time, this technique should provide the best answer to the clinician’s requests.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
KJ-P was responsible for the design of the study, collection, and analysis of patients’ data, their interpretation, and drafted the initial manuscript. JP participated in patients’ data collection and analysis. AS-P was responsible for the clinical evaluation of patients, collection and security of patients data, participated in drafting the initial manuscript, and critically revised its final version. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zaki-Dizaji M, Akrami SM, Abolhassani H, Rezaei N, Aghamohammadi A. Ataxia telangiectasia syndrome: Moonlighting ATM. Expert Rev Clin Immunol. (2017) 13:1155–72. doi: 10.1080/1744666X.2017.1392856
2. Lee JH, Paul TT. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat Rev Mol Cell Biol. (2021) 22:796–814. doi: 10.1038/s41580-021-00394-2
3. Bott L, Lebreton JP, Thumerelle C, Cuvellier JC, Deschildre JC, Sardet A. Lung disease in ataxia-telangiectasia. Acta Paediatr. (2007) 96:1021–4. doi: 10.1111/j.1651-2227.2007.00338.x
4. McGrath-Morrow SA, Gower WA, Rothblum-Oviatt C, Brody AS, Langston C, Fan LL, et al. Evaluation and management of pulmonary disease in ataxia-telangiectasia. Pediatr Pulmonol. (2010) 45:847–59. doi: 10.1002/ppul.21277
5. Weiss B, Krauthammer A, Soudack M, Lahad A, Sarouk I, Somech R, et al. Liver disease in pediatric patients with ataxia telangiectasia: A novel report. J Pediatr Gastroenterol Nutr. (2016) 62:550–5. doi: 10.1097/MPG.0000000000001036
6. Donath H, Woelke S, Theis M, Hess U, Knop V, Herrmann E, et al. Progressive liver disease in patients with ataxia-telangiectasia. Front Pediatr. (2019) 7:458. doi: 10.3389/fped.2019.00458
7. Voss S, Pietzner J, Hoche F, Taylor AMR, Last JI, Schubert R, et al. Growth retardation and growth hormone deficiency in patients with ataxia telangiectasia. Growth Factors. (2014) 32:123–9. doi: 10.3109/08977194.2014.939805
8. Nissenkorn A, Levy-Shraga Y, Banet-Levi Y, Lahad A, Sarouk I, Modan-Moses D. Endocrine abnormalities in ataxia telangiectasia: Findings from a national cohort. Pediatr Res. (2016) 79:889–94. doi: 10.1038/pr.2016.19
9. Szczawińska-Popłonyk A, Olejniczak K, Tąpolska-Jóźwiak K, Boruczkowski M, Jończyk-Potoczna K, Małdyk J, et al. Cutaneous and systemic granulomatosis in ataxia-telangiectasia: A clinic-pathological study. Adv Dermatol Allergol. (2020) 37:760–5. doi: 10.5114/ada.2020.100485
10. Woelke S, Valesky E, Bakhtiar S, Pommering H, Pfeffermann JM, Schubert R, et al. Treatment of granulomas in patients with ataxia telangiectasia. Front Immunol. (2018) 9:2000. doi: 10.3389/fimmu.2018.02000
11. Szczawińska-Popłonyk A, Ossowska L, Jończyk-Potoczna K. Granulomatous liver disease in ataxia-telangiectasia with the hyper-IgM phenotype: A case report. Front Pediatr. (2020) 8:570330. doi: 10.3389/fped.2020.570330
12. Van Os NJH, Jansen AFM, van Deuren M, Haraldsson A, van Driel NTM, Etzioni A, et al. Ataxia-telangiectasia: Immunodeficiency and survival. J Clin Immunol. (2017) 178:45–55. doi: 10.1016/j.clim.2017.01.009
13. Van Os NJH, Haaxma CA, van der Flier M, Merkus PJFM, van Deuren M, de Groot IJM, et al. Ataxia telangiectasia: Recommendations for multidisciplinary treatment. Dev Med Child Neurol. (2017) 59:680–9. doi: 10.1111/dmcn.13424
14. McGrath-Morrow SA, Rothblum-Oviatt CC, Wright J, Schlechter H, Lefton-Greif MA, Natale VA, et al. Multidisciplinary management of ataxia-telangiectasia: Current perspectives. J Multidiscip Health. (2021) 14:1637–44. doi: 10.2147/JMDH.S295486
15. Bhatt JM, Bush A, van Gerven M, Nissenkorn A, Renke M, Yarlett L, et al. ERS statement on the multidisciplinary respiratory management of ataxia telangiectasia. Eur Respir Rev. (2015) 24:565–81. doi: 10.1183/16000617.0066-2015
16. McGrath-Morrow SA, Lederman HM, Aherrera AD, Lefton-Greif MA, Crawford TO, Ryan T, et al. Pulmonary function in children and young adults with ataxia telangiectasia. Pediatr Pulmonol. (2014) 49:84–90. doi: 10.1002/ppul.22760
17. Driessen GJ, Ijspert H, Weemaes CMR, Haraldsson A, Trip M, Warris A, et al. Antibody deficiency in patients with ataxia telangiectasia is caused by disturbed B- and T-cell homeostasis and reduced immune repertoire diversity. J Allergy Clin Immunol. (2013) 131:1367–75. doi: 10.1016/j.jaci.2013.01.053
18. Manson DE, Sikka S, Reid B, Roifman C. Primary immunodeficiencies: A pictorial immunology primer for radiologists. Pediatr Radiol. (2000) 30:501–10. doi: 10.1007/s002470000237
19. Bazregari S, Azizi G, Tavakol M, Asgardoon MH, Kiaee F, Tavakolinia N, et al. Evaluation of infectious and non-infectious complications in patients with primary immunodeficiency. Centr Eur J Immunol. (2017) 42:336–41. doi: 10.5114/ceji.2017.72825
20. Bellman LA, Liu YT. Lung ultrasound for diagnosis of pneumonia in children. Acad Emerg Med. (2020) 27:245–6. doi: 10.1111/acem.13920
21. Bobillo-Perez S, Girona-Alarcon M, Rodriguez-Fanjul J, Jordan I, Balaguer Gargallo M. Lung ultrasound in children: What does it give us? Paediatr Respir Rev. (2020) 36:136–41. doi: 10.1016/j.prrv.2019.09.006
22. Musolino AM, Toma P, Supino MC, Scialanga B, Mesturino A, Scateni S, et al. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: A prospective study. Pediatr Pulmonol. (2019) 54:1479–86. doi: 10.1002/ppul.24426
23. Stadler JAM, Andronikou S, Zar HJ. Lung ultrasound for the diagnosis of community- acquired pneumonia in children. Pediatr Radiol. (2017) 47:1412–7. doi: 10.1007/s00247-017-3910-1
24. Bloise S, La Regina DP, Pepino D, Iovine E, Laudisa M, Di Mattia G, et al. Lung ultrasound compared to chest X-ray for the diagnosis of CAP in children. Pediatr Int. (2021) 63:448–53. doi: 10.1111/ped.14469
25. Tangsinmankong N, Wayne AS, Howenstine MS, Washington KR, Longston C, Gatti RA, et al. Lymphocytic interstitial pneumonitis, elevated IgM concentration, and hepatosplenomegaly in ataxia-telangiectasia. J Pediatr. (2001) 138:939–41. doi: 10.1067/mpd.2001.113356
26. Meadows O, Sarkodieh J. Ultrasound evaluation of persistent cervical lymph nodes in young children. Clin Radiol. (2021) 76:e9–315. doi: 10.1016/j.crad.2020.12.021
27. Zakaria OM, Moussa A, AlSadhan R, Sultan TA, Eid AF, Daoud MY, et al. Reliability of sonoelastography in predicting pediatric cervical lymph node malignancy. Pediatr Surg Int. (2018) 34:885–90. doi: 10.1007/s00383-018-4301-x
28. Mentzel HJ, Glutig K, Grager S, Kruger PC, Waginger M. Ultrasound elastography in children: Nice to have for scientific studies or arrived in clinical routine? Mol Cell Pediatr. (2022) 9:11. doi: 10.1186/s40348-022-00143-1
29. Weinstock MS, Patel NA, Smith LP. Pediatric cervical lymphadenopathy. Pediatr Rev. (2018) 39:433–43. doi: 10.1542/pir.2017-0249
30. Park JE, Ryu YJ, Kim JY, Kim YH, Park JY, Lee H, et al. Cervical lymphadenopathy in children: A diagnostic tree analysis model based on ultrasonographic and clinical findings. Eur Radiol. (2020) 30:4475–85. doi: 10.1007/s00330-020-06794-w
31. Pecora F, Abate L, Scavone S, Petrucci I, Costa F, Caminiti C, et al. Management of infectious lymphadenitis in children. Children. (2021) 8:860. doi: 10.3390/children8100860
32. Rankovic N, Todorovic J, Simic R. Clinical and ultrasound characteristics of pediatric lateral neck masses. PLoS One. (2021) 16:e0251563. doi: 10.1371/journal.pone.0251563
33. Szczawińska-Popłonyk A, Grześk E, Schwartzmann E, Materna-Kiryluk A, Małdyk J. Case report: Autoimmune lymphoproliferative syndrome versus chronic active Epstein Barr virus infection in children: A diagnostic challenge. Front Pediatr. (2021) 9:798959. doi: 10.3389/fped.2021.798959
34. Grant CN, Aldrink J, Lautz TB, Tracey ET, Rhee DS, Baertschiger EM, et al. Lymphadenopathy in children: A streamlined approach for the surgeon: A report from the APSA cancer committee. J Pediatr Surg. (2021) 56:274–81. doi: 10.1016/j.jpedsurg.2020.09.058
35. Pitsidianakis G, Vassalou EE, Vasarmidi E, Bolaki M, Klontzas ME, Xirouchaki N, et al. Performance of lung ultrasound for monitoring interstitial lung disease. J Ultrasound Med. (2022) 41:1077–84. doi: 10.1002/jum.15790
36. Volpicelli G. Lung ultrasound B-lines in interstitial lung disease: Moving from diagnosis to prognostic stratification. Chest. (2020) 158:1323–4. doi: 10.1016/j.chest.2020.05.528
37. Szczawinska-Poplonyk A, Jonczyk-Potoczna K, Mikos M, Ossowska L, Langfort R. Granulomatous lymphocytic interstitial lung disease in a spectrum of pediatric primary immunodeficiencies. Pediatr Dev Pathol. (2021) 24:504–12. doi: 10.1177/10935266211022528
38. Iovine E, Nenna R, Bloise S, La Regina DP, Pepino D, Petrarca L, et al. Lung ultrasound: Its findings and new applications in neonatology and pediatric diseases. Diagnostics. (2021) 11:652. doi: 10.3390/diagnostics11040652
39. Schroeder SA, Swift M, Sandoval C, Langston C. Interstitial lung disease in patients with ataxia telangiectasia. Pediatr Pulmonol. (2005) 39:537–43. doi: 10.1002/p.pul.202009
40. Szczawińska-Popłonyk A, Jończyk-Potoczna K, Ossowska L, Brêborowicz A, Bartkowska-Śniatkowska A, Wachowiak J. Cytomegalovirus pneumonia as the first manifestation of severe combined immunodeficiency. Cent Eur J Immunol. (2014) 39:392–5. doi: 10.5114/ceji.2014.45953
41. De Rose DU, Auriti C, Lozzi S, Coltella L, Piccioni L, Rossi S, et al. Severe herpes virus 6 interstitial pneumonia in an infant with three variants in genes predisposing to lung disease. J Med Virol. (2021) 93:5182–7. doi: 10.1002/jmv27016
42. Cirillo E, Polizzi A, Soresina A, Prencipe R, Giardino C, Cancrini C, et al. Progressive depletion of B and T lymphocytes in patients with ataxia telangiectasia: Results of the Italian primary immunodeficiency network. J Clin Immunol. (2022) 42:783–97. doi: 10.1007/s10875-022-01234-4
43. Liszewski MC, Hersman W, Altes TA, Ohno Y, Ciet P, Warfield SK, et al. Magnetic resonance imaging of pediatric lung parenchyma, airways, vasculature, ventilation, and perfusion, State of the art. Radiol Clin N Am. (2013) 51:555–82. doi: 10.1016/j.rcl.2013.04.004
44. Liszewski MC, Ciet P, Lee EY. MR imaging of lungs and airways in children: Past and present. Magn Reson Imaging Clin N Am. (2019) 27:201–25. doi: 10.1016/j.mric.2019.01.002
45. Liszewski MC, Ciet P, Winant AJ, Lee EY. Lung disease and airway imaging: Magnetic resonance imaging versus computed tomography. Pediatr Radiol. (2022). doi: 10.1007/s00247-022-05386-8 [Epub ahead of print].
46. Liszewski MC, Ciet P, Winant AJ, Lee EY. Pediatric large airway imaging: Evolution and revolution. Pediatr Radiol. (2022). doi: 10.1007/s00247-022-05377-9 [Epub ahead of print].
47. Sodhi KS, Ciet P, Vasanawala S, Biederer J. Practical protocol for lung magnetic resonance imaging and common clinical indications. Pediatr Radiol. (2022) 52:295–311. doi: 10.1007/s00247-021-05090-z
48. Abdel Razek AA, Gaballa G, Elashry R, Elkhamary S. Diffusion-weighted MRI imaging of mediastinal lymphadenopathy in children. Jpn J Radiol. (2015) 33:449–54. doi: 10.1007/s11604-015-0434-1
49. Sodhi KS. Lung MRI in children: The road less travelled. Indian J Radiol Imaging. (2021) 31:237–41. doi: 10.1055/s-0041-1729126
50. Montella S, Mollica C, Finocchi A, Pession A, Pietrogrande MC, Trizzino A, et al. Non invasive assessment of lung disease in ataxia telangiectasia by high-field magnetic resonance imaging. J Clin Immunol. (2013) 33:1185–91. doi: 10.1007/s10875-013-9933-y
51. Sahama I, Sinclair K, Pannek K, Lavin M, Rose S. Radiological imaging in ataxia telangiectasia: A review. Cerebellum. (2014) 13:521–30. doi: 10.1007/s12311-014-0557-4
Keywords: imaging, ataxia-telangiectasia, magnetic resonance, ultrasound, lymphadenopathy, children
Citation: Jończyk-Potoczna K, Potoczny J and Szczawińska-Popłonyk A (2022) Imaging in children with ataxia-telangiectasia—The radiologist’s approach. Front. Pediatr. 10:988645. doi: 10.3389/fped.2022.988645
Received: 07 July 2022; Accepted: 29 August 2022;
Published: 16 September 2022.
Edited by:
Rita Consolini, University of Pisa, ItalyReviewed by:
Martin Francis Lavin, The University of Queensland, AustraliaCopyright © 2022 Jończyk-Potoczna, Potoczny and Szczawińnska-Popłonyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katarzyna Jończyk-Potoczna, am9uY3p5a0B1bXAuZWR1LnBs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.