- 1Department of Epidemiology and Cancer Control, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 2Division of Epidemiology, Biostatistics, and Environmental Health, School of Public Health, University of Memphis, Memphis, TN, United States
- 3Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 4Department of Diagnostic Imaging, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 5Department of Radiology, University of Tennessee Health Science Center, Memphis, TN, United States
- 6Department of Psychology, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 7Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 8Division of Cardiovascular Diseases, Institute for Cardiovascular Science, University of Tennessee Health Science Center, Memphis, TN, United States
Purpose: Adult survivors of childhood acute lymphoblastic leukemia (ALL) have impaired adaptive physical function and poor health-related quality of life (HRQoL). Obesity may contribute to these impairments by increasing the physiological cost of walking. Due to treatment exposures during ALL therapy, survivors’ cost of walking may be more impacted by obesity than the general population. Therefore, we examined associations between obesity, persistent motor neuropathy, and energy cost of walking; and examined associations between energy cost of walking, adaptive physical function, and HRQoL, in adult survivors of childhood ALL vs. community controls.
Methods: Obesity was measured via body mass index (BMI) and body fat percentage. The physiological cost index (PCI) was calculated from the six-minute walk test. Adaptive physical functioning was measured using two tests: the timed up and go (TUG) test and the physical performance test. Persistent motor neuropathy was measured using the modified total neuropathy score; HRQoL was measured using the Short-Form-36 questionnaire. The associations between obesity and PCI were evaluated using multivariable linear regressions in adult survivors of childhood ALL (n = 1,166) and community controls (n = 491). Then, the associations between PCI, adaptive physical functioning and peripheral neuropathy were examined using multivariable linear regressions. Finally, to determine the association between obesity, and neuropathy on PCI, while accounting for potential lifestyle and treatment confounders, a three model, sequential linear regression was used.
Results: Obese individuals (BMI > 40 kg/m2 and excess body fat percentage [males: >25%; females: >33%]) had higher PCI compared to those with normal BMI and body fat percentage (0.56 ± 0.01 vs. 0.49 ± 0.009 beats/meter p < .01; and 0.51 ± 0.007 vs. 0.48 ± .0006 beats/meter p < .01, respectively). Treatment exposures did not attenuate this association. Increased PCI was associated with longer TUG time in survivors, but not community controls (6.14 ± 0.02 s vs. 5.19 ± 0.03 s, p < .01). Survivors with PCI impairment >95th percentile of community controls had lower HRQoL compared to un-impaired ALL survivors: 46.9 ± 0.56 vs. 50.4 ± 1.08, respectively (p < .01).
Conclusion: Obesity was associated with increased PCI. Survivors with high PCI had disproportionately worse adaptive physical function and HRQoL compared to controls. Survivors with increased energy costs of walking may benefit from weight loss interventions.
Introduction
Due to advances in cancer therapy and supportive care, the 5-year survival rate of childhood acute lymphoblastic leukemia (ALL) now exceeds 90%, with ALL representing 19% of all long-term childhood cancer survivors (1). Over the past six decades, therapy for ALL has not only improved survival, but has also been refined to minimize long-term morbidities and optimize health for survivors (2). Nevertheless, survivors of childhood ALL, when compared to their peers, remain at increased risk for chronic health conditions, including obesity, that interfere with activities of daily living (3).
Obesity is a prevalent public health problem associated with chronic disease, including metabolic disease, cardiovascular disease, secondary cancers, and type II diabetes (4–6). It is particularly concerning in ALL survivors, as past treatment exposures confer risk for comorbidities, including diabetes and cardiovascular disease. Obesity perpetuates these chronic health conditions. Although obesity may be a modifiable condition, it may be difficult to manage in adult survivors of childhood ALL, particularly in ALL survivors with neuromusculoskeletal problems (i.e., persistent motor neuropathy) that interfere with health optimizing behaviors, including engagement in physical activity (PA) (7). Additionally, ALL survivors have increased sedentary behavior compared to peers, which may independently increase risk for these chronic health conditions (8).
Energy cost is the relative oxygen cost required to perform an activity or to move the body through space (9). In the general population, adults with obesity have a higher energy cost of performing activities compared to those without obesity (10). Higher energy cost of activity changes the perception of exertion while moving, increases fatiguability, and reduces time spent in PA (11). This results in a reciprocal association between PA and obesity: obesity contributes to reduced PA because movement is difficult; less movement reduces overall energy expenditure and, if not countered by reduced energy intake, results in weight gain (12, 13). Excess body weight taxes the body’s systems. Initially, among overweight persons, only vigorous activity may seem difficult. however, this impairment may interfere if job demands, or leisure activities, require vigorous organ system responses. Subsequently, as body weight increases, less intensive activities, e.g., walking, housework, grocery shopping, or getting out of a chair, become difficult, limiting participation in important life roles, and eventually interfering with quality of life (14).
When compared to peers, adult survivors of childhood ALL spend significantly less time in moderate to vigorous PA (15). They are also at an increased risk for difficulties with performing activities of daily living that require negotiating the environment outside of the home, and have a lower physical component summary score (PCS), a measure of physical health-related quality of life (HRQoL) (16). Peripheral neuropathy is a prevalent outcome in ALL survivors, and is related to cancer therapy (13). Associations between peripheral neuropathy, activities of daily living, and HRQoL are well known (17–20). However, the impact of obesity, and peripheral neuropathy, on the energy costs of daily activity, adaptive physical function, and HRQoL, have not been explored in this population. Understanding these associations have the potential to provide information to help tailor interventions designed to increase PA and/or promote weight loss, which have previously produced inconsistent results (21). Thus, the aims of this study were to examine associations between obesity, persistent motor neuropathy and energy cost of walking, and to examine associations between energy cost of walking, adaptive physical function, and HRQoL in adult survivors of childhood ALL.
Materials and methods
Study population
Participants included members of the St. Jude Lifetime Cohort (22), assembled to assess health outcomes in aging survivors of childhood malignancies. For these analyses, we included any adult survivors (≥18 years of age) of childhood ALL treated at St. Jude Children’s Research Hospital (since it’s opening) that were at least 10 years from their original diagnosis (1962–2012). Participants also must have completed an on-campus assessment [e.g., self-reported questionnaires and a functional assessment (n = 1,166, age range 18.0–65.5 years)]. A comparison group (community controls) was also recruited from parents/relatives of current pediatric patients, and adult friends of survivors. Controls were not first-degree relatives of St. Jude Lifetime Cohort participants, had no history of childhood cancer, but did not have to be free of other chronic disease (n = 491, age range 18.2–70.2 years). The St. Jude Children’s Research Hospital Institutional Review Board approved all procedures. Written informed consent was obtained on all participants prior to testing.
Anthropometrics
Height in centimeters (cm) and weight in kilograms (kg) were measured using a wall-mounted stadiometer and electronic scale (Model 5002, ScaleTronix, Inc., Wheaton, IL). Body mass index (BMI) was calculated and categorized as underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), obese grade I (30.0–34.9 kg/m2), obese grade II (35.0–39.9 kg/m2), and obese grade III (≥40.0 kg/m2). Percent body fat was measured via the three site technique (males: pectoral, abdominal, and thigh; females: triceps, suprailiac, and thigh) by trained exercise physiologists, using the Harpenden skinfold caliper (Baty, West Sussex, United Kingdom) (23). The three site skinfold technique is a valid alternative to dual energy x-ray absorptiometry in childhood cancer survivors (24). Survivors and controls were categorized as having normal body fat (males: <27.5%; females: <39.9%) or excess body fat (males: ≥27.5%; females: ≥39.9%) (25).
Outcomes
To measure the energy cost of walking, the physiological cost index (PCI) was calculated (maximal heart rate while walking—resting heart rate)/(meters walked) (beats per meter) from the six-minute walk test (6 MWT). While the PCI calculation from the 6 MWT is not been specifically validated in adult survivors of childhood cancer, it is a valid measure of walking efficiency in children with cystic fibrosis (26), persons with traumatic brain injury (27) or stroke (28), non-disabled older adults (29), persons with amputation (30), and survivors of polio (31). Before the test, participants were asked to sit quietly for five minutes to acquire resting heart rate. Participants were then instructed to walk as fast as they could around a 41-meter track. Heart rate (measured using the Masimo Rad-5 handheld pulse oximeter, Irvine, CA) was taken at two-, four-, and six-minutes. To meet the assumption that the participants’ heart rate was in steady state, maximal heart rate was defined as the highest heart rate at the four-minute or six-minute measurement.
To assess HRQoL, participants completed the Medical Outcomes Survey Short-Form-36 (SF-36), which has been previously validated in adult survivors of childhood cancer (32). The SF-36 contains eight subscales: physical function, role physical, vitality, bodily pain, general health, social functioning, role emotional and mental health; and two summary scores: PCS and mental component summary (MCS). For these analyses, we included subscale scores and summary scores. Raw scores were calculated and converted into T-scores: with the general population mean 50 and standard deviation 10. Higher scores indicated better HRQoL.
Adaptive physical functioning was assessed using a 7-item physical performance test (PPT) and a timed up-and-go (TUG) test. The PPT is an examination of fine and gross motor skills designed to simulate activities of daily living. More specifically, the examiner evaluates the participant on how fast they can write a sentence, simulate eating by moving five beans into an empty bowl with a spoon, lift an object onto a shelf, put on and remove a jacket, pick up a coin from the floor, and turn 360 degrees and walk 50 feet. The PPT is more sensitive than traditional self-reports in detecting loss of function (33). The TUG test evaluates balance and mobility, measured as the time required to stand up from a seated position, walk three meters, turn around, walk back three meters, and sit down again. The TUG has excellent reliability (intraclass correlation coefficient: 0.93) and good discriminate validity (area under the curve: 0.65) (34).
Other measures
Diagnosis and treatment information were obtained from medical records by trained abstractors and included type of individual chemotherapeutic agents and if survivors received cranial radiation therapy. Demographic information such as smoking status and educational attainment were obtained from questionnaires that the participants complete during their St. Jude Lifetime Cohort evaluation.
Physical activity levels were determined using six self-report items from the National Health and Nutrition Examination Survey (35). The questions asked if the participant spent at least ten minutes doing vigorous PA, on how many days per week they did these activities, and for how many total minutes per day; identical questions were asked for moderate physical activities. Weekly minutes of vigorous activity were multiplied by six; weekly minutes of moderate activities were multiplied by three and summed to get metabolic equivalent minutes per week. For analysis, PA was dichotomized into meeting (≥450 metabolic equivalent minutes per week) or not meeting the 2018 Centers for Disease Control and Prevention (CDC) guidelines (36).
Peripheral nervous system integrity was evaluated with the modified Total Neuropathy Scale (37) consisting of self-reported sensory and motor symptoms, and quantitative testing. Protective sensation was evaluated with a 4.17 log force Semmes Weinstein Monofilament. Vibration was measured using a Bioesthesiometer with a threshold of 9.0 volts (Bio-Medical Instrument Company, Newbury, OH). Manual muscle testing was performed on the fingers, ankles, and wrists (37). Reflex testing was performed in the ankles, knees, brachioradialis, biceps, and triceps tendons. A total score of 24 was possible: having fewer or no symptoms or measured deficits equated to having lower scores (37).
Statistical analysis
Descriptive statistics of participants vs. non-participants characteristics and survivors vs. community controls were calculated and compared using chi-squared tests or Fischer’s exact test for categorical variables, and two sample t-tests for continuous variables. Multivariable linear regressions were used to examine the associations of BMI and percent body fat with PCI in survivors and community controls. The models were adjusted for potential confounders (age, sex, race, and smoking status) that changed the beta estimate for the association between BMI or body fat percentage and PCI by more than ten percent. Initial models included interaction terms (BMI × group and percent body fat × group) which were not significant. Due to the changing trends in PA over the decades, as well as the marked increase in 5-year survival starting in the 1980s for ALL, a supplemental analysis limited survivors to those treated after 1980 to see if results differed when those treated in the 1960s and 1970s were not included (38, 39). To determine the effects of neuropathy and obesity on the energy cost of walking among survivors, while accounting for potential confounders of these associations, three sequential regression models were used (40). The first model regressed past treatment exposures and prevalent neuropathy on PCI; the second regressed past treatment exposures, BMI, and prevalent neuropathy on PCI, and the third model regressed treatment exposures, BMI, prevalent neuropathy, and lifestyle factors on PCI (40). Multivariable linear models, adjusted for potential confounders (age, sex, race, smoking status, and PA), and that included a PCI by group (survivor vs. control) interaction term were used to determine associations between PCI (as both a continuous measure and as two separate dichotomies (>90th percentile (impaired) vs. ≤90th percentile (not impaired); >95th percentile (severely impaired) vs. ≤95th percentile (not severely impaired), adaptive physical functioning and HRQoL. Variables that modified associations between PCI and either adaptive physical functioning or HRQoL were retained (41). Data were analyzed with SAS version 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of participants
Among 1,521 adult ALL survivors potentially eligible for analysis, 1,166 (76.7%) participants completed an on campus visit and a functional assessment that included the 6 WMT, while the remaining 355 eligible survivors refused to participate, could not be contacted, completed a survey only, or did not complete the 6 MWT (Supplementary Figure S1). Compared to non-participants, participants were older, more likely to have received glucocorticoids, 6-mercaptopurine, cytarabine, doxorubicin, daunorubicin, and etoposide, but less likely to have received cranial irradiation (Supplementary Table S1).
Compared to community controls, survivors were older and more likely to be male, more likely to report current smoking, and less likely to report adequate PA, and had lower educational attainment. Survivors were also more likely to be categorized as obese grade I, obese grade II, and were more likely to have excess body fat vs. community controls (Table 1).

Table 1. Demographic characteristics of acute lymphoblastic leukemia survivors and the community controls.
Physiological cost index
Means (±SE) of the PCI in survivors and community controls, categorized by BMI and body fat percentage, are shown in Table 2. After removing nine survivors and seven community controls who could not complete the 6 MWT (see Table 2 footnote for reasons), the PCI was significantly higher in individuals classified by BMI with grade III obesity compared to those with normal weight; and those with excess body fat percentage, compared to those who were normal. The PCI was also significantly higher in survivors compared to community controls in the model with BMI as an independent predictor of PCI, but not in the model when body fat percentage was used as the independent variable. There was no interaction between survivor status and either BMI or body fat percentage; interaction terms were not included in final models. Limiting the models to survivors treated after 1980 did not significantly change the results (Supplementary Table S2).
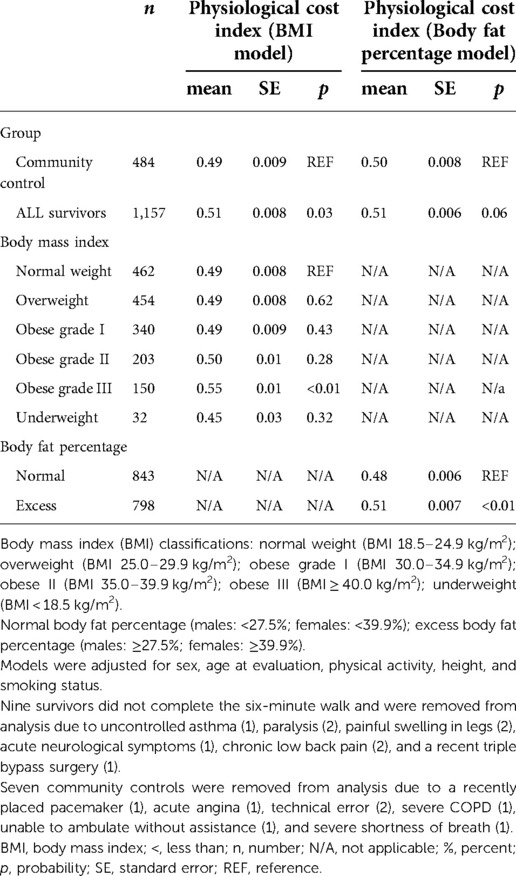
Table 2. Associations between physiological cost index and body mass index or body fat percentage in adult survivors of childhood acute lymphoblastic leukemia vs. community controls.
Treatment exposures, persistent motor neuropathy, body composition, and physiological cost index
Table 3 summarizes associations between persistent motor neuropathy, and PCI in three sequential multivariable models (A, B, and C) in survivors. After adjustment for treatment exposures age at analysis, age at diagnosis, and sex, survivors with persistent motor neuropathy had a higher energy cost of walking than those without persistent motor neuropathy (0.57 ± 0.04 vs. 0.52 ± 0.03 beats/meter) (Model A). With additional adjustment for the BMI categories, the association between persistent motor neuropathy and PCI was attenuated (Model B). After adjustment for all variables in models A and B and for lifestyle factors (smoking, PA), both grade III obesity and neuropathy were associated with the PCI (Model C).
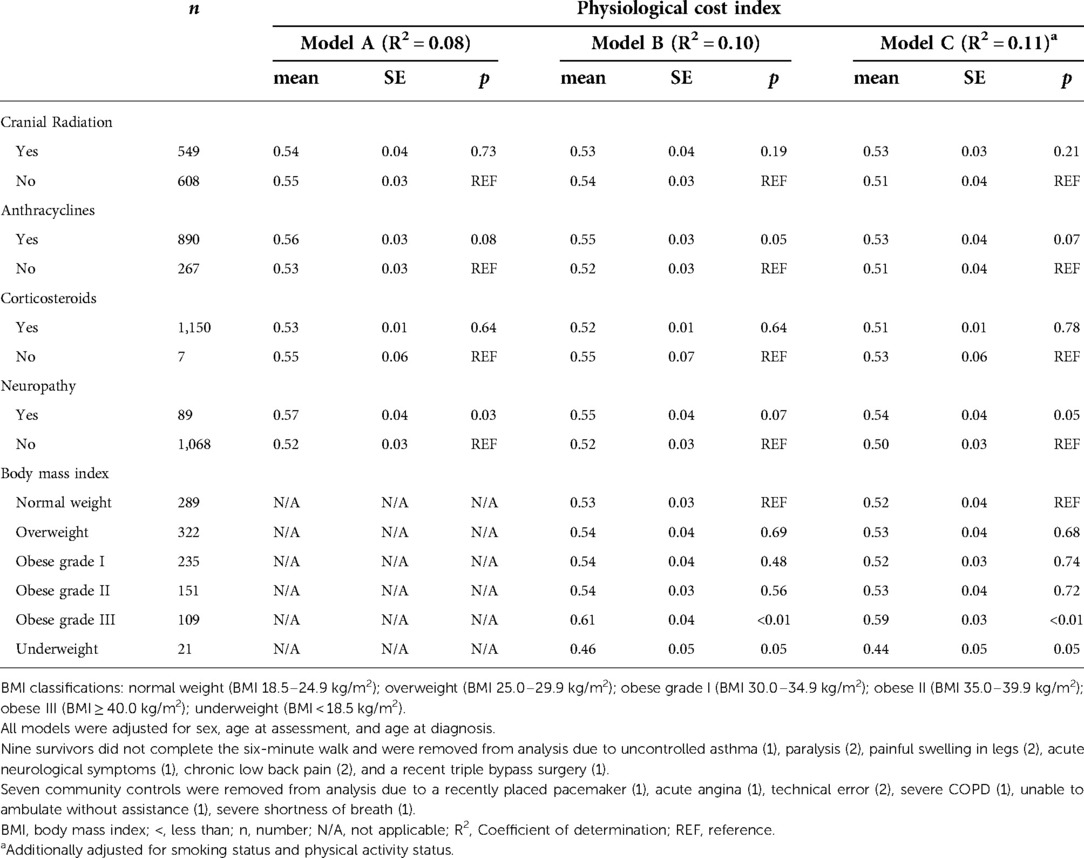
Table 3. Sequential linear regressions examining associations between treatment exposures, BMI, prevalent neuropathy, and lifestyle factors on physiological cost index in adult survivors of childhood acute lymphoblastic leukemia.
Quality of life and adaptive physical function
Table 4 shows associations between the PCI, group (survivors or community controls), PA status, and the PCS and MCS of the SF-36. Survivors had significantly lower MCS and PCS scores than community controls. However, scores were not impacted by the energy cost of walking, in models with or without interaction terms (PCI by group status). Participants who met CDC PA guidelines had higher scores on the MCS and PCS. Results were similar for the other SF-36 subscales. Adaptive physical function was measured with PPT and TUG time (Table 4). Compared to community controls, survivors scored lower on the PPT. There was no association between the PCI, PPT, or SF-36 scores. Survivors with higher PCI scores had significantly longer TUG times compared to community controls with higher PCI scores.
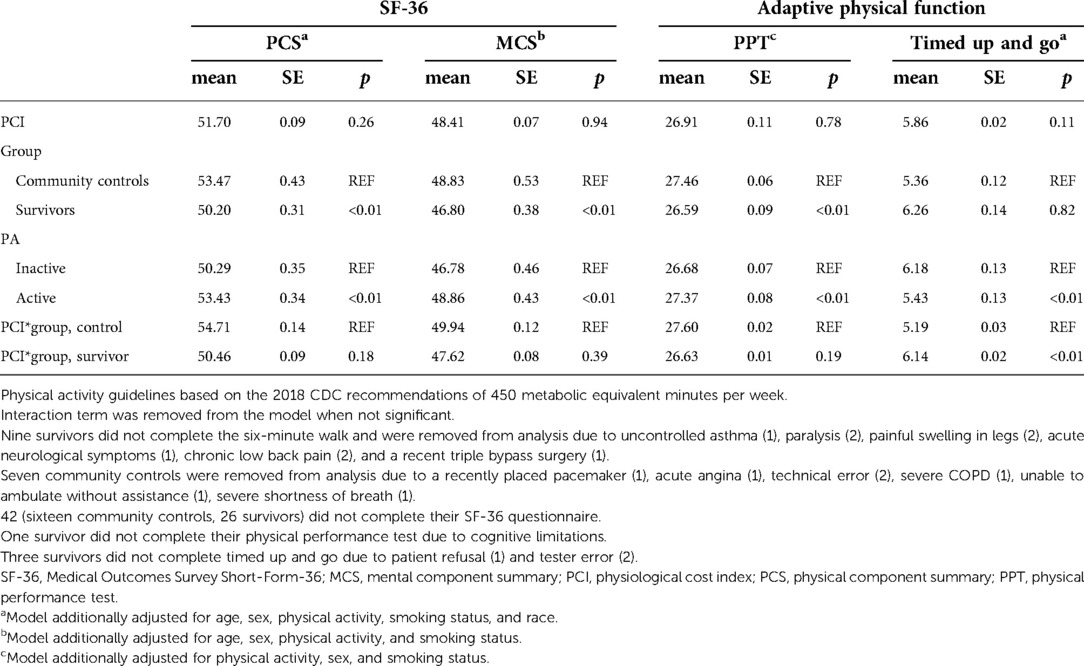
Table 4. Associations between the physiological cost index, SF-36 component summaries, and adaptive physical function in adult survivors of acute lymphoblastic leukemia vs. community controls.
Associations between PCI, quality of life, and adaptive physical function
To further assess HRQoL, participants were categorized by PCI as impaired (>90th percentile) and not impaired (<90th percentile), as well as severely impaired (>95th percentile) and not severely impaired (<95th percentile). Impairment was associated with lower PCS scores in survivors but not community controls (Figure 1A). Severe impairment was also associated with lower PCS in survivors but not community controls (Figure 1B).
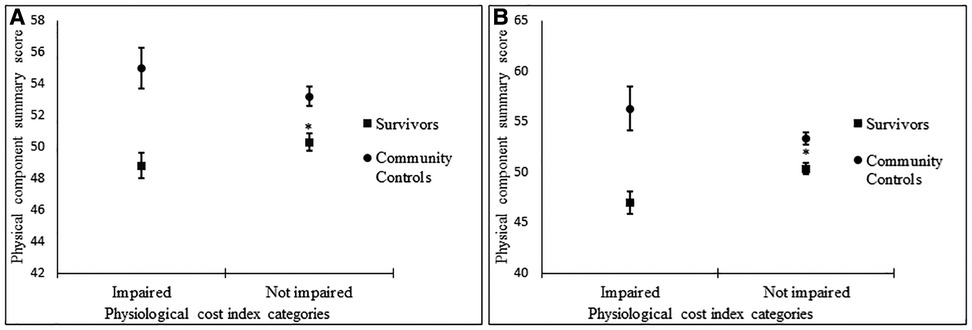
Figure 1. Associations between impaired vs. not impaired physiological cost index and physical component summary score (A), severely impaired vs. not impaired physiological cost index, physical component summary score (B).
Discussion
Adult survivors of childhood ALL who are morbidly obese (BMI ≥ 40 kg/m2) have an increased energy cost of walking. This association persists after accounting for treatment exposures and other host factors, including persistent symptoms of motor neuropathy. Moreover, impaired waking efficiency impacts activities of daily life, as survivors who have increased energy cost of walking take longer to complete the TUG, an indicator of general mobility and future functional decline (42). Decreased walking efficiency also impacts perceived well-being among survivors as evident by the lower physical HRQoL reported by survivors with the most impaired energy cost of walking (>90th percentile and >95th percentile) compared to survivors not impaired. Community controls whose energy cost of walking is the most impaired do not report impaired HRQoL, suggesting that survivors, compared to persons without a history of childhood ALL, have more difficulty compensating for obesity during day-to-day activities. Because obesity is potentially modifiable, these data support the need for tailored weight loss interventions that consider the specific energy costs of adult survivors of childhood ALL.
Our finding of increased energy cost of walking among young adult survivors of childhood ALL, compared to community controls, is supported by a study that examined energy cost of walking in younger ALL survivors, but that did not consider obesity or neuropathy as risk factors. Warner et al. compared differences in heart rate at rest and during a treadmill walking test [set speed of 2 kilometers/hour (km/h)] in children and adolescents after treatment for ALL (median age 12.3 years; range 7.2–18.2 years) and sibling controls (43). In their study, heart rate differences were greater among ALL survivors [mean: 112 beats per min (bpm) range: 85–134 bpm] than siblings (mean: 101 bpm; range 75–128 bpm, p < 0.01). As stated, the impact of obesity on this association was not examined, however, survivors had higher BMI values [1.27 standard deviation score (SDS)] than siblings (0.62 SDS). Our additional findings that both obesity and peripheral neuropathy are independently associated with energy cost of walking are supported by studies in populations with neuromuscular disease, that identified associations between gait patterns and energy cost of walking, and by studies in the general population, that observed independent effects of obesity and gait kinematics, on energy cost of walking.
Abnormal gait mechanics, predicted by degree of neuromuscular impairment, are associated with the energy cost of walking in studies that include persons with multiple sclerosis (n = 33, mean age 41 ± 1.7 years) and those with Charcot Marie Tooth Disease (n = 8, median age 34 ± 9.7 years; 37.5% male) (44, 45). Obesity was not included as a risk factor in either of these studies. In studies of the general population, obesity increases the energy cost of walking by 10%–33% (10, 46), independent of abnormal gait biomechanics (47). Adult survivors of childhood ALL are at an increased risk for obesity (3), which confers increased demand, and peripheral motor neuropathy (48, 49), which appears to limit their ability to respond to increased demand (48, 49). Thus, to promote PA in adult survivors of childhood ALL, those who are obese, or have peripheral neuropathy, will need an intervention that addresses both impairments.
In our analysis, when obesity is measured by body fat percentage, rather than BMI, the impact of excess mass on the energy cost of walking is similar between adult ALL survivors and community controls. This suggests that BMI is a measure that does not adequately characterize obesity in adult ALL survivors. As we have previously shown, BMI misclassifies a large proportion of childhood cancer survivors (47% males; 53% females) as obese, when compared to the gold standard (body fat percentage) (24). Alternatively, it may be that body composition is less impactful on energy costs of walking than actual body size among survivors, whose muscle quality may be impaired (50), resulting in altered gait dynamics and reduced gait efficiency. The latter hypothesis is supported by Browning et al. who examined the effects of both excess weight measured by BMI and body composition, measured by dual energy x-ray absorptiometry, on the energy cost of walking at set speeds (0.5, 0.75, 1.00, 1.25, 1.50, or 1.75 m/s) in 39 otherwise healthy persons, some of whom who were obese (mean age 24.5 ± 5.1 years; 48.7% obese; 51.2% male) (51). Participants with obesity (BMI > 30 kg/m2) had 10% higher energy cost of walking, whereas body composition only explained a small part of the increase (r2 = 0.15). In another study, Griffin et al. evaluated the impact of adding lead weights to a waist band (0, 10, 20, or 30% of body weight) on energy cost of walking in eight healthy individuals (mean weight 68.7 ± 12.5 kg; mean age 26.0 ± 5.0 years; 50% male) (52). Adding 30% of body weight increased cost of walking by 47 ± 17%. Survivors of childhood ALL also have impaired lower body strength compared to their peers (50), which could further limit their capacity to compensate for excess weight. Hunter et al. reported a significant association between isometric quadriceps strength and energy cost of walking in 66 overweight premenopausal women (mean age 34.6 ± 6.2 years). They showed an increase of 36 newtons of quadriceps force was associated with a lower peak oxygen uptake requirement [VO2 (mL/kg/min) β −0.37; p < 0.01] at a set walking speed of 4.84 km/h (53). Thus, in addition to addressing obesity and neuropathy to increase ease of movement and promote PA in adult survivors of childhood ALL, increasing muscle strength should be further researched as another component to raise walking efficiency.
Although our study is not the first to report lower adaptive physical function (slower TUG time) or lower scores on measures of HRQoL among ALL survivors compared to community controls (50, 54), our data adds to existing literature by documenting that deficits in adaptive physical function and HRQoL are associated with the energy cost of movement in this vulnerable population. Deficits in adaptive physical function and HRQoL are problematic and relevant. In the general adult population (n = 200, age range 20–50 years, 44.5% male), slower TUG time is associated with higher scores on the cumulative illness rating scale and with lower scores on the PCS SF-36 Summary Scale (55). Among older adults, increasing time on the TUG is associated with risk of future falls, hospitalizations, and functional decline (56–58). When dichotomized, a decline below 50 on the PCS SF-36 Summary Scale is associated with a 58% (95% CI, 30%–91%) increased risk of mortality in women; (59) and when evaluated as a continuous measure, every three point decrement on the PCS increased risk for mortality by 27% (60). Thus, because increased energy cost of walking from obesity is associated both adaptive physical function and HRQoL, early weight management strategies in survivors of childhood ALL, if successful, are likely to have a significant impact on long term health.
Our study has some important strengths. First, we have a large, well characterized study population with not only detailed clinical data, but also detailed disease, treatment, and lifestyle information. This allows for robust analysis and control of confounders of our estimates. Second, our study has a control group, which allows for direct comparisons to a representative sample of the general population, as well as relevant categorizations of impaired energy cost using control percentile scores. Third, objective measures of predictors (BMI and body fat percentage) and outcomes (adaptive physical functioning) limit measurement bias associated with non-objective measures and self-report.
Our study is, however, not without limitations. These data are cross-sectional: temporal associations between obesity, energy cost of walking, adaptive physical function and HRQoL cannot be definitively determined. For example, in an older population, poor physical function is associated with increasing energy costs performing submaximal activities, and survivors can develop functional deficits during treatment that persist through their childhood. More research should be done to exam the temporality between function and energy costs. In addition, our sample was treated at one institution (St. Jude Children’s Research Hospital) between 1962 and 2012; our data may not be generalizable to adult survivors of ALL treated more recently, or at other institutions with different treatment regimens. Furthermore, not every eligible participant agreed to take part in the study. This may bias our estimates of the association between obesity and energy cost of walking. For example, if non-participants were healthier, and less obese, the direction of the association between obesity and the energy cost of walking would be away from the null. Conversely, if the non-participants were less healthy and more obese, the direction of the association would be towards the null. In our study, while we used a self-report questionnaire widely used in population cohorts to characterize PA (35), these data are prone to error when compared to data collected by doubly labelled water or accelerometry (61, 62). Additionally, using calipers to ascertain body fat percentage increases measurement error, thus increase the risk of misclassification bias. Finally, our study looked at the associations between obesity, neuropathy, and energy costs of walking, however, other factors could be impacting energy costs of walking in our survivors. Some examples include muscle weakness, balance, and compensation for chronic pain (63, 64). Future studies should examine these exposures impact on energy costs of walking.
However, using calipers for body fat percentage is valid replacement for the dual energy x-ray absorptiometry in both the general population and childhood cancer survivors (24, 65). Finally, while the PCI is a valid measure of the energy cost of walking, and easy to do across clinical settings, it is not the gold standard, which would include evaluating energy consumption during walking using a portable metabolic cart.
Nevertheless, this study provides important information about the impact of obesity on daily function in adult survivors of childhood ALL. Among survivors, but not among community controls, obesity increased the energy cost of walking, suggesting that the survivors have difficulty compensating for excess weight. Unfortunately, due to their increased energy cost of walking, survivors have disproportionally worse adaptive physical function and HRQoL, compared to community controls. Tailored interventions, accessible to those with increased energy cost of walking, with a focus on weight loss and decreasing the burden of peripheral neuropathy, are needed for this vulnerable population. Providing adaptive equipment or exercise modifications to help manage neuropathy, accompanied by behavioral strategies that promote weight loss and encourage physical activity, should be considered.
Data availability statement
The data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by St. Jude Children’s Research Hospital Human Research Protection Program (HRPP) and the Institutional Review board (IRB). The patients/participants provided their written informed consent to participate in this study.
Author contributions
KKN conceived the study; MDW and KKN provided the study design and manuscript draft; MDW and KKN provided statistical analysis; MDW and KKN acquired data; MMH and LLR designed and conducted the SJLIFE cohort study; MDW and KKN interpreted data; all authors contributed to manuscript revision.
Funding
This study was supported by research funding by the St. Jude Lifetime Cohort Study [NIH U01 CA195547 (MMH and KKN)], Cancer Center Support (CORE) (NIH CA 21765) (CR), and American Lebanese Syrian Associated Charities.
Acknowledgments
The authors acknowledge Tracie Gatewood for her assistance preparing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.976012/full#supplementary-material.
References
1. SEER Cancer Statistics Review, 1975–2014 (2017). Available from: https://seer.cancer.gov/csr/1975_2014/ (Accessed January 1, 2022).
2. Mulrooney DA, Hyun G, Ness KK, Bhakta N, Pui CH, Ehrhardt MJ, et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia: a retrospective analysis of the St Jude Lifetime Cohort Study. Lancet. (2019) 6:e306–e16. doi: 10.1016/S2352-3026(19)30050-X
3. Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, Stovall M, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. (2003) 21:1359–65. doi: 10.1200/JCO.2003.06.131
4. Gurney JG, Ness KK, Sibley SD, O’Leary M, Dengel DR, Lee JM, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. (2006) 107:1303–12. doi: 10.1002/cncr.22120
5. Archives of Physical Medicine and Rehabilitation, Oeffinger KC, Buchanan GR, Eshelman DA, Denke MA, Andrews TC, et al. Cardiovascular risk factors in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. (2001) 23:424–30. doi: 10.1097/00043426-200110000-00007
6. Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five–year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. (2008) 111:5515–23. doi: 10.1182/blood-2007-10-117150
7. Ness KK, DeLany JP, Kaste SC, Mulrooney DA, Pui CH, Chemaitilly W, et al. Energy balance and fitness in adult survivors of childhood acute lymphoblastic leukemia. Blood. (2015) 125:3411–9. doi: 10.1182/blood-2015-01-621680
8. Lemay V, Caru M, Samoilenko M, Drouin S, Mathieu M-E, Bertout L, et al. Physical activity and sedentary behaviors in childhood acute lymphoblastic leukemia survivors. J Pediatr Hematol Oncol. (2020) 42:53–60. doi: 10.1097/MPH.0000000000001594
9. Bernardi M, Macaluso A, Sproviero E, Castellano V, Coratella D, Felici F, et al. Cost of walking and locomotor impairment. J Electromyogr Kinesiol. (1999) 9:149–57. doi: 10.1016/s1050-6411(98)00046-7
10. Browning RC, Kram R. Energetic cost and preferred speed of walking in obese vs. normal weight women. Obes Res. (2005) 13:891–9. doi: 10.1038/oby.2005.103
11. Wert DM, Brach JS, Perera S, VanSwearingen J. The association between energy cost of walking and physical function in older adults. Arch Gerontol Geriat. (2013) 57:198–203. doi: 10.1016/j.archger.2013.04.007
12. Brown DW, Balluz LS, Heath GW, Moriarty DG, Ford ES, Giles WH, et al. Associations between recommended levels of physical activity and health-related quality of life - findings from the 2001 Behavioral Risk Factor Surveillance System (BRFSS) survey. Prev Med. (2003) 37:520–28. doi: 10.1016/S0091-7435(03)00179-8
13. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB, Health A, et al. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. (2004) 52:502–9. doi: 10.1111/j.1532-5415.2004.52154.x
14. Monteiro F, Ponce DAN, Silva H, Pitta F, Carrilho AJF. Physical function, quality of life, and energy expenditure during activities of daily living in obese, post-bariatric surgery, and healthy subjects. Obes Surg. (2017) 27:2138–44. doi: 10.1007/s11695-017-2619-4
15. Howell CR, Wilson CL, Ehrhardt MJ, Partin RE, Kaste SC, Lanctot JQ, et al. Clinical impact of sedentary behaviors in adult survivors of acute lymphoblastic leukemia: a report from the St. Jude Lifetime Cohort study. Cancer. (2018) 124:1036–43. doi: 10.1002/cncr.31162
16. Zeltzer LK, Lu Q, Leisenring W, Tsao JC, Recklitis C, Armstrong G, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. (2008) 17:435–46. doi: 10.1158/1055-9965.EPI-07-2541
17. Bjornard KL, Gilchrist LS, Inaba H, Diouf B, Hockenberry MJ, Kadan-Lottick NS, et al. Peripheral neuropathy in children and adolescents treated for cancer. Lancet Child Adolesc Health. (2018) 2:744–54. doi: 10.1016/S2352-4642(18)30236-0
18. Varedi M, Lu L, Howell C, Partin R, Hudson M, Pui C-H, et al. Peripheral neuropathy, function and quality of life in adult survivors of childhood acute lymphoblastic leukemia. Arch Phys Med Rehabil. (2017) 98:e91–2. doi: 10.1016/j.apmr.2017.08.293
19. Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. (2014) 22:2261–69. doi: 10.1007/s00520-014-2255-7
20. Kandula T, Farrar MA, Cohn RJ, Mizrahi D, Carey K, Johnston K, et al. Chemotherapy-induced peripheral neuropathy in long-term survivors of childhood cancer: clinical, neurophysiological, functional, and patient-reported outcomes. JAMA Neurol. (2018) 75:980–88. doi: 10.1001/jamaneurol.2018.0963
21. Cohen JE, Wakefield CE, Cohn RJ. Nutritional interventions for survivors of childhood cancer. Cochrane Database Syst Rev. (2016) 2016:CD009678. doi: 10.1002/14651858.CD009678.pub2
22. Hudson MM, Ness KK, Nolan VG, Armstrong GT, Green DM, Morris EB, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. (2011) 56:825–36. doi: 10.1002/pbc.22875
23. Jackson AS, Pollock ML. Practical assessment of body composition. Phys Sportsmed. (1985) 13:76–90. doi: 10.1080/00913847.1985.11708790
24. Karlage RE, Wilson CL, Zhang N, Kaste S, Green DM, Armstrong GT, et al. Validity of anthropometric measurements for characterizing obesity among adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. (2015) 121:2036–43. doi: 10.1002/cncr.29300
25. Heo M, Faith MS, Pietrobelli A, Heymsfield SB. Percentage of body fat cutoffs by sex, age, and race-ethnicity in the US adult population from NHANES 1999–2004. Am J Clin Nutr. (2012) 95:594–602. doi: 10.3945/ajcn.111.025171
26. Okuro RT, de Oliveira Ribeiro MAG, Ribeiro JD, Minsky RC, Schivinski CIS. Alternative indexes to estimate the functional capacity from the 6-minute walk test in children and adolescents with cystic fibrosis. Respir Care. (2017) 62:324–32. doi: 10.4187/respcare.04625
27. Merritta C, Cherian B, Macaden AS, John JA. Measurement of physical performance and objective fatigability in people with mild-to-moderate traumatic brain injury. Int J Rehabil Res. (2010) 33:109–14. doi: 10.1097/MRR.0b013e32832e6b37
28. Delussu AS, Morone G, Iosa M, Bragoni M, Paolucci S, Traballesi M. Concurrent validity of physiological cost Index in walking over ground and during robotic training in subacute stroke patients. Biomed Res Int. (2014) 20146. doi: 10.1155/2014/384896
29. Peebles KC, Woodman-Aldridge AD, Skinner MA. The physiological cost index in elderly subjects during treadmill and floor walking. N Z J Physiother. (2003) 31:11–7.
30. Hagberg K, Tranberg R, Zügner R, Danielsson A. Reproducibility of the physiological cost index among individuals with a lower-limb amputation and healthy adults. Physiother Res Int. (2011) 16:92–100. doi: 10.1002/pri.477
31. Hachisuka K, Makino K, Wada F, Saeki S, Yoshimoto N. Oxygen consumption, oxygen cost and physiological cost index in polio survivors: a comparison of walking without orthosis, with an ordinary or a carbon-fibre reinforced plastic knee-ankle-foot orthosis. J Rehabil Med. (2007) 39:646–50. doi: 10.2340/16501977-0105
32. Reulen RC, Zeegers MP, Jenkinson C, Lancashire ER, Winter DL, Jenney ME, et al. The use of the SF-36 questionnaire in adult survivors of childhood cancer: evaluation of data quality, score reliability, and scaling assumptions. Health Qual Life out. (2006) 4:77. doi: 10.1186/1477-7525-4-77
33. Rozzini R, Frisoni GB, Bianchetti A, Zanetti O, Trabucchi M. Physical Performance Test and Activities of Daily Living scales in the assessment of health status in elderly people. J Am Geriatr Soc. (1993) 41:1109–13. doi: 10.1111/j.1532-5415.1993.tb06460.x
34. Lin MR, Hwang HF, Hu MH, Wu HD, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one-leg stand, functional reach, and Tinetti balance measures in community-dwelling older people. J Am Geriatr Soc. (2004) 52:1343–8. doi: 10.1111/j.1532-5415.2004.52366.x
35. Chen T, Clark J, Riddles MK, Mohadjer LK, Fakhouri THI. National health and nutrition examination survey, 2015–2018: sample design and estimation procedures. National center for health statistics. Vital Health Stat. (2020) 2:1–35.
36. Ojha RP, Oancea SC, Ness KK, Lanctot JQ, Srivastava DK, Robison LL, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. (2013) 60:856–64. doi: 10.1002/pbc.24348
37. Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp K. The Modified Total Neuropathy Score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol. (2006) 4:9–16.
38. Harber MP, Metz M, Peterman JE, Whaley MH, Fleenor BS, Kaminsky LA. Trends in cardiorespiratory fitness among apparently healthy adults from the Ball State Adult Fitness Longitudinal Lifestyle STudy (BALL ST) cohort from 1970 to 2019. PLoS One. (2020) 15:e0242995. doi: 10.1371/journal.pone.0242995
39. Pui CH, Evans WE. A 50-year journey to cure childhood acute lymphoblastic leukemia. Semin Hematol. (2013) 50:185–96. doi: 10.1053/j.seminhematol.2013.06.007
40. Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. (1986) 51:1173–82. doi: 10.1037//0022-3514.51.6.1173
41. Steyerberg EW, Eijkemans MJ, Harrell FE Jr., Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. (2000) 19:1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8%3C1059::aid-sim412%3E3.0.co;2-0
42. Wennie Huang WN, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc. (2010) 58:844–52. doi: 10.1111/j.1532-5415.2010.02820.x
43. Warner JT, Bell W, Webb DK, Gregory JW. Daily energy expenditure and physical activity in survivors of childhood malignancy. Pediatr Res. (1998) 43:607–13. doi: 10.1203/00006450-199805000-00008
44. Olgiati R, Burgunder JM, Mumenthaler M. Increased energy cost of walking in multiple sclerosis: effect of spasticity, ataxia, and weakness. Arch Phys Med Rehabil. (1988) 69:846–9.3178452
45. Menotti F, Felici F, Damiani A, Mangiola F, Vannicelli R, Macaluso A. Charcot-Marie-Tooth 1A patients with low level of impairment have a higher energy cost of walking than healthy individuals. Neuromuscular Disord. (2011) 21:52–7. doi: 10.1016/j.nmd.2010.09.008
46. Freyschuss U, Melcher A. Exercise energy expenditure in extreme obesity: influence of ergometry type and weight loss. Scand J Clin Lab Invest. (1978) 38:753–9. doi: 10.1080/00365517809104883
47. Browning RC, McGowan CP, Kram R. Obesity does not increase external mechanical work per kilogram body mass during walking. J Biomech. (2009) 42:2273–78. doi: 10.1016/j.jacc.2008.12.068
48. Gilchrist L, Tanner L. Gait patterns in children with cancer and vincristine neuropathy. Pediatr Phys Ther. (2016) 28:16–22. doi: 10.1097/PEP.0000000000000208
49. Tay CG, Lee VWM, Ong LC, Goh KJ, Ariffin H, Fong CY. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. (2017) 64:e26471. doi: 10.1002/pbc.26471
50. Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. (2007) 49:975–81. doi: 10.1002/pbc.21091
51. Browning RC, Baker EA, Herron JA, Kram R. Effects of obesity and sex on the energetic cost and preferred speed of walking. J Appl Physiol. (2006) 100:390–8. doi: 10.1152/japplphysiol.00767.2005
52. Griffin TM, Roberts TJ, Kram R. Metabolic cost of generating muscular force in human walking: insights from load-carrying and speed experiments. J Appl Physiol. (2003) 95:172–83. doi: 10.1152/japplphysiol.00944.2002
53. Hunter GR, McCarthy JP, Bryan DR, Zuckerman PA, Bamman MM, Byrne NM. Increased strength and decreased flexibility are related to reduced oxygen cost of walking. Eur J Appl Physiol. (2008) 104:895–901. doi: 10.1007/s00421-008-0846-z
54. Khan RB, Hudson MM, Ledet DS, Morris EB, Pui CH, Howard SC, et al. Neurologic morbidity and quality of life in survivors of childhood acute lymphoblastic leukemia: a prospective cross-sectional study. J Cancer Surviv. (2014) 8:688–96. doi: 10.1007/s11764-014-0375-1
55. Kear BM, Guck TP, McGaha AL. Timed up and go (TUG) test: normative reference values for ages 20 to 59 years and relationships with physical and mental health risk factors. J Prim Care Community Health. (2017) 8:9–13. doi: 10.1177/2150131916659282
56. Eagles D, Perry JJ, Sirois MJ, Lang E, Daoust R, Lee J, et al. Timed up and go predicts functional decline in older patients presenting to the emergency department following minor traumadagger. Age Ageing. (2017) 46:214–18. doi: 10.1093/ageing/afw184
57. Viccaro LJ, Perera S, Studenski SA. Is timed up and go better than gait speed in predicting health, function, and falls in older adults? J Am Geriatr Soc. (2011) 59:887–92. doi: 10.1111/j.1532-5415.2011.03336.x
58. Russell MA, Hill KD, Blackberry I, Day LL, Dharmage SC. Falls risk and functional decline in older fallers discharged directly from emergency departments. J Gerontol A Biol Sci Med Sci. (2006) 61:1090–5. doi: 10.1093/gerona/61.10.1090
59. Kroenke CH, Kubzansky LD, Adler N, Kawachi I. Prospective change in health-related quality of life and subsequent mortality among middle-aged and older women. Am J Public Health. (2008) 98:2085–91. doi: 10.2105/AJPH.2007.114041
60. Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health. (2013) 16:993–1000. doi: 10.1016/j.jval.2013.06.022
61. Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. (2003) 37:197–206; discussion 06. doi: 10.1136/bjsm.37.3.197
62. Rikli RE. Reliability, validity, and methodological issues in assessing physical activity in older adults. Res Q Exercise Sport. (2000) 71(Suppl 2):89–96. doi: 10.1080/02701367.2000.11082791
63. Marques NR, LaRoche DP, Hallal CZ, Crozara LF, Morcelli MH, Karuka AH, et al. Association between energy cost of walking, muscle activation, and biomechanical parameters in older female fallers and non-fallers. Clin Biomech (Bristol, Avon). (2013) 28:330–36. doi: 10.1016/j.clinbiomech.2013.01.004
64. IJmker T, Houdijk H, Lamoth CJ, Jarbandhan AV, Rijntjes D, Beek PJ, et al. Effect of balance support on the energy cost of walking after stroke. Arch Phys Med Rehabil. (2013) 94:2255–61. doi: 10.1016/j.apmr.2013.04.022
Keywords: childhood cancer, fitness, obesity, quality of life, acute lymphoblastic leukemia
Citation: Wogksch MD, Finch ER, Nolan VG, Smeltzer MP, Mzayek F, Goodenough CG, Pui C, Inaba H, Mulrooney DA, Kaste SC, Brinkman TM, Lanctot JQ, Srivastava DK, Jefferies JL, Armstrong GT, Robison LL, Hudson MM and Ness KK (2022) Energy cost of walking in obese survivors of acute lymphoblastic leukemia: A report from the St. Jude Lifetime Cohort. Front. Pediatr. 10:976012. doi: 10.3389/fped.2022.976012
Received: 22 June 2022; Accepted: 10 October 2022;
Published: 28 October 2022.
Edited by:
Amanda Wurz, University of the Fraser Valley, CanadaReviewed by:
Sabine Marie Pierre Verschueren, KU Leuven, BelgiumVesile Yildiz Kabak, Hacettepe University, Turkey
© 2022 Wogksch, Finch, Nolan, Smeltzer, Mzayek, Goodenough, Pui, Inaba, Mulrooney, Kaste, Brinkman, Lanctot, Srivastava, Jefferies, Armstrong, Robison, Hudson and Ness. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kirsten K. Ness S2lyaS5OZXNzQHN0anVkZS5vcmc=
Specialty Section: This article was submitted to Pediatric Oncology, a section of the journal Frontiers in Pediatrics
Abbreviations ALL, Acute lymphoblastic leukemia; 6 MWT, six-minute walk test; BMI, body mass index; HRQoL, Health-related quality of life; MCS, mental component summary score; PA, physical activity; PCI, physiological cost index; PCS, physical component summary score; SF-36, Medical Outcomes Survey Short-Form-36; PPT, physical performance test; TUG, timed up and go.
 Matthew D. Wogksch
Matthew D. Wogksch Emily R. Finch1
Emily R. Finch1 Vikki G. Nolan
Vikki G. Nolan Matthew P. Smeltzer
Matthew P. Smeltzer Fawaz Mzayek
Fawaz Mzayek Chelsea G. Goodenough
Chelsea G. Goodenough Hiroto Inaba
Hiroto Inaba Daniel A. Mulrooney
Daniel A. Mulrooney Tara M. Brinkman
Tara M. Brinkman Deo Kumar Srivastava
Deo Kumar Srivastava John L. Jefferies
John L. Jefferies Leslie L. Robison
Leslie L. Robison Melissa M. Hudson
Melissa M. Hudson Kirsten K. Ness
Kirsten K. Ness