
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 03 October 2022
Sec. Pediatric Urology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.973016
 Qiang Zhang1,2,3,4
Qiang Zhang1,2,3,4 Zhi-Cheng Zhang1,2,3,4
Zhi-Cheng Zhang1,2,3,4 Xue-Yu He1,2,3,4
Xue-Yu He1,2,3,4 Zhen-Min Liu1,2,3,4
Zhen-Min Liu1,2,3,4 Guang-Hui Wei1,2,3,4
Guang-Hui Wei1,2,3,4 Xing Liu1,2,3,4*
Xing Liu1,2,3,4*Background: Investigations regarding the association between maternal smoking and specific urogenital teratogenesis exist. However, an integrated systematic review and meta-analysis studying the relationship by encompassing the whole urogenital system is essential.
Objective: Even though many studies about inborn urogenital malformations have been conducted, its etiologic factors and exact pathogenesis are still unclear. Our aim is to assess the risk of congenital urogenital malformations in offspring of smoking pregnant women.
Results: The meta-analysis, covering 41 case-control and 11 cohort studies, suggested that maternal smoking was associated with an increased risk of urogenital teratogenesis (odds ratio [OR] = 1.13, 95% confidence interval [CI]: 1.04–1.23, p = 0.005), cryptorchidism (OR = 1.18, 95%CI: 1.12-1.24, p = 0.0001), hypospadias (OR = 1.16, 95%CI: 1.01-1.33, p = 0.039), and kidney malformations (OR = 1.30, 95%CI: 1.14-1.48, p = 0.0001). Moreover, paternal smoking during the mother’s pregnancy was also significantly associated (OR = 1.26, 95%CI: 1.03-1.55, p = 0.028). The association between smoking > 10 cigarettes/day was evident but was not significant (OR = 1.24, 95%CI:0.81-1.88, p = 0.323).
Conclusion: Our results showed that maternal smoking during pregnancy increased the risk of congenital urogenital malformations. In numerous epidemiological studies, maternal smoking during pregnancy has a significant role in fetal development. Therefore, quitting tobacco use may be an effective method for reducing the risk of congenital urogenital malformation in pregnant women.
The etiology of congenital malformations involves various genetic and environmental factors. However, the associations of environmental factors have been rarely characterized in the previous study (1). Maternal smoking, particularly during the gestation period, is a substantial risk factor for congenital malformations (2). Maternal smoking during pregnancy influenced the occurrence of congenital abnormalities (3). Due to the small prevalence of urogenital teratogenesis, a specific abnormality may be categorized as a genetic or gross defect (4). The mechanism of association between maternal smoking during pregnancy and congenital malformations has remained unclear. It was reported in several studies (5, 6) that maternal smoking during the gestation period was related to the increased risk of low birthweight (LBW) and spontaneous abortion. Regarding spontaneous abortion, the toxic effect of cigarette smoking during pregnancy on the fetus may cloud the teratogenic effect (7). Hence, the urogenital malformation rate of offspring among smoking pregnant women was relatively high (8). Moreover, the study (9) has shown that maternal smoking during pregnancy was significantly related to congenital urinary tract defects. Although the relationship between maternal smoking during pregnancy and specific urogenital malformations, such as urinary tract defects, cryptorchidism, hypospadias, and kidney defects in offspring seem to be established, an integrated systematic meta-analysis of the relationship considering the whole urogenital system is essential.
Maternal smoking during pregnancy is regarded as an adverse birth outcome. Tobacco smoke is composed of more than 2,000 compounds (8). Nicotine is a key ingredient in cigarettes. Also, nicotine replacement therapy has been proposed in countless studies. However, the safety and effectiveness of the method are unclear. The nicotine ingestion of pregnant women had a dose-response effect on the bloodstream of the embryo (10). As a result, the umbilical artery blood flow speed alters, affecting the fetal cardiovascular system (11). Nicotine, increases vasoconstriction and endothelial injury, causing hypoxia, which leads to abnormal fetal morphology (12). Hypoxia is associated with, elevated levels of environmental pollutants, and maternal smoking during pregnancy. Smoking during pregnancy is the most common fetal toxic exposure across the countries, which reduces fetal growth and increases the risk of some placental complications and fetal abnormalities (7, 13, 14).
Maternal smoking-associated teratogenesis may be related to hormone levels (15). Maternal smoking during pregnancy may cause metabolic derangement, that could contribute to urogenital teratogenesis (16). In male births, cryptorchidism and hypospadias are the most common urogenital malformations. Even though many studies about inborn urogenital malformations have been conducted, its etiologic factors and exact pathogenesis are still unclear. Many studies evaluated the interrelation between maternal smoking during pregnancy and specific urinary abnormalities. Still, there is no systematic review regarding the relationship between maternal smoking and urogenital teratogenesis. Therefore, we undertook this review and meta-analysis to evaluate the association of maternal smoking during pregnancy with the risk of congenital urogenital malformations.
The studies that met the following criteria were selected: (1) the case group or the exposure group included babies diagnosed with congenital urogenital malformations; (2) the control group comprised babies without congenital urogenital malformations; (3) maternal smoking during pregnancy or paternal smoking during mother’s pregnancy or other words similar to smoking was investigated; and (4) the study was the cohort, case-control, or other designs.
The studies that met the subsequent criteria were excluded: (1) reviewed and repeated articles; (2) non-English and unable to access full articles; and (3) articles without providing valuable data.
Guidelines for PRISMA (17) were followed in this study, and no similar research was found. In PubMed, Cochrane Library, Embase, Science Direct, and Web of Science Database, we searched for relevant, English-language studies until February 22, 2022. The search term was ((((((gestational smoking) OR gestational cigarette exposure) OR gestational tobacco exposure) OR nicotine exposure)) AND ((((((((congenital anomalies) OR Congenital Abnormality) OR Congenital Defects) OR Congenital Defect) OR Fetal Malformations) OR Fetal Malformation) OR Fetal Anomalies) OR Fetal Anomaly)). Moreover, we identified studies based on reference literature.
(Figure 1) displays flow diagram of the process of study identification and included. We assessed every selected study carefully. Two reviewers independently extracted the first author, year of publication, country, research type, number of congenital urogenital malformations, and maternal or paternal smoking during pregnancy, respectively in each group, as well as the count of cigarettes/day, type of congenital urogenital malformation, the ORs and CIs. The third author independently reviewed all of the selected articles. Additionally, we described the included study’s characteristics (Supplementary Table 1). Eventually, the quality assessment of studies was conducted based on the Newcastle-Ottawa Scale (NOS) (18).
Due to diversity in the follow-up span, maternal or paternal characteristics, and inclusion criteria for case offspring, heterogeneity may occur in data analysis (19). Heterogeneity plays an essential role in statistical analysis and could lead to sampling errors (20). To assess heterogeneity between studies, the X2 test was used, as well as the I2 statistic of inconsistency. We combined data using the random effects model when there was heterogeneity, otherwise, we used the fixed effects model. Using existing data in included studies, we calculated the independent OR, ES, and 95%CI of the relationship between maternal or paternal smoking during pregnancy and the risk of congenital urogenital malformations. There was significance for p-values of < 0.05. An Egger’s test was conducted to assess publication bias, and the results were tested for stability using a sensitivity analysis. Calculations were made using STATA/MP 17.0 software (StataCorp, College Station, TX, United States).
The following databases were searched: PubMed, Cochrane Library, Embase, Science Direct, and Web of Science Database, covering 41 case-control articles (1, 3, 4, 9, 15, 21–56) and 11 cohort articles (7, 57–66). (Figure 2), from the 19 articles (1, 3, 4, 7, 9, 24, 25, 30, 34, 38, 49, 52, 57–61, 64, 65), suggested that maternal smoking was associated with an increased risk of urogenital teratogenesis (OR = 1.13, 95%CI: 1.04–1.23, p = 0.005). We combined data using the random effects model when there was high heterogeneity (I2 = 63.8%). Based on the sensitivity analysis (Supplementary Figure 8), the results were stable. Using Egger’s test for publication bias, we found publication bias (p = 0.001). Perhaps, the results need careful interpretation.
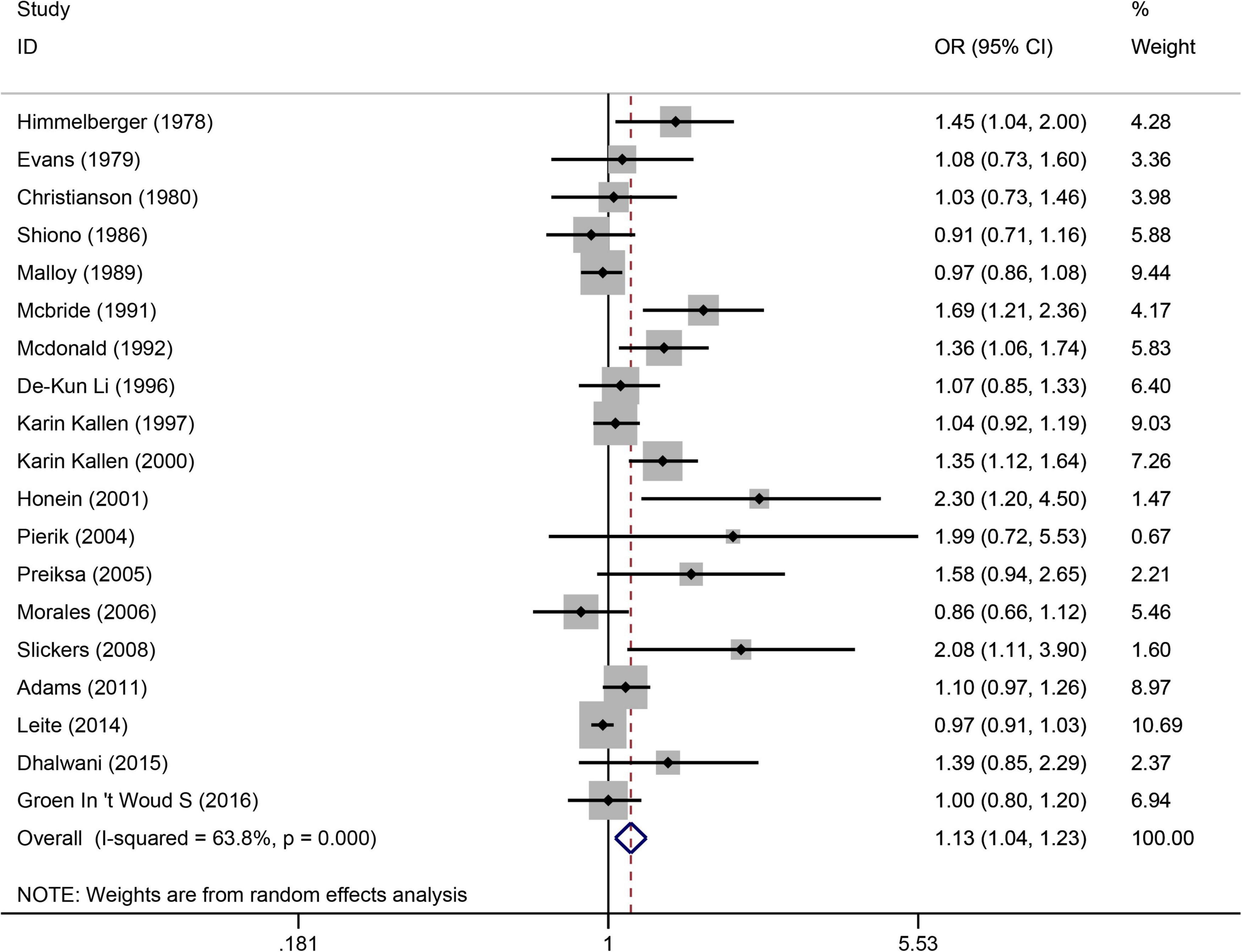
Figure 2. Forest plot for the relationship between maternal smoking during pregnancy and the risk of congenital urogenital malformations. Studies are sorted according to the sequence of publication time.
(Figure 3) shows the relationship between maternal smoking during pregnancy and the increased risk of cryptorchidism. A total of 21 studies (4, 22, 25–29, 31, 34, 36, 39, 40, 42–44, 46, 48, 52–54, 63) showed that maternal smoking during pregnancy increased the risk of cryptorchidism by 1.18 times (OR = 1.18, 95%CI:1.12–1.24, p = 0.0001). As there was no significant heterogeneity (I2 = 45.8%, p = 0.012), a fixed effects model was used. Based on Egger’s test (p = 0.689), there was free publication bias.
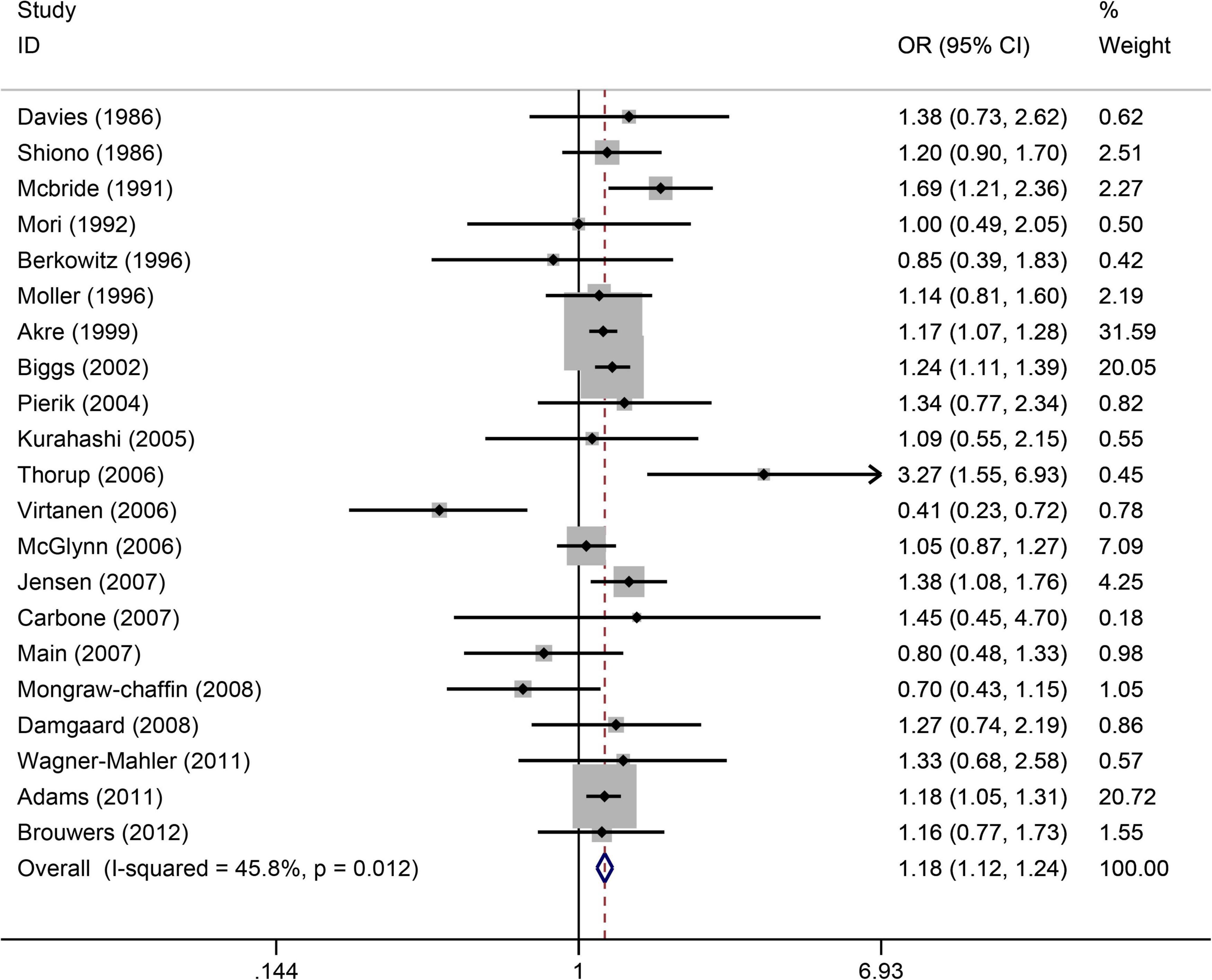
Figure 3. Forest plot for between maternal smoking during pregnancy and the risk of cryptorchidism. Studies are sorted by the sequence of publication time.
(Figure 4) displays the significantly increased risk of hypospadias associated with maternal smoking during pregnancy. There were 12 studies (21, 34, 35, 37, 41, 45, 47, 50–52, 55, 66) to evaluate the association between maternal smoking during pregnancy and the risk of hypospadias (OR = 1.16, 95%CI: 1.01–1.33, p = 0.039). The random effects model was used as there was significant heterogeneity (I2 = 53.4%). Sensitivity analysis (Supplementary Figure 9) showed the stability of the results. The Egger’s test was used to assess publication bias, which showed no evidence of publication bias (p = 0.712).
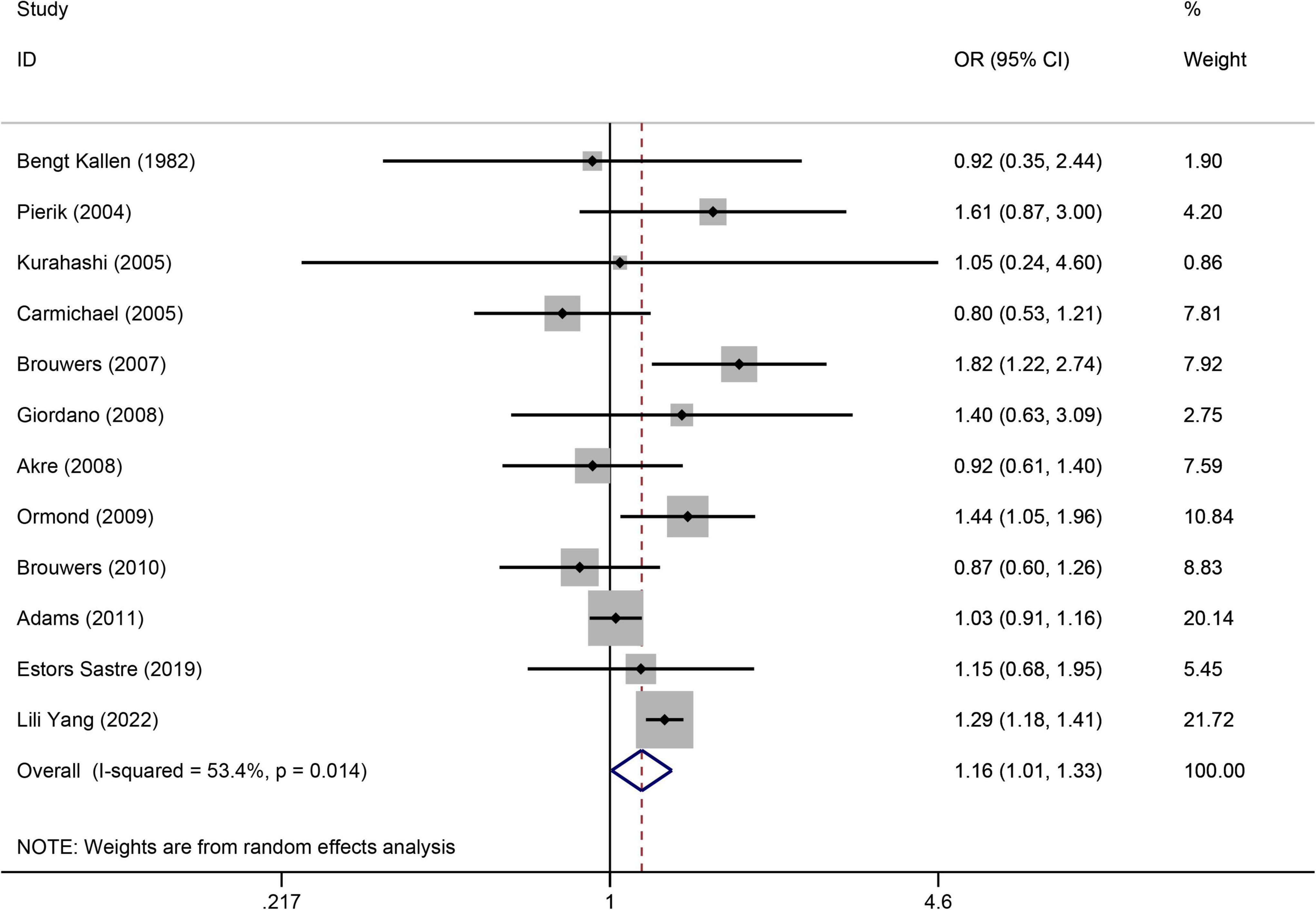
Figure 4. Forest plot for the relationship between maternal smoking during pregnancy and the risk of hypospadias. Studies were sorted by the sequence of publication time.
(Figure 5) displays a significant increased risk of having a baby with kidney malformations in the six articles (32, 33, 49, 59, 60, 62) when maternal smoking during pregnancy (OR = 1.30, 95%CI: 1.14–1.48, p = 0.0001). Due to the heterogeneity (I2 = 43%), we used the fax effects model. We evaluated the publication bias using Egger’s test and found that there is no publication bias (p = 0.331).
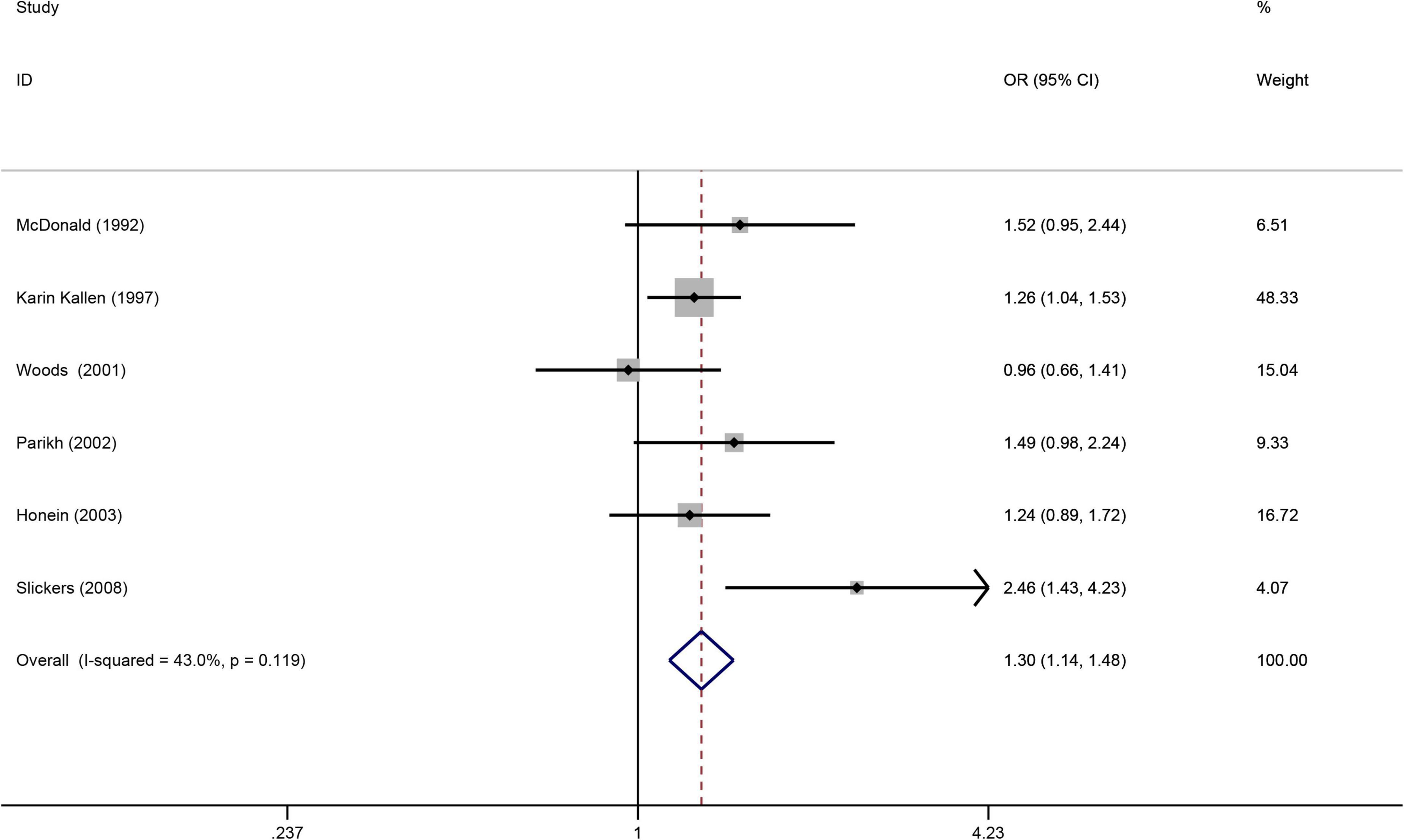
Figure 5. Forest plot for the relationship between maternal smoking during pregnancy and the risk of kidney malformations. Studies were sorted by the sequence of publication time.
(Figure 6) shows the significant association of risk of congenital urogenital malformations when paternal smoking during a mother’s pregnancy. A total of 13 case-control articles (9, 28, 34–36, 41–43, 49, 51, 53–55) showed that paternal smoking during a mother’s pregnancy increased the risk of congenital urogenital malformations by 1.26 times (OR = 1.26, 95%CI: 1.03–1.55, p = 0.028). Due to the high heterogeneity (I2 = 70.3%), we used the random effects model. A sensitivity analysis confirmed the robustness of the results (Supplementary Figure 10). We evaluated the publication bias using Egger’s test and found that there is no publication bias (p = 0.071).

Figure 6. Forest plot for the increased risk of congenital urogenital malformations when paternal smoking during a mother’s pregnancy. Studies were sorted by the sequence of publication time.
(Figure 7) presents the non-significant association of >10 cigarettes/day with maternal smoking during pregnancy. A total of 9 case-control articles (15, 23, 28, 42, 43, 45, 49, 55, 56) were used to evaluate the relationship between amount of >10 cigarettes/day and risk of congenital urogenital malformations (OR = 1.24, 95%CI:0.81–1.88, p = 0.323). We combined data using the random effects model when there was high heterogeneity (I2 = 86.9%). Using Egger’s test for publication bias, we found no publication bias (p = 0.331). A sensitivity analysis confirmed the stability of the results (Supplementary Figure 11).

Figure 7. Forest plot for the non-significant association of > 10 cigarettes/day when maternal smoking during pregnancy. Studies were sorted by the sequence of publication time.
With the growing incidence of urogenital teratogenesis, growing attention is directed toward the field of urogenital health. To our knowledge, the present study was the first systematic meta-analysis encompassing the whole urogenital system, that revealed an increased risk of urogenital teratogenesis in maternal smoking.
Our meta-analysis concluded that maternal smoking during pregnancy correlated with the increased risk of urogenital teratogenesis. The risk of genetic urogenital malformations in offspring of smoking pregnant women was found to be 1.13 times higher compared to non-smoking pregnant women. Maternal smoking over the gestation period was associated with an increased risk of cryptorchidism and hypospadias in the offspring. Moreover, we showed an increased association between paternal smoking during a mother’s pregnancy and urogenital teratogenesis. The included studies had statistical heterogeneity, and we conducted data analysis by using a random effects model. Moreover, sensitivity analysis showed that the results did not change before and after the analysis, which confirmed their stability.
The underlying mechanism of maternal smoking-induced congenital malformations remains unclear. Several studies have suggested possible mechanisms. The increased levels of carboxyhemoglobin in maternal and fetal blood are directly linked to fetal hypoxia and developmental defect associated with smoking during pregnancy (57). The teratogenic effect may be a sign of fetal hypoxia (67). In addition, the instability of natural killer cell activity and thyroid function is associated with increased thyroglobulin level, which is known to be elevated in smoking pregnant women. This causes low immunity and increases vulnerability to infection (68). Maternal smoking may affect the body’s hormone levels by disturbing the gonadal axis, leading to congenital malformations (69, 70). Thus, further research is required to elucidate the mechanisms at work.
The incidence of cryptorchidism is related not only to genetic factors but also to parental environmental factors. Several studies (25, 29, 31, 43) have reported that maternal smoking during pregnancy increases the risk of cryptorchidism. Smoking causes impaired placental function, and cryptorchidism is associated with impaired placental functioning (31). Due to androgen-dependent male sexual differentiation, exposure to an environment that affects androgen homeostasis during fetal life may contribute to cryptorchidism (36). Changing levels of endogenous estrogen in smoking mothers may be associated with cryptorchidism in offspring. The report indicates that the levels of human chorionic gonadotropin and epidermal growth factor were lower in smokers than in non-smokers (71). A significant increase in cryptorchidism risk was also observed among sons whose mothers consumed oral contraceptives during pregnancy (54). There are pieces of evidence that the use of analgesics, such as paracetamol or paracetamol during pregnancy, especially in the first trimester, may increase the risk of cryptorchidism (72, 73). However, the potential mechanism of how drugs mediate cryptorchidism needs further study.
The relationship between maternal smoking with teratogenic hypospadias is uncertain. Tissue fusion is critical to the formation and function of organs and tissues during embryonic development, including the heart, neural tube, face, and urethra (74). Disruption of Shh and Fgf signals during urethral development leads to failure of urethral plate fusion (75). There are higher rates of congenital heart disease and cleft lip in infants of smoking mothers (24, 64). It has been reported that there may be associations between maternal heavy smoking and neural tube defects (59, 76). Studies have found that maternal smoking is related to birth defects of hypospadias (34). One study demonstrated that nicotine intake by smoking pregnant women modulates fibrosis by changing the function of fibrocytes (77). The fibrocyte growth factors play an indispensable role in the development of the urethral plate and the formation of hypospadias (78). A case reported in one study showed that hypoxia might result in hypospadias (79). However, the biological mechanisms underlying hypospadias caused by nicotine or other chemicals in tobacco remain unclear.
We observed the association between maternal smoking and renal defects, although a low number of studies was included. It has been reported that smoking during pregnancy is associated with renal malformation (49, 60). The reduction of ureteric branching and nephron number, through ureteric β-catenin signaling, is observed in the hypoxic kidney (80, 81). The prevalence rate of kidney malformations is so low that there are few reports about renal teratogenesis associated with maternal smoking. Due to the association between maternal smoking during pregnancy and spontaneous abortion or stillbirth (3, 5, 82), there is a weak association between maternal smoking during live birth and congenital kidney abnormalities. Possibly, the toxic effects of cigarette smoking during pregnancy clouded the teratogenic effect (7). Kidney malformations account for a small part of teratogenesis. Further studies are required with large sample size.
Paternal smoking leads to passive smoking; however, there were no reports on the harmful effects of passive smoking on urogenital teratogenesis. Our results suggested that passive smoking plays an important role in fetal development compared with active smoking. We speculate that non-smoking mothers may be more sensitive to alien smoking. Paternal smoking during pregnancy is associated with urethral stricture, possibly due to passive smoking (83). More research is needed to determine how passive smoking affects urogenital malformations.
The study had some limitations. First, the relationship between maternal medication use during pregnancy and urogenital malformations was not assessed. Some drugs may have potential etiological effects on urogenital development; however, this relationship cannot be assessed due to the limited number of cases. There are potential deviations from our results. Second, the association between the use of assisted reproductive technology in smoking mothers and urogenital malformations was not evaluated in our study. Demand for assisted reproductive technology increases as human male reproductive health declines. Some studies have reported that assisted reproductive technology increases the risk of congenital urogenital malformations. After a careful review of mothers’ characteristics, we found that information about assisted reproductive technology was not collected in the included studies. Thus, this relationship assessment was not performed. Confounding factors, such as alcoholic beverage use, age, education level, vegetarian diet, and history of infertility, had not always been considered in the study. Third, we did not find a dose-response effect between the quantity of smoking and the risk of urogenital teratogenesis. This might be due to the lack of continuous dose level measurements. Perhaps, studies with a higher sample size were used to assess associations between maternal heavy smoking and urogenital teratogenesis.
An increased risk of congenital urogenital malformation is associated with smoking among pregnant women. There is a gradual increase in evidence of the harmful, comprehensive fetal toxic effects of smoking, warning pregnant women not to smoke at all during pregnancy.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
QZ designed the study, analyzed the data, and completed the manuscript. Z-CZ designed the study and assisted in drafting and revising the manuscript. X-YH and Z-ML independently searched and extracted the data. G-HW helped revise the manuscript. XL served as the corresponding author, provided financial support, and assisted with drafting and revising the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (No. 81970571).
We would like to acknowledge the support of the Department of Urology of Children’s Hospital of Chongqing Medical University, the financial support of the National Natural Science Foundation of China, and we would like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.973016/full#supplementary-material
CI, confidence interval; OR, odds ratio.
1. Groen In’t Woud S, Renkema KY, Schreuder MF, Wijers CH, van der Zanden LF, Knoers NV, et al. Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: a case-control study. Birth Defects Res A Clin Mol Teratol. (2016) 106:596–603. doi: 10.1002/bdra.23500
2. Xuan Z, Zhongpeng Y, Yanjun G, Jiaqi D, Yuchi Z, Bing S, et al. Maternal active smoking and risk of oral clefts: a meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:680–90. doi: 10.1016/j.oooo.2016.08.007
3. Himmelberger DU, Brown BW Jr., cohen EN. Cigarette smoking during pregnancy and the occurrence of spontaneous abortion and congenital abnormality. Am J Epidemiol. (1978) 108:470–9. doi: 10.1093/oxfordjournals.aje.a112645
4. Shiono PH, Klebanoff MA, Berendes HW. Congenital malformations and maternal smoking during pregnancy. Teratology. (1986) 34:65–71. doi: 10.1002/tera.1420340109
5. Kline J, Stein ZA, Susser M, Warburton D. Smoking: a risk factor for spontaneous abortion. N Engl J Med. (1977) 297:793–6. doi: 10.1056/NEJM197710132971501
6. Li DK, Daling JR. Maternal smoking, low birth weight, and ethnicity in relation to sudden infant death syndrome. Am J Epidemiol. (1991) 134:958–64. doi: 10.1093/oxfordjournals.aje.a116180
7. Morales-Suárez-Varela MM, Bille C, Christensen K, Olsen J. Smoking habits, nicotine use, and congenital malformations. Obstetr Gynecol. (2006) 107:51–7. doi: 10.1097/01.AOG.0000194079.66834.d5
8. Abel EL. Smoking and pregnancy. J Psycho Drugs. (1984) 16:327–38. doi: 10.1080/02791072.1984.10472303
9. Li DK, Mueller BA, Hickok DE, Daling JR, Fantel AG, Checkoway H, et al. Maternal smoking during pregnancy and the risk of congenital urinary tract anomalies. Am J Public Health. (1996) 86:249–53. doi: 10.2105/AJPH.86.2.249
10. Lindblad A, Marsál K, Andersson KE. Effect of nicotine on human fetal blood flow. Obstetr Gynecol. (1988) 72:371–82.
11. Bruner JP, Forouzan I. Smoking and buccally administered nicotine. Acute effect on uterine and umbilical artery doppler flow velocity waveforms. J Reprod Med. (1991) 36:435–40.
12. Quinton AE, Cook CM, Peek MJ. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG. (2008) 115:780–4. doi: 10.1111/j.1471-0528.2008.01691.x
13. O’Callaghan MJ, Harvey JM, Tudehope DI, Gray PH. Aetiology and classification of small for gestational age infants. J Paediatr Child Health. (1997) 33:213–8. doi: 10.1111/j.1440-1754.1997.tb01582.x
14. Meis PJ, Michielutte R, Peters TJ, Wells HB, Sands RE, Coles EC, et al. Factors associated with term low birthweight in Cardiff, Wales. Paediatr Perinatal Epidemiol. (1997) 11:287–97. doi: 10.1111/j.1365-3016.1997.tb00006.x
15. Källén K. Role of maternal smoking and maternal reproductive history in the etiology of hypospadias in the offspring. Teratology. (2002) 66:185–91. doi: 10.1002/tera.10092
16. Håkonsen LB, Ernst A, Ramlau-Hansen CH. Maternal cigarette smoking during pregnancy and reproductive health in children: a review of epidemiological studies. Asian J Androl. (2014) 16:39–49. doi: 10.4103/1008-682X.122351
17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Lo CK, Mertz D, Loeb M. Newcastle-ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20. Borenstein M, Higgins JP, Hedges LV, Rothstein HR. Basics of meta-analysis: I(2) is not an absolute measure of heterogeneity. Res Synth Methods. (2017) 8:5–18. doi: 10.1002/jrsm.1230
21. Källén B, Winberg J. An epidemiological study of hypospadias in Sweden. Acta Paediatr Scand Suppl. (1982) 293:1–21. doi: 10.1111/j.1651-2227.1982.tb09577.x
22. Davies TW, Williams DR, Whitaker RH. Risk factors for undescended testis. Int J Epidemiol. (1986) 15:197–201. doi: 10.1093/ije/15.2.197
23. Källén B. Case control study of hypospadias, based on registry information. Teratology. (1988) 38:45–50. doi: 10.1002/tera.1420380107
24. Malloy MH, Kleinman JC, Bakewell JM, Schramm WF, Land GH. Maternal smoking during pregnancy: no association with congenital malformations in Missouri 1980-83. Am J Public Health. (1989) 79:1243–6. doi: 10.2105/AJPH.79.9.1243
25. McBride ML, Van den Steen N, Lamb CW, Gallagher RP. Maternal and gestational factors in cryptorchidism. Int J Epidemiol. (1991) 20:964–70. doi: 10.1093/ije/20.4.964
26. Mori M, Davies TW, Tsukamoto T, Kumamoto Y, Fukuda K. Maternal and other factors of cryptorchidism – a case-control study in Japan. Kurume Med J. (1992) 39:53–60. doi: 10.2739/kurumemedj.39.53
27. Berkowitz GS, Lapinski RH. Risk factors for cryptorchidism: a nested case-control study. Paediatr Perinatal Epidemiol. (1996) 10:39–51. doi: 10.1111/j.1365-3016.1996.tb00024.x
28. Møller H, Skakkebaek NE. Risks of testicular cancer and cryptorchidism in relation to socio-economic status and related factors: case-control studies in Denmark. Int J Cancer. (1996) 66:287–93. doi: 10.1002/(SICI)1097-0215(19960503)66:3<287::AID-IJC2>3.0.CO;2-V
29. Akre O, Lipworth L, Cnattingius S, Sparén P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology (Cambridge Mass). (1999) 10:364–9. doi: 10.1097/00001648-199907000-00005
30. Källén K. Multiple malformations and maternal smoking. Paediatr Perinatal Epidemiol. (2000) 14:227–33. doi: 10.1046/j.1365-3016.2000.00269.x
31. Biggs ML, Baer A, Critchlow CW. Maternal, delivery, and perinatal characteristics associated with cryptorchidism: a population-based case-control study among births in Washington State. Epidemiology (Cambridge Mass). (2002) 13:197–204. doi: 10.1097/00001648-200203000-00015
32. Parikh CR, McCall D, Engelman C, Schrier RW. Congenital renal agenesis: case-control analysis of birth characteristics. Am J Kidney Dis. (2002) 39:689–94. doi: 10.1053/ajkd.2002.31982
33. Honein MA, Moore CA, Watkins ML. Subfertility and prepregnancy overweight/obesity: possible interaction between these risk factors in the etiology of congenital renal anomalies. Birth Defects Res A Clin Mol Teratol. (2003) 67:572–7. doi: 10.1002/bdra.10077
34. Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect. (2004) 112:1570–6. doi: 10.1289/ehp.7243
35. Carmichael SL, Shaw GM, Laurent C, Lammer EJ, Olney RS. Hypospadias and maternal exposures to cigarette smoke. Paediatr Perinatal Epidemiol. (2005) 19:406–12. doi: 10.1111/j.1365-3016.2005.00680.x
36. Kurahashi N, Kasai S, Shibata T, Kakizaki H, Nonomura K, Sata F, et al. Parental and neonatal risk factors for cryptorchidism. Med Sci Monit. (2005) 11:Cr274–83.
37. Kurahashi N, Sata F, Kasai S, Shibata T, Moriya K, Yamada H, et al. Maternal genetic polymorphisms in CYP1A1, GSTM1 and GSTT1 and the risk of hypospadias. Mol Hum Reprod. (2005) 11:93–8. doi: 10.1093/molehr/gah134
38. Preiksa RT, Zilaitiene B, Matulevicius V, Skakkebaek NE, Petersen JH, Jørgensen N, et al. Higher than expected prevalence of congenital cryptorchidism in Lithuania: a study of 1204 boys at birth and 1 year follow-up. Hum Reprod (Oxford Engl). (2005) 20:1928–32. doi: 10.1093/humrep/deh887
39. McGlynn KA, Graubard BI, Klebanoff MA, Longnecker MP. Risk factors for cryptorchism among populations at differing risks of testicular cancer. Int J Epidemiol. (2006) 35:787–95. doi: 10.1093/ije/dyl024
40. Virtanen HE, Tapanainen AE, Kaleva MM, Suomi AM, Main KM, Skakkebaek NE, et al. Mild gestational diabetes as a risk factor for congenital cryptorchidism. J Clin Endocrinol Metab. (2006) 91:4862–5. doi: 10.1210/jc.2006-1420
41. Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, Roeleveld N. Risk factors for hypospadias. Eur J Pediatr. (2007) 166:671–8. doi: 10.1007/s00431-006-0304-z
42. Carbone P, Giordano F, Nori F, Mantovani A, Taruscio D, Lauria L, et al. The possible role of endocrine disrupting chemicals in the aetiology of cryptorchidism and hypospadias: a population-based case-control study in rural Sicily. Int J Androl. (2007) 30:3–13. doi: 10.1111/j.1365-2605.2006.00703.x
43. Jensen MS, Toft G, Thulstrup AM, Bonde JP, Olsen J. Cryptorchidism according to maternal gestational smoking. Epidemiology (Cambridge Mass). (2007) 18:220–5. doi: 10.1097/01.ede.0000254061.90686.9f
44. Main KM, Kiviranta H, Virtanen HE, Sundqvist E, Tuomisto JT, Tuomisto J, et al. Flame retardants in placenta and breast milk and cryptorchidism in newborn boys. Environ Health Perspect. (2007) 115:1519–26. doi: 10.1289/ehp.9924
45. Akre O, Boyd HA, Ahlgren M, Wilbrand K, Westergaard T, Hjalgrim H, et al. Maternal and gestational risk factors for hypospadias. Environ Health Perspect. (2008) 116:1071–6. doi: 10.1289/ehp.10791
46. Damgaard IN, Jensen TK, Petersen JH, Skakkebaek NE, Toppari J, Main KM. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLoS One. (2008) 3:e3051. doi: 10.1371/journal.pone.0003051
47. Giordano F, Carbone P, Nori F, Mantovani A, Taruscio D, Figà-Talamanca I. Maternal diet and the risk of hypospadias and cryptorchidism in the offspring. Paediatr Perinatal Epidemiol. (2008) 22:249–60. doi: 10.1111/j.1365-3016.2007.00918.x
48. Mongraw-Chaffin ML, Cohn BA, Cohen RD, Christianson RE. Maternal smoking, alcohol consumption, and caffeine consumption during pregnancy in relation to a son’s risk of persistent cryptorchidism: a prospective study in the child health and development studies cohort, 1959-1967. Am J Epidemiol. (2008) 167:257–61. doi: 10.1093/aje/kwm311
49. Slickers JE, Olshan AF, Siega-Riz AM, Honein MA, Aylsworth AS. Maternal body mass index and lifestyle exposures and the risk of bilateral renal agenesis or hypoplasia: the national birth defects prevention study. Am J Epidemiol. (2008) 168:1259–67. doi: 10.1093/aje/kwn248
50. Ormond G, Nieuwenhuijsen MJ, Nelson P, Toledano MB, Iszatt N, Geneletti S, et al. Endocrine disruptors in the workplace, hair spray, folate supplementation, and risk of hypospadias: case-control study. Environ Health Perspect. (2009) 117:303–7. doi: 10.1289/ehp.11933
51. Brouwers MM, van der Zanden LF, de Gier RP, Barten EJ, Zielhuis GA, Feitz WF, et al. Hypospadias: risk factor patterns and different phenotypes. BJU Int. (2010) 105:254–62. doi: 10.1111/j.1464-410X.2009.08772.x
52. Adams SV, Hastert TA, Huang Y, Starr JR. No association between maternal pre-pregnancy obesity and risk of hypospadias or cryptorchidism in male newborns. Birth Defects Res A Clin Mol Teratol. (2011) 91:241–8. doi: 10.1002/bdra.20805
53. Wagner-Mahler K, Kurzenne JY, Delattre I, Bérard E, Mas JC, Bornebush L, et al. Prospective study on the prevalence and associated risk factors of cryptorchidism in 6246 newborn boys from Nice area, France. Int J Androl. (2011) 34:e499–510. doi: 10.1111/j.1365-2605.2011.01211.x
54. Brouwers MM, de Bruijne LM, de Gier RP, Zielhuis GA, Feitz WF, Roeleveld N. Risk factors for undescended testis. J Pediatr Urol. (2012) 8:59–66. doi: 10.1016/j.jpurol.2010.11.001
55. Estors Sastre B, Campillo Artero C, González Ruiz Y, Fernández Atuan RL, Bragagnini Rodríguez P, Frontera Juan G, et al. Occupational exposure to endocrine-disrupting chemicals and other parental risk factors in hypospadias and cryptorchidism development: a case-control study. J Pediatr Urol. (2019) 15:520.e1–520.e8. doi: 10.1016/j.jpurol.2019.07.001
56. Kjersgaard CL, Arendt LH, Ernst A, Søndergaard Lindhard M, Olsen J, Henriksen TB, et al. Lifestyle in pregnancy and hypospadias in sons: a study of 85,923 mother-son pairs from two danish pregnancy cohorts. Clin Epidemiol. (2022) 14:149–57. doi: 10.2147/CLEP.S335877
57. Evans DR, Newcombe RG, Campbell H. Maternal smoking habits and congenital malformations: a population study. Br Med J. (1979) 2:171–3. doi: 10.1136/bmj.2.6183.171
58. Christianson RE. The relationship between maternal smoking and the incidence of congenital anomalies. Am J Pidemiol. (1980) 112:684–95. doi: 10.1093/oxfordjournals.aje.a113041
59. McDonald AD, Armstrong BG, Sloan M. Cigarette, alcohol, and coffee consumption and congenital defects. Am J Public Health. (1992) 82:91–3. doi: 10.2105/AJPH.82.1.91
60. Källen K. Maternal smoking and urinary organ malformations. Int J Epidemiol. (1997) 26:571–4. doi: 10.1093/ije/26.3.571
61. Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects: validity of birth certificate data for effect estimation. Public Health Rep. (2001) 116:327–35. doi: 10.1016/S0033-3549(04)50054-7
62. Woods SE, Raju U. Maternal smoking and the risk of congenital birth defects: a cohort study. J Am Board Fam Pract. (2001) 14:330–4.
63. Thorup J, Cortes D, Petersen BL. The incidence of bilateral cryptorchidism is increased and the fertility potential is reduced in sons born to mothers who have smoked during pregnancy. J Urol. (2006) 176:734–7. doi: 10.1016/j.juro.2006.03.042
64. Leite M, Albieri V, Kjaer SK, Jensen A. Maternal smoking in pregnancy and risk for congenital malformations: results of a Danish register-based cohort study. Acta Obstetr Gynecol Scand. (2014) 93:825–34. doi: 10.1111/aogs.12433
65. Dhalwani NN, Szatkowski L, Coleman T, Fiaschi L, Tata LJ. Nicotine replacement therapy in pregnancy and major congenital anomalies in offspring. Pediatrics. (2015) 135:859–67. doi: 10.1542/peds.2014-2560
66. Yang L, Wang H, Yang L, Zhao M, Guo Y, Bovet P, et al. Maternal cigarette smoking before or during pregnancy increases the risk of birth congenital anomalies: a population-based retrospective cohort study of 12 million mother-infant pairs. BMC Med. (2022) 20:4. doi: 10.1186/s12916-021-02196-x
67. Fantel AG, Juchau MR, Burroughs CJ, Person RE. Studies of embryotoxic mechanisms of niridazole: evidence that oxygen depletion plays a role in dysmorphogenicity. Teratology. (1989) 39:243–51. doi: 10.1002/tera.1420390306
68. Castellazzi AM, Maccario R, Moretta A, De Amici M, Gasparoni A, Chirico G, et al. Effect of active and passive smoking during pregnancy on natural killer-cell activity in infants. J Allergy Clin Immunol. (1999) 103:172–3. doi: 10.1016/S0091-6749(99)70542-7
69. Virtanen HE, Sadov S, Toppari J. Prenatal exposure to smoking and male reproductive health. Curr Opin Endocrinol Diabetes Obesity. (2012) 19:228–32. doi: 10.1097/MED.0b013e3283537cb8
70. Ratcliffe JM, Gladen BC, Wilcox AJ, Herbst AL. Does early exposure to maternal smoking affect future fertility in adult males? Reprod Toxicol (Elmsford NY). (1992) 6:297–307. doi: 10.1016/0890-6238(92)90192-V
71. Lindqvist P, Grennert L, Marsál K. Epidermal growth factor in maternal urine – a predictor of intrauterine growth restriction? Early Hum Dev. (1999) 56:143–50. doi: 10.1016/S0378-3782(99)00037-7
72. Jensen MS, Rebordosa C, Thulstrup AM, Toft G, Sørensen HT, Bonde JP, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology (Cambridge Mass). (2010) 21:779–85. doi: 10.1097/EDE.0b013e3181f20bed
73. Kristensen DM, Hass U, Lesné L, Lottrup G, Jacobsen PR, Desdoits-Lethimonier C, et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod (Oxford Engl). (2011) 26:235–44. doi: 10.1093/humrep/deq323
74. Ray HJ, Niswander L. Mechanisms of tissue fusion during development. Development. (2012) 139:1701–11. doi: 10.1242/dev.068338
75. Cohn MJ. Development of the external genitalia: conserved and divergent mechanisms of appendage patterning. Dev Dyn. (2011) 240:1108–15. doi: 10.1002/dvdy.22631
76. Choi NW, Klaponski FA. On neural-tube defects: an epidemiological elicitation of etiological factors. Neurology. (1970) 20:399–400.
77. Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB J. (2012) 26:4778–87. doi: 10.1096/fj.12-206458
78. Haid B, Pechriggl E, Nägele F, Dudas J, Webersinke G, Rammer M, et al. FGF8, FGF10 and FGF receptor 2 in foreskin of children with hypospadias: an analysis of immunohistochemical expression patterns and gene transcription. J Pediatr Urol. (2020) 16:.e1–41. doi: 10.1016/j.jpurol.2019.10.007
79. Goldwurm S, Biondi A. Case of congenital hypotransferrinemia suggests that tissue hypoxia during fetal development may cause hypospadias. Am J Med Genet. (2000) 95:287–90. doi: 10.1002/1096-8628(20001127)95:3<287::AID-AJMG18>3.0.CO;2-H
80. Wilkinson LJ, Neal CS, Singh RR, Sparrow DB, Kurniawan ND, Ju A, et al. Renal developmental defects resulting from in utero hypoxia are associated with suppression of ureteric β-catenin signaling. Kidney Int. (2015) 87:975–83. doi: 10.1038/ki.2014.394
81. Phua YL, Gilbert T, Combes A, Wilkinson L, Little MH. Neonatal vascularization and oxygen tension regulate appropriate perinatal renal medulla/papilla maturation. J Pathol. (2016) 238:665–76. doi: 10.1002/path.4690
82. Goujard J, Rumeau C, Schwartz D. Smoking during pregnancy, stillbirth and abruptio placentae. Biomedicine. (1975) 23:20–2.
Keywords: maternal smoking, congenital urogenital malformations, hypospadias, cryptorchidism, meta-analysis, paternal smoking
Citation: Zhang Q, Zhang Z-C, He X-Y, Liu Z-M, Wei G-H and Liu X (2022) Maternal smoking during pregnancy and the risk of congenital urogenital malformations: A systematic review and meta-analysis. Front. Pediatr. 10:973016. doi: 10.3389/fped.2022.973016
Received: 19 June 2022; Accepted: 24 August 2022;
Published: 03 October 2022.
Edited by:
Pierluigi Marzuillo, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Giorgia Sebastiani, Hospital Clinic of Barcelona, SpainCopyright © 2022 Zhang, Zhang, He, Liu, Wei and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing Liu, ZHIubGl1eDAyMTdAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.