
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 26 September 2022
Sec. Pediatric Surgery
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.970876
This article is part of the Research Topic Case Reports in Pediatric Surgery 2022 View all 21 articles
 Xiangang Xiong1,2†
Xiangang Xiong1,2† Yonghua Lou3†
Yonghua Lou3† Teng Zhou1,2
Teng Zhou1,2 Zebing Zheng1,2
Zebing Zheng1,2 Yuanmei Liu1,2
Yuanmei Liu1,2 Rui Liu1,2
Rui Liu1,2 Kaizhi Zhang1,2
Kaizhi Zhang1,2 Yuan Gong1,2
Yuan Gong1,2 Chengyan Tang1,2
Chengyan Tang1,2 Zhu Jin1,2*
Zhu Jin1,2*The accessory hepatic lobe (AHL) is a rare congenital malformation of the hepatic tissue, among which the giant AHL is the rarest in children. Patients without complications are usually asymptomatic, and most auxiliary examinations cannot provide a definitive preoperative diagnosis. Surgical procedure is the only recommended management for patients who suffered from the complications of AHL. We report the case of a rare pediatric giant AHL torsion combined with left hepatic vein branch thrombosis which was successfully treated by laparoscopic lobectomy followed by excision of AHL.
An accessory hepatic lobe (AHL) is a rare congenital malformation of the liver, and even rarer in the case of AHL with a stalk. When it is large and lacks anatomical fixation by hepatic ligaments, it is susceptible to rotation or even torsion, which can lead to ischemic infarction (1, 2). It is universally acknowledged that any symptomatic giant AHL in children is the rarest. Azmy (3) first described a neonatal case of Beckwith–Wiedemann syndrome combined with an accessory hepatic lobe torsion in 1980. Among 23 reported cases of pediatric AHL, only 3 have undergone laparoscopic resection of twisted AHL (4–6). We report the case of a rare pediatric giant AHL torsion combined with left hepatic vein branch thrombosis which was successfully treated by laparoscopic lobectomy followed by excision of AHL.
A 12-year-old male child was admitted to the emergency room with abdominal pain; the child presented with persistent vague pain in the right abdomen without cause, accompanied by vomiting a gastric content. Vital signs were stable, and a tough mass about 19 cm × 10 cm × 6 cm in size detected in the right lower and middle abdomen was movable and obviously painful. Laboratory tests showed only mild elevation of alanine aminotransferase and portal aminotransferase. Abdominal Doppler ultrasound revealed a hypoechoic mass measuring approximately 146 × 44 mm connected to the liver was identified in the left outer lobe of the liver. On 25 April 2021, the abdominal enhancement computed tomography (CT) was performed in the Emergency Department of the Affiliated Hospital of Zunyi Medical University, suggesting a large mass of approximately 170 × 95 × 40 mm in size protruding into the pelvis and attached to the left lobe of the liver by a twisted stalk (Figures 1A,B). The abdominal pain and vomiting disappeared after the child was hospitalized. On 27 April 2021, a re-examination of the abdomen by contrast-enhanced CT revealed that the twisted AHL had spontaneously reverted to its location (Figure 1C). The AHL was visible as non-uniform reinforcement in the inferior portion, with density similar to the liver parenchyma (Figure 2A). The computed tomography angiography (CTA) illustrated that the left hepatic artery, the left hepatic vein branch, and the left branch of the portal vein were observed within the mass, but multiple filling defects were recognized inside the branch of the left hepatic vein (Figures 2A,B).
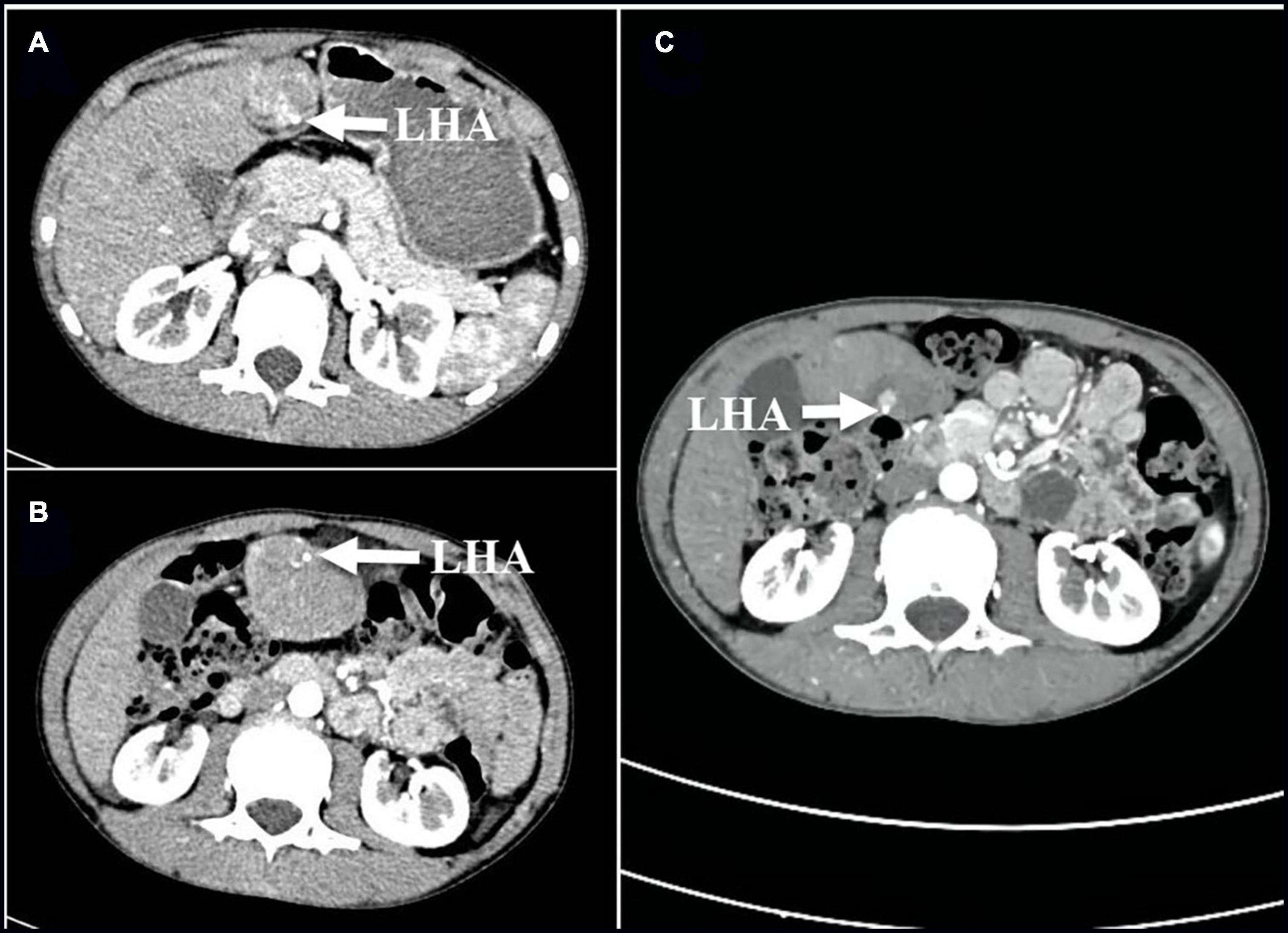
Figure 1. (A,B) Cross-sectional images of the first enhanced CT scan before admission on 25 April 2021 show 180° torsion of the left hepatic artery (arrow); (C) On 27 April 2021, compared with the previous CT scan, the enhanced CT scans repeated after the disappearance of abdominal pain symptoms suggested that the twisted hepatic artery has reset itself (arrows). LHA, left hepatic artery; CT, computed tomography.
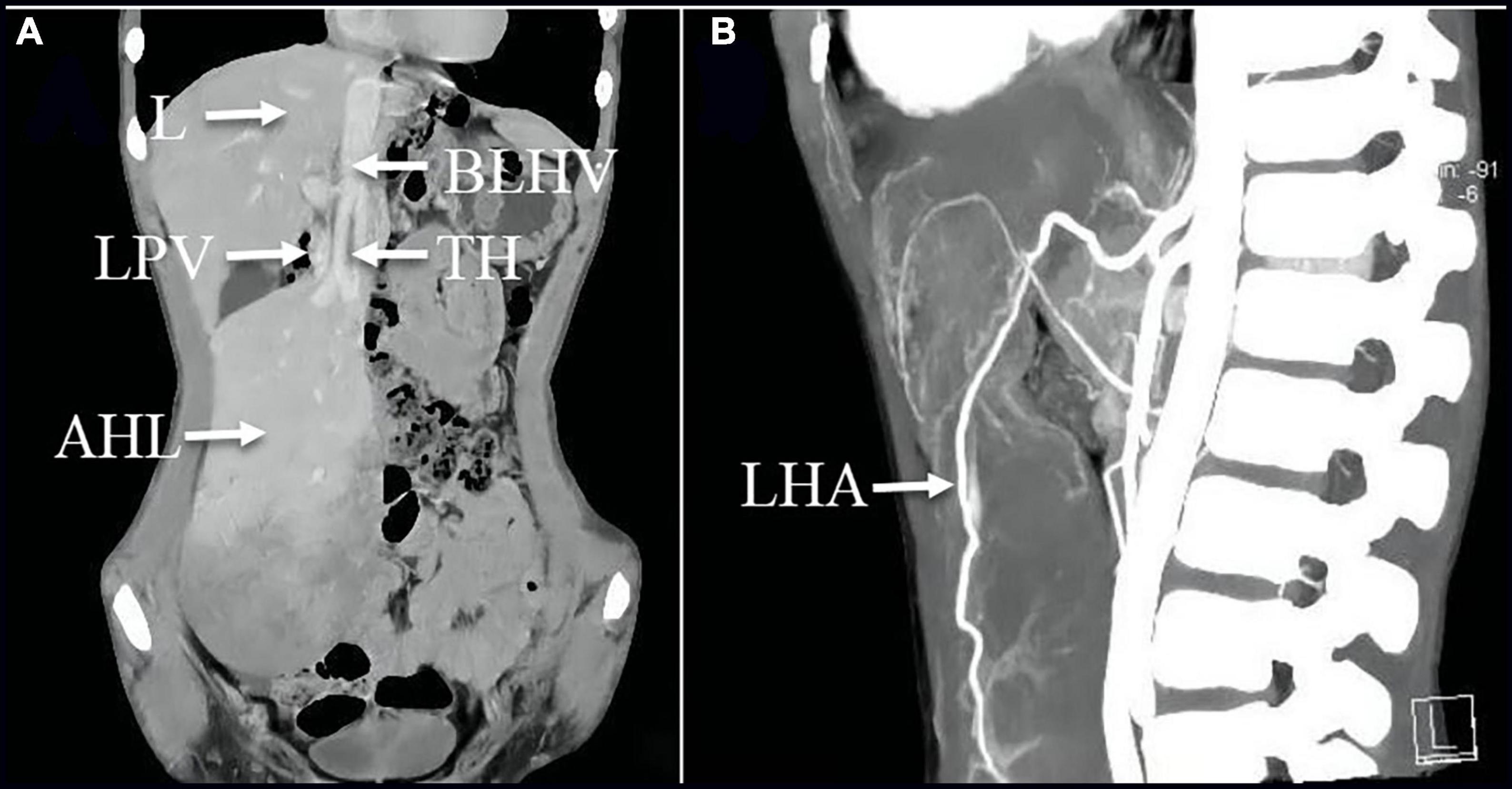
Figure 2. (A) Coronal image of the enhanced CT scan on 27 April 2021 revealed the mass was visible as non-uniform reinforcement in the inferior portion; (A,B) CTA images illustrated that the left hepatic artery, the branch of the left hepatic vein, and the left branch of the portal vein were observed within the mass, but multiple filling defects are recognized inside the branch of the left hepatic vein. AHL, accessory hepatic lobe; TH, thrombosis; L, liver; LPV, left hepatic portal vein; BLHV, the branch of the left hepatic vein; LHA, left hepatic artery.
The patient was placed in the supine position, and laparoscopy was performed using a 30°camera (STORZ). A total of four 5 mm trocars were placed at the following port sites: an infraumbilical, a left upper quadrant, and two right upper quadrant ports. During the laparoscopic procedure, it was diagnosed that a giant AHL of an estimated size of 18 × 10 × 6 cm was visible at the left side of the hepatic round ligament, with only a 2 cm in diameter liver tissue forming a stalk adjacent to the left liver, through which the branch of the left hepatic vein, the left branch of the portal vein, the left hepatic artery, and the bile duct were passing through (Figure 3A). The twisted stalk had repositioned itself. Partial infarction had occurred in the lower part of the giant AHL entering the pelvis, with an excellent flow of blood in the upper part. Careful dissection by using a 5-mm harmonic scalpel (Ethicon) was performed on blood vessels and bile duct ligated with the use of Hem-o-lock clips (Weck) through the stalk, and ligation of the stalk facilitated resection of the giant AHL. However, owing to the large size of the AHL, an incision of 6 cm in length was made in the midline of the abdomen to remove the AHL, weighing 552 g, from the abdominal cavity. The branch of the left hepatic vein was dissected in vitro, and a large amount of thrombosis was obtained (Figures 3B,C). The histopathology result demonstrated the irregular structure of the hepatic lobules, dilated hepatic sinusoids, and disordered vascular distribution in the portal area and interlobular (Figure 3D). The prognosis was satisfactory at the postoperative 2-month follow-up.
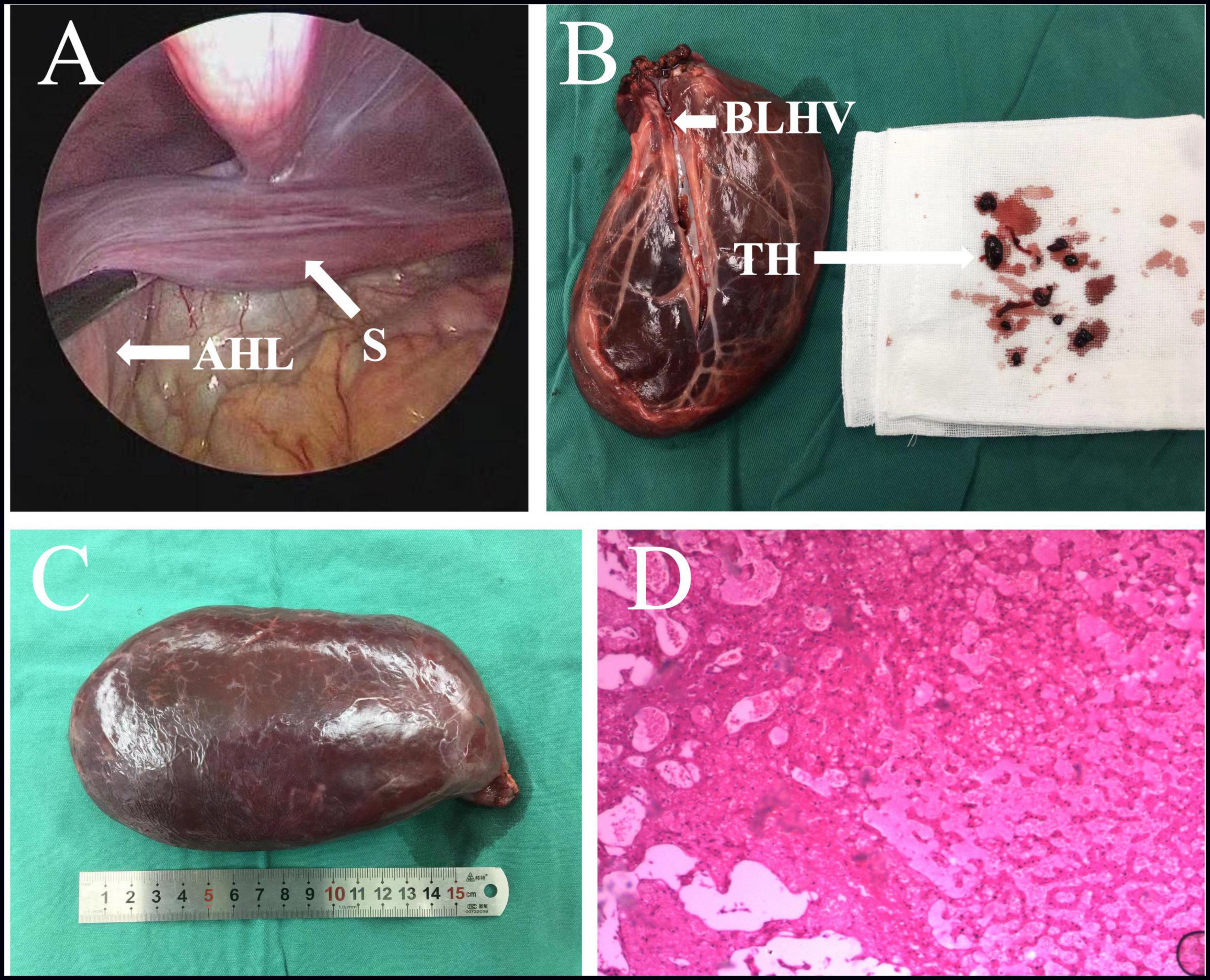
Figure 3. (A) Intraoperative images suggested the AHL has repositioned itself, with only a stalk of approximately 2 cm in diameter liver tissue forming an attachment to the left liver. (B,C) Excision of large AHL with thrombosis of a branch of the left hepatic vein. (D) The histopathology result demonstrated the irregular structure of the hepatic lobules, dilated hepatic sinusoids, and disordered vascular distribution in the portal area and interlobular. AHL, accessory hepatic lobe; S, stalk; TH, thrombosis; BLHV, branch of the left hepatic vein.
The exact pathogenesis of AHL remains unknown. One theory states that it is associated with a malformation of the caudal foregut of the endoderm and an abnormal division of the tissue buds during the 3rd week of gestation (7). AHL is categorized on the basis of size and its attachment to the liver: (1) large AHL (weight >30 g), (2) small AHL (weight <30 g), (3) ectopic AHL without hepatic attachment, and (4) microscopic AHL in the gallbladder wall (8). Among the 23 pediatric AHL cases reported in the literature, the age ranged from 1 day to 15 years, the male to female ratio was 11:12, and laparoscopic resection of AHL torsion has been reported (4–6). AHL was typically an incidental finding on radiographic imaging or autopsy, and most cases were also associated with persistent defects of anterior abdominal wall (4, 5). Corbitt et al. (4) reported that 41% of children with AHL are associated with abdominal wall defects, including umbilical bulge, Beckwith–Wiedemann syndrome, umbilical hernia, and cloacal exstrophy, stemming from abnormal development of the AHL that may obstruct the closure of the umbilical ring (4). A torsion of the large AHL may result in venous thrombosis, but such a large venous thrombosis has not been observed in the reported pediatric cases. Another report suggests that the risk of malignancy in AHLs is higher than in healthy liver tissue because of impaired blood supply and bile duct drainage (9, 10). In some instances, on imaging, it is a possibility to consider the AHL as a tumor or abscess (11). The patient was diagnosed with giant AHL which is extremely rare in children.
Patients with uncomplicated AHL are usually asymptomatic. Large AHL torsion with strangulation of the supplying vessels often resulting in severe hepatic ischemia is possibly caused by strenuous exercise or trauma (12). In that case, most patients are presented with sudden severe right-sided abdominal pain, probably accompanied by nausea or vomiting. As for palpation, an enlarged tender mass might be detected (5, 13). The AHL should not be reversed before ligation to prevent dislodgement of the thrombus (2). AHL torsion could be misdiagnosed as any other acute abdominal disease or intra-abdominal tumor. In this case, the giant AHL is attached to the left lobe of the liver through a stalk formed by normal liver tissue, with vague pain in the right mid-lower abdomen and lacked a history of trauma. Laparoscopic exploration revealed infarction of the lower part of giant AHL, but the twisted giant AHL had repositioned itself.
Most reports do not provide a definitive preoperative diagnosis. Ultrasonography, CT, and magnetic resonance imaging (MRI) are recommended for the diagnosis of large diameter AHL, while the auxiliary examinations could not detect the small diameter AHL. Some researchers proved that ultrasonography had a limited diagnostic value in the diagnosis of AHL (6). MRI indicates superior diagnostic value to CT, as the vascular system extending from the healthy liver through the stalk into the giant AHL is more likely to be detected by dynamic contrast-enhanced MRI (2). However, it has been suggested that for complete stalk torsion, a contrast enhancement CT scan, especially CTA, is more useful than ultrasonography for diagnosis (14). Ultrasonography, plain CT scan, and MRI have also been reported to be non-specific for diagnostic imaging findings in pediatric AHL patients (5). Hepatic infarcts often showed as hypoechoic, non-vascular areas on ultrasonography (15). The typical AHL demonstrated on CT is a mass with a narrow base or broad bottom attached to the healthy liver, with the Hounsfield unit consistent with healthy liver tissue (16). This case was in accordance with the CT presentation of the AHL, and the CTA was applied subsequently for further evaluation of the vascular system. CTA revealed thrombosis in a left hepatic vein branch. However, ultrasonography only indicates hypoechoic mass and was of limited diagnostic value in this case. CT is of better diagnostic value than ultrasound for AHL.
Most patients with an AHL will not demand medical treatment. A close follow-up would be advisable in asymptomatic cases. Surgical management is only necessary for those individuals suffering from complications such as torsion, rupture, and pedunculated AHL. Prompt management and emergency excision or laparoscopy should be recommended in cases of suspected AHL torsion (6, 17). Even among individuals presenting with complications, most do well following surgery (4–6). As this disease is relatively rare, torsion of the giant pedunculated AHL was already evident at the onset of abdominal pain in this child, but the diagnosis on early imaging failed and emergent operation was not performed. During examination, the torsion has reset itself and the abdominal pain disappeared. The child eventually underwent laparoscopy-assisted resection of the AHL.
This case is the first report of a rare stalk giant AHL torsion along with the left hepatic vein branch thrombosis. The AHL was resected by laparoscopic-assisted surgery, thus minimizing disruption to the abdominal cavity. When it is large and lacks anatomical fixation by hepatic ligaments, it is susceptible to rotation or even torsion, which can lead to ischemic infarction. The AHL needs to be differentiated by pediatricians from any other abdominal disease. We recommend laparoscopy as the first-choice treatment modality for complicated AHL.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
XX and YL wrote the first draft of the manuscript. ZJ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (81760099) and the Fund of Zunyi Science and Technology Bureau (Zunshi Kehe HZ 2019No. 104).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AHL, accessory hepatic lobe; TH, thrombosis; L, liver; LPV, left portal vein; BLHV, the branch of the left hepatic vein; S, stalk; LHA, left hepatic artery; CT, computed tomography; MRI, magnetic resonance imaging.
1. Umehara M, Sugai M, Kudo D, Hakamada K, Sasaki M, Munakata H. Torsion of an accessory lobe of the liver in a child: report of a case. Surg Today. (2009) 39:80–2. doi: 10.1007/s00595-008-3772-0
2. Khan AM, Hundal R, Manzoor K, Dhuper S, Korsten MA. Accessory liver lobes: a diagnostic and therapeutic challenge of their torsions. Scand J Gastroenterol. (2006) 41:125–30. doi: 10.1080/00365520500377862
3. Azmy A, Boddy SA, Eckstein HB. Torsion of gall bladder, embedded in an accessory lobe of liver in a neonate with Beckwith syndrome. Z Kinderchir Grenzgeb. (1980) 30:277–9. doi: 10.1055/s-2008-1066372
4. Corbitt N, Rellinger EJ, Hernanz-Schulman M, Chung DH. Accessory hepatic lobes in the pediatric population: a report of three cases of torsion and literature review. J Pediatr Surg Case Rep. (2017) 16:15–8. doi: 10.1016/j.epsc.2016.09.003
5. Nagano A, Onishi S, Kedoin C, Matsui M, Murakami M, Sugita K, et al. A rare case of accessory liver lobe torsion in a pediatric patient who showed recurrent epigastralgia and who was treated by elective laparoscopic resection. Surg Case Rep. (2021) 7:143. doi: 10.1186/s40792-021-01231-6
6. Salisbury SM, Yi CE, Merianos DJ, Sapra A, Anselmo DM. Laparoscopic resection of a torsed accessory hepatic lobe: case report and literature review. J Pediatr Surg Case Rep. (2013) 1:214–7. doi: 10.1016/j.epsc.2013.06.006
7. Carrabetta S, Piombo A, Podesta R, Auriati L. Torsion and infarction of accessory liver lobe in young man. Surgery. (2009) 145:448–9. doi: 10.1016/j.surg.2007.12.016
8. Yankol Y, Mecit N, Kanmaz T, Acarli K, Kalayoglu M. A very original case of a mini accessory liver lobe with its own gallbladder. Surg Radiol Anat. (2015) 37:535–8. doi: 10.1007/s00276-014-1380-9
9. Wang X, Zhang Q, Xu K. Hepatocellular carcinoma arising from left accessory liver lobe supplied by the branch of left hepatic artery: a case report. Medicine. (2019) 98:e16912. doi: 10.1097/MD.0000000000016912
10. Kubota K, Kita J, Rokkaku K, Iwasaki Y, Sawada T, Imura J, et al. Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol. (2007) 13:4270–3. doi: 10.3748/wjg.v13.i31.4270
11. Al-Anwar S, Askari A, Alvi A. Right Iliac fossa pain due to torsion and ischaemia of accessory liver lobe. J Surg Case Rep. (2019) 2019:rjz101. doi: 10.1093/jscr/rjz101
12. Koplewitz BZ, Manson DE, Ein SH. Posttraumatic torsion of accessory lobe of the liver and the gallbladder. Pediatr Radiol. (1999) 29:799–802. doi: 10.1007/s002470050698
13. Kostov DV, Kobakov GL. Accessory hepatic lobe. Surg Radiol Anat. (2011) 33:819–22. doi: 10.1007/s00276-011-0818-6
14. Nagavalli A, Polanski S, Peterson CM, Birkholz JH, Burdette AS. The liver twist: a case of accessory liver lobe torsion presenting after mild trauma. Radiol Case Rep. (2021) 16:2817–23. doi: 10.1016/j.radcr.2021.04.057
15. Park AH, Tran TA, Neychev V. Accessory liver lobe attached to the wall of the gallbladder. Cureus. (2019) 11:e6113. doi: 10.7759/cureus.6113
16. Wang Y, Junlin L, Zhang WG, Chen JH, He Y, Chen JM. Accessory lobe of right liver mimicking a pulmonary tumor in an adult male. Ann Thorac Surg. (2010) 89:e9–10. doi: 10.1016/j.athoracsur.2009.10.010
Keywords: accessory hepatic lobe, accessory hepatic lobe torsion, left hepatic vein branch thrombosis, child, liver
Citation: Xiong X, Lou Y, Zhou T, Zheng Z, Liu Y, Liu R, Zhang K, Gong Y, Tang C and Jin Z (2022) Case report: A case of giant accessory hepatic lobe torsion combined with left hepatic vein branch thrombosis in a child. Front. Pediatr. 10:970876. doi: 10.3389/fped.2022.970876
Received: 16 June 2022; Accepted: 05 September 2022;
Published: 26 September 2022.
Edited by:
Luca Pio, St. Jude Children’s Research Hospital, United StatesReviewed by:
Filippo Parolini, ASST Spedali Civili di Brescia, ItalyCopyright © 2022 Xiong, Lou, Zhou, Zheng, Liu, Liu, Zhang, Gong, Tang and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu Jin, MTMxMTEyNDAwMDZAZnVkYW4uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.