
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 12 October 2022
Sec. Pediatric Orthopedics
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.970309
This article is part of the Research Topic Case Reports in Pediatric Orthopedics 2022 View all 14 articles
The Giant Cell tumor (GCT) is a benign, locally aggressive lesion that cause bone destruction and shows a malignant potential. It is a relatively common skeletal tumor that is therefore typically seen in young adults. Few cases are described in literature of GCT in the immature skeleton, and the metatarsal is an unusual location for a primary bone GCT, especially in pediatric age. Therefore, there are very few data reported regarding the management protocol of GCT in metatarsal bones. We report a case about the use of no vascularized fibular graft for an original Y-shaped reconstruction of the metatarsal bone after Giant Cell Tumor resection in a 9 years-old patient, and performed a literature review about metatarsal bone reconstruction in skeletally immature patient.
The Giant Cell tumor (GCT) is a benign, locally aggressive lesion that cause bone destruction and shows a malignant potential with occasional capacity to metastasize. Pulmonary metastases occur in about 2% of patients with GCT (1). It is a relatively common skeletal tumor that usually occurs in young adults, accounting for 4%–9.5% of all primary osseous tumors and 18%–23% of benign bone tumors (2, 3), but it can rarely occur also in skeletally immature patients (4). This tumor develops almost exclusively in the epiphysis of long bones, next to the adjacent joint. The most common location is around the knee region (distal femur, proximal tibia), but distal radius, fibular head, proximal humerus and sacrum can also be frequently involved (5). Its histogenesis is unclear. It presents a typical, but not specific, microscopic structure that justify the different terms used in the past to identify it: myeloid sarcoma, tumor of myeloplexus, osteoblastoclastoma and osteoclastoma. It contains a prominent and diffuse osteoclast-type giant cell component, uniformly distributed in a population of mononuclear plump epithelioid or spindle cells (6–8). The metatarsal is an unusual location for a primary bone GCT, especially in the immature skeleton. We report a case about the use of fibular graft for the reconstruction of the metatarsal bone after Giant Cell Tumor resection in a pediatric patient and performed a literature review about metatarsal bone reconstruction in skeletally immature patient.
A review of the literature was performed using common databases (Pubmed, Google Scholar), searching for “metatarsal reconstruction, pediatric giant cell tumor, en-bloc resection, non-vascularized fibular graft”. The literature research was focused on cases of GCT of metatarsal bone in pediatric age treated with wide resection and reconstruction with free fibular autograft.
A 9-years-old male, with a history of leukemia, presented to our hospital with a localized pain and a 3 cm area of swelling of the upper part of the right forefoot, first noticed 3 months earlier. There was no history of trauma, fever or any symptoms influencing his general health. Pain also got worse by walking. On examination, there was a fixed swelling area occupying the dorsal and the inner side of IV metatarsus, which was firm to hard consistency. There were no other specific abnormal findings on either of his feet on standard physical examinations.
The routine hematological examination was within normal limit. The patient was subdued a standard x-ray (Figure 1A) of the right foot, that have highlighted the presence of an expansile, osteolytic lesion, which expands and destroys the overlying cortex, without periosteal reaction, involving the base and the middle third of the IV metatarsus and extending into the soft tissue. Distally the growth plate appeared disrupted. Magnetic resonance imaging (Figure 1B) was suggestive of GCT and the biopsy confirmed the diagnosis. Chest x-rays ruled out lung metastasis. The patient underwent wide local resection, local adjuvant phenol therapy and a Y-shaped reconstruction with a no vascularized fibular autograft. The dorsal approach was used for the resection with incision including biopsy track (Figure 2A). The length of the required fibular graft was estimated preoperatively, and it was osteotomized out from the distal part of the ipsilateral fibula (Figure 2B). Fibula periosteum of the donor site was maintained intact and closed at the end of the harvest. The bone graft was synthesized, using a percutaneous intramedullary Kirschner-wire, from the head of the IV metatarsus to the V metatarsus proximal metaphysis, obtaining an inverted Y shaped construct and maintaining the tarso-metatarsal joint function (Figures 3A,B). The V metatarsal proximal medial cortex was scraped to allow an adequate fitting of the graft and to enhance osteointegration. The IV to V metatarsus junction, blocked by the K-wire was also reinforced by a vicryl bone suture. The patient's postoperative course was unremarkable and he was discharged after 5 days from surgery. A cast was applied for 2 months and the Kirschner wire was removed 50 days after surgery, without anesthesia. The patient began to walk, increasing weight-bearing progressively on the affected leg with crutches 70 days after surgery and then progressed to full weight-bearing walk, without pain, 4 months after surgery and after a radiographic control. Clinical and radiographic follow-up was performed at 1, 3, 6, 12 and 24 months (Figures 4A,B). Bone union at the proximal and distal junction sites occurred in 6 weeks. There was no evidence of peroneal nerve injury and the patient has achieved a full recovery of functions and normal activities at 12 months from surgery. At two years clinical and radiographic follow-up the patient showed normal walking without pain, no local recurrences or metastases and evidence of new, almost complete, ossification of the fibular bone gap.
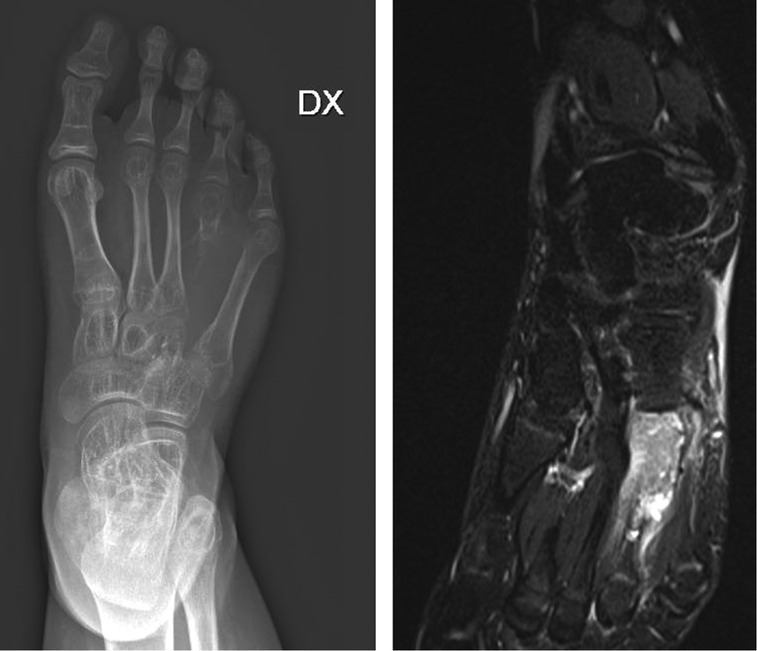
Figure 1. Pre-operative x-ray A/P (A) and MRI (B) view showing an expansile, lytic, encapsulated and iso-intense destructive lesion of the fourth metatarsus with soft tissue involvement.
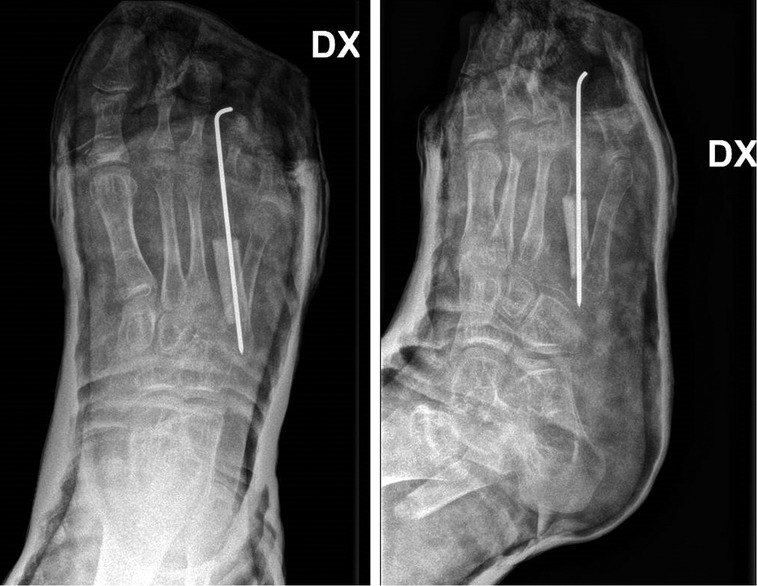
Figure 3. Post-operative x-ray A/P (A) and lateral (B) view showing reconstruction of IV metatarsal by fibular graft fixed to the base of V metatarsal (inverted Y shaped construct) using Kirschner’s wire and preserving tarso-metatarsal joint.
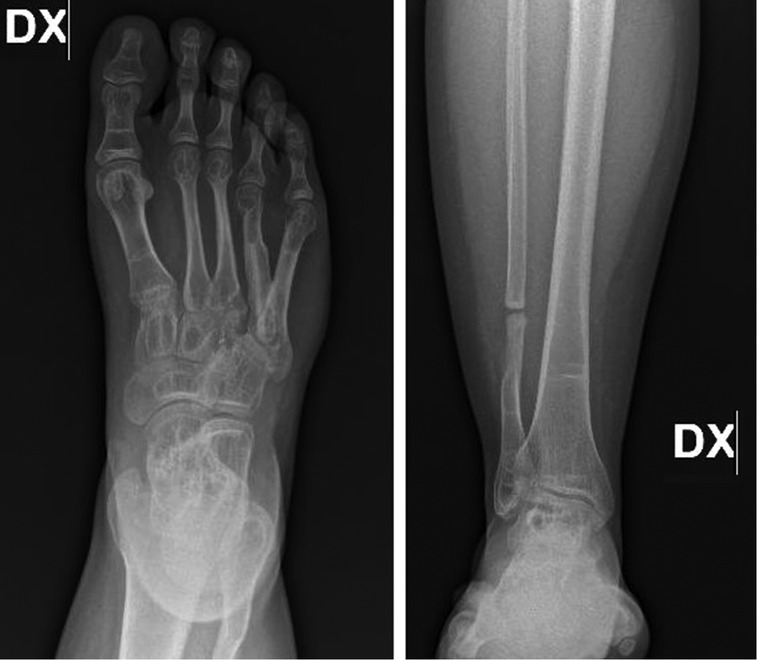
Figure 4. Follow-up x-ray of the foot and leg respectively taken at 12 months (A) and 24 months (B) showing incorporation of graft with no evidence of recurrences or other lesions and the donor site with an almost full recovery of the bone fibular gap.
Our case represents a combination of unusual location and age-group for GCT. Very few cases are described in literature of GCT in the immature skeleton, with an incidence from 1.8% to 10.6% (9, 10). In that case, the tumor is commonly found in bone metaphysis (4). Moreover, Bone GCT is rarely found in the metatarsal bones (11–13), it has more aggressive behavior and tends to grow faster in the foot (or hand) than in other bones, especially in young patients (14). Therefore, there are very few data reported regarding the management protocol of GCT in metatarsal bones. The goals of therapy are eradication of the tumor, to prevent damage to adjacent joint, and to prevent local recurrence. Different surgical procedures are adopted to treat this tumor, from intralesional curettage and bone grafting to wide resection with or without the use of several local adjuvants (phenol, liquid nitrogen, methyl methacrylate, hydrogen peroxide and alcohol) in order to control recurrences (5). The choice of treatment depends on both the site and the type of lesion. In our case, the patient presented a post-operative diagnosis of high grade GCT of bone (Campanacci grade 3 tumor) (15) with a thinned and partially destroyed overlying cortex, therefore it was impossible to carry out an intralesional curettage and wide resection was the treatment of choice.
Wide local resection is associated with a lower recurrence rate (5, 16), but has greater morbidity and higher rates of surgical complications when compared with intralesional curettage. However, metatarsal involvement makes wide resection of the lesion difficult as there is a little space between the rays of the foot. Looking at Enneking staging system and foot compartments (17) a radical resection of an extraosseous metatarsal lesion is difficult to obtain. In that case, the use of local adjuvants associated with surgical en-bloc resection can represent an effective solution in order to avoid amputation and control recurrence rate, as reported in literature (2). There are very few data reported about the reconstruction of metatarsal bones after GCT resection. Treatments described in literature may vary from resection alone to graft reconstruction from iliac crest or fibula. Fixation techniques may include both K-wires and plates.
Sheridan et al. (18), in 2020, in their retrospective review of 10 pediatric cases with non-traumatic primary bone defects, demonstrated that the use of non-vascularized fibular autograft is successful in the reconstruction of large bone defects secondary to malignant or benign pediatric bone tumors, reporting the largest known series of malignant pediatric tumors treated with this technique to date.
Rengsen et al. (19), in 2013, described a case of GCT of the 2nd metatarsal in a 14 years old girl. After resection, the metatarsal was reconstructed with a non-vascularized fibular graft, fixed with a dynamic compression plate. The outcome was good.
Compared to Rengsen procedure, our technique allows to remove the k-wire without further surgery and anesthesia and with less risks of hardware infection. Moreover, the use of a K wire through the growth plate did not caused damages of the cartilage. As already demonstrated by Guzzanti et al. in 1994 (20) the passage of a small, smooth hardware across the physis, then removed, do not stimulate physeal growth disorders. The tarso-metatarsal joint was maintained intact, avoiding walking impairment. In order to obtain that, an inverted Y shaped construct was chosen. Despite it was not an anatomical construct, pediatric orthopedic surgeons are used to Y-shaped metatarsal as a variant of post-axial polydactyly. The original construct was so carefully planned to avoid walking impairment, tarso-metatarsal joint disfunction and, at the same time, further surgeries to remove hardwares. Furthermore, one of the advantages of this technique is the remodeling capacity of the fibula and its relative ease of harvest. The periosteum preservation and closure has allowed a recovery of the bone gap. The closed membrane creates a biological chamber that permits revascularization and produces growth factors. The membrane also gathers growth factors with osteogenic potential. Looking at similarities with Masquelet technique, it's important to underline that there isn't bone union in cases with induced membrane, like periosteum, removal (21).
The proximal meta-epiphysis of the metatarsus is an unusual place for primary bone GCT and it is important to know atypical locations in order to perform a proper diagnosis and treatment, especially in young patients. GCT should be conceded in the differential diagnosis of destroying lesions in the immature skeleton. This case study assessed the good outcomes with no recurrences of metatarsal GCT treated with resection and original reconstructive technique that shows to be a valid surgical option in the treatment of this particular and difficult condition.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
MG conceived the idea and edited the manuscript. MF performed the majority of the writing. SC made the literature review and contributed to manuscript drafting. FF, AGA, CZ and RMT made the revision of the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer [FL] declared a past co-authorship with the author(s) [MF] to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br. (1998) 80(1):43–7. doi: 10.1302/0301-620X.80B1.0800043
2. Manaster BJ, Doyle AJ. Giant cell tumors of bone. Radiol Clin North Am. (1993) 31:299–323. doi: 10.1016/S0033-8389(22)02859-7
3. Mirra J.M. Bone tumors: Clinical, radiologic and pathologic correlations. 2nd ed. Philadelphia, PA: Lea / Febiger (1989).
4. Hoeffel JC, Galloy MA, Grignon Y, Chastagner P, Floquet J, Mainard L, et al. Giant cell tumor of bone in children and adolescents. Rev Rhum Engl Ed. (1996) 63(9):618–23.8938873
5. Bridge JA, Neff JR, Mouron BJ. Giant cell tumor of bone. Chromosomal analysis of 48 specimens and review of the literature. Cancer Genet Cytogenet. (1992) 58(1):2–13. doi: 10.1016/0165-4608(92)90125-R
6. Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. (2006) 37(1):35–51. doi: 10.1016/j.ocl.2005.08.005
7. Cavanna L, Biasini C, Monfredo M, Maniscalco P, Mori M. Giant cell tumor of bone. Oncologist. (2014) 19(11):1207. doi: 10.1634/theoncologist.2014-0267
8. Huvos AG, editor. Bone tumors: Diagnosis, treatment,and prognosis. Philadelphia, PA: Saunders (1991); Stewart MJ. The histogenesis of myeloid sarcoma. Lancet (1922); 2:1106–8.
9. Picci P, Manfrini M, Zucchi V, Gherlinzoni F, Rock M, Bertoni F, et al. Giant-cell tumor of bone in skeletally immature patients. J Bone Joint Surg Am. (1983) 65(4):486–90. doi: 10.2106/00004623-198365040-00009
10. Schutte HE, Taconis WK. Giant cell tumor in children and adolescents. Skeletal Radiol. (1993) 22(3):173–6. doi: 10.1007/BF00206148
11. Ly JQ, Arnett GW, Beall DP. Case 122: giant cell tumor of the second metatarsal. Radiology. (2007) 245:288–91. doi: 10.1148/radiol.2451031776
12. Aaron AD, Kenan S, Klein MJ, Hausman MR, Abdelwahab IF, Lewis MM. Case report 810: giant cell tumor of the first metatarsal. Skeletal Radiol. (1993) 22:543–5. doi: 10.1007/BF00209107
13. Wang EH, Arbatin JJ. Allograft reconstruction of a large giant cell tumor of the first metatarsal: a case report. Foot Ankle Int. (2008) 29:97–100. doi: 10.3113/FAI.2008.0097
14. Biscaglia R, Bacchini P, Bertoni F. Giant cell tumor of the bones of the hand and foot. Cancer. (2000) 88:2022–32. doi: 10.1002/(SICI)1097-0142(20000501)88:9%3C2022::AID-CNCR6%3E3.0.CO;2-Y
15. Campanacci M, Bladini N, Boriani S, Sudanese A. Giant cell tumor of bone. J Bone Joint Surg Am. (1987) 69:106–14. doi: 10.2106/00004623-198769010-00018
16. Lausten GS, Jensen PK, Schiodt T, Lund B. Local recurrences in giant cell tumour of bone. Long-term follow up of 31 cases. Int Orthop. (1996) 20(3):172–6. doi: 10.1007/s002640050057
17. Enneking WF. A system of staging musculoskeletal neoplasm. Clin Orthop Relat Res. (1986) 204:9–24. doi: 10.1097/00003086-198603000-00003
18. Sheridan GA, Cassidy JT, Donnelly A, Noonan M, Kelly PM, Moore DP, et al. Non-vascularised fibular autograft for reconstruction of paediatric bone defects: an analysis of 10 cases. Strateg Trauma Limb Reconstr. (2020) 15(2):84–90. doi: 10.5005/jp-journals-10080-1462
19. Rengsen P, Tiong KL, Teo YM, Goh TC, Sivapathasundram N. Reconstruction of the second metatarsal with non-vascularized fibular graft following en-bloc resection for giant celll tumour: a case report. Malays Orthop J. (2013) 7(3):15–7. doi: 10.5704/MOJ.1311.001
20. Guzzanti V, Falciglia F, Gigante A, Fabbriciani C. The effect of intra-articular ACL reconstruction on the growth plates of rabbits. J Bone Joint Surg Br. (1994) 76(6):960–3. doi: 10.1302/0301-620X.76B6.7983128
Keywords: pediatric giant cell tumor, foot tumors, metacarpal replacement, en-bloc resection, non-vascularized fibular graft
Citation: Florio M, Careri S, Zoccali C, Aulisa AG, Falciglia F, Toniolo RM and Giordano M (2022) Reconstruction of metatarsal bone after giant cell tumor resection with no vascularized fibular graft in a pediatric patient: Case report and review of literature. Front. Pediatr. 10:970309. doi: 10.3389/fped.2022.970309
Received: 15 June 2022; Accepted: 20 September 2022;
Published: 12 October 2022.
Edited by:
Marco Rainer Kesting, University Hospital Erlangen, GermanyReviewed by:
Sherif Kheir, Ain Shams University, Egypt© 2022 Florio, Careri, Zoccali, Aulisa, Falciglia, Toniolo and Giordano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Careri c2lsdmlhLmNhcmVyaUBvcGJnLm5ldA==
Specialty Section: This article was submitted to Pediatric Orthopedics, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.