
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 26 August 2022
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.959419
This article is part of the Research Topic Case Reports in Pediatric Infectious Diseases 2022 View all 10 articles
 Chunjiao Han1,2†
Chunjiao Han1,2† Tongqiang Zhang2†
Tongqiang Zhang2† Yidi Zhao1
Yidi Zhao1 Lili Dong2
Lili Dong2 Xiaole Li2
Xiaole Li2 Jiafeng Zheng2
Jiafeng Zheng2 Wei Guo2*
Wei Guo2* Yongsheng Xu2*
Yongsheng Xu2* Chunquan Cai3*
Chunquan Cai3*With the rapid increase in the number of infections, children with Staphylococcus aureus (S. aureus) infection secondary to Influenza A virus (IAV), appear to have a great possibility of causing severe complications and illness. Despite some cases and research findings regarding the death of children with IAV and S. aureus, coinfection included, there were few details about successful treatment of pleural empyema and necrotizing pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA) infection following IAV. In this case report, we describe the clinical symptoms and treatment of a teenager with pleural empyema and necrotizing pneumonia related to S. aureus secondary infection who was initially infected by IAV. This case highlights the importance of early recognition and application of thoracoscopy for this potentially fatal pleural empyema caused by MRSA and IAV coinfection. We conclude that this is a significant case that contributes to raising awareness regarding rarely occurring severe respiratory infections by MRSA in a child with normal immune function after IAV. In addition, further studies are needed to explore risk factors for IAV coinfection with S. aureus.
Retrospective studies of samples from the four pandemic influenza outbreaks of the last century have identified secondary bacterial infections as the fatal cause of co-morbidity and co-mortality, which reportedly manifested especially in the following week after viral infection symptoms are manifested, in 40–95% of influenza A -associated cases (1). IAV infections are associated with increased susceptibility to secondary bacterial infections, such as S. aureus and Streptococcus infections, wherein morbidity and mortality increase significantly (2). Children with IAV and S. aureus coinfection appear to have a great possibility of causing severe complications, and the mortality rate is very high as well. However, the details about the successful treatment of complications caused by coinfection of IAV and S. aureus have been rarely reported. In the present study, we report a unique case of pleural empyema and necrotizing pneumonia related to MRSA in a 13-year-old boy who was initially infected by IAV. Meanwhile, based on previous literature reports, we summarized the clinical characteristics and treatment of IAV and S. aureus coinfection.
A previously healthy 13-year-old boy was hospitalized with a three-day history of cough and fever. He was diagnosed with IAV infection in the local hospital. On admission, he was in respiratory distress and complained of left-sided chest pain. Laboratory indicators were shown in Table 1. His oxygen saturation level was 89–94% with an oxygen supply and he could not even lie down. The computed tomography (CT) scan of his chest showed atelectasis and a small amount of pleural effusion (Figure 1A). He was provided with oxygen support, anti-infection therapy and nutritional support. A diagrammatic representation of the treatment and outcome was presented in Figure 2.

Figure 1. (A) CT scan of his chest, show the left lung and the right lower lobe consolidation, with atelectasis in the lower lobe of the left lung. The left pleural cavity showed a small amount of pleural effusion, and the lumen of the bronchial branch of the left lower lobe was not unobstructed. (B) Bronchoscopy, show the basal segment of left lower lobe, the bronchial mucosa was rough, a large number of yellow and white mucus plugs were found in the opening, and the ventilation was not smooth. (C) Chest CT scan. Indicate consolidation of left and right upper and lower lobes with signs of atelectasis and multiple cavities in the left lung, partially wrapped left pneumothorax. (D) Thoracoscopy, show the lung surface covered with a yellow purulent moss-like layer.
The following day, the patient was not doing well and developed a stridor; hence, the decision was made to perform a flexible bronchoscopy with bronchoalveolar lavage (BAL) under ECG monitoring and respiratory oxygen support after employing local anesthesia (Figure 1B). Because the patient’s severe pneumonia progressed rapidly and even endangered his life, an early and accurate etiological diagnosis was very important for the implementation of pathogen-specific treatment. Therefore, the next-generation sequencing analysis from BAL fluid was performed, and it indicated S. aureus as the infectious pathogen residing in the patient’s lungs. Blood culture for microbial infection was also found positive for S. aureus. On the fourth day of hospitalization, the chest X-ray showed no improvement, so the bronchoscope was continued to be used for BAL. Tracheal aspirate showed heavy growth of MRSA sensitive to linezolid, vancomycin, and rifampicin. Meanwhile, it was resistant to tetracyclines and quinolones.
Unfortunately, the patient was very ill, remained pyrexial (38.8°C) and developed a left-sided pyopneumothorax on day 8 of hospitalization with recurring fever, and the body temperature increased to 38.8°C. The patient complained of chest pain related to breathing abnormality, accompanied by apparent breath-holding. B-scan ultrasound and CT imaging (Figure 1C) of thorax revealed massive bilateral pleural effusions and multiple cavities in the left lung. Based on the laboratory and imaging diagnostic investigations, thoracentesis was performed on the patient’s left chest, and the chest tube was placed on the same side.
Even after 12 days of hospitalization and treatment, the patient’s condition did not improve due to poor drainage of empyema as a result of cellulose and thick pus accumulation. The patient underwent thoracoscopy to diagnose and treat pleural effusion (Figure 1D). After thoracoscopic investigations of the pleural cavity, the lung surface was cleared of the thick yellow moss-like layer of pus, and the left chest cavity was repeatedly flushed until the outflow was clear. At the end of the surgical procedure, the light bloody liquid could be seen in the chest bottle, and the chest tube was connected to the closed thoracic drainage system to prevent further pleural effusions.
On the 19th day of hospitalization, the chest drainage tube was removed. His cough condition was improved, and his breathing rate was stable without the occurrence of shortness of breath. The patient was discharged without any complaints of breathing discomfort. The patient was followed up for a chest X-ray examination after ten months of hospital discharge. The prognosis was good and the patient was in good physical condition.
Influenza is considered as a known potential risk factor for Staphylococcal diseases (3). There are strong and consistent pieces of evidence of epidemiologically and clinically important interactions between the influenza virus and secondary bacterial respiratory pathogens (4). A study in the United States (5) showed that S. aureus was the most common bacterial pathogen (44%) among the 36 children who died of bacterial co-infection reported during the 2004–2007 influenza season, while MRSA accounted for 60%. The complication of co-infection progresses rapidly, and children cannot get accurate and timely treatment, which is one of the reasons for its high mortality rate.
There are several hypothetical mechanisms of bacterial co-infection secondary to influenza virus infection. It is reported that the influenza virus can increase the adhesion of bacteria by destroying the epithelial layer of the tracheobronchial tree and neuraminidase activity (6, 7). At the same time, Panton-Valentine leukocidin (PVL) is a pore-forming cytotoxin produced by S. aureus genes, acting synergistically to induce a strong lytic effect on host defense cells, notably with poly-morphonuclear leucocytes but especially on neutrophils (8). Previously infected influenza virus can enhance the proinflammatory and cytotoxic effects of PVL on neutrophils. Disintegration of the epithelial airway results in hemorrhage and tissue damage, which leads to the development of necrotizing pneumonia (7). In addition, nasal carriage of S. aureus is a significant risk factor for secondary staphylococcal pneumonia in IAV (9). Therefore, children with a history of S. aureus infection should be more alert to the risk of IAV and S. aureus co-infection. Finelli et al. found that compared with children without S. aureus infection, children with S. aureus infection were more prone to pneumonia and acute respiratory distress syndrome during the influenza season (5).
We reviewed 16 clinical reports of IAV and S. aureus coinfection; however, five of them lack detailed clinical information (Table 2). Reviewing the literature of 16 children with IAV and S. aureus co-infection, we can find that S. aureus is almost always characterized by MRSA. Most of these children are older than 10 years old, which is consistent with the result of Finelli et al. (5). Complications in these children mainly include sepsis, empyema, DIC, pneumothorax, lung abscess, emphysema, necrotizing pneumonia, and so on. However, most detection of virulence factors of S. aureus were missing. Two cases presented PVL (+) and one presented PVL (−). A study suggested that an early confirmed presence of the PVL toxin is particularly important in choosing antibiotics and administrating immunoglobulin that inhibits PVL toxin release in early stage (10). Notably, the application of bronchoscopy was important for early detection of the patient’s respiratory conditions. However, bronchoscopic results have rarely been reported and utilized in treating children with S. aureus co-infection that is secondary to IAV. In this case, we removed the obstruction of the trachea and lung with the assistance of bronchoscopy findings. Moreover, we could wash the diseased area of alveoli through alveolar lavage and subsequently analyzed the BAL fluid for bacterial infection. Bronchoscopy has also been reported in the case of Sharp et al. (12). Copious cloudy secretions, fibrinous debris, and patchy plaques were found in the main bronchi and distal trachea. We should pay more attention to the application opportunity of bronchoscope. Thoracoscopy was also the key for this potentially fatal pleural empyema caused by MRSA and IAV coinfection when patient developed pleural adhesion and multiple cavities. In addition, the application of drugs was also critical. In our case, we covered the positive cocci in the initial treatment. We timely adjusted antibiotics by detecting the drug resistance of S. aureus, which effectively controlled the S. aureus infection. Due to the application of flexible bronchoscopy, thoracoscopy and adequate anti-infective treatment, we could successfully relieve the patient’s uncomfortable respiratory conditions, and let the patient be discharged within 3-weeks of hospitalization.
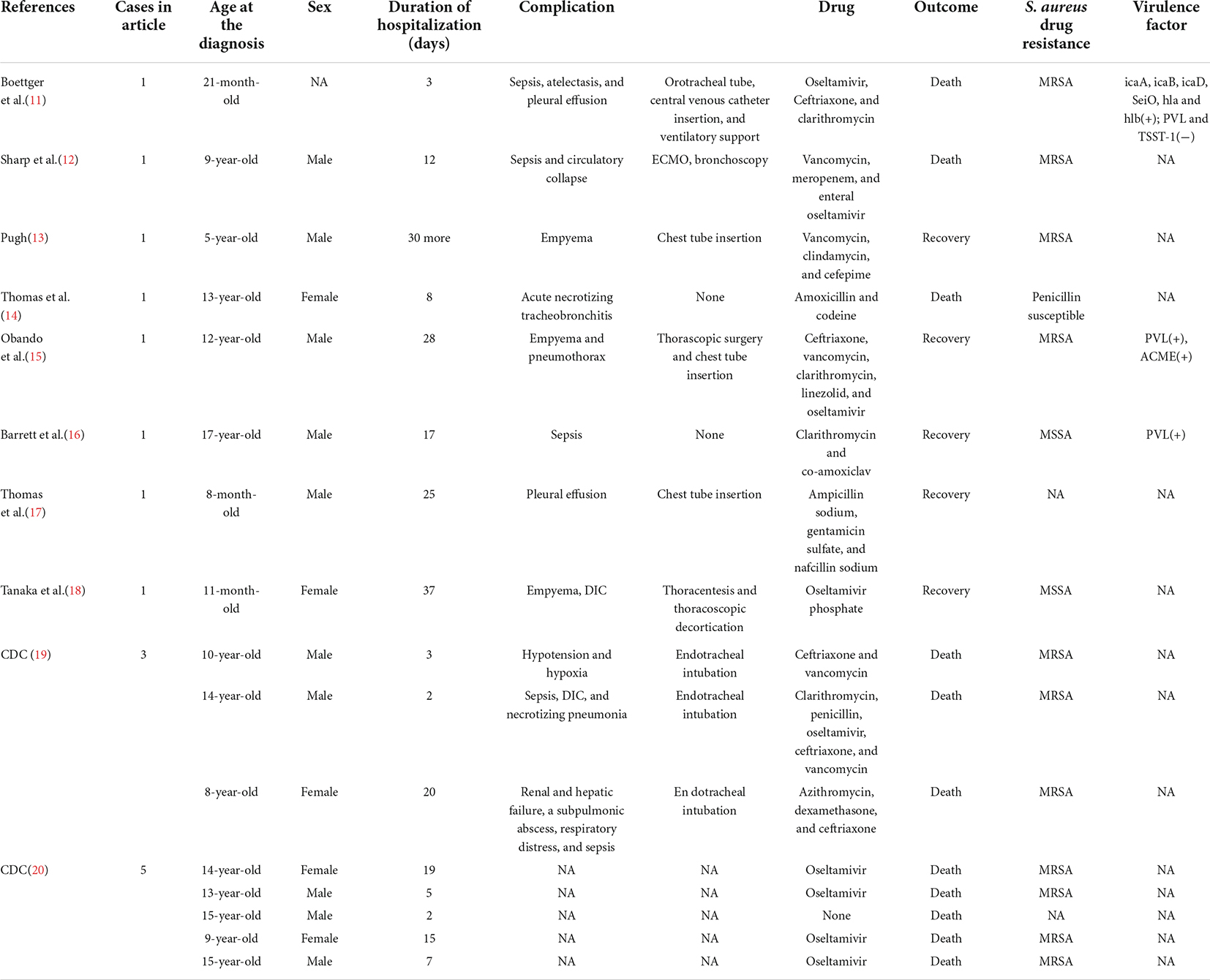
Table 2. The clinical characteristics and results of the literature review of the IAV and S. aureus coinfection.
In conclusion, due to the synergistic pathogenic effects between the influenza virus and coinfecting respiratory bacteria, we should raise awareness regarding the rarely occurring severe respiratory infections by S. aureus, following influenza for early diagnosis and rapid recovery from respiratory complications in children. In addition, this study suggests a need for further research about risk factors of secondary S. aureus infection of IAV.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consents were obtained from the parents for publication of this report.
TZ and XL cared for patients. YZ and LD collected the data. CH and JZ drafted the article. WG and YX revised it for intellectual content. CH and CC approved the final completed article. All authors read and approved the final manuscript.
This work was supported by the Tianjin Natural Science Foundation (Grant no. 21JCYBJC00460) and Tianjin Science and Technology Planning Project (Grant no. 20JCZXJC00170), the Science and Technology Training Project of Tianjin Health Committee (Grant no. RC20020), and General Project of Tianjin Children’s Hospital (Grant no. Y2020013). We are grateful for the financial support from the “Tianjin Medical Key Discipline (Specialty) Construction Project” (Grant no. TJYXZDXK-040A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. McCullers JA. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol. (2014) 12:252–62. doi: 10.1038/nrmicro3231
2. Borgogna TR, Hisey B, Heitmann E, Obar JJ, Meissner N, Voyich JM. Secondary bacterial pneumonia by Staphylococcus aureus following influenza a infection is SaeR/S dependent. J Infect Dis. (2018) 218:809–13. doi: 10.1093/infdis/jiy210
3. Reed C, Kallen AJ, Patton M, Arnold KE, Farley MM, Hageman J, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. (2009) 28:572–6. doi: 10.1097/INF.0b013e31819d8b71
4. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. (2006) 6:303–12. doi: 10.1016/s1473-3099(06)70466-2
5. Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L, et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics. (2008) 122:805–11. doi: 10.1542/peds.2008-1336
6. Peltola VT, McCullers JA. Respiratory viruses predisposing to bacterial infections: role of neuraminidase. Pediatr Infect Dis J. (2004) 23(Suppl. 1):S87–97. doi: 10.1097/01.inf.0000108197.81270.35
7. Niemann S, Ehrhardt C, Medina E, Warnking K, Tuchscherr L, Heitmann V, et al. Combined action of influenza virus and Staphylococcus aureus panton-valentine leukocidin provokes severe lung epithelium damage. J Infect Dis. (2012) 206:1138–48. doi: 10.1093/infdis/jis468
8. El Haddad L, Moineau S. Characterization of a novel panton-valentine leukocidin (PVL)-encoding staphylococcal phage and its naturally PVL-lacking variant. Appl Environ Microbiol. (2013) 79:2828–32. doi: 10.1128/aem.03852-12
9. Mulcahy ME, McLoughlin RM. Staphylococcus aureus and influenza A virus: partners in coinfection. mBio. (2016) 7:e2068–2016. doi: 10.1128/mBio.02068-16
10. Boan P, Tan HL, Pearson J, Coombs G, Heath CH, Robinson JO. Epidemiological, clinical, outcome and antibiotic susceptibility differences between PVL positive and PVL negative Staphylococcus aureus infections in Western Australia: a case control study. BMC Infect Dis. (2015) 15:10. doi: 10.1186/s12879-014-0742-6
11. Boettger BC, Rezende TFT, Teixeira NB, Pignatari ACC, Kiffer CRV. Case report of a child with influenza and fatal community-associated methicillin-resistant Staphylococcus aureus sepsis. Rev Soc Bras Med Trop. (2020) 53:e20200050. doi: 10.1590/0037-8682-0050-2020
12. Sharp JK, Hereth J, Fasanello J. Bronchoscopic findings in a child with pandemic novel H1N1 influenza A and methicillin-resistant Staphylococcus aureus. Pediatr Pulmonol. (2011) 46:92–5. doi: 10.1002/ppul.21306
13. Pugh CP. Empyema necessitans a rare complication of methicillin-resistant Staphylococcus aureus empyema in a child. Pediatr Infect Dis J. (2020) 39:256–7. doi: 10.1097/inf.0000000000002555
14. Thomas P, Riffelmann M, Schweiger B, Dominik S, von König CH. Fatal influenza A virus infection in a child vaccinated against influenza. Pediatr Infect Dis J. (2003) 22:201–2.
15. Obando I, Valderrabanos ES, Millan JA, Neth OW. Necrotising pneumonia due to influenza A (H1N1) and community-acquired methicillin-resistant Staphylococcus aureus clone USA300: successful management of the first documented paediatric case. Arch Dis Child. (2010) 95:305–6. doi: 10.1136/adc.2009.175281
16. Barrett NA, Armstrong-James D, Edgeworth J, Wyncoll D. Novel H1N1 influenza and panton-valentine leukocidin Staphylococcus aureus necrotizing pneumonia. Br J Hosp Med (Lond). (2010) 71:350–1. doi: 10.12968/hmed.2010.71.6.48456
17. Mausbach TW, Cho CT. Pneumonia and pleural effusion. Association with influenza A virus and Staphylococcus aureus. Am J Dis Child. (1976) 130:1005–6. doi: 10.1001/archpedi.1976.02120100095016
18. Tanaka K, Mizobuchi T, Fujiwara T, Saito T, Hiramoto R, Iwai N. Successful thoracoscopic treatment of severe bilateral empyema in an infant. Gen Thorac Cardiovasc Surg. (2007) 55:130–3. doi: 10.1007/s11748-006-0083-5
19. Centers for Disease Control and Prevention [CDC]. Severe methicillin-resistant Staphylococcus aureus community-acquired pneumonia associated with influenza–Louisiana and Georgia, December 2006-January 2007. MMWR Morb Mortal Wkly Rep. (2007) 56:325–9.
Keywords: Staphylococcus aureus, influenza A virus, pleural empyema, necrotizing pneumonia, bronchoscopy
Citation: Han C, Zhang T, Zhao Y, Dong L, Li X, Zheng J, Guo W, Xu Y and Cai C (2022) Successful treatment of pleural empyema and necrotizing pneumonia caused by methicillin-resistant Staphylococcus aureus infection following influenza A virus infection: A case report and literature review. Front. Pediatr. 10:959419. doi: 10.3389/fped.2022.959419
Received: 01 June 2022; Accepted: 05 August 2022;
Published: 26 August 2022.
Edited by:
Hans Van Rostenberghe, Universiti Sains Malaysia (USM), MalaysiaReviewed by:
Frieder Schaumburg, University of Münster, GermanyCopyright © 2022 Han, Zhang, Zhao, Dong, Li, Zheng, Guo, Xu and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Guo, Z3Vvd2VpNzk2NTZAMTI2LmNvbQ==; Yongsheng Xu, eHh5eXNzQDEyNi5jb20=; Chunquan Cai, MTUxMjI2NTYzMTNAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.