- 1Department of Pediatrics, Ospedale Pediatrico “Giovanni XXIII,” University of Bari, Bari, Italy
- 2Department of Radiology, Ospedale Pediatrico “Giovanni XXIII,” University of Bari, Bari, Italy
- 3Department of Pediatric Surgery, Istituto “Giannina Gaslini,” University of Genoa, Genoa, Italy
Hypertrophic Pyloric Stenosis (HPS) represents a relatively rare occurrence beyond infancy. Here, we present the case of a barely 3-year-old boy diagnosed with late-onset HPS and successfully treated with extra-mucosal pyloromyotomy. We review the literature, challenging the principle that more aggressive surgical approaches should be preferred over less invasive ones.
Introduction
Hypertrophic pyloric stenosis (HPS) represents the most common cause of gastric outlet obstruction (GOO) in infants, being a rare occurrence beyond infancy.
Here, we present the case of a barely 3-year-old child, who was admitted to the Emergency Department with symptoms of persistent non-bilious vomiting over the past 15 days. Abdominal ultrasound demonstrated thickening of the pylorus, while an upper GI series revealed obstruction to gastric emptying.
Endoscopic balloon dilation (EBD), despite providing symptomatic improvement, failed to normalize gastric emptying. He was treated with extra mucosal pyloromyotomy, the gold standard of treatment in infantile forms, with a good outcome and fast recovery.
After reviewing the literature, we found that conservative pyloric surgery, which represents the gold standard treatment for infantile forms, is also effective and safe in late-onset forms of HPS and should be preferred over more invasive surgical approaches whenever possible.
Case description
A 2-year-and-11-month-old male was admitted to the emergency department for persistent non-bilious vomiting episodes, which had started 15 days before, and became more frequent over time. Episodes typically occurred 4–5 h after his meals and were preceded by hypersalivation and rumination. A weight loss of 3.5 kg had occurred over the last 4 weeks, while bowel function had remained regular. One month before, he had suffered from a self-limited upper respiratory tract infection. Past medical and family history was otherwise unremarkable.
On admission, physical examination was normal, with a soft, symmetric, non-tender abdomen with no palpable masses, with no signs of significant dehydration. Arterial Blood Gas analysis showed signs of mild metabolic alkalosis (pH 7.49, HCO3 28 mmol/L). Within 2 h of being admitted to the hospital, however, the patient presented some projectile, non-bilious vomiting. An abdominal X-ray showed significant gastric distension, with no signs of pneumoperitoneum, while an abdominal ultrasound (US) showed a thickened pylorus with a single-wall thickness of 7 mm and a length of 16 mm. An upper GI series (Figure 1) revealed an accumulation of contrast medium in the dilated gastric antrum (shoulder sign), a narrow string-like pyloric channel (string sign), and delayed gastric emptying, with only a modest amount of contrast medium passing through pylorus over the next 60 min. An urgent CT scan was performed to exclude extrinsic causes of gastric outlet obstruction (GOO), showing an elongated pyloric canal (approximately 20 mm) without a visible lumen and a thickened wall (single wall thickness of 6 mm). These measurements were later confirmed by an abdominal MRI (Figure 2).
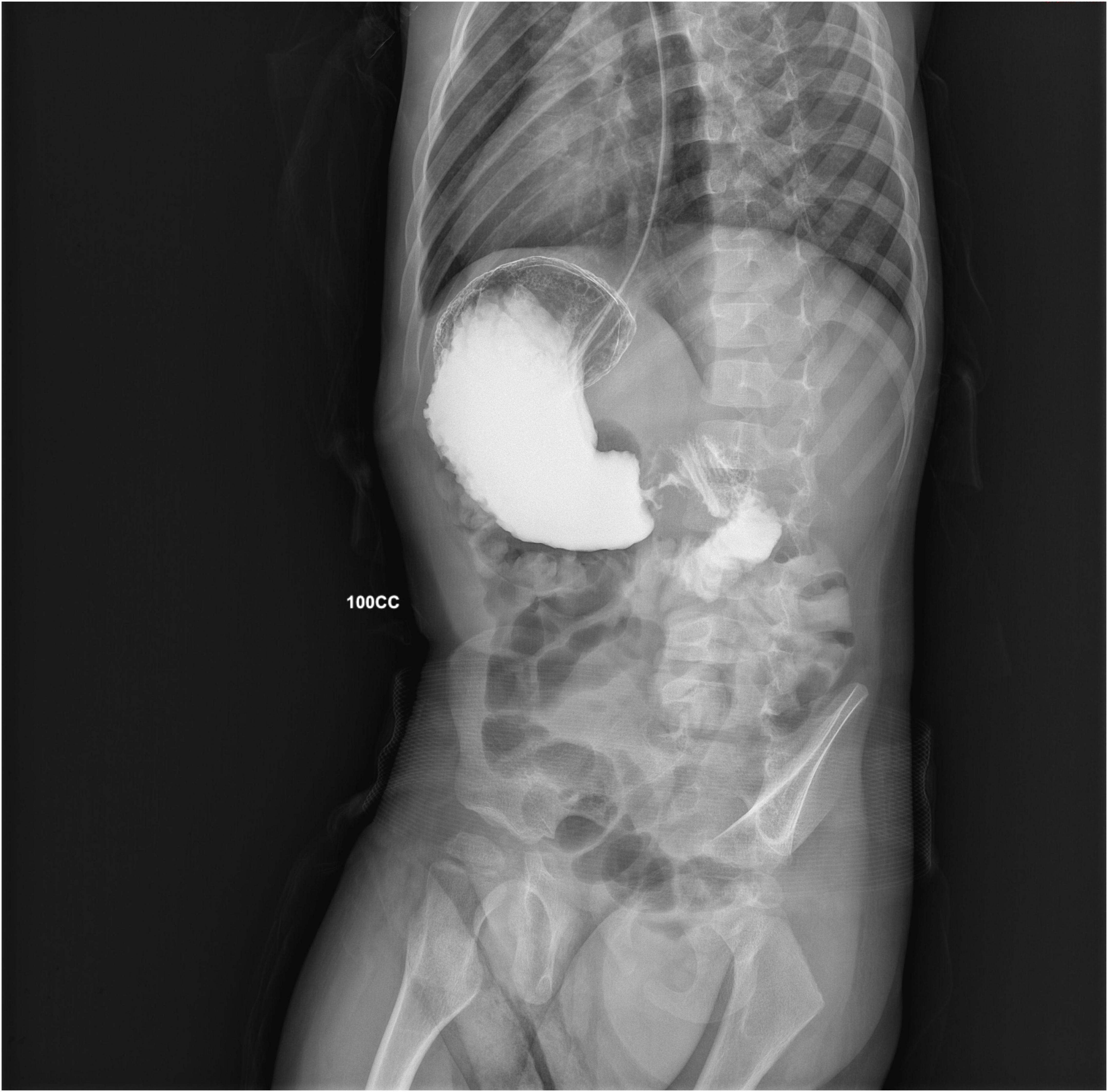
Figure 1. A right anterior oblique projection from the GI series, showing accumulation of contrast medium into a dilated antrum (shoulder sign) and a narrow string-like pyloric channel (string sign).
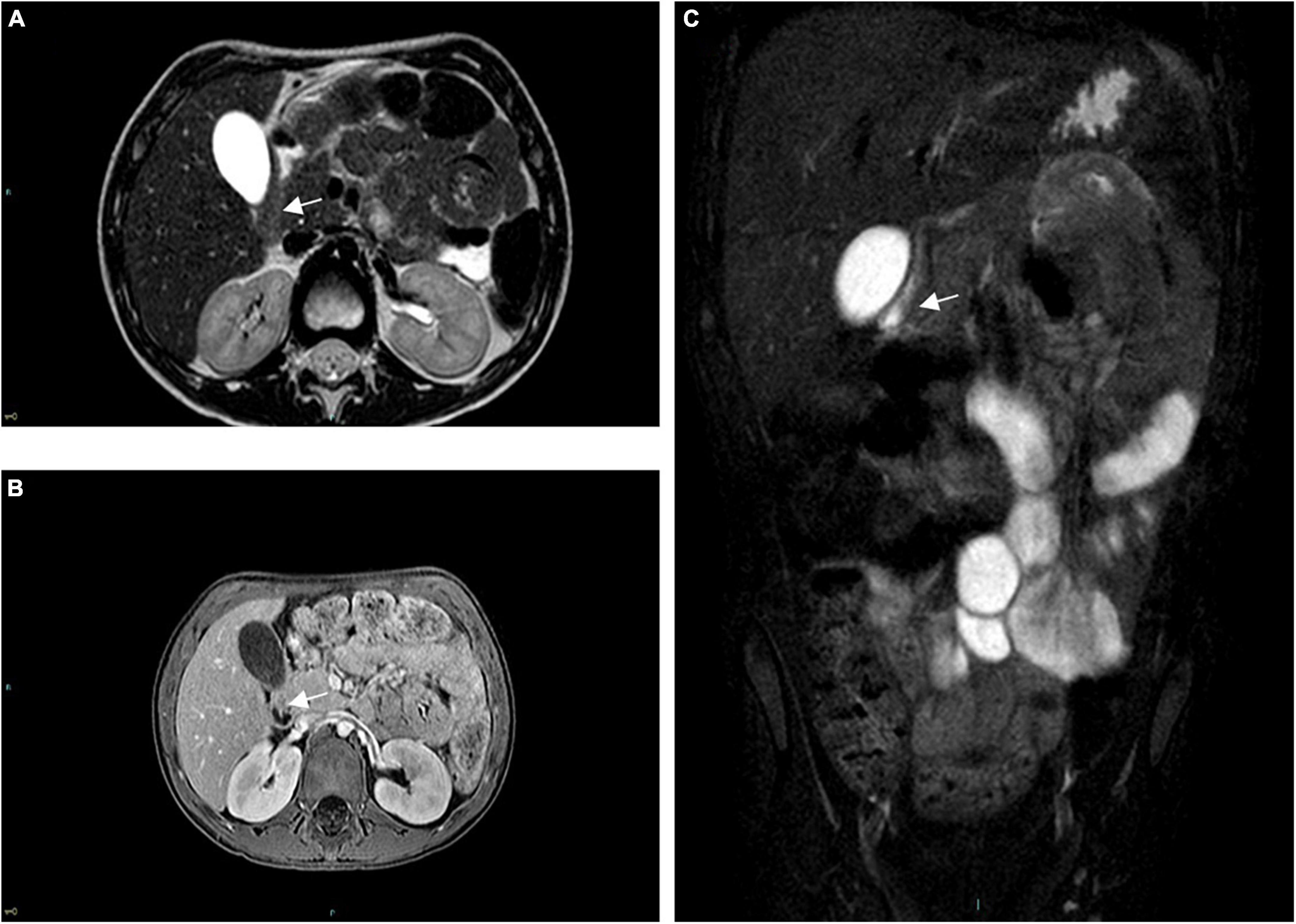
Figure 2. Pre-operative abdominal MRI. (A) Axial TSE-T2w, (B) axial GRE-T1w, and (C) coronal turbo-spin echo T2w FAT-SAT sequences are shown, all demonstrating a thickened and narrowed pylorus (arrow), without a visible lumen.
A nasogastric tube was placed, and the patient was started on parenteral nutrition. Upper endoscopy was performed 48 h later, showing distal esophagitis with no evidence of gastric ulcers, mucosal webs, or foreign bodies. Endoscopic balloon dilation (EBD) was attempted, and biopsies were performed, testing negative for Helicobacter pylori infection and showing no inflammatory infiltrate. The patient was started on PPIs and alginate, and 48 h after the procedure, a normal-caloric liquid formula was administered with good tolerance.
Despite significant clinical improvement, with no more episodes of vomiting reported, the post-operative GI series performed on the 5th post-operative day still showed delayed gastric emptying (complete emptying requiring approximately 4 h) and the persistence of a narrowed pyloric outlet. The patient was, therefore, referred to surgery. A laparoscopic extra mucosal pyloromyotomy was performed with prompt normalization of transpyloric transit time (as demonstrated by a barium swallow) and good tolerance to refeeding 24 h postoperatively.
Over the subsequent 6 months, the patient has shown good clinical conditions with no more episodes of vomiting, or other relevant symptoms reported during follow-up evaluations. Weight loss was completely recovered over the first 30 days from hospital discharge.
Discussion
Gastric outlet obstruction refers to a group of heterogeneous conditions that prevent or delay the passage of gastric content into the duodenum (1). It occurs in approximately 2–5 per 1,000 infants and children per year (2). Non-bilious vomiting, dehydration, failure to thrive, and weight loss are the most common clinical manifestations.
Hypertrophic pyloric stenosis is, by far, the most common cause of gastric outlet obstruction (GOO) in young infants, with an incidence of 1–4 in 1,000 live births (3). It is defined as hyperplasia of the smooth muscle fibers of the pylorus, causing a narrowing of its lumen. HPS usually appears within the first 2–8 weeks of life (4). More than 90% of HPS cases occur between 3 and 10 weeks of age, being instead a rare finding after 12 weeks (4, 5). Males are more commonly affected with a male-to-female ratio of 4:1 (6). Its etiopathogenesis is almost invariably idiopathic, with genetic, hormonal, and environmental factors all playing a role.
When HPS is excluded, the incidence of GOO falls dramatically (1 in 100,000 births by some estimates (7)). These non-hypertrophic, least common causes of GOO can be classified into three groups: congenital (esophageal aplasia, atresia, diaphragms, and webs; luminal obstructions); secondary (to acid peptic diseases, neoplasm, chemical injury, or foreign body ingestion); or primary acquired (Jodhpur disease), characterized by defects in pyloric motility with no hypertrophy (7–9).
HPS beyond infancy is a rare occurrence (7, 10, 11). Its etiology in older patients is controversial. Most authors, however, seem to agree that late-onset HPS is due to the persistence of the infantile form, which becomes clinically evident only at a later stage, when a triggering event, such as inflammation, edema, or spasms, precipitates pyloric occlusion (12, 13).
If GOO is suspected, an early patient assessment should include blood gas analysis, electrolytes, and fluid resuscitation; placement of a nasogastric tube allows for prompt gastric decompression. Abdominal ultrasound is the first-line examination (14), allowing the identification of a thickened pylorus (transverse section > 3 mm and length > 12 mm (15, 16)). An upper GI series allows to rule out other potential causes of projectile vomiting, such as malrotations and severe gastroesophageal reflux (14). In HPS, the pyloric lumen appears narrowed, with evidence of a string-like passage between the antrum and the duodenum (string sign), occasionally with a duplicated appearance due to puckering of the mucosa (double-track sign) (17). MRI and CT scans prove helpful to rule out extrinsic causes of obstruction. Total parenteral nutrition is indicated and should be initiated upon diagnosis confirmation, while PPIs may be of help in patients with persistent episodes of vomiting to prevent esophagitis (18). Pyloromyotomy has become the standard of care for infantile HPS; postoperatively, feeding can be resumed within 6 h, and the patient is discharged within 2 days. Laparoscopy is rapidly gaining ground over the open approach, although no systematic review has yet shown a clear advantage of one over the other, neither in terms of efficacy nor when it comes to post-operative complications (19).
When it comes to late-onset HPS, the data in the literature are conflicting, and several different procedures have been reported (20) (Table 1).
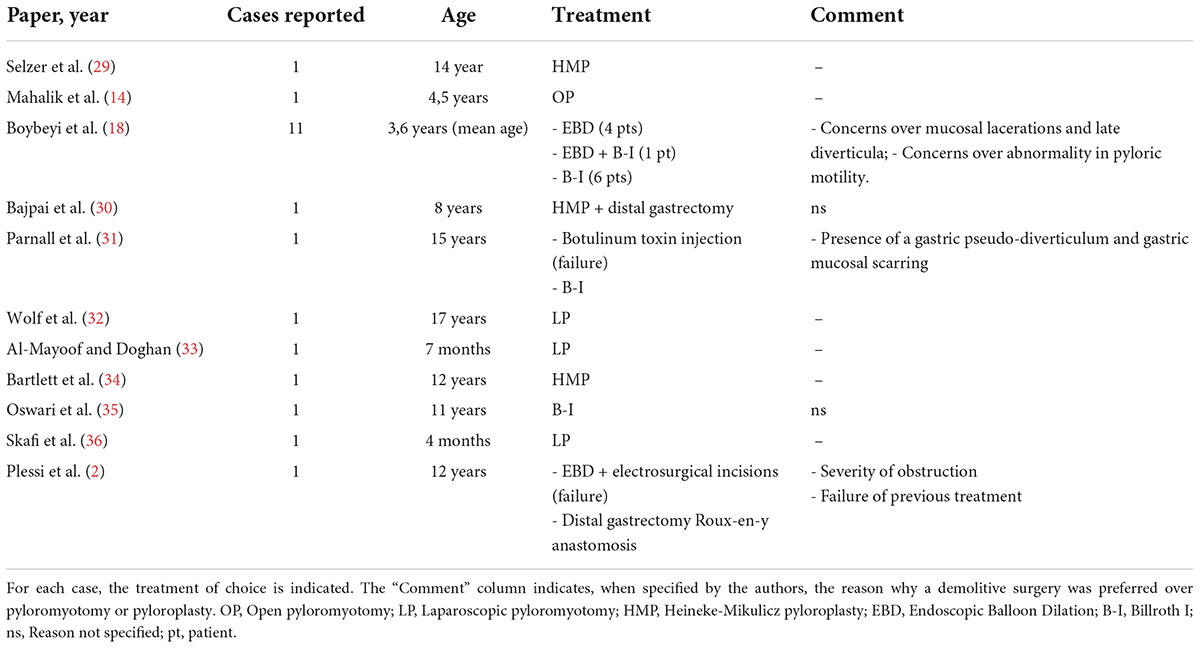
Table 1. Published case reports and one case series (Boybeyi et al.) (18) of late-onset HPS.
Boybeyi et al. suggested a possible treatment algorithm, indicating gastrectomy followed by Billroth I reconstruction whenever two consecutive EBDs attempts have failed or in those circumstances in which EBD cannot be performed (18). The rationale of gastrectomy, as compared to less invasive approaches, is related to possible complications, such as mucosal lacerations, pyloric scarring, diverticula formation, abnormal post-operative pyloric motility, and function (13, 18, 21–23). However, most of these concerns appear to be based on anecdotal evidence and are not corroborated by recent data (24–27).
Other authors underline some other limitations of less invasive procedures, such as the inability to visualize the gastric and duodenal mucosa, or the impossibility to obtain large histologic specimens (23, 26), which should be, however, evaluated on a case-to-case basis.
Some studies reporting on gastric emptying after conservative pyloric surgery seem to agree that conservative pyloric surgery produces no alterations in gastric emptying or intragastric distribution of meals and does not increase the risk of complications, such as gastric and duodenal ulcerations (27, 28).
In our case, gastric emptying did not improve significantly after EBD but underwent complete normalization after pyloromyotomy, with no return of symptoms after 6 months from the procedure.
The case report herein presented shows that a high degree of suspicion for HPS should be maintained even beyond infancy, to promptly identify and treat it.
Endoscopic balloon dilation represents a relatively safe, minimally invasive first-line approach in late-onset forms of HPS. As to the surgical approach to be adopted in refractory forms, the evidence is still insufficient. Pyloromyotomy is the gold standard treatment in infants. However, its efficacy and possible complications in older patients are yet to be established. The adoption of more invasive strategies, including distal gastrectomy and subsequent Billroth I reconstruction, should be decided on an individual basis and should be reserved for those cases, in which: (a) there are doubts on adequate pyloric motility and functionality (e.g., Jodhpur disease, scarring); (b) pyloromyotomy/pyloroplasty is made technically difficult by anatomy or by the thickness of the pylorus; (c) full-thickness biopsies are required to exclude other diseases, such as neoplasms; or (d) more conservative approaches have failed, or relapses have occurred.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
OI and GV drafted the initial manuscript, and reviewed and revised the manuscript. GM and MW carried out the surgical procedure and critically reviewed the manuscript, providing important insights concerning the surgical aspects of the cases. SC and FC collected the data, conducted the endoscopic study, and reviewed and revised the manuscript. RF and PG contributed to the conception and design of the report and wrote sections of the manuscript. SP conducted the radiological studies, selected the images, drafted their description, and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding
“Bruno Trambusti” Pediatric Department funding for clinical research received to cover publication fees.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Otjen JP, Iyer RS, Phillips GS, Parisi MT. Usual and unusual causes of pediatric gastric outlet obstruction. Pediatr Radiol. (2012) 42:728–37. doi: 10.1007/s00247-012-2375-5
2. Plessi C, Sica M, Molinaro F, Fusi G, Rossi F, Costantini M, et al. Diagnosis and treatment of primary hypertrophic pyloric stenosis (HPS) in older children. J Pediatr Surg Case Rep. (2021) 69:101860. doi: 10.1016/j.epsc.2021.101860
4. Pedersen RN, Garne E, Loane M, Korsholm L, Husby S, EUROCAT Working Group. Infantile hypertrophic pyloric stenosis: A comparative study of incidence and other epidemiological characteristics in seven European regions. J Matern Fetal Neonatal Med. (2008) 21:599–604. doi: 10.1080/14767050802214824
5. Aboagye J, Goldstein SD, Salazar JH, Papandria D, Okoye MT, Al-Omar K, et al. Age at presentation of common pediatric surgical conditions: Reexamining dogma. J Pediatr Surg. (2014) 49:995–9. doi: 10.1016/j.jpedsurg.2014.01.039
6. Oussama S, Fadi I, Mohammad A, Ranad G, Falaou S, Merhi BA, et al. Case report of late onset hypertrophic pyloric stenosis in lebanese infant. SAR J Med Case Rep. (2020) 1:29–31.
7. Sharma K, Agrawal P, Toshniwal H. Acquired gastric outlet obstruction during infancy and childhood: A report of five unusual cases. J Pediatr Surg. (1997) 32:928–30. doi: 10.1016/S0022-3468(97)90654-0
8. Sharma K, Ranka P, Goyal P, Dabi D. Gastric outlet obstruction in children: An overview with report of Jodhpur disease and Sharma’s classification. J Pediatr Surg. (2008) 43:1891–7. doi: 10.1016/j.jpedsurg.2008.07.001
9. Kajal P, Bhutani N, Kadian YS. Primary acquired gastric outlet obstruction (Jodhpur disease). J Pediatr Surg Case Rep. (2019) 40:6–9. doi: 10.1016/j.epsc.2018.09.009
10. Abuhandan M, Çaksen H, Eskiçubuk S. A case of acquired gastric outlet obstruction diagnosed at 16 years of age. Pediatr Surg Int. (2014) 20:148–50. doi: 10.1007/s00383-003-1108-0
11. Feng J, Gu W, Li M, Yuan J, Weng Y, Wei M, et al. Rare causes of gastric outlet obstruction in children. Pediatr Surg Int. (2005) 21:635–40. doi: 10.1007/s00383-005-1472-z
12. Hellan M, Lee T, Lerner T. Diagnosis and therapy of primary hypertrophic pyloric stenosis in adults: Case report and review of the literature. J Gastrointest Surg. (2006) 10:265–9. doi: 10.1016/j.gassur.2005.06.003
13. Quigley RL, Pruitt SK, Pappas TN, Akwari O. Primary hypertrophic pyloric stenosis in the adult. Arch Surg. (1990) 125:1219–21. doi: 10.1001/archsurg.1990.01410210145025
14. Mahalik S, Prasad A, Sinha A, Kulshrestha R. Delayed presentation of hypertrophic pyloric stenosis: A rare case. J Pediatr Surg. (2010) 45:e9–11. doi: 10.1016/j.jpedsurg.2009.11.012
15. Blumhageen J, Krauter D, Rosenbaum D, Weinberger E. Sonographic diagnosis of hypertrophic pyloric stenosis. Am J Roentgenol. (1988) 150:1367–70. doi: 10.2214/ajr.150.6.1367
16. Dias C, Swinson S, Torrão S, Gonçalves L, Kurochka S, Vaz CP, et al. Hypertrophic pyloric stenosis: Tips and tricks for ultrasound diagnosis. Insights Imaging. (2012) 3:247–50. doi: 10.1007/s13244-012-0168-x
17. Cohen H, Schenchter S, Mestel A, Eaton D, Haller J. Ultrasonic “double track” sign in hypertrophic pyloric stenosis. J Ultrasound Med. (1987) 6:139–43. doi: 10.7863/jum.1987.6.3.139
18. Boybeyi O, Karnak I, Ekinci S, Ciftci AO, Akçören Z, Tanyel FC, et al. Late-onset hypertrophic pyloric stenosis: Definition of diagnostic criteria and algorithm for the management. J Pediatr Surg. (2010) 45:1777–83. doi: 10.1016/j.jpedsurg.2010.04.014
19. Staerkle RF, Lunger F, Fink L, Sasse T, Lacher M, von Elm E, et al. Open versus laparoscopic pyloromyotomy for pyloric stenosis. Cochrane Database Syst Rev. (2021) 3:CD012827. doi: 10.1002/14651858.CD012827.pub2
20. Lone YA, Hushain D, Chana RS, Khan RA, Sachdeva S, Mushtaq E. Primary acquired gastric outlet obstruction in children: A retrospective single center study. J Pediatr Surg. (2019) 54:2285–90. doi: 10.1016/j.jpedsurg.2019.02.056
21. Ikenaga T, Honmyo U, Takano S, Murakami A, Harada K, Mizumoto S, et al. Primary hypertrophic pyloric stenosis in the adult. J Gastroenterol Hepatol. (1992) 7:524–6. doi: 10.1111/j.1440-1746.1992.tb01032.x
22. Danikas D, Geis WP, Ginalis EM, Gorcey SA, Stratoulias C. Laparoscopic pyloroplasty in idiopathic hypertrophic pyloric stenosis in an adult. JSLS. (2000) 4:173–5.
23. Brahos GJ, Mack E. Adult hypertrophic pyloric stenosis managed by double pyloroplasty. JAMA. (1980) 243:1928–9. doi: 10.1001/jama.243.19.1928
24. McCann JC, Dean MA. Hypertrophy of pyloric muscle in adult; experiences with conservative and radical surgical treatment. Surg Gynecol Obstet. (1950) 90:535–42.
25. MacDonald JAE. Adult hypertrophic pyloric stenosis. Br J Surg. (1973) 60:73–5. doi: 10.1002/bjs.1800600122
26. Christiansen KH. Idiopathic hypertrophic pyloric stenosis in the adult. Arch Surg. (1962) 85:207. doi: 10.1001/archsurg.1962.01310020037008
27. Lüdtke FE, Bertus M, Michalski S, Dapper FD, Lepsien G. Long-term analysis of ultrasonic features of the antropyloric region 17-27 years after treatment of infantile hypertrophic pyloric stenosis. J Clin Ultrasound. (1994) 22:299–305. doi: 10.1002/jcu.1870220503
28. Sun WM, Doran SM, Jones KL, Davidson G, Dent J, Horowitz M. Long-term effects of pyloromyotomy on pyloric motility and gastric emptying in humans. Am J Gastroenterol. (2000) 95:92–100. doi: 10.1111/j.1572-0241.2000.01705.x
29. Selzer D, Croffie J, Breckler F, Rescorla F. Hypertrophic pyloric stenosis in an adolescent. J Laparoendosc Adv Surg Tech. (2009) 19:451–2. doi: 10.1089/lap.2008.0276
30. Bajpai M, Singh A, Panda SS, Chand K, Rafey AR. Hypertrophic pyloric stenosis in an older child: A rare presentation with successful standard surgical management. Case Rep. (2013) 2013:bcr2013201834. doi: 10.1136/bcr-2013-201834
31. Parnall T, Caldwell K, Noel J, Russell J, Reyes C. Hypertrophic pyloric stenosis in a 15-year-old male. J Pediatr Surg Case Rep. (2016) 15:33–6. doi: 10.1016/j.epsc.2016.09.006
32. Wolf L, Nijagal A, Flores A, Buchmiller T. Late-onset hypertrophic pyloric stenosis with gastric outlet obstruction: Case report and review of the literature. Pediatr Surg Int. (2016) 32:1013–6. doi: 10.1007/s00383-016-3955-5
33. Al-Mayoof AF, Doghan IK. Late onset infantile hypertrophic pyloric stenosis. J Pediatr Surg Case Rep. (2018) 30:22–4. doi: 10.1016/j.epsc.2017.11.001
34. Bartlett E, Carlisle E, Mak G. Gastric outlet obstruction in a 12 year old male. J Pediatr Surg Case Rep. (2018) 31:57–9. doi: 10.1016/j.epsc.2017.12.012
35. Oswari H, Kresnawati W, Yani A, Handjari D, Alatas F. Abdominal injury-induced gastric outlet obstruction in primary hypertrophic pyloric stenosis in adolescent. Indian J Surg. (2020) 83:264–264. doi: 10.1007/s12262-020-02097-y
Keywords: pyloromyotomy, laparoscopy, hypertrophic pyloric stenosis, vomit, surgery
Citation: Iacoviello O, Verriello G, Castellaneta S, Palladino S, Wong M, Mattioli G, Giordano P, Francavilla R and Cristofori F (2022) Case report: Late-onset hypertrophic pyloric stenosis in a 3-year-old boy: It is never too late. Front. Pediatr. 10:949144. doi: 10.3389/fped.2022.949144
Received: 20 May 2022; Accepted: 28 July 2022;
Published: 16 August 2022.
Edited by:
Pedro Gutierrez-Castrellon, Hospital General Dr. Manuel Gea González, MexicoReviewed by:
Ivana I. Kavecan, University of Novi Sad, SerbiaAllen F. Browne, Independent Researcher, Falmouth, ME, United States
Jose Francisco Gonzalez-Zamora, National Institute of Pediatrics, Mexico
Doina Anca Plesca, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2022 Iacoviello, Verriello, Castellaneta, Palladino, Wong, Mattioli, Giordano, Francavilla and Cristofori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Onofrio Iacoviello, by5pYWNvdmllbGxvQGdtYWlsLmNvbQ==
 Onofrio Iacoviello
Onofrio Iacoviello Giuseppe Verriello1
Giuseppe Verriello1 Michela Wong
Michela Wong Girolamo Mattioli
Girolamo Mattioli Paola Giordano
Paola Giordano Ruggiero Francavilla
Ruggiero Francavilla Fernanda Cristofori
Fernanda Cristofori