
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pediatr. , 18 July 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.945090
 Emily J. J. Horn-Oudshoorn1
Emily J. J. Horn-Oudshoorn1 Ronny Knol1
Ronny Knol1 Suzan C. M. Cochius-den Otter2
Suzan C. M. Cochius-den Otter2 Arjan B. te Pas3
Arjan B. te Pas3 Stuart B. Hooper4
Stuart B. Hooper4 Calum T. Roberts4,5
Calum T. Roberts4,5 Neysan Rafat6
Neysan Rafat6 Thomas Schaible6
Thomas Schaible6 Willem P. de Boode7
Willem P. de Boode7 Robin van der Lee7
Robin van der Lee7 Anne Debeer8
Anne Debeer8 Florian Kipfmueller9
Florian Kipfmueller9 Charles C. Roehr10,11,12
Charles C. Roehr10,11,12 Irwin K. M. Reiss1
Irwin K. M. Reiss1 Philip L. J. DeKoninck4,13*
Philip L. J. DeKoninck4,13*Background: Infants with a congenital diaphragmatic hernia (CDH) and expected mild pulmonary hypoplasia have an estimated survival rate of 90%. Current guidelines for delivery room management do not consider the individual patient's disease severity, but an individualized approach with spontaneous breathing instead of routine mechanical ventilation could be beneficial for the mildest cases. We developed a resuscitation algorithm for this individualized approach serving two purposes: improving the success rate by structuring the approach and providing a guideline for other centers.
Methods: An initial algorithm was discussed with all local stakeholders. Afterwards, the resulting algorithm was refined using input from international experts.
Results: Eligible CDH infants: left-sided defect, observed to expected lung-to-head ratio ≥50%, gestational age at birth ≥37.0 weeks, and no major associated structural or genetic abnormalities. To facilitate fetal-to-neonatal transition, we propose to start stabilization with non-invasive respiratory support and to adjust this individually.
Conclusions: Infants with mild CDH might benefit from an individualized approach for neonatal resuscitation. Herein, we present an algorithm that could serve as guidance for centers implementing this.
Around 70% of all infants with a congenital diaphragmatic hernia (CDH) are detected during prenatal screening (1–3). This provides an opportunity for early referral to specialized centers, additional diagnostic procedures, and individualized counseling. For isolated cases, postnatal outcomes largely depend on the extent of the pulmonary disease (4, 5). Antenatal ultrasound measurement of the contralateral lung, expressed as the observed to expected lung-to-head ratio (o/e LHR), is the most validated method to estimate the severity of pulmonary hypoplasia (4, 5). Liver position and defect-side are additional independent predictors of postnatal outcomes (3, 4, 6). Based on these parameters, one can distinguish a group with a relatively mild degree of pulmonary hypoplasia, corresponding with an estimated survival rate of 90% (4, 5). Current guidelines on delivery room management apply to all neonates with CDH and do not take the individual neonate's disease severity into account. An example of this is initial mechanical ventilation, which might be too aggressive for infants with expected mild pulmonary hypoplasia, given the favorable outcomes, the risk of ventilator-induced lung injury, and the stress caused by intubation (3–5, 7, 8). A more individualized approach has the potential to avoid overtreatment and risks of intubation.
The Erasmus MC implemented a trial of spontaneous breathing for a specific subset of infants (isolated left-sided CDH, o/e LHR ≥50%, and intra-abdominal liver position) in December 2014 (9). A retrospective single-center audit recently demonstrated that the spontaneous breathing approach (SBA) was feasible, but 60% of cases still required intubation in the first hours after birth (10). On the other hand, there was an apparent decrease in the total length of hospital stay in successful cases and, more importantly, there were no adverse effects of the delayed intubation in cases that failed the SBA (10). These results justify further evaluation of this approach. Yet, the low success rate in this small series highlights that optimal case selection is challenging and emphasizes the need for a standardized management algorithm (10). Meanwhile, other centers have already implemented the SBA or are interested. For these reasons, we developed a resuscitation algorithm that serves two purposes: improving the success rate by structuring the approach and providing a guideline for centers that consider implementation.
Algorithm development was a two-step process: first, it was drafted and discussed by all stakeholders that are involved in the care of CDH infants in the Erasmus MC (i.e., neonatal nurses, neonatologists, obstetricians, pediatric intensivist, and pediatric surgeons); second, the resulting algorithm was optimized with input from international experts on neonatal resuscitation, CDH management, and fetal/neonatal physiology. Medical ethical approval for prospective data collection was obtained in the Erasmus MC as the initiating center (MEC-2021-0304) and will be obtained in all centers that start data collection.
Only CDH infants with expected mild pulmonary hypoplasia are considered candidates. We propose the following eligibility criteria depicted in Table 1. We recommend discussing the initial ventilation strategy for each case during a multidisciplinary meeting around 30 weeks of gestation, involving all caregivers.
The primary aim of perinatal stabilization of infants with a CDH is to establish adequate oxygenation whilst avoiding hypoxia, hyperoxia, and high peak airway pressures (9, 14). In the abovementioned series, reasons for intubation in the delivery room were low SpO2-levels, absence of breathing movements, or signs of respiratory distress (10). To facilitate the fetal-to-neonatal transition, and, thus, the success of the SBA, we suggest to start stabilization with non-invasive respiratory support and to adjust this individually (Figure 1). It is, however, not clear whether the fetal-to-neonatal transition in these infants is more favorably supported by additional FiO2 and/or continuous distending airway pressures (high flow or CPAP). To enable implementation in other centers, we leave it up to the centre's discretion to decide whether high flow or CPAP is more feasible within their local logistics and standard of care. To minimize the negative effects of potential abdominal distension associated with non-invasive respiratory support, we recommend early insertion of an oro-/nasogastric tube.
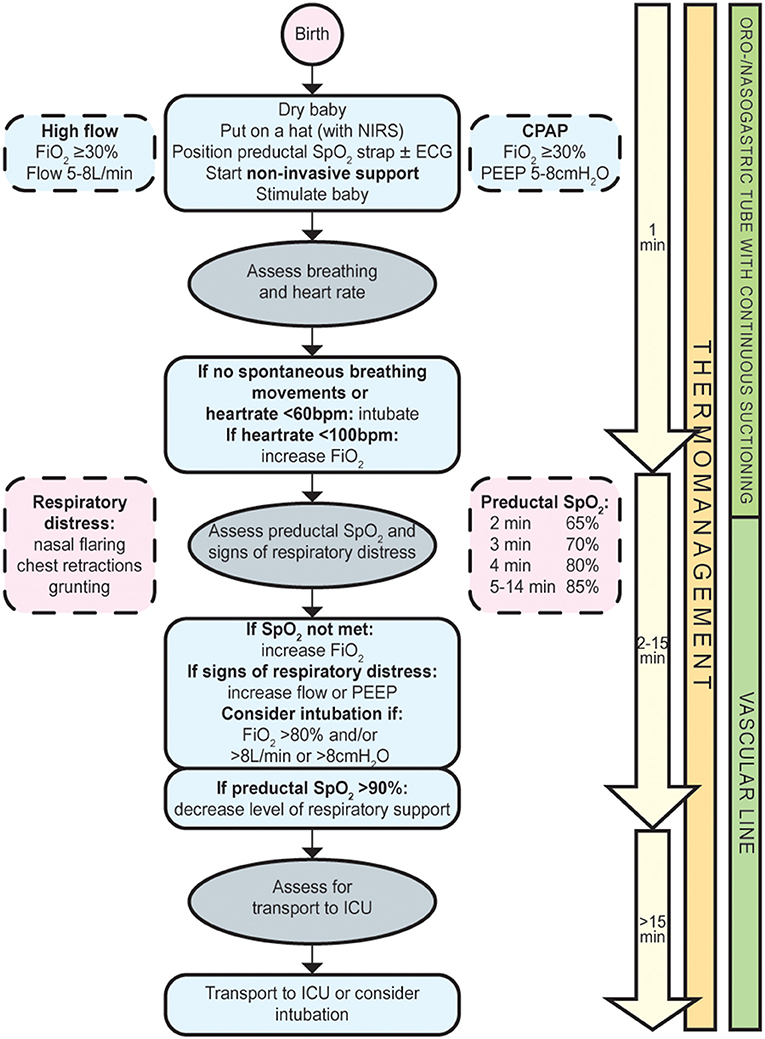
Figure 1. Flowchart spontaneous breathing approach for infants with a congenital diaphragmatic hernia.
We recommend to:
- Initiate nasal high flow or CPAP and subsequently titrate up or down by continuously evaluating the infant's respiratory status using the European Neonatal Life Support guidelines (15);
- Consider intubation in case of insufficient spontaneous breathing movements, heartrate <60/min, FiO2 > 80%, flow >8 L/min, or CPAP > 8 cmH2O;
- Decrease the level of respiratory support if preductal SpO2 >90%;
- Insert an oro-/nasogastric tube with continuous suctioning.
This resuscitation algorithm presents an individualized approach for infants with a CDH and predicted mild pulmonary hypoplasia. We acknowledge that the proposed algorithm is based on expert-opinion and low-grade, single-center evidence (Scottish Intercollegiate Guidelines Network criteria, grade of recommendation D) (16). Ideally, this strategy should be tested in a randomized controlled trial. However, the lack of equipoise in centers that have already implemented the SBA would pose a challenge for reaching a sufficient sample size to evaluate the full extent of the various clinically relevant outcomes. Instead, prospective observational data collection of CDH infants cared for with the SBA is in progress within the framework of an international research consortium: the very mild CDH—SBA consortium (VeSBA). We share our algorithm, so that the SBA may be adopted by other centers and we invite their contribution to this prospective registry. We emphasize that strict adherence to the algorithm is not a prerequisite to join the VeSBA consortium and local adaptations are obviously acceptable.
Current guidelines on delivery room management for infants with a CDH do not take into account the individual patient's disease severity. However, the spontaneous breathing approach is an individualized approach for infants with a relatively mild CDH that could prevent overtreatment in this specific subgroup.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Medical ethical approval for prospective data collection within the international VeSBA registry was obtained in the Erasmus MC as the initiating centre (MEC-2021-0304) and will be obtained in all centres that start data collection. Informed consent for prospective data collection will be obtained for each patient cared for with the spontaneous breathing approach.
EH-O, RK, SC, IR, and PD were all involved in the conception of this manuscript and the design of the algorithm. AP, SH, CTR, NR, TS, WB, RL, AD, FK, and CCR contributed to the algorithm. EH-O drafted the initial manuscript, which was critically reviewed by RK, SC, AP, SH, CTR, NR, TS, WB, RL, AD, FK, CCR, IR, and PD. All authors have approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
EH-O and PD are supported by a grant from Sophia Children's Hospital Foundation (SSWO, grant S19-12).
The authors thank Dr. R. C. J. de Jonge, E. E. Langenberg, Dr. N. van Paassen, Profs. Drs. D. Tibboel, and R. M. H. Wijnen from the Erasmus MC for their contribution to this algorithm. This proposal has been endorsed by the European Scientific Collaboration on Neonatal Resuscitation Research (ESCNR), a section of the European Society for Pediatric Research (ESPR), and the CDH EURO Consortium.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CDH, congenital diaphragmatic hernia; o/e LHR, observed to expected lung-to-head ratio; SBA, spontaneous breathing approach.
1. Burgos CM, Frenckner B, Luco M, Harting MT, Lally PA, Lally KP, et al. Prenatally versus postnatally diagnosed congenital diaphragmatic hernia - side, stage, and outcome. J Pediatr Surg. (2019) 54:651–5. doi: 10.1016/j.jpedsurg.2018.04.008
2. Gallot D, Boda C, Ughetto S, Perthus I, Robert-Gnansia E, Francannet C, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol. (2007) 29:276–83. doi: 10.1002/uog.3863
3. Cordier AG, Russo FM, Deprest J, Benachi A. Prenatal diagnosis, imaging, and prognosis in congenital diaphragmatic hernia. Semin Perinatol. (2020) 44:51163. doi: 10.1053/j.semperi.2019.07.002
4. Jani J, Nicolaides KH, Keller RL, Benachi A, Peralta CF, Favre R, et al. Observed to expected lung area to head circumference ratio in the prediction of survival in fetuses with isolated diaphragmatic hernia. Ultrasound Obstet Gynecol. (2007) 30:67–71. doi: 10.1002/uog.4052
5. Snoek KG, Peters NCJ, van Rosmalen J, van Heijst AFJ, Eggink AJ, Sikkel E, et al. The validity of the observed-to-expected lung-to-head ratio in congenital diaphragmatic hernia in an era of standardized neonatal treatment; a multicenter study. Prenat Diagn. (2017) 37:658–65. doi: 10.1002/pd.5062
6. Dekoninck P, Gratacos E, Van Mieghem T, Richter J, Lewi P, Ancel AM, et al. Results of fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia and the set up of the randomized controlled TOTAL trial. Early Hum Dev. (2011) 87:619–24. doi: 10.1016/j.earlhumdev.2011.08.001
7. Deprest JA, Flemmer AW, Gratacos E, Nicolaides K. Antenatal prediction of lung volume and in-utero treatment by fetal endoscopic tracheal occlusion in severe isolated congenital diaphragmatic hernia. Semin Fetal Neonatal Med. (2009) 14:8–13. doi: 10.1016/j.siny.2008.08.010
8. Mullassery D, Ba'ath ME, Jesudason EC, Losty PD. Value of liver herniation in prediction of outcome in fetal congenital diaphragmatic hernia: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2010) 35:609–14. doi: 10.1002/uog.7586
9. Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus – 2015 update. Neonatology. (2016) 110:66–74. doi: 10.1159/000444210
10. Cochius-den Otter SCM, Horn-Oudshoorn EJJ, Allegaert K, DeKoninck PLJ, Peters NCJ, Cohen-Overbeek TE, et al. Routine intubation in newborns with congenital diaphragmatic hernia. Pediatrics. (2020) 146:e20201258. doi: 10.1542/peds.2020-1258
11. DeKoninck P, Gomez O, Sandaite I, Richter J, Nawapun K, Eerdekens A, et al. Right-sided congenital diaphragmatic hernia in a decade of fetal surgery. BJOG. (2015) 122:940–6. doi: 10.1111/1471-0528.13065
12. Tsao K, Allison ND, Harting MT, Lally PA, Lally KP. Congenital diaphragmatic hernia in the preterm infant. Surgery. (2010) 148:404–10. doi: 10.1016/j.surg.2010.03.018
13. Akinkuotu AC, Cruz SM, Cass DL, Lee TC, Cassady CI, Mehollin-Ray AR, et al. An evaluation of the role of concomitant anomalies on the outcomes of fetuses with congenital diaphragmatic hernia. J Pediatr Surg. (2016) 51:714–7. doi: 10.1016/j.jpedsurg.2016.02.008
14. Reiss I, Schaible T, van den Hout L, Capolupo I, Allegaert K, van Heijst A, et al. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus. Neonatology. (2010) 98:354–64. doi: 10.1159/000320622
15. Madar J, Roehr CC, Ainsworth S, Ersdal H, Morley C, Rüdiger M, et al. European resuscitation council guidelines 2021: newborn resuscitation and support of transition of infants at birth. Resuscitation. (2021) 161:291–326. doi: 10.1016/j.resuscitation.2021.02.014
Keywords: congenital diaphragmatic hernia, intubation, spontaneous breathing approach, neonatal resuscitation, birth
Citation: Horn-Oudshoorn EJJ, Knol R, Cochius-den Otter SCM, te Pas AB, Hooper SB, Roberts CT, Rafat N, Schaible T, de Boode WP, van der Lee R, Debeer A, Kipfmueller F, Roehr CC, Reiss IKM and DeKoninck PLJ (2022) Spontaneous breathing approach in mild congenital diaphragmatic hernia: A resuscitation algorithm. Front. Pediatr. 10:945090. doi: 10.3389/fped.2022.945090
Received: 16 May 2022; Accepted: 27 June 2022;
Published: 18 July 2022.
Edited by:
Hans Fuchs, University of Freiburg Medical Center, GermanyReviewed by:
Andrea Conforti, Bambino Gesù Children's Hospital (IRCCS), ItalyCopyright © 2022 Horn-Oudshoorn, Knol, Cochius-den Otter, te Pas, Hooper, Roberts, Rafat, Schaible, de Boode, van der Lee, Debeer, Kipfmueller, Roehr, Reiss and DeKoninck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philip L. J. DeKoninck, cC5kZWtvbmluY2tAZXJhc211c21jLm5s
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.