
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 08 November 2022
Sec. Pediatric Neurology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.944067
 Zhongchen Luo1
Zhongchen Luo1 Beibei You1
Beibei You1 You Zhang2
You Zhang2 Jiao Tang3,4
Jiao Tang3,4 Zehong Zheng5
Zehong Zheng5 Yuling Jia1
Yuling Jia1 Li Wang1
Li Wang1 Dan Zeng1
Dan Zeng1 Hong Li1
Hong Li1 Xiuhong Wang1,6*
Xiuhong Wang1,6*
Background: Extrauterine growth restriction among the very-low birth weight preterm infants (VLBWPIs) is associated with poorer cognitive development outcome, while the rapid weight gain in infancy increases the long-term risk of obesity and noncommunicable disease among VLBWPIs. However, the results of research on the association between early postnatal growth velocity and neurodevelopmental outcomes in VLBWPIs are still limited and controversial.
Objective: We aimed to explore the association between the growth velocity in early postnatal and neurodevelopmental impairment (NDI) among VLBWPIs.
Methods: This study was a secondary analysis of a previously published prospective cohort. It was based on data on 1,791 premature infants with a birth weight of less than 1500 g, registered in the database of the Premature Baby Foundation of Taiwan between 2007 and 2011. A binary logistic regression model was used to evaluate the association between the weight gain velocity in different periods [from birth to 6 months corrected age (CA), 6 to 12 months CA, and 12 to 24 months CA] and NDI, respectively. The generalized additive model and the smooth curve fitting (penalized spline method) were used to address nonlinearity, and a two-piece-wise binary logistic regression model was added to explain the nonlinearity further.
Results: Nonlinearities were observed between NDI and the weight gain velocity from birth to 6 months CA [inflection point 20.36, <inflection point: odds ratio (OR) = 0.75, 95% confidence interval (CI) 0.67–0.84, >inflection point: OR = 1.01, 95% CI 0.97–1.05], 6–12 months CA [inflection point 9.44, <inflection point: OR = 0.89, 95% CI 0.84–0.94, >inflection point: OR = 1.05, 95% CI 1.05–(1.00, 1.11)], and 12–24 months CA [inflection point 16.00, <inflection point: OR = 0.93, 95% CI 0.88–0.98, >inflection point: OR = 1.75, 95% CI 1.05–(0.96, 3.08)].
Conclusion: The neurodevelopmental benefits from a rapid weight gain velocity from birth to 24 months CA might be limited once the growth pace reaches an optimum level. It would help find a pattern of growth that facilitates optimal neurodevelopment, yet minimizes negative health consequences associated with overnutrition further.
Advanced neonatal care has dramatically decreased the mortality of very-low birth weight preterm infants (VLBWPIs, with a birth weight ≤1500 g and gestational age at birth <37 weeks) in recent decades (1–3), and the rate of VLBWPIs was estimated to account for 0.8% of live births in Taiwan (about 200,000 infants in total born each year) (1). The weight gain plays a significant role in neurodevelopment among VLBWPIs. Extrauterine growth restriction among VLBWPIs is associated with poorer cognitive development outcome (4–6). However, the correlation between the early weight gain velocity after birth and neurodevelopmental impairment (NDI) in preterm infants remains controversial. A large number or studies reported that there is a positive association between postnatal weight growth with neurocognitive outcomes in different periods among low-weight preterm infants (7, 8). However, other articles revealed a nonlinear association between them. Pylipow et al. (9) revealed a nonlinear association between the postnatal growth velocity in the first four postnatal months of infants with intrauterine growth restriction (<2211 g at ≥37 weeks’ gestation) and later cognitive function. Infants weight gain ranged from 1,059 to 5,119 g had lower achieved cognitive testing scores apparent at both extremes (an inverted J-shape), with both extremes associated with negative effects. Kim et al. (10) examined the growth velocity at different time periods after birth to school age in relation to neurocognitive outcomes among small for gestational age preterm infants, and only postnatal weight gain in the neonatal intensive care unit (NICU) was positively correlated with good neurodevelopmental outcomes. Additionally, Meyers et al. (11) reported that linear growth-restricted infants born <29 weeks with weight gain out of proportion to linear growth was associated with poorer 2-year neurodevelopmental outcomes, and infants with high body mass index (BMI) were more likely to have neurodevelopmental impairment compared with those with low-to-normal BMI. These inconsistencies may be attributable to the disparity in population, design, adjustment for covariates, especially the timing of measurement, different outcome indicators of neurodevelopmental impairment, or calculation of the weight gain velocity.
In addition, the rapid early postnatal weight growth pace among VLBWPIs is positively associated with a higher risk of overweight/obesity and another noncommunicable disease. The rapid weight gain in infancy increases the long-term risk of obesity and noncommunicable disease among low-weight preterm infants including insulin resistance and metabolic syndrome, cardiovascular risk, adiposity, higher blood pressure, insulin resistance, dyslipidemia, and endothelial dysfunction (12, 13). It is noteworthy that in line with advances in medical and nutritional care during the past decade, the weight growth pace of preterm infants in the postnatal period is rapid and the rate of short-term extrauterine growth restriction is reduced (14, 15). Overemphasizing the prevention of failure to thrive postnatally and ignoring the effect of rapid acceleration of growth on metabolic syndrome later in life may be also a harmful pattern of growth. Achieving a pattern of growth that facilitates optimal neurodevelopment, yet minimizes negative health consequences associated with overnutrition, is desirable. The pattern of growth required to achieve this balance is unknown.
Based on the above, we performed a secondary analysis based on a published cohort study to explore the association between the early postnatal weight gain velocity and neurodevelopmental impairment at 24 months corrected age (CA) in the VLBWIs, thus providing more evidence in support of the association between the two variables and helping find a pattern of growth that facilitates optimal neurodevelopment yet minimizes negative health consequences associated with overnutrition.
This study uses secondary analysis of a previously published prospective cohort study (16). The data were retrieved from the “PLOS ONE” database (https://journals.plos.org/). Since the data uploader has waived all copyright, a secondary analysis without infringing on the authors’ rights when the data was used. (Data from: Chung-Ting Hsu, Chao-Huei Chen, Ming-Chih Lin, Teh-Ming Wang, Ya-Chi Hsu, 2017. Postdischarge body weight and neurodevelopmental outcomes among VLBWPIs in Taiwan: A nationwide cohort study. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0192574). The interesting independent variable in the present work is the weight gain velocity in different periods (from birth to 6 months CA, 6 to 12 months CA, and 12 to 24 months CA). The dependent variable is NDI (dichotomous variable: 0 = Non-NDI, 1 = NDI).
The original study nonselectively and consecutively collected data on VLBWPIs (with a birth weight of less than 1500 g, and with a birth weight ≤1500 g and gestational age at birth less than 37 weeks) registered at the Premature Baby Foundation of Taiwan between 2007 and 2011 (16). All 21 hospitals located across the island of Taiwan participated in the data collection (17). Inclusion criteria were premature infants with a birth weight less than 1500 g, and exclusion criteria included (1) babies with advanced intraventricular hemorrhage (grade III and grade IV), (2) chromosome anomalies, (3) death before the end of follow-up, and (4) without BSID-II scores or complete follow-up data. The data were obtained from the Taiwan Premature Infant Developmental Collaborative Study Group, which was established by the Premature Baby Foundation of Taiwan. The local ethics committee of Taiwan Premature Infant Developmental Collaborative Study Group approved this original study (16). Informed consent was waived because that data were accessed anonymously in this study.
As body weight gain is a key indicator of the degree of nutrition and catch-up growth of neonates, early postnatal growth in most cases is defined by weight growth (18, 19). There are many methods to calculate the weight gain velocity include grams/kilogram/day (g/kg/d), grams/day (g/d), and change in z scores (20). However, z score differences based on cross-sectional growth charts can be confused with distorted reference data, leading to a false reading of growth velocity of VLBWPIs (21). According to a systematic review, one of the frequently used methods to calculate the weight gain velocity was g/d (20). Considering the order of magnitude of uniform units and make the results easier to understand and to guide clinical practice, we defined the weight gain velocity of weight as g/d (W2 − W1)/(t2 − t1) in this study.
The first 6 months might be the key period of neurodevelopment in moderately preterm infants (8). The first 12 months of life are the most sensitive period of catch-up in weight and length for very preterm infants. It would be significant in determining later cognitive function (22). Therefore, we have chosen the time points above for data analysis.
Our interesting outcome variable was NDI. The detailed process of measure of NDI is defined as any of the following conditions: Mental Developmental Index (MDI) score below 70, Psychomotor Developmental Index (PDI) below 70, cerebral palsy, visual impairment, or hearing impairment (16, 23). MDI and PDI scores were generated by BSID-II (24, 25). Cerebral palsy was defined as the presence of any of the following disorders: spastic tetraparesis, spastic hemiparesis, spastic diplegia, spastic dyskinesia, or hypotonia at 24 months CA by the neurologist. Visual impairment is defined as amblyopia or blindness in any eye at 24 months CA by an ophthalmologist. Hearing impairment is defined as more than 30 decibels (dB) hearing loss in any ear at 24 months CA by an otologist. All children who had visual or hearing impairments would be followed up by an ophthalmologist or otologist (16).
Covariates were selected in our study according to the previous literature (26–28). Based on the above principles, therefore, the following variables were used as covariates: (1) continuous variables: gestation age and birth bodyweight; (2) categorical variables: gender, small for gestational age, intraventricular hemorrhage mild, persistent pulmonary hypertension of newborn, hemodynamic significant patent ductus arteriosus, necrotizing enterocolitis, chronic lung disease, respiratory distress syndrome, surfactant use, indomethacin use, and extrauterine growth retardation. Chronic lung disease was defined as needing respiratory support with oxygen or positive pressure ventilation at the postmenstrual age of 36 weeks. Hemodynamic significant patent ductus arteriosus was defined as needing medical intervention or surgical ligation (16). Small for gestational age was defined as a birth body weight below the 10th percentile of the standard fetal growth curve (29). Extrauterine growth retardation was defined as a weight below the 3rd percentile of the growth curve at discharge (30).
The interval between each follow-up was at 6, 12, and 24 months CA. Monitoring indicators at each follow-up included body weight and neurological and psychomotor performance at 24 months CA. According to the selection criteria, the study initially collected 4,636 participants in this study; afterward, 1,093 participants were excluded due to chromosome anomalies (n = 24), severe intraventricular hemorrhage (grade III–grade IV) (n = 360), or death (n = 709). At 24 months CA, 1,752 infants were not followed up. Finally, a total of 1,791 participants were left for data analysis. Details of recruitment and follow-up were described in the original literature (16).
Continuous variables are expressed as mean (standard deviation) (Gaussian distribution) or median (min, max) (Skewed distribution), and categorical variables are given as frequencies and percentages. χ2 (categorical variables), Student’s t-test (normal distribution), or Man–Whitney U test (skewed distribution) were used to detect the differences among different NDI (binary variable). We used univariate and multivariate binary logistic regression models to test the connection between the weight gain velocity from birth to 6 months CA, 6 to 12 months CA, and 12 to 24 months CA and NDI with three distinct models, respectively. Model 1 is the nonadjusted model with no covariates adjusted. Model 2 is the minimally adjusted model with only sociodemographic variables adjusted [gender (male, female), gestational age at birth, birth body weight]. Model 3 is the fully adjusted model with covariates presented in Table 1 adjusted.
To test the robustness of our results, we performed a sensitivity analysis. We converted the weight gain velocity in different periods into a categorical variable according to the tercile and calculated the P for trend in order to verify the results of the weight gain velocity as the continuous variable and to examine the possibility of nonlinearity.
To account for the nonlinear relationship between the weight gain velocity in different periods and NDI, we also used the generalized additive model and the smooth curve fitting (penalized spline method) to further explore the shape of their relations. In addition, a two-piece-wise binary logistic regression model was also used to explain the nonlinearity further.
All the analyses were performed with the statistical software packages R (http://www.R-project.org, the R Foundation) and EmpowerStats (http://www. empowerstats.com, X & Y Solutions, Inc., Boston, MA, United States). P values less than 0.05 (two-sided) were considered statistically significant.
The authors presented baseline demographics and clinical characteristics of included participants in Table 1. The population (n = 1791) at baseline, of whom 52.15% % were male, had a mean gestational age of 29.55 ± (2.50) weeks. Compared with infants who got an NDI, those who are non-NDI have a higher weight gain velocity from birth to 6 months CA. However, there is no difference between the two groups in the weight gain velocity from 6 to 12 months CA and 12 to 24 months CA. In addition, infants who were not followed up had a greater prevalence of necrotizing enterocolitis, respiratory distress syndrome, and chronic lung disease, and had less surfactant, indomethacin use, and extrauterine growth retardation (weight < 3rd percentile at discharge). However, gender, gestational age at birth, birth body weight, small for gestational age, extrauterine growth retardation (weight < 10th percentile at discharge), mild intraventricular hemorrhage, persistent pulmonary hypertension of newborn, significant patent ductus arteriosus, etc., were proved to be similar among the 1,752 infants who were not followed up and the 1,791 that were left (16).
For the weight gain velocity from birth to 6 months CA, in the fully adjusted model, 1 g/d increase of weight gain velocity was related to 5% decrease in risk of NDI [odds ratio (OR) = 0.95, 95% confidence interval (CI) 0.92–0.99, p < 0.01]; 1 g/d increase of weight gain velocity from 6 to 12 months CA was related to 3% decrease in risk of NDI (OR = 0.97, 95% CI 0.94, 1.00, p = 0.049); and 1 g/d increase of weight gain velocity from 12 to 24 months CA was related to 5% decreases in risk of NDI (OR = 0.95, 95% CI 0.90, 0.99, p = 0.02). All results are statistically significant (Table 2).
To verify the robustness of our findings, a sensitivity analysis was performed. We first convert different periods of the weight gain velocity from continuous variables to categorical variables (according to tercile), then put these categorical variables back into the model. The results (Table 2) show that after the weight gain velocity from 6 to 12 and 12 to 24 months CA were transformed into categorical variables, the trend of the effect sizes in different groups is equidistant, but P for trend (P = 0.30) are inconsistent with the result (P < 0.05) when the weight gain velocity is a continuous variable, suggesting that the clarification of nonlinearity between the weight gain velocity on NDI is necessary.
Through the generalized additive model and smooth curve fitting, we observed that from birth to 6 months CA and 6 to 12 months CA, the correlation between the weight gain velocity and NDI is nonlinear. However, there is no nonlinear relationship between the weight gain velocity from 12 to 24 months CA and NDI (Table 3). In our study, the P for log-likelihood ratio test of the relationship between NDI and both of the weight gain velocity from birth to 6 months CA and from 6 to 12 months CA was less than 0.01, so we used a two-piece-wise model to fitting the correlation between the weight gain velocity during this two periods and NDI. By recursive algorithm, the inflection points obtained first were 20.36 and 9.44 in those two sensitive periods, respectively. Then, the effect sizes and confidence interval on the left and right of the inflection point were calculated by a two-piece-wise binary logistic regression model. The results revealed that there are ceiling effects between the weight gain velocity from birth to 6 months CA and 6 to 12 months CA and NDI. For the weight gain velocity from birth to 6 months CA, on the left side of the inflection point, the rate is negatively associated with the risk of NDI (inflection point 20.36, <inflection point: OR = 0.75, 95% CI 0.67–0.84, >inflection point: OR = 1.01, 95% CI 0.97–1.05) (Figure 1); for the weight gain velocity from 6 to 12 months CA, the rate is negatively associated with the risk of NDI in the interval where the rate is less than 9.44 g/d (inflection point 9.44, <inflection point: OR = 0.89, 95% CI 0.84–0.94, >inflection point: OR = 1.05, 95% CI 1.00–1.11) (Figure 2), and for the weight gain velocity from 12 to 24 months CA, the rate is also negatively associated with the risk of NDI when the rate less than 16.00 g/d [inflection point 16.00, <inflection point: OR = 0.93, 95% CI 0.88–0.98, >inflection point: OR = 1.75, 95% CI 1.05 (0.96–3.08)] (Figure 3).
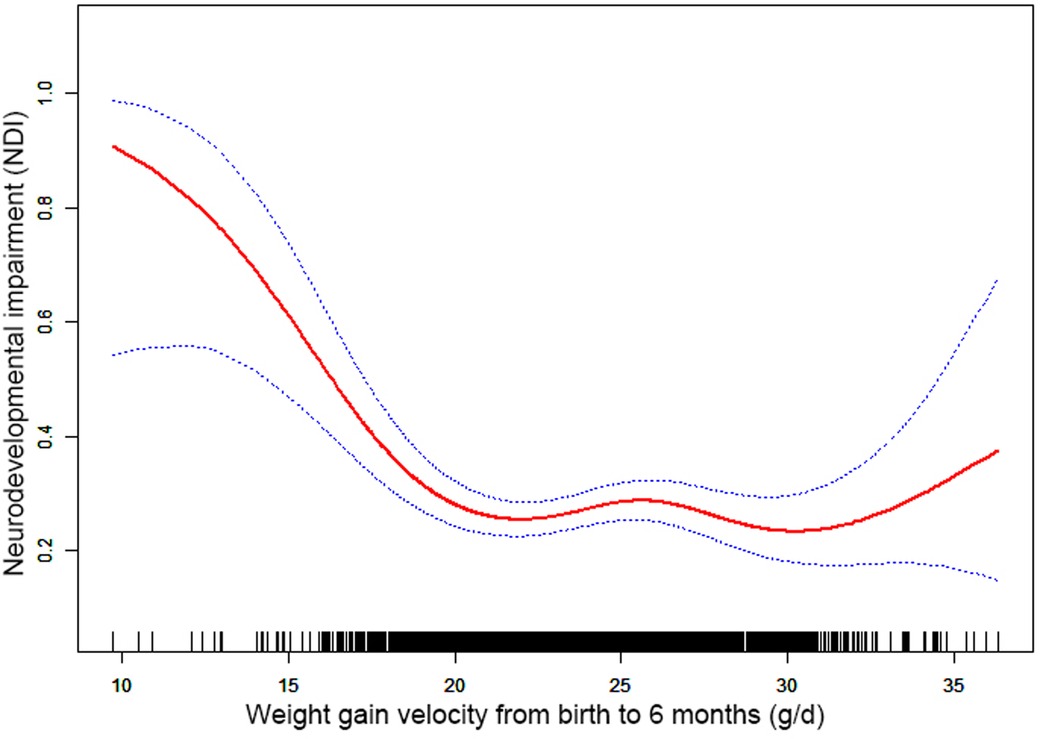
Figure 1. Nonlinearity of weight gain velocity from birth to 6 months CA on neurodevelopmental impairment.
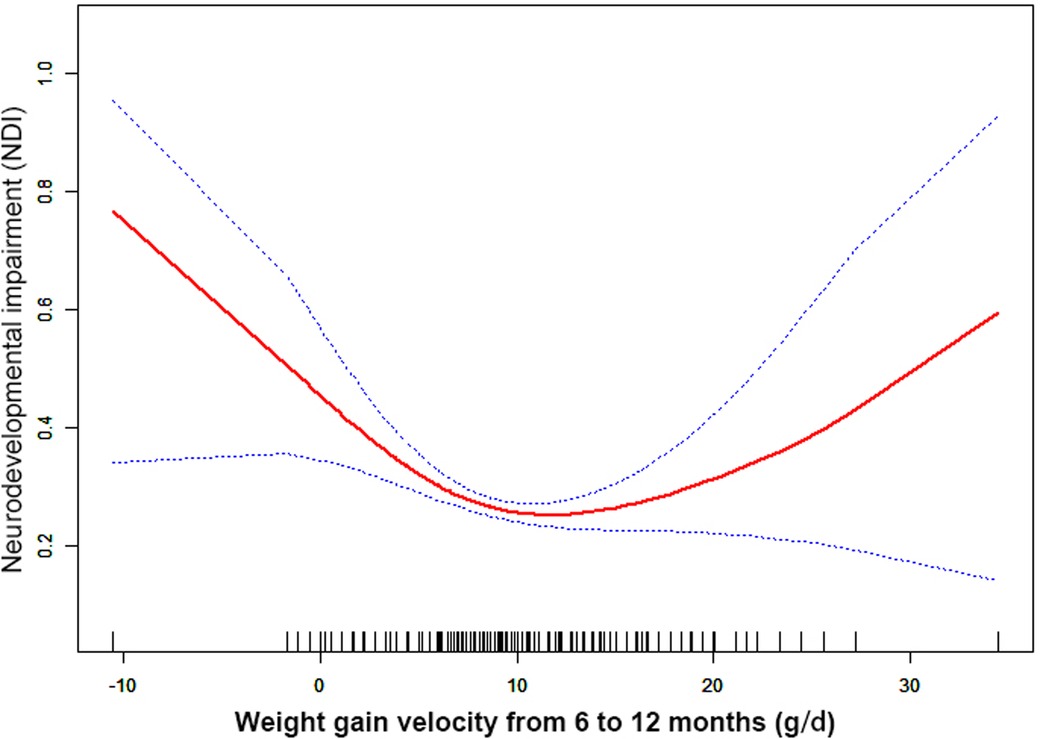
Figure 2. Nonlinearity of weight gain velocity from 6 to 12 months CA on neurodevelopmental impairment.
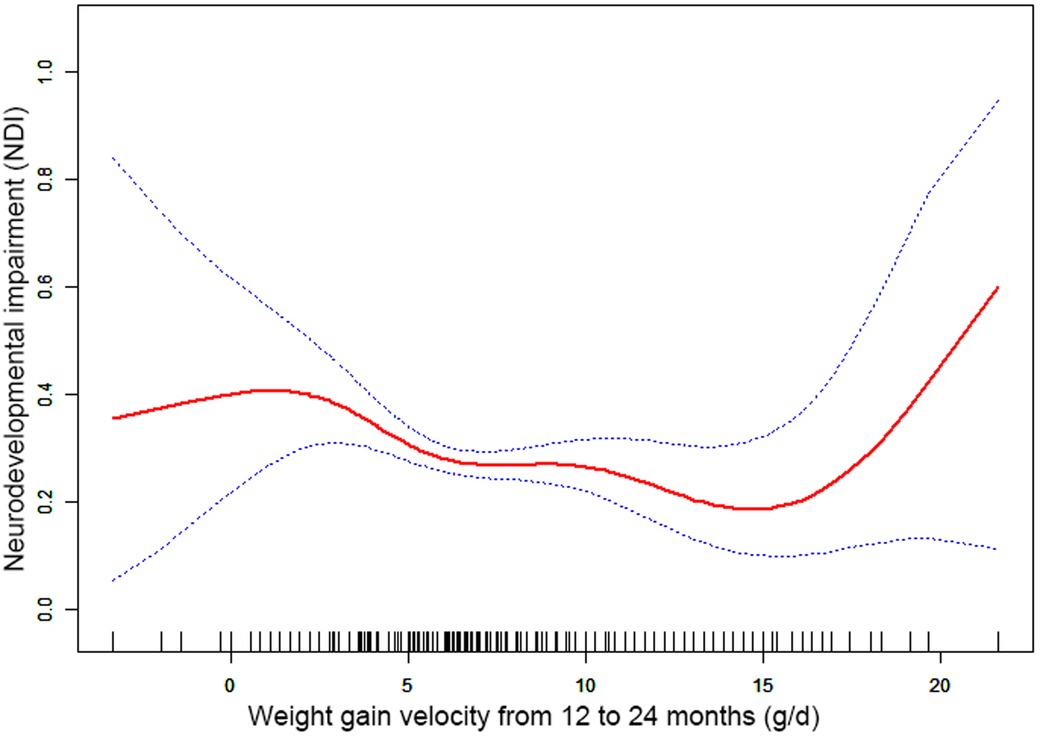
Figure 3. Nonlinearity of weight gain velocity from 12 to 24 months CA on neurodevelopmental impairment.
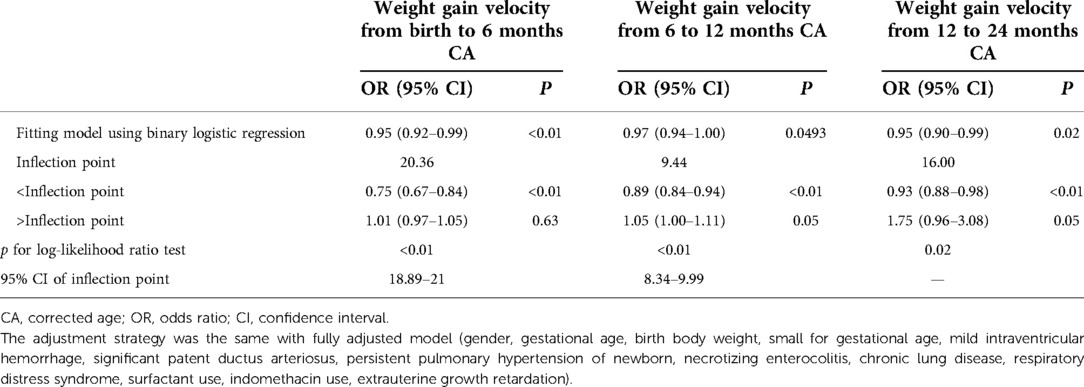
Table 3. Nonlinearity of weight gain velocity in different periods on neurodevelopmental impairment.
In this study, the results revealed that there were different relationships between the different periods of the weight gain velocity and neurodevelopmental outcomes in VLBWPIs. Surprisingly, we find it is not a simple linear relationship, but nonlinearity between early postnatal growth and NDI in the first year of CA. From birth to 6 months CA, a ceiling effect exists. If the rate is less than 20.36 g/d, the higher the weight gain velocity, the less risk of NDI. From 6 to 12 months CA, the higher the weight gain velocity, the less risk of NDI when the rate is less than 9.44 g/d. Moreover, we also found there is a nonlinear relationship between the weight gain velocity from 12 to 24 months CA and NDI, and the higher weight gain velocity is related with less risk of NDI when the rate is less than 16.00 g/d. The results did not appear to be fully compatible with other literature studies on this theme. Belfort et al. (31) conducted a study to identify the association between the linear slopes of weight growth from 1 week of age to term, term to 4 months, and 4–12 months, and the MDI and PDI scores at 18 months CA of 613 infants’ gestation age less than 33 weeks by linear regression analysis, finding that only the period from term to 4 months was the sensitive period of postnatal growth for preterm infants relative to neurodevelopment. There is no increase in indicators associated with MDI or PDI scores from 4 to 12 months and the faster weight gain, and linear growth from term to 4 months are associated with increased PDI scores at 18 months CA in preterm infants. Sammallahti et al. (32) identified weight growth between birth, 5 and 20 months CA, and 56 months to predicted neurocognitive abilities at 26 years of age, and the results showed that faster birth-to-5-months weight growth was the sensitive period associated with higher intelligence quotient (IQ), but no associations between neurocognitive after 5 months CA were identified. The above studies all found the linear relationship between weight growth and neurodevelopmental outcome among prematurity in the early stage after birth, but did not detect any associations between those two variables later in life. The widespread growth restriction in early postnatal might be the primary factor of those distinctions. Additionally, the first year of life, a sensitive period of human brain growth with the highest rate (33), is the key period for catch-up growth and later intelligence of VLBWPIs (34). The growth of very-low-weight preterm infants at discharge remains below the expected developmental level despite aggressive nutrition supplementation (35). Approximately one-third of preterm infants within the first 3 months after discharge would suffer a variety of feeding difficulties until the first year of their life (36, 37). Therefore, during the beginning months of postnatal, VLBWPIs need a higher growth velocity to achieve the body demand of catch-up growth. However, there might be a nonlinearity between both the weight gain velocity during the first year in postnatal and NDI, so those studies fail to detect it just by linear regression analysis. Nevertheless, there are still a lot of unknown fields about the mechanisms of relationships between the different periods of the weight gain velocity and neurodevelopmental outcomes in VLBWPIs that need to be explored.
Other studies stated that there might be a nonlinear association between early postnatal growth and neurodevelopmental outcome. Pylipow et al. revealed that an inverted J-shape curve existed between the postnatal growth rate in the first 4 postnatal months of infants with intrauterine growth restriction and later cognitive function (7). Taine et al. performed a systematic review of the relationship between early postnatal growth before age 3 years and neurodevelopmental outcome in children born moderately preterm or small for gestational age at term, and they found that few articles revealed a plateau for IQ with higher weight gain. It may suggest a possible ceiling effect (8). The possible reasons for this inconsistency may be related to the study population, study design, adjustment for covariates, especially the timing or tool of measurement (NICU hospitalization or postnatal; at gestational age or chronological age, different outcome indicators of neurodevelopmental impairment), or calculation (g/kg/d, g/d, cm/week, or change in z scores) of the weight gain velocity (20, 21, 38).
Furthermore, although the brain, corticospinal tract growth, and neurodevelopment in preterm infants after early postnatal energy- and protein-supplemented diet may be improved (39, 40), and malnutrition in a sensitive period during early neonatal life may lead to long-term neurodevelopment impairment (33, 41), but, conversely, rapid weight gain velocity among VLBWPIs promote higher long-term risk of overweight/obesity and other noncommunicable diseases, such as insulin resistance and metabolic syndrome, cardiovascular risk, adiposity, higher blood pressure, insulin resistance, dyslipidemia, and endothelial dysfunction (12, 13). The present study revealed that there are ceiling effects between the weight gain velocity from birth to 6 months CA and 6 to 24 months CA and NDI. For the weight gain velocity from birth to 6 months CA, 1 g/d increase of weight gain velocity is related to 25% decreases in risk of NDI when the rate is less than 20.36 g/d. For the weight gain velocity from 6 to 12 months CA, 1 g/d increase of weight gain velocity was related to 11% decreases in risk of NDI when the rate is less than 9.44 g/d. For the weight gain velocity from 12 to 24 months CA, 1 g/d increase of weight gain velocity was related to 7% decreases in risk of NDI the rate is less than 16.00 g/d. It means the neurodevelopmental benefits from a rapid weight gain velocity from birth to 24 months CA might be limited once the growth pace reaches an optimum level. Confirming the sensitive period of the relationship between weight gain velocity and neurodevelopment can help find a pattern of growth that facilitates optimal neurodevelopment, yet minimizes negative health consequences associated with overnutrition. According to these results of the study, we could keep the weight gain velocity of VLBWPIs close to those optimum levels in corresponding window period instead of gaining weight as fast as possible, as a result, to get enough the neurodevelopment benefits and simultaneously decrease the risk of long-term metabolic disorders. However, more evidence on the practical aspects for action should be explored deeply.
Our study has some strengths listed as follows. A strength of our study is the large sample size that allows such analysis, whereas most prior studies were limited to small numbers. Compared with previous research, the research on the nonlinearity addressing is a significant improvement. It may propose a new insight into the association between those two variables and give some clue for further studies on this theme, and then give more evidence on the practical aspects for action finally. Additionally, this study is an observational study and therefore susceptible to potential confounding, so we used strict statistical adjustment to minimize residual confounders. We tested the robustness of the results through a series of sensitivity analyses (target independent variable transformation, subgroup analysis, log-likelihood ratio test, etc.) to ensure the reliability of the results.
Our research has the following shortcomings. First, our findings can be generalized to VLBWPIs only, and the relationship of the weight gain velocity on NDI may be different in babies with advanced intraventricular hemorrhage (grade III and grade IV) or chromosome anomalies. Second, as in all observational studies, even though known potential confounders factors were controlled for, there might have been still uncontrolled confounders. Third, it should be noticed that the causality between the weight gain velocity and NDI among VLBWPIs could not be addressed and the bidirectional interrelation may exist between these two variables. Further studies regarding identifying the mechanism of the relationship between weight gain velocity and NDI should be recommended.
We explore the association between the weight gain velocity in different periods of early postnatal (from birth to 6 months CA, 6 to 12 months CA, and 12 to 24 months CA) and neurodevelopmental impairment among VLBWPIs. The results show there is not simple linear relationship between the weight gain velocity form birth to 12 months CA and NDI among VLBWPIs. Ceiling effects was identified between less NDI and higher weight gain velocity in this group during birth to 6 months CA and 6 to 12 months CA. For the weight gain velocity from birth to 6 months CA, 1 g/d increase of weight gain velocity is related to 25% decrease in risk of NDI. For the weight gain velocity from 6 to 12 months CA, 1 g/d increase of weight gain velocity was related to 11% decrease in risk of NDI. For the weight gain velocity from 12 to 24 months CA, 1 g/d increase of weight gain velocity was related to 7% decrease in risk of NDI; the rate is less than 16.00 g/d. Assessing causality between the NDI and the weight gain velocity in an observational study is very difficult. But at the minimum, the exposure occurrence before the outcome and potential confounding variables were assessed and controlled. The results reveal that the neurodevelopmental benefits from a rapid weight gain velocity from birth to 12 months CA might be limited once the growth pace reaches an optimum level, while it may not be limited with the weight gain velocity after 12 months CA. It provides new insight into the association between the early postnatal the weight gain velocity and neurodevelopmental outcome. Further studies regarding identifying the mechanism of the relationship between weight gain velocity and NDI should be recommended to help us find the pattern of manage nutrition and weight gain curves that facilitate optimal neurodevelopment, yet minimizes negative health consequences associated with overnutrition further.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
ZL designed the work, acquired and interpreted the data, and drafted the work and substantively revised the article. BY, YZ, and JT contributed to the methodology and interpretation of data. ZZ and YJ contributed to the formal analysis. LW critically revised this work for important intellectual content. HL and DZ supervised and critically revised this work for important intellectual content. XW contributed to the conception and design of the study, supervision and revised the work critically for important intellectual content, and communicated with the Editorial Office and all other co-authors. All authors contributed to the article and approved the submitted version.
This research was funded by the Guizhou Education Department Youth Science and Technology Talents Growth Project (Grant No. KY[2022]240) and the Special Nursing Project of Guizhou Medical University in 2020 (YJ20063).
The authors would like to thank to the Premature Baby Foundation of Taiwan and the Taiwan Premature Infant Developmental Collaborative Study Group and the authors of the “Post-discharge body weight and neurodevelopmental outcomes among very low birth weight infants in Taiwan: A nationwide cohort study” for providing the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
NDI, neurodevelopmental impairment; OR, odds ratio; 95% CI, 95% confidence interval; VLBWPIs, very-low birth weight preterm infants; MDI, Mental Developmental Index; PDI, Psychomotor Developmental Index
1. Su YY, Wang SH, Chou HC, Chen CY, Hsieh WS, Tsao PN, et al. Morbidity and mortality of very low birth weight infants in Taiwan—changes in 15 years: a population based study. J Formos Med Assoc. (2016) 115(12):1039–45. doi: 10.1016/j.jfma.2016.10.011
2. Lee J H, Youn Y, Chang Y S. Short- and long-term outcomes of very low birth weight infants in Korea: Korean neonatal network update in 2019. Clin Exp Pediatr. (2020) 63(8):284–90. doi: 10.3345/cep.2019.00822
3. Alqurashi MA. Survival rate of very low birth weight infants over a quarter century (1994–2019): a single-institution experience. J Neonatal Perinatal Med. (2021) 14(2):253–60. doi: 10.3233/NPM-200595
4. Franz AR, Pohlandt F, Bode H, Mihatsch WA, Sander S, Kron M, et al. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics. (2009) 123(1):e101–9. doi: 10.1542/peds.2008-1352
5. Shah PS, Wong KY, Merko S, Bishara R, Dunn M, Asztalos E, et al. Postnatal growth failure in preterm infants: ascertainment and relation to long-term outcome. J Perinat Med. (2006) 34(6):484–9. doi: 10.1515/JPM.2006.094
6. Rafei R E, Jarreau PH, Norman M, Maier RF, Barros H, Van Reempts P, et al. Association between postnatal growth and neurodevelopmental impairment by sex at 2 years of corrected age in a multi-national cohort of very preterm children. Clin Nutr. (2021) 40(8):4948–55. doi: 10.1016/j.clnu.2021.07.005
7. Castanys-Muñoz E, Kennedy K, Castañeda-Gutiérrez E, Forsyth S, Godfrey KM, Koletzko B, et al. Systematic review indicates postnatal growth in term infants born small-for-gestational-age being associated with later neurocognitive and metabolic outcomes. Acta Paediatr. (2017) 106(8):1230–8. doi: 10.1111/apa.13868
8. Taine M, Charles MA, Beltrand J, Rozé JC, Léger J, Botton J, et al. Early postnatal growth and neurodevelopment in children born moderately preterm or small for gestational age at term: a systematic review. Paediatr Perinat Epidemiol. (2018) 32(3):268–80. doi: 10.1111/ppe.12468
9. Pylipow M, Spector LG, Puumala SE, Boys C, Cohen J, Georgieff MK. Early postnatal weight gain, intellectual performance, and body mass index at 7 years of age in term infants with intrauterine growth restriction. J Pediatr. (2009) 154(2):201–6. doi: 10.1016/j.jpeds.2008.08.015
10. Kim YJ, Shin SH, Lee ES, Jung YH, Lee YA, Shin CH, et al. Impact of size at birth and postnatal growth on metabolic and neurocognitive outcomes in prematurely born school-age children. Sci Rep. (2021) 11(1):6836. doi: 10.1038/s41598-021-86292-1
11. Meyers JM, Tan S, Bell EF, Duncan AF, Guillet R, Stoll BJ, et al. Neurodevelopmental outcomes among extremely premature infants with linear growth restriction. J Perinatol. (2019) 39(2):193–202. doi: 10.1038/s41372-018-0259-8
12. Corpeleijn W E, Kouwenhoven S M, van Goudoever J B. Optimal growth of preterm infants. World Rev Nutr Diet. (2013) 106:149–55. doi: 10.1159/000342584
13. Singhal A. Long-term adverse effects of early growth acceleration or catch-up growth. Ann Nutr Metab. (2017) 70(3):236–40. doi: 10.1159/000464302
14. Toftlund LH, Halken S, Agertoft L, Zachariassen G. Catch-up growth, rapid weight growth, and continuous growth from birth to 6 years of age in very-preterm-born children. Neonatology (2018) 114(4):285–93. doi: 10.1159/000489675
15. Ceratto S, Savino F, Vannelli S, De Sanctis L, Giuliani F. Growth assessment in preterm children from birth to preschool age. Nutrients. (2020) 12(7):1941. doi: 10.3390/nu12071941
16. Hsu CT, Chen CH, Lin MC, Wang TM, Hsu YC. Post-discharge body weight and neurodevelopmental outcomes among very low birth weight infants in Taiwan: a nationwide cohort study. PLoS One. (2018) 13(2):e192574. doi: 10.1371/journal.pone.0192574
17. Tsai LY, Chen YL, Tsou KI, Mu SC. Taiwan Premature infant developmental collaborative study group. The impact of small-for-gestational-age on neonatal outcome among very-low-birth-weight infants. Pediatr Neonatol. (2015) 56(2):101–7. doi: 10.1016/j.pedneo.2014.07.007
18. Beyerlein A, Ness AR, Streuling I, Hadders-Algra M, von Kries R. Early rapid growth: no association with later cognitive functions in children born not small for gestational age. Am J Clin Nutr. (2010) 92(3):585–93. doi: 10.3945/ajcn.2009.29116
19. Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. (2005) 6(2):143–54. doi: 10.1111/j.1467-789X.2005.00183.x
20. Fenton TR, Chan HT, Madhu A, Griffin IJ, Hoyos A, Ziegler EE, et al. Preterm infant growth velocity calculations: a systematic review. Pediatrics. (2017) 139(3):e20162045. doi: 10.1542/peds.2016-2045
21. Rochow N, Landau-Crangle E, So HY, Pelc A, Fusch G, Däbritz J, et al. Z-score differences based on cross-sectional growth charts do not reflect the growth rate of very low birth weight infants. PLoS One. (2019) 14(5):e216048. doi: 10.1371/journal.pone.0216048
22. Rijken M, Wit JM, Le Cessie S, Veen S. Leiden follow-up project on prematurity. The effect of perinatal risk factors on growth in very preterm infants at 2 years of age: the Leiden follow-up project on prematurity. Early Hum Dev. (2007) 83(8):527–34. doi: 10.1016/j.earlhumdev.2006.10.002
23. Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. (2006) 117(4):1253–61. doi: 10.1542/peds.2005-1368
24. Bayley N. Bayley scales of infant development. 2nd ed. San Antonio, TX: The Psychological Corporation (1993).
26. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. (2005) 115(3):696–703. doi: 10.1542/peds.2004-0569
27. Frondas-Chauty A, Simon L, Branger B, Gascoin G, Flamant C, Ancel PY. Early growth and neurodevelopmental outcome in very preterm infants: impact of gender. Arch Dis Child Fetal Neonatal Ed. (2014) 99(5):F366–72. doi: 10.1136/archdischild-2013-305464
28. Neubauer AP, Voss W, Kattner E. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. (2008) 167(1):87–95. doi: 10.1007/s00431-007-0435-x
29. Hsieh WS, Wu HC, Jeng SF, Liao HF, Su YN, Lin SJ. Nationwide singleton birth weight percentiles by gestational age in Taiwan, 1998-2002. Acta Paediatr Taiwan. (2006) 47(1):25–33. PMID: 17016966
30. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
31. Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. (2011) 128(4):e899–906. doi: 10.1542/peds.2011-0282
32. Sammallahti S, Heinonen K, Andersson S, Lahti M, Pirkola S, Lahti J. Growth after late-preterm birth and adult cognitive, academic, and mental health outcomes. Pediatr Res. (2017) 81(5):767–74. doi: 10.1038/pr.2016.276
33. Gale CR, O'Callaghan FJ, Bredow M, Martyn CN. Longitudinal study of parents and children study team. The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics. (2006) 118(4):1486–92. doi: 10.1542/peds.2005-2629
34. Hack M, Breslau N. Very low birth weight infants: effects of brain growth during infancy on intelligence quotient at 3 years of age. Pediatrics. (1986) 77(2):196–202. doi: 10.1542/peds.77.2.196
35. Kumar P, Perino J, Bowers L, Welch B, Albert V, Drenckpohl D. Cumulative impact of multiple evidence based strategies on postnatal growth of extremely-low-birth-weight infants. Clin Nutr. (2021) 40(6):3908–13. doi: 10.1016/j.clnu.2021.05.018
36. DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early- and late-preterm infants. Clin Pediatr (Phila). (2011) 50(10):957–62. doi: 10.1177/0009922811409028
37. Pridham K, Steward D, Thoyre S, Brown R, Brown L. Feeding skill performance in premature infants during the first year. Early Hum Dev. (2007) 83(5):293–305. doi: 10.1016/j.earlhumdev.2006.06.004
38. Nash A, Dunn M, Asztalos E, Corey M, Mulvihill-Jory B, O'Connor DL. Pattern of growth of very low birth weight preterm infants, assessed using the WHO growth standards, is associated with neurodevelopment. Appl Physiol Nutr Metab. (2011) 36(4):562–9. doi: 10.1139/h11-059
39. Dabydeen L, Thomas JE, Aston TJ, Hartley H, Sinha SK, Eyre JA. High-energy and -protein diet increases brain and corticospinal tract growth in term and preterm infants after perinatal brain injury. Pediatrics. (2008) 121(1):148–56. doi: 10.1542/peds.2007-1267
40. Christmann V, Roeleveld N, Visser R, Janssen AJ, Reuser JJ, van Goudoever JB, et al. The early postnatal nutritional intake of preterm infants affected neurodevelopmental outcomes differently in boys and girls at 24 months. Acta Paediatr. (2017) 106(2):242–9. doi: 10.1111/apa.13669
Keywords: neurodevelopmental outcomes, weight gain velocity, very low birth weight premature infants, early postnatal, prospective cohort study
Citation: Luo Z, You B, Zhang Y, Tang J, Zheng Z, Jia Y, Wang L, Zeng D, Li H and Wang X (2022) Nonlinear relationship between early postnatal weight gain velocity and neurodevelopmental outcomes in very-low birth weight preterm infants: A secondary analysis based on a published prospective cohort study. Front. Pediatr. 10:944067. doi: 10.3389/fped.2022.944067
Received: 14 May 2022; Accepted: 13 October 2022;
Published: 8 November 2022.
Edited by:
Anna Maria Lavezzi, University of Milan, ItalyReviewed by:
Bernard Branger, Centre Hospitalier Universitaire d'Angers, France© 2022 Luo, You, Zhang, Tang, Zheng, Jia, Wang, Zeng, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuhong Wang d2FuZ3hpdWhvbmdAZ21jLmVkdS5jbg==
Specialty Section: This article was submitted to Pediatric Neurology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.