
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 06 September 2022
Sec. Pediatric Hematology and Hematological Malignancies
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.941318
 Saralee Harnchoowong1
Saralee Harnchoowong1 Sirisucha Soponkanaporn1*
Sirisucha Soponkanaporn1* Soamarat Vilaiyuk1
Soamarat Vilaiyuk1 Butsabong Lerkvaleekul1
Butsabong Lerkvaleekul1 Samart Pakakasama2
Samart Pakakasama2Introduction: Hemophagocytic lymphohistiocytosis (HLH) is a potentially life-threatening condition. This study aimed to evaluate treatment outcomes and identify prognostic-related factors in Thai children with HLH.
Materials and methods: We retrospectively reviewed the medical records of 76 pediatric patients with HLH who were treated at Ramathibodi Hospital between January 2004 and December 2019. Treatment outcomes were defined as early mortality (death within 30 days after diagnosis) and early treatment response (resolution of all clinical features and normalization of at least one HLH-related laboratory parameter within 4 weeks).
Results: The overall mortality rate was 38% (29/76), with an early mortality rate of 45% (13/29). Malignancy-associated HLH had the highest mortality rate (88%), followed by primary HLH (56%). The predictors of early mortality were central nervous system (CNS) involvement [OR 13 (95%CI 2–83), p = 0.007] and platelet counts <44 × 106/mm3 [OR 8 (95%CI 1.3–49), p = 0.024]. The predictors of early treatment response were no CNS involvement [OR 6.6 (95%CI 1.5–28.8), p = 0.011], platelet counts more than 44 × 106/mm3 [OR 8 (95%CI 2.1–30.9), p = 0.003], and total bilirubin levels <1.8 mg/dL [OR 4 (95%CI 1.1–14.8), p = 0.036]. In the mixed-model analysis, platelet counts in non-survivors increased significantly less than those in survivors, with a mean difference in platelet changes between the two groups of 94.6 × 106/mm3 (p = 0.003).
Conclusion: The independent predictors of early mortality in children with HLH were CNS involvement and low baseline platelet counts. A slow rate of platelet increases during the first week after diagnosis was also associated with mortality.
Hemophagocytic lymphohistiocytosis (HLH) is a rare life-threatening condition characterized by persistent activation of T lymphocytes and macrophages, leading to the hypersecretion of pro-inflammatory cytokines, including interleukin (IL)-1, IL-6, IL-18, tumor necrosis factor (TNF), and interferon-gamma (IFNγ) (1). HLH is classified as primary and secondary HLH. Primary HLH is caused by genetic mutations affecting granule-dependent natural killer cell cytotoxicity and cytotoxic T lymphocytes. The main subtype of primary HLH is familial HLH (fHLH), which had specific gene mutations. Known genotypes that have been reported in fHLH are PRF1, UNC13D, STX11, and STXBP2 genes (2–5). The other primary HLH subtype is related to primary immune deficiencies with defects in vesicle or lysosome synthesis, trafficking, and release, leading to T cell, macrophage, and neutrophil dysfunctions (6). Primary HLH occurs most commonly in patients with younger age, a family history of HLH, or recurrent episodes (7). Secondary HLH is an acquired condition that may be triggered by infections (infection-associated hemophagocytic syndrome or IAHS), rheumatic diseases (macrophage activation syndrome or MAS), and malignancies (malignancy-associated HLH or M-HLH). The common underlying rheumatic diseases in children are systemic juvenile idiopathic arthritis (SJIA), systemic lupus erythematosus (SLE), and Kawasaki disease (8–11). However, recent studies showed that some patients with secondary HLH contained heterozygous fHLH-related mutations which associated with poor outcome (12, 13).
The treatment of HLH varies across subtypes, and outcomes often depend on how early patients are diagnosed. It is challenging to differentiate HLH from sepsis and active underlying disease because the symptoms are quite similar. In the late stage of HLH, patients rapidly deteriorate and have multiple organ dysfunction, causing high morbidity and mortality. The overall mortality rate in children with HLH is 20–40%, with higher mortality in specific subtypes (50% in primary HLH and 56% in M-HLH) and lower mortality rates in patients with MAS (8%) (1, 8, 14). Early identification of high-risk patients is essential for improving treatment outcomes. Previous studies identified risk factors of poor treatment outcomes in children with HLH and found that central nervous system (CNS) involvement was associated with a lower survival rate (15). A study of non-malignancy-associated secondary HLH showed that factors related to worse prognosis included low levels of hemoglobin, platelets, and albumin (16). Prolonged partial thromboplastin time (PTT), high levels of lactate dehydrogenase, and increased ferritin also predicted mortality in children with HLH (17–19).
In addition to initial laboratory findings, sequential changes in laboratory parameters can help predict treatment outcomes. However, only a few studies have investigated changes in laboratory markers. Lin et al. found that a reduction in ferritin by <50% was associated with higher mortality than ferritin decreases by more than 96% (20). In an adult study, patients with a reduction in ferritin by more than 35% from the peak level had a higher chance of survival, whereas patients with an increase in ferritin by more than 35% were likely to die (21). In addition, a study in children with HLH found that platelet normalization within 2 weeks was a predictor of disease resolution (22). However, no study has investigated the association between the magnitude of change in hematologic laboratory parameters and treatment outcomes. Here, we aimed to evaluate disease outcomes and identify predictive factors for treatment outcomes and mortality risk in Thai children with HLH.
This was a retrospective cohort study. Patients under 18 years diagnosed with HLH between January 2004 and December 2019 at Ramathibodi Hospital were included. The diagnosis of HLH was based on HLH-2004 criteria, which required molecular genetic diagnostic confirmation or fulfillment of at least five out of the following criteria: (1) fever, (2) splenomegaly, (3) cytopenia for at least two lineages (hemoglobin level <9 g/dL, neutrophil count <1,000/mm3, or platelet count <100 × 106/mm3), (4) triglyceride level more than 265 mg/dL or fibrinogen level <150 mg/dL, (5) evidence of hemophagocytosis in bone marrow, spleen, or lymph nodes, (6) low or absent natural killer cell activity, (7) ferritin level more than 500 ng/mL, and (8) soluble CD25 level more than 2,400 U/mL (23). Because assessments for natural killer cell activity and soluble CD25 levels were not available, we included patients with at least five out of the other six criteria. Patients with primary HLH were diagnosed by positive molecular genetic confirmation, a family history of HLH, or young age of onset with recurrent episodes. The diagnosis of MAS in SJIA patients was based on the 2016 classification criteria of MAS in SJIA, including febrile patients with ferritin levels more than 684 ng/mL and any of two of the following: (1) platelet count <181 × 106/mm3, (2) aspartate aminotransferase (AST) level more than 48 U/L, (3) triglyceride level more than 156 mg/dL, and (4) fibrinogen level <360 mg/dL (24). Patients were diagnosed with SJIA according to the International League of Associations for Rheumatology classification (25). This study was approved by the Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Thailand (MURA2020/393) and conducted following the principles of the Declaration of Helsinki.
The medical records were retrospectively reviewed. Demographic data and baseline clinical characteristics, including age, gender, underlying disease, duration of disease, initial manifestations, and treatment (medications and platelet transfusions during 1 week after diagnosis), were obtained. CNS involvement was indicated when patients had symptoms of seizure, irritability, altered consciousness, or paralysis. Hemorrhagic symptoms were defined as mucosal, gastrointestinal, or respiratory tract bleeding. Triggers of secondary HLH were determined based on physician's assessments. Laboratory parameters, including complete blood count, erythrocyte sedimentation rate, C-reactive protein, liver function test, lactate dehydrogenase, triglyceride, ferritin, fibrinogen, PTT, and d-dimer levels, were collected at the time of diagnosis and 1 week after diagnosis.
The primary outcome was the early mortality rate, defined as death that occurred within 30 days after diagnosis. Overall mortality was evaluated as death occurring during the follow-up period. Early treatment response was defined as the resolution of all clinical manifestations and normalization of HLH-related laboratory findings either complete blood count or other HLH-related laboratory parameters within 4 weeks (16).
Descriptive statistics of demographic data and baseline laboratory parameters were expressed as a number with percentage and median with interquartile range (IQR). Comparisons between groups were performed using the chi-square or Fisher's exact test for categorical data and Mann–Whitney U test or Kruskal–Wallis test for continuous data. Predictors of treatment outcomes were analyzed using logistic regression, and the strength of predictors was described using the odds ratio (OR). Receiver operating characteristics (ROC) curve analysis was applied to select the optimal cut-off values of parameters. Associations between changes in laboratory parameters during 1 week after diagnosis and mortality were performed by mixed-model analysis. Survival was estimated by the Kaplan–Meier method with the log-rank test. A p-value <0.05 was considered statistically significant. The analysis was performed using IBM SPSS 24.0 statistical software.
Among 76 patients with HLH, 41 (54%) were females. The median age at diagnosis was 6.1 years (IQR 2–11.6). The most common HLH subtypes were IAHS, followed by MAS, primary HLH, and M-HLH (Table 1). The major causes of IAHS were viral infections with 45% Epstein–Barr virus (EBV), 24% dengue virus, and 13% cytomegalovirus (CMV). The common underlying rheumatic diseases in MAS were SJIA (67%) and SLE (33%). All cases of M-HLH occurred in patients with hematologic malignancies, mainly lymphoid malignancies (75%).
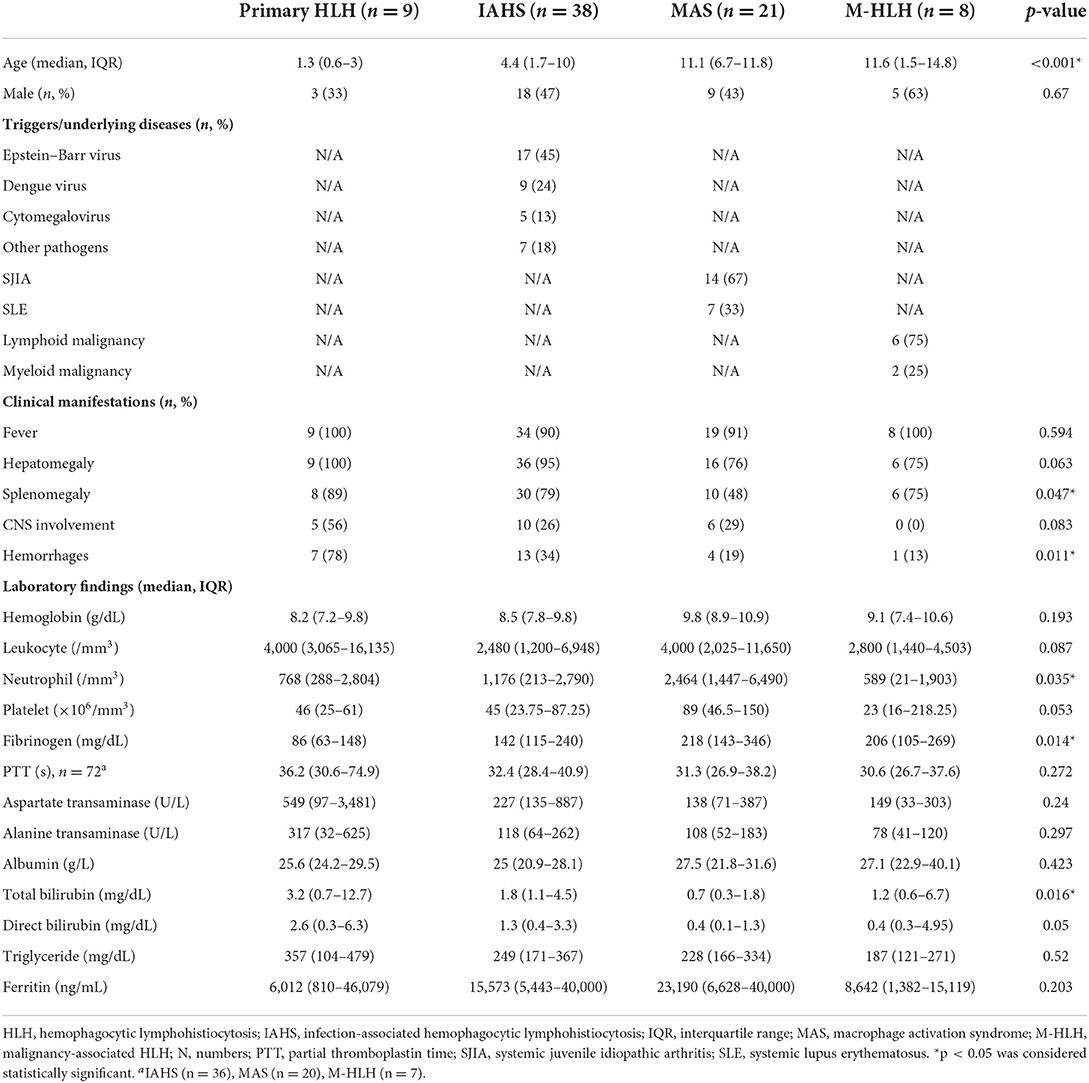
Table 1. Baseline clinical features and laboratory findings in HLH patients with different subtypes.
Clinical characteristics and laboratory findings at the time of diagnosis were described in Table 1. Fever (92%) and hepatomegaly (88%) were commonly found in HLH patients. Patients with MAS had a lower prevalence of splenomegaly (48%) than patients with other HLH subtypes (p = 0.047). CNS involvement tended to be more prevalent (56%) in patients with primary HLH when compared to other HLH subtypes (p = 0.083). Among patients with clinical features of CNS involvement (n = 21), 5/6 patients who underwent CSF evaluation had pleocytosis or high protein level. Moreover, 10/14 patients who underwent brain imaging had abnormal results. In contrast, 14 patients without clinical features of CNS involvement had CSF evaluation or brain imaging which may be due to suspicious of infection or bleeding, and all patients had normal results. Patients with primary HLH had a higher prevalence of hemorrhage (78%) than patients with other subtypes (p = 0.011). The prevalence of hemorrhagic symptoms in MAS patients was 19%. SLE patients were more likely to have hemorrhagic symptoms (43%) compared with SJIA patients (7%). Laboratory findings showed that most HLH patients were thrombocytopenic (75%) and hyperferritinemic (93%) at the time of diagnosis. For primary HLH, patients had lower fibrinogen levels (p = 0.014) and higher total bilirubin levels (p = 0.016) than patients with other subtypes. Patients with MAS had significantly higher neutrophil counts (p = 0.035) and tended to have higher platelet counts (p = 0.053) compared with patients with other subtypes.
Twenty-five (33%) patients were treated according to the HLH 2004 protocol (26). Seven out of nine patients (78%) with primary HLH received the HLH 2004 protocol, and the remaining two died before receiving all medications in the protocol. Allogeneic hematopoietic stem cell transplantation was performed in two patients with primary HLH. Approximately 68% of IAHS patients required intravenous immunoglobulin (IVIG) combined with other immunosuppressive therapies, such as cyclosporine and etoposide, whereas 32% of patients were treated with IVIG monotherapy (Table 2). Patients with MAS were mainly treated with high-dose corticosteroids and cyclosporine with or without IVIG. Half of the patients with M-HLH received chemotherapies according to underlying malignancies, and the other half were treated with the HLH 2004 protocol.
The overall mortality rate was 38% (29/76), with early death in 45% (13/29) (Table 2). The causes of death were infections (41%), uncontrolled bleeding (31%), and multiple organ failure (28%). M-HLH and primary HLH patients had high mortality rates of 88 and 56%, respectively. Among IAHS patients, the mortality rate of EBV and dengue-associated IAHS was 32, and 33%, respectively. In MAS patients, the overall mortality rate was 24%. SLE patients had a significantly higher mortality rate (57%) than SJIA patients (7%) (p = 0.025). For early mortality, there was no significant difference between HLH types. Patients with MAS tended to respond to treatment earlier than patients with other subtypes (early treatment response rate 81%, p = 0.05).
We compared clinical features and laboratory findings between patients with and without early mortality, as shown in Supplementary Table 1. Then, we included the notable clinical manifestations and laboratory parameters classified using cut-off values from ROC curve analysis in the univariate analysis. The sensitivity, specificity and area under the ROC curve (AUC) values of the predictors in the regression model were shown in Supplementary Table 2. We found that CNS involvement, platelet counts <44 × 106/mm3, PTT more than 35 s, and total bilirubin (TB) levels more than 1.8 mg/dL were predictors of early mortality. In the multivariate analysis, CNS involvement and platelet counts <44 × 106/mm3 were independent predictors of early mortality with an OR of 13 [95% confidence interval (CI) 2–83, p = 0.007] and 8 [95%CI 1.3–49, p = 0.024], respectively (Table 3). Primary HLH was also included in the univariate regression analysis and the result showed OR 2.8 (95% CI 0.6–13.3), p = 0.183. We therefore did not include this factor in the multivariate regression analysis.
The frequencies of CNS involvement and hemorrhagic symptoms were significantly higher in non-survivors than in survivors (48 vs. 15%, p = 0.002 and 48 vs. 23%, p = 0.025, respectively). Therefore, we included these two factors in the survival analysis. Overall survival was decreased in patients with CNS involvement (p < 0.001) and hemorrhagic symptoms (p = 0.009) (Figure 1).
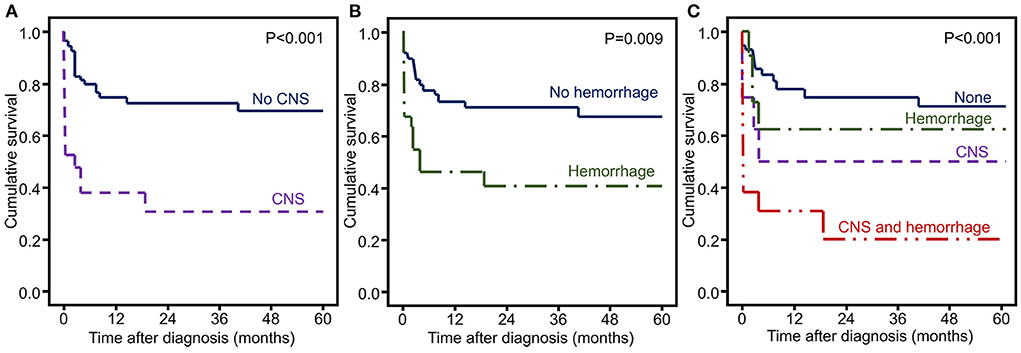
Figure 1. Kaplan–Meier curves of HLH patients with significant risk factors of mortality. (A) CNS involvement, (B) hemorrhagic symptoms, and (C) CNS and hemorrhagic symptoms.
Predictors related to early treatment response in the univariate analysis were no CNS involvement, platelet counts more than 44 × 106/mm3, PTT <35 s, and TB levels <1.8 mg/dL. Subsequent multivariate analysis was performed, and the remaining predictors were no CNS involvement, platelet counts more than 44 × 106/mm3, and TB levels <1.8 mg/dL (Table 4).
We evaluated associations between changes in laboratory parameters during 1 week after diagnosis and mortality using mixed-model analysis. Non-survivors exhibited a significantly less improvement in platelet counts after the first week [mean increase 25.1 × 106/mm3 (standard deviation 53.3)] than survivors (mean increase 119.7 × 106/mm3 [standard deviation 169.4]) despite requiring more platelet transfusions during the first week than survivors [median (IQR) unit/kg, 1 (0.6–1.8) vs. 0 (0–0.4), p < 0.001]. The mean difference in platelet changes between the two groups was 94.6 × 106/mm3 (95% CI 29.9–159.4, p = 0.003). Lymphocyte counts decreased in non-survivors but slightly increased in survivors. However, the difference was not statistically significant. Interestingly, ferritin levels decreased in both survivor and non-survivor groups, and the changes were not different between the two groups (Figure 2).
Figure 2. Associations between changes in laboratory parameters and mortality using mixed-model analysis. (A) Platelet count, (B) lymphocyte count, and (C) ferritin level.
Our study demonstrated that mortality in HLH depends on the underlying disease that triggered this condition, particularly malignancies. Additionally, we revealed the predictive roles of mortality in HLH represented by CNS involvement (including seizures, irritability, altered consciousness, and paralysis) and changes in platelet counts. The predictors of early mortality were CNS involvement and platelet counts <44 × 106/mm3, and the independent predictors of early treatment response were no CNS involvement, platelet counts more than 44 × 106/mm3, and TB levels <1.8 mg/dL.
Consistent with previous reports, the two most common HLH types in this study were IAHS and MAS (16, 26). The most common pathogens triggering IAHS in previous studies were EBV and CMV (27, 28), whereas EBV followed by dengue virus were the most common pathogens triggering IAHS in our study. This might be because Thailand is in the endemic area of dengue infection. This was confirmed by the fact that most dengue-associated HLH cases were reported in South-East Asia (29, 30). The most frequent underlying rheumatic diseases in MAS were SJIA, followed by SLE. Patients with MAS had higher neutrophil counts and tended to have higher platelet counts. One potential explanation is that most MAS patients in this study were SJIA patients with higher baseline neutrophil and platelet counts than patients with other diseases. All MAS patients received high-dose corticosteroids, and more than 50% of patients required additional IVIG or cyclosporine, which was in line with previous studies (9, 31). Regarding M-HLH patients, most simultaneously had malignancies, predominately lymphoid malignancies, and 50–60% of patients were treated with the HLH protocol as first-line therapy (14, 27, 32).
The overall mortality rate in this study was 38%, and nearly half of deaths occurred within 30 days. This was similar to the 20–40% mortality rate and the majority of deaths occurring within 30 days in previous studies (1, 16, 22, 28). M-HLH had the highest mortality rate among all HLH types. Our 88% mortality rate of M-HLH was higher than the 56% mortality rate in the previous study of pediatric M-HLH (14). This might be because of the longer time to diagnosis and lower initial platelet counts in our patients than in patients from the previous study. The highest mortality among HLH subtypes was supported by numbers from previous studies in both children and adults (14, 27, 32–35). Primary HLH had the second highest mortality among HLH subtypes, despite the advanced treatment protocol. The mortality rate was approximately similar to that reported in a previous study (1). Interestingly, the mortality rate of dengue-associated HLH in our study (33%) was higher than the mortality rate of 14.6% in a recent meta-analysis (29). All dengue-associated non-survivors in our study had highly elevated AST and ALT, which were previously reported as risk factors of mortality (30). The other patients with dengue-associated HLH had milder disease courses and required only IVIG monotherapy. For MAS patients, those with SLE had a higher mortality rate than SJIA patients, potentially because of more hemorrhagic symptoms. Additionally, the hemorrhagic symptoms in our SLE patients were pulmonary hemorrhage symptoms, which are associated with high mortality (36). The reason for a higher incidence of hemorrhage may be lower platelet counts in patients with SLE than in patients with SJIA, although there was no statistical difference. The mortality rate reported in SLE patients with MAS was 5–16% (10, 37), which was lower than in our study. These differences might be because of lower platelet counts and higher rates of hemorrhagic symptoms in our SLE patients. The mortality rate of MAS in SJIA patients in our study was consistent with that reported in previous studies (9, 38, 39).
CNS involvement has been reported in 10–73% of HLH patients depending on the definition (15, 40, 41), which was similar to our study. Kim et al. found that positive neurological symptoms, elevated leukocytes in cerebrospinal fluid, and brain imaging abnormalities were associated with inferior survival compared with no CNS involvement (15). In addition, Zhao et al. (41) found that among HLH patients with CNS involvement, the presence of neurological symptoms, including seizures, irritability, somnolence, and unconsciousness, was the strongest prognostic factor for mortality compared with abnormal cerebrospinal fluid or abnormal brain imaging. Our study also showed that CNS involvement was associated with increased mortality rates in the survival analysis and was a predictor of early mortality. Moreover, absence of CNS involvement was a predictor of early treatment response. Although this study could not show statistical difference across the different HLH subtypes due to limited number of patients, patients with primary HLH had the highest proportion of CNS involvement. Also, more than half of those patients died within 30 days. From a neuropathologic study in children with HLH, the pathology in CNS can be staged based on the distribution pattern of lymphocytes and histiocytes or macrophage infiltration, finally leading to tissue necrosis. The more pronounced clinical symptoms were associated with more advanced stages of pathology (42). Therefore, CNS symptoms might reflect the severity of the disease and predict treatment outcomes.
Hemorrhagic symptoms were also associated with increased mortality in our study. The platelet count was one of the laboratory parameters associated with the risk of bleeding. Severe thrombocytopenia with a cut-off ranging from 30 to 75 × 106/mm3 was associated with early mortality and poor treatment outcomes in children with HLH (16, 18, 19, 43). Similarly, our results found that a platelet count <44 × 106/mm3 was another predictor of early death, and a platelet count more than this cut-off was a predictor of early treatment response. Thrombocytopenia in HLH results from hemophagocytosis and cytokine-induced bone marrow suppression, accompanied by excessive platelet destruction due to disseminated intravascular coagulation and hypersplenism (44). It reflects the severity of the condition (1, 44, 45).
Our study also found that patients with early mortality had higher levels of AST, ALT, TB, and direct bilirubin compared with patients without early mortality. In the ROC curve analysis, AST and ALT required very high cut-off values (>1,000 U/L) to increase the risk of early mortality; therefore, it might be impractical to use these parameters to identify patients at risk. Given that hyperbilirubinemia was previously reported as a potential predictor of treatment outcomes (17, 18, 22), we included TB levels in the logistic regression. TB levels <1.8 mg/dL were an independent predictor of early treatment response. All of these parameters, including PTT, were related to hepatic dysfunction; therefore, hepatic dysfunction might be more frequent in patients with poor treatment outcomes. Cytokines from macrophages, including IL-2, IFNγ, and TNF, cause inflammation in the liver, leading to sinusoidal dilatation, congestion, and elevated liver enzymes. In addition, lymphohistiocytic infiltration causes bile duct injury and cholestasis (46, 47). Hepatic injury in HLH results from excessive immune activation; therefore, its severity may affect treatment outcomes.
Previous studies reported that hyperferritinemia was associated with poor outcomes in both children and adults with HLH (19, 33, 43, 48, 49). Ferritin production is a compensatory mechanism that sequesters free Fe2+, which is released during red blood cell degradation in cytokine-mediated hemophagocytic processes. Thus, hyperferritinemia reflects the severity of cytokine storms (50). The cut-off values of ferritin ranged from more than 2,000 to 50,000 ng/mL (19, 33, 43, 48, 49). Baseline serum ferritin levels in this study were not statistically different between patients with and without early mortality; however, a significantly higher percentage of patients with early mortality had <35% reduction in ferritin levels over 1 week. Therefore, serial ferritin measurements during the disease course may provide a greater clinical benefit than the baseline measurement (51). Lin et al. (20) reported that ferritin decreases by <50% during 10 weeks after diagnosis were associated with an increased risk of mortality.
Our study also assessed changes in parameters from routine and basic laboratory data, such as hemoglobin, lymphocyte counts, and platelet counts, that can be used to identify high-risk patients. The increase in platelet counts during 1 week after diagnosis in non-survivors was less than that in survivors, despite more platelet transfusions. Refractory thrombocytopenia is likely owing to the vigorous consumption of platelets. This not only reflected the severity of HLH but also the increased risk of bleeding, leading to worse outcomes. Lymphocyte counts in the non-survivor group tended to decrease but were slightly increased in the survivor group. This might be explained by the fact that patients with lymphopenia were at a higher risk of infection, which was one of the causes of death in HLH.
The strength of this study is that our hospital is a tertiary care center that receives referrals from all over the country. We also have all types of specialists who see a variety of HLH patients. In addition, this study identified alterations in laboratory parameters during the disease course as predictive factors of treatment outcomes and early mortality. However, this study also has some limitations. First, genetic testing was not performed in patients with secondary HLH. Thus, we could not evaluate whether different mutations affect treatment outcome or not. Second, evaluation of CSF and brain imaging were lacking in most patients without clinical signs and symptoms of CNS involvement. We are aware that evaluation of CNS involvement which was mainly based on CNS signs and symptoms may not be adequate to identify patients with CNS involvement. Moreover, this study was performed in a single center and had a small sample size. Also, because of its retrospective design, some values were missing. Therefore, additional multi-center, prospective studies are recommended.
In conclusion, CNS involvement and low initial platelet counts are predictors of early mortality in children with HLH. Additionally, a slow rate of platelet increases during the first week after diagnosis was associated with mortality. Recognizing CNS symptoms at the initial presentation and serial monitoring of platelet counts during treatment courses may help identify the subgroups of HLH patients with a poor prognosis who need more intensive therapeutic modalities.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand (MURA2020/393). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
SS contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SH and SS. The first draft of the manuscript was written by SH and SS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
We receive funding from Faculty of Medicine Ramathibodi Hospital, Mahidol University for open access publication fees.
We would like to thank Mr. Nattawut Unwanatham for his help in performing the data analysis. In addition, we would like to express our great appreciation to Prof. Dr. Pat Mahachoklertwattana and Associate Prof. Dr. Pornthep Tanpawpong for his scientific input and constructive recommendations during the preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.941318/full#supplementary-material
AST, aspartate aminotransferase; CMV, cytomegalovirus; CNS, central nervous system; CI, confidence interval; EBV, Ebstein–Barr virus; fHLH, familial hemophagocytic lymphohistiocytosis; HLH, hemophagocytic lymphohistiocytosis; IAHS, infection-associated hemophagocytic syndrome; IFN, interferon; IL, interleukin; IQR, interquartile range; IVIG, intravenous immunoglobulin; MAS, macrophage activation syndrome; M-HLH, malignancy-associated hemophagocytic syndrome; OR, odd ratio; PTT, partial thromboplastin time; ROC, receiver operating characteristics; SJIA, systemic juvenile idiopathic arthritis; SLE, systemic lupus erythematosus; TB, total bilirubin; TNF, tumor necrosis factor.
1. Brisse E, Matthys P, Wouters CH. Understanding the spectrum of haemophagocytic lymphohistiocytosis: update on diagnostic challenges and therapeutic options. Br J Haematol. (2016) 174:175–87. doi: 10.1111/bjh.14144
2. Stepp SE, Dufourcq-Lagelouse R, Le Deist F, Bhawan S, Certain S, Mathew Porunelloor A, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. (1999) 286:1957–59. doi: 10.1126/science.286.5446.1957
3. Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell. (2003) 115:461–73. doi: 10.1016/S0092-8674(03)00855-9
4. zur Stadt U, Schmidt S, Kasper B, Beutel K, Diler A.S, Henter J-I, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. (2005) 14:827–34. doi: 10.1093/hmg/ddi076
5. Côte M, Ménager MM, Burgess A, Mahlaoui N, Picard C, Schaffner C, et al. Munc18-2 deficiency causes familial hemophagocytic lymphohistiocytosis type 5 and impairs cytotoxic granule exocytosis in patient NK cells. J Clin Invest. (2009) 119:3765–73. doi: 10.1172/JCI40732
6. Faitelson Y, Grunebaum E. Hemophagocytic lymphohistiocytosis and primary immune deficiency disorders. Clin Immunol. (2014) 155:118–25. doi: 10.1016/j.clim.2014.09.008
7. Morimoto A, Nakazawa Y, Ishii E. Hemophagocytic lymphohistiocytosis: pathogenesis, diagnosis, and management. Pediatr Int. (2016) 58:817–25. doi: 10.1111/ped.13064
8. Henderson LA, Cron RQ. Macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in childhood inflammatory disorders: diagnosis and management. Paediatr Drugs. (2020) 22:29–44. doi: 10.1007/s40272-019-00367-1
9. Minoia F, Davì S, Horne A, Mahlaoui N, Picard C, Schaffner C, et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. (2014) 66:3160–9. doi: 10.1002/art.38802
10. Borgia RE, Gerstein M, Levy DM, Silverman ED, Hiraki LT. Features, treatment, and outcomes of macrophage activation syndrome in childhood-onset systemic lupus erythematosus. Arthritis Rheumatol. (2018) 70:616–24. doi: 10.1002/art.40417
11. García-Pavón S, Yamazaki-Nakashimada MA, Báez M, Borjas-Aguilar KL, Murata C. Kawasaki disease complicated with macrophage activation syndrome: a systematic review. J Pediatr Hematol Oncol. (2017) 39:445–51. doi: 10.1097/MPH.0000000000000872
12. Miao Y, Zhu HY, Qiao C, Xia Y, Kong Y, Zou YX, et al. Pathogenic gene mutations or variants identified by targeted gene sequencing in adults with hemophagocytic lymphohistiocytosis. Front Immunol. (2019) 10:395. doi: 10.3389/fimmu.2019.00395
13. Kaufman KM, Linghu B, Szustakowski JD, Husami A, Yang F, Zhang K, et al. Whole-exome sequencing reveals overlap between macrophage activation syndrome in systemic juvenile idiopathic arthritis and familial hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. (2014) 66:3486–95. doi: 10.1002/art.38793
14. Huang Z, Jia Y, Zuo Y, Wu J, Lu A, Zhang L. Malignancy-associated hemophagocytic lymphohistiocytosis in children: a 10-year experience of a single pediatric hematology center. Hematology. (2020) 25:389–99. doi: 10.1080/16078454.2020.1833505
15. Kim MM, Yum MS, Choi HW, Ko T-S, Im HJ, Seo J-J, et al. Central nervous system (CNS) involvement is a critical prognostic factor for hemophagocytic lymphohistiocytosis. Korean J Hematol. (2012) 47:273–80. doi: 10.5045/kjh.2012.47.4.273
16. Pan H, Wang G, Guan E, Song L, Song A, Liu X, et al. Treatment outcomes and prognostic factors for non-malignancy associated secondary hemophagocytic lymphohistiocytosis in children. BMC Pediatr. (2020) 20:288. doi: 10.1186/s12887-020-02178-7
17. Luo ZB, Chen YY, Xu XJ, Zhao N, Tang YM. Prognostic factors of early death in children with hemophagocytic lymphohistiocytosis. Cytokine. (2017) 97:80–5. doi: 10.1016/j.cyto.2017.03.013
18. Dao AT, Luong VT, Nguyen TT, Huynh QTV, Phan TT, Lam MT, et al. Risk factors for early fatal outcomes among children with hemophagocytic lymphohistiocytosis (HLH): a single-institution case-series in Vietnam. Pediatr Hematol Oncol. (2014) 31:271–81. doi: 10.3109/08880018.2013.858198
19. Kaya Z, Bay A, Albayrak M, Kocak U, Yenicesu I, Gursel T. Prognostic factors and long-term outcome in 52 Turkish children with hemophagocytic lymphohistiocytosis. Pediatr Crit Care Med. (2015) 16:e165–73. doi: 10.1097/PCC.0000000000000449
20. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. (2011) 56:154–5. doi: 10.1002/pbc.22774
21. Abou Shaar R, Eby CS, van Dorp S, de Witte T, Otrock ZK. Increasing ferritin predicts early death in adult hemophagocytic lymphohistiocytosis. Int J Lab Hematol. (2021) 43:1024–31. doi: 10.1111/ijlh.13489
22. Bin Q, Gao JH, Luo JM. Prognostic factors of early outcome in pediatric hemophagocytic lymphohistiocytosis: an analysis of 116 cases. Ann Hematol. (2016) 95:1411–8. doi: 10.1007/s00277-016-2727-6
23. Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. (2007) 48:124–31. doi: 10.1002/pbc.21039
24. Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European league against rheumatism/American college of rheumatology/paediatric rheumatology international trials organisation collaborative initiative. Arthritis Rheumatol. (2016) 68:566–76. doi: 10.1002/art.39332
25. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31:390–2.
26. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. (2021) 120:172–9. doi: 10.1016/j.jfma.2020.03.025
27. Pan H, Huo Y, Sun L. Comparison between clinical features and prognosis of malignancy- and non-malignancy-associated pediatric hemophagocytic lymphohistiocytosis. BMC Pediatr. (2019) 19:468. doi: 10.1186/s12887-019-1702-5
28. Oguz MM, Sahin G, Altinel Acoglu E, Polat E, Yucel H, Oztek Celebi FZ, et al. Secondary hemophagocytic lymphohistiocytosis in pediatric patients: a single center experience and factors that influenced patient prognosis. Pediatr Hematol Oncol. (2019) 36:1–16. doi: 10.1080/08880018.2019.1572253
29. Giang HTN, Banno K, Minh LHN, Trinh LT, Loc LT, Eltobgy A, et al. Dengue hemophagocytic syndrome: a systematic review and meta-analysis on epidemiology, clinical signs, outcomes, and risk factors. Rev Med Virol. (2018) 28:e2005. doi: 10.1002/rmv.2005
30. Kan FK, Tan CC, Von Bahr Greenwood T, Khalid KE, Supramaniam P, Myrberg IH, et al. Dengue infection complicated by hemophagocytic lymphohistiocytosis: experiences from 180 patients with severe dengue. Clin Infect Dis. (2020) 70:2247–55. doi: 10.1093/cid/ciz499
31. Pal P, Bathia J, Giri PP, Roy M, Nandi A. Macrophage activation syndrome in pediatrics: 10 years data from an Indian center. Int J Rheum Dis. (2020) 23:1412–6. doi: 10.1111/1756-185X.13915
32. Lehmberg K, Sprekels B, Nichols KE, Woessmann W, Muller I, Suttorp M, et al. Malignancy-associated haemophagocytic lymphohistiocytosis in children and adolescents. Br J Haematol. (2015) 170:539–49. doi: 10.1111/bjh.13462
33. Otrock ZK, Eby CS. Clinical characteristics, prognostic factors, and outcomes of adult patients with hemophagocytic lymphohistiocytosis. Am J Hematol. (2015) 90:220–4. doi: 10.1002/ajh.23911
34. Hayden A, Park S, Giustini D, Lee AY, Chen LY. Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev. (2016) 30:411–20. doi: 10.1016/j.blre.2016.05.001
35. Posas-Mendoza TF, McLeod C, Davis W, Zakem J, Quinet R. Etiologies and management of haemophagocytic lymphohistiocytosis: is it time for an updated protocol and targeted treatments? Rheumatology. (2021) 60:2927–33. doi: 10.1093/rheumatology/keaa741
36. Santos-Ocampo AS, Mandell BF, Fessler BJ. Alveolar hemorrhage in systemic lupus erythematosus: presentation and management. Chest. (2000) 118:1083–90. doi: 10.1378/chest.118.4.1083
37. Parodi A, Davì S, Pringe AB, Pistorio A, Ruperto N, Magni-Manzoni S, et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: a multinational multicenter study of thirty-eight patients. Arthritis Rheum. (2009) 60:3388–99. doi: 10.1002/art.24883
38. Naveen R, Jain A, Muhammed H, Gupta L, Misra DP, Lawrence A, et al. Macrophage activation syndrome in systemic lupus erythematosus and systemic-onset juvenile idiopathic arthritis: a retrospective study of similarities and dissimilarities. Rheumatol Int. (2021) 41:625–31. doi: 10.1007/s00296-020-04763-6
39. Aytaç S, Batu ED, Ünal S, Bilginer Y, Çetin M, Tuncer M, et al. Macrophage activation syndrome in children with systemic juvenile idiopathic arthritis and systemic lupus erythematosus. Rheumatol Int. (2016) 36:1421–9. doi: 10.1007/s00296-016-3545-9
40. Horne A, Trottestam H, Aricò M, Maarten Egeler R, Filipovich AH, Gadner H, et al. Frequency and spectrum of central nervous system involvement in 193 children with haemophagocytic lymphohistiocytosis. Br J Haematol. (2008) 140:327–35. doi: 10.1111/j.1365-2141.2007.06922.x
41. Zhao YZ, Zhang Q, Li ZG, Zhang L, Lian H-Y, Ma H-H, et al. Central nervous system involvement in 179 Chinese children with hemophagocytic lymphohistiocytosis. Chin Med J. (2018) 131:1786–92. doi: 10.4103/0366-6999.237409
42. Henter JI, Nennesmo I. Neuropathologic findings and neurologic symptoms in twenty-three children with hemophagocytic lymphohistiocytosis. J Pediatr. (1997) 130:358–65. doi: 10.1016/S0022-3476(97)70196-3
43. Trottestam H, Berglöf E, Horne A, Onelöv E, Beutel K, Lehmberg K, et al. Risk factors for early death in children with haemophagocytic lymphohistiocytosis. Acta Paediatr. (2012) 101:313–8. doi: 10.1111/j.1651-2227.2011.02501.x
44. Valade S, Mariotte E, Azoulay E. Coagulation disorders in hemophagocytic lymphohistiocytosis/macrophage activation syndrome. Crit Care Clin. (2020) 36:415–26. doi: 10.1016/j.ccc.2019.12.004
45. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How i treat hemophagocytic lymphohistiocytosis. Blood. (2011) 118:4041–52. doi: 10.1182/blood-2011-03-278127
46. de Kerguenec C, Hillaire S, Molinié V, Gardin C, Degott C, Erlinger S, et al. Hepatic manifestations of hemophagocytic syndrome: a study of 30 cases. Am J Gastroenterol. (2001) 96:852–7. doi: 10.1111/j.1572-0241.2001.03632.x
47. Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood. (2005) 105:1648–51. doi: 10.1182/blood-2004-08-2997
48. Yoon JH, Park SS, Jeon YW, Lee SE, Cho BS, Eom KS, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. (2019) 104:269–76. doi: 10.3324/haematol.2018.198655
49. Ruscitti P, Cipriani P, Ciccia F, Masedu F, Liakouli V, Carubbi F, et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: Analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun Rev. (2017) 16:16–21. doi: 10.1016/j.autrev.2016.09.016
50. Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol. (2019) 10:119. doi: 10.3389/fimmu.2019.00119
51. Vilaiyuk S, Sirachainan N, Wanitkun S, Pirojsakul K, Vaewpanich J. Recurrent macrophage activation syndrome as the primary manifestation in systemic lupus erythematosus and the benefit of serial ferritin measurements: a case-based review. Clin Rheumatol. (2013) 32:899–904. doi: 10.1007/s10067-013-2227-1
Keywords: hemophagocytic lymphohistiocytosis (HLH), macrophage activation syndrome (MAS), mortality rate, prognostic factor, pediatric patients
Citation: Harnchoowong S, Soponkanaporn S, Vilaiyuk S, Lerkvaleekul B and Pakakasama S (2022) Central nervous system involvement and thrombocytopenia as predictors of mortality in children with hemophagocytic lymphohistiocytosis. Front. Pediatr. 10:941318. doi: 10.3389/fped.2022.941318
Received: 11 May 2022; Accepted: 18 August 2022;
Published: 06 September 2022.
Edited by:
Pietro Merli, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Alix Eden Seif, Children's Hospital of Philadelphia, United StatesCopyright © 2022 Harnchoowong, Soponkanaporn, Vilaiyuk, Lerkvaleekul and Pakakasama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sirisucha Soponkanaporn, c2lyaXN1Y2hhcnNAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.