
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 06 October 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.934505
Background: Studies investigating the relationship between gestational dyslipidemia and small for gestational age (SGA) have reported differing results. This review was performed to determine whether maternal lipid levels during pregnancy were associated with SGA.
Methods: Literature searches for relevant studies were conducted systematically from establishment until February 2022 with PubMed, Embase, Cochrane Library and Web of Science. Risk of bias was assessed with the Newcastle-Ottawa Scale and 11-item checklist. According to the classification of GHD parameters, meta-analyses reporting cases regarding total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C) were performed respectively. If I2 ≥ 50%, considered to demonstrate substantial heterogeneity, the random effect model was employed. Otherwise, a fixed effect model was employed.
Results: Eight studies (14,213 pregnancies) were included. Decreased levels of TC (MD −0.13; 95% CI −0.24 to −0.02), TG (MD −0.09; 95% CI −0.14 to −0.03) and LDL-C (MD −0.12; 95% CI −0.23 to −0.00) were risk factors for SGA infant birth. No evident association was observed between HDL-C and delivery of SGA (MD −0.08; 95% CI −0.19 to 0.02).
Conclusion: Gestations complicated with dyslipidemia, especially lower concentrations of TC, TG and LDL-C, were at significantly higher risk of delivery of SGA.
Systematic review registration: [www.crd.york.ac.uk/prospero], identifier [CRD42022304648].
Considered as one of the major adverse birth outcomes, small for gestational age (SGA) is defined as birthweight less than 10th percentile for gestational age (1). The mortality rates of infants with SGA are 6–9 times higher than those with normal birthweight. Moreover, neonates with SGA are at a higher risk of complications such as respiratory distress syndrome, hypothermia, metabolic disorders, retinopathy and necrotizing enterocolitis. They are also more likely to be diagnosed with secondary to metabolic syndromes including obesity, type 2 diabetes mellitus, hypertension, insulin resistance and hyperlipidemia in adulthood (2–4). Limited effective treatments for SGA and the irreversibility of SGA emphasize the necessity of strengthening the prediction and prevention of SGA.
It has been well-established that SGA is associated with low pre-pregnancy body mass index (5), sleep disturbances (6), short stature (7), serum plasma protein A levels (8), smoking (9) and maternal antioxidant levels (10). Recently, gestational dyslipidemia has also been reported to be a risk factor for SGA infant delivery (11–18).
Lipid parameters, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C) and high-density lipoprotein-cholesterol (HDL-C), gradually increase from the 12th week of gestation and keep growing through the second and third trimesters in normal pregnancy (19–22). Adapted to physiological metabolism, lipid levels change to meet the demand of fetal growth. Dyslipidemia is one of the principal metabolic disorders during gestation (23). Dyslipidemia has been regarded as a risk factor for many adverse health outcomes, especially type 2 diabetes and cardiovascular disease (24, 25). Preceding reviews have revealed that gestational dyslipidemia is associated with increased incidence of preterm delivery, hypertensive disorders and gestational diabetes mellitus,(26–28) but epidemiological evidence regarding SGA is conflicting. Some studies showed there was no association between maternal lipid levels during pregnancy and delivery of SGA,(12, 16, 17) whereas other reports found that gestational dyslipidemia could lead to SGA infant birth (11–15, 18).
Therefore, this systematic reviewed was performed to explore the relationship between maternal lipid levels during pregnancy and delivery of SGA.
The study protocol was registered on PROSPERO as CRD42022304648. This systematic review was performed in accordance with the PRISMA (29) and MOOSE (30) guidelines. Literature searches for relevant studies were conducted systematically from establishment until February 2022 with the following databases: PubMed, Embase, Cochrane Library and Web of Science. The search combined related titles and abstracts, keywords and word variants for “lipid” or “lipoprotein” or “triglycerides” or “cholesterol” and “small for gestational age” (Supplementary Table 1). The search and eligibility criteria were limited to human studies published in English language. Review articles, book chapters, conference proceedings, case reports, and thesis dissertations were excluded.
We included the articles with the following criteria: (1) cohort studies, cross-sectional studies and case-control studies. (2) Studies with outcomes regarding both maternal lipid levels during pregnancy and SGA, and we excluded the articles with the following criteria: (1) articles with only antenatal and postpartum maternal lipid concentrations. (2) Articles without available data. (3) Studies with unspecific measurements for lipid parameters.
The primary outcome was SGA. Infants with birthweight between 10th and 90th percentile were classified as appropriate for gestational age (AGA), and those having weight below 10th percentile for gestational age were SGA. With maternal blood samples taken, serum was assayed for lipid parameters including TC, TG, LDL-C and HDL-C respectively. Owing to diverse methods for serum analysis, we accepted the method specified by authors. As lipid parameters were classified into TC, TG, LDL-C and HDL-C, meta-analyses were performed separately.
Full texts of relevant studies were screened by two authors (YW and ZFC) for eligibility against the inclusion and exclusion criteria. When confronted with discrepancies, consensus was reached in consultation with another reviewer (FZ). With included studies double screened, the same two review authors extracted data in relation to study characteristics and maternal and fetal outcomes independently. In case of over one study reporting information on the basis of the identical cohort with the same endpoints, we included the most comprehensive and informative report.
Quality assessment of eligibility cohort and case-control studies was carried out with the Newcastle-Ottawa Scale (NOS) (31). This scale consisted of 3 broad aspects which were selection of patients, comparability of study groups and ascertainment of exposure or outcome. The scores of this system range from 0 to 9. We gave points when the studies met relevant condition. The included studies were judged as having a high (scores of 0–3), medium (scores of 4–6), or low risk of bias (scores of 7–9) respectively. Publications judged as having a low risk of bias were included in this analysis. 11-item checklist was applied to assess the quality of included cross-sectional studies, which was recommended by the Agency for Healthcare Research and Quality (AHRQ) (32). Each item was scored “0” if answered “No” or “Unclear”; when it was answered “Yes,” the item was scored “1.” We considered articles as having low quality (scores of 0–3), moderate quality (scores of 4–7) or high quality (scores of 8–11).
Data analysis was performed with Review Manager (version 5.3) software. Because of the involvement of continuous data (mean and standard deviation), mean and standard deviation (SMD) with 95% CI were used to show the final analysis. During data extraction, median and interquartile range provided by some studies were converted to SMD (33). Heterogeneity between the trials was explored by the Higgins I2 statistics, estimating the percentage of variability across studies that was owing to heterogeneity rather than chance. If I2 ≥ 50%, considered to demonstrate substantial heterogeneity, the random effect model was employed. Otherwise, a fixed effect model was employed.
Of a total of 2,377 identified articles, 39 were assessed in terms of their eligibility for inclusion, and 8 studies were considered appropriate to be selected in this systematic review (Table 1 and Figure 1). These 8 studies (including 14,213 pregnancies) reported maternal lipid levels in pregnancies with SGA infants compared with those with infants born AGA. The articles were published between 2012 and 2021. They differed in sample size, ranging from 119 to 5,282. Four of these included publications were cohort studies (12–14, 17), two were case-control studies (15, 18) and two were cross-sectional studies (11, 16). They were conducted in different countries, Netherlands (12), China (13), Turkey (16), Japan (18), Korea (14), Canada (15), German (17) and Egypt (11) separately. Among the selected studies, three reported maternal lipid profile during early pregnancy (12, 14, 16), three during mid-pregnancy (15, 17, 18), and one during mid and late pregnancy (13) in detail, yet one study didn’t mention this information (11).
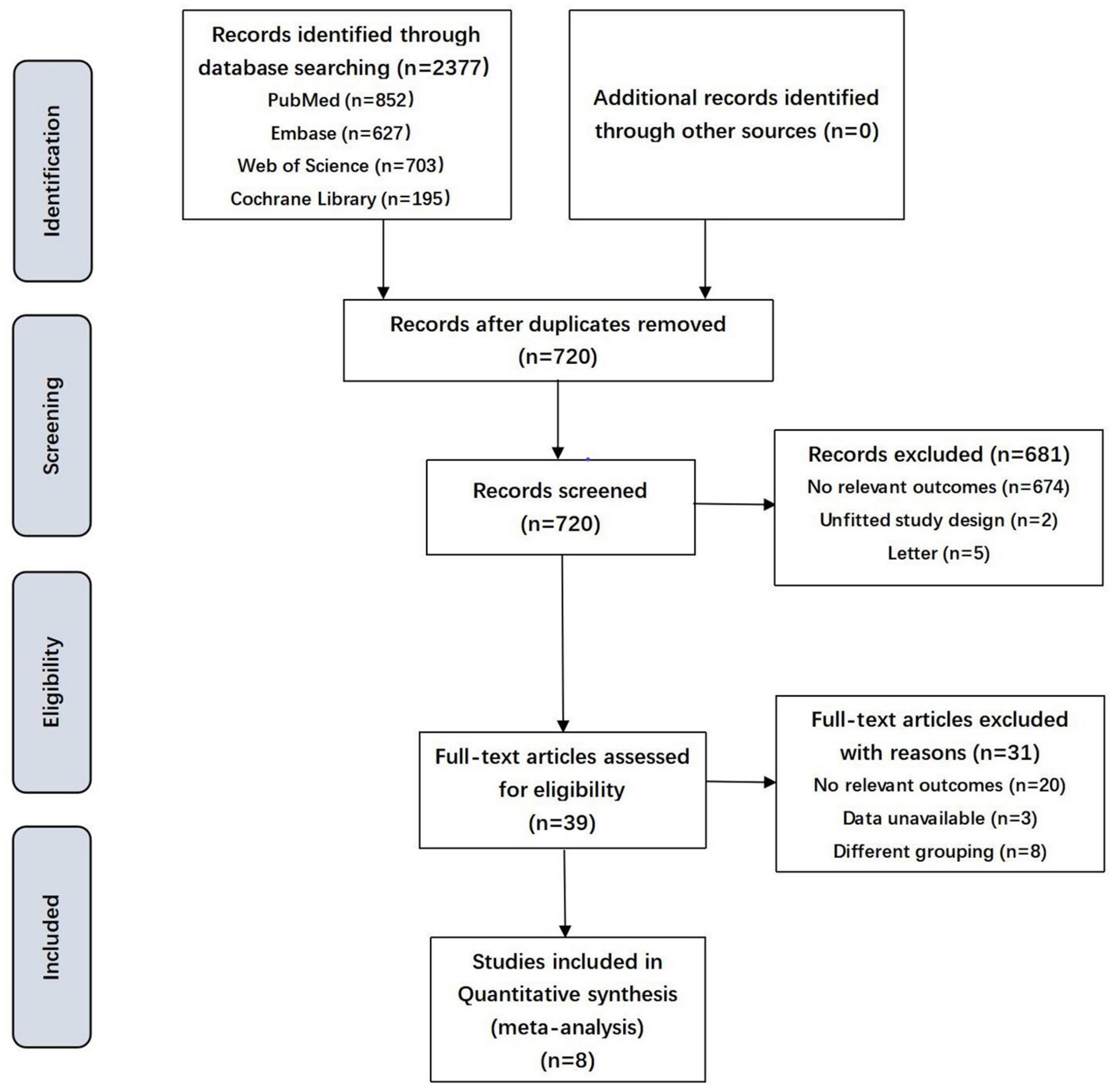
Figure 1. Flowchart showing the selection process of the included studies. SGA, small for gestational age.
Quality assessment of the eight publications performed with NOS and 11-item checklist was presented in Table 2. All the selected cohort and case-control studies showed good quality with regard to selection, comparability and outcome with NOS scores ≥ 5. Two cross-sectional studies also had low a risk of bias with a greater number of “Yes” answers than “No” or “Unclear” answers to the 11 items. The main weaknesses of these publications were their trial design, restricted study populations, lack of adequate information regarding the follow-up outcome, differences in the methods for measurements and heterogeneity in the period of maternal blood samples taken.
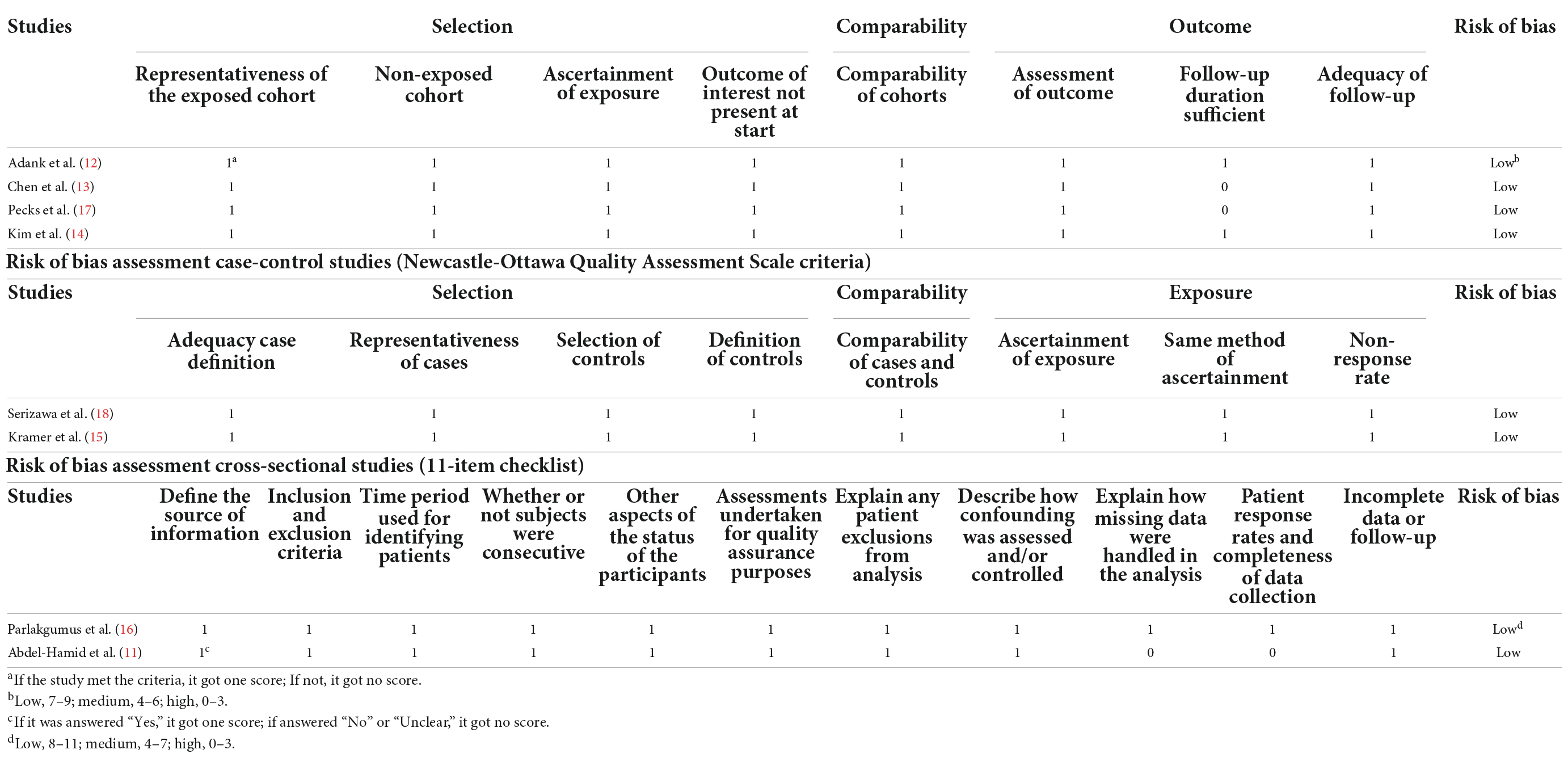
Table 2. Risk of bias assessment cohort studies (Newcastle-Ottawa Quality Assessment Scale criteria).
Six studies (12,385 pregnancies) explored TC concentrations in gestations with SGA neonates compared with AGA group. Considering all pregnancies, decreased concentrations of TC was associated with higher risk of SGA infant delivery (SMD −0.13; 95% CI −0.24 to −0.02; P = 0.03) (Figure 2).
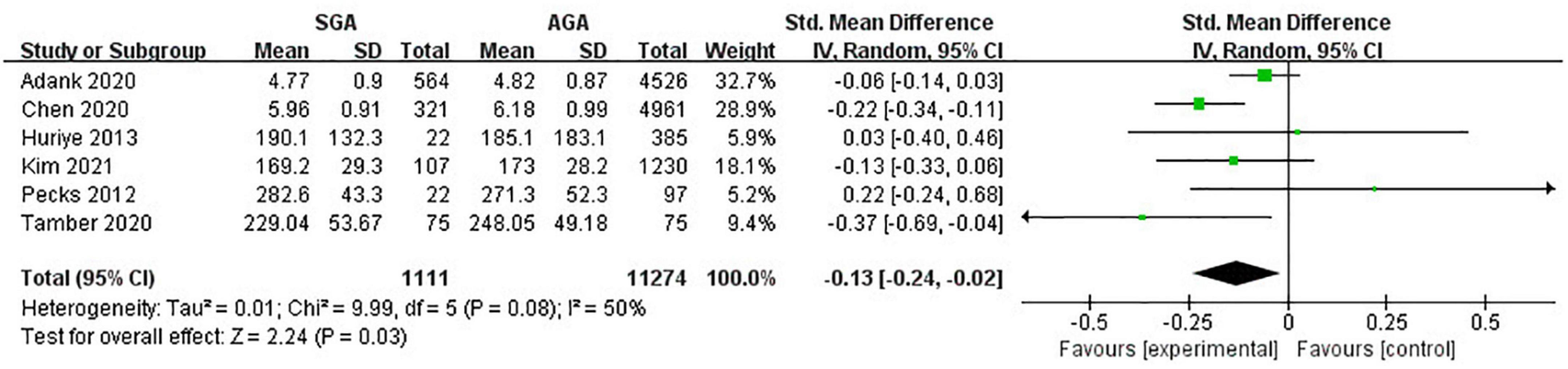
Figure 2. Forest plot exploring the association between maternal TC levels and delivery of SGA. TC, total cholesterol; SGA, small for gestational age.
Eight studies (14,213 pregnancies) explored the maternal concentrations of TG in pregnancies with infants born SGA compared to those born AGA. In all, pregnant women with lower levels of TG were at a higher risk of giving birth to SGA infants (SMD −0.09; 95% CI −0.14 to −0.03; P = 0.002) (Figure 3).
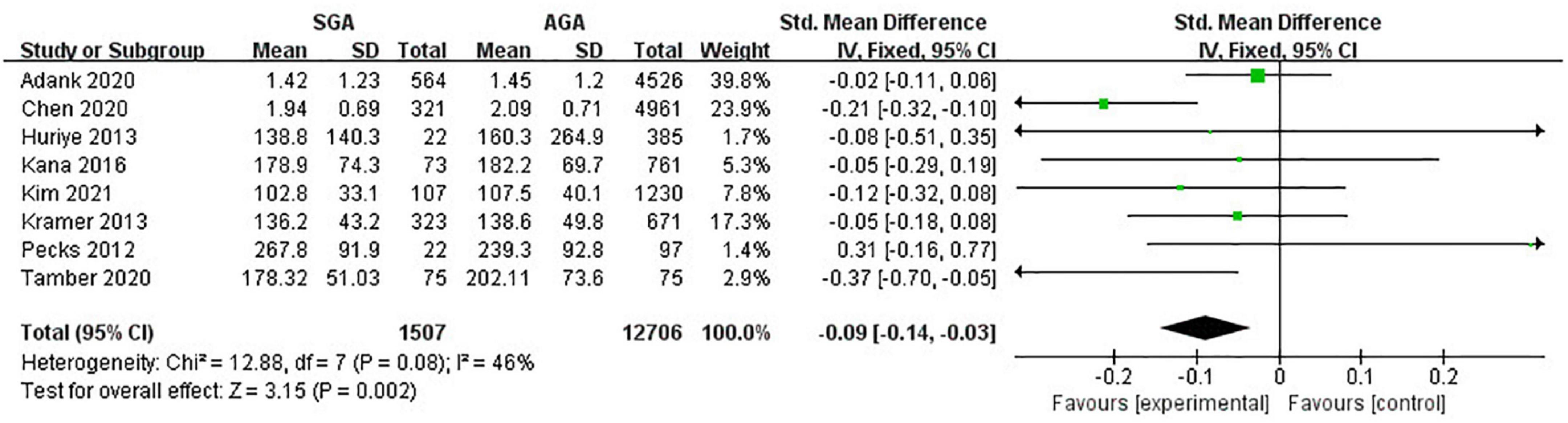
Figure 3. Forest plot exploring the association between maternal TG levels and delivery of SGA. TG, triglycerides; SGA, small for gestational age.
Seven studies (13,219 twin pregnancies) explored the maternal levels of LDL-C in gestations with SGA infants in comparison with those with AGA ones. It turned out that increased odds of giving birth to infants with SGA were associated with decreased levels of LDL-C (SMD −0.12; 95% CI −0.23 to −0.00; P = 0.05) (Figure 4).
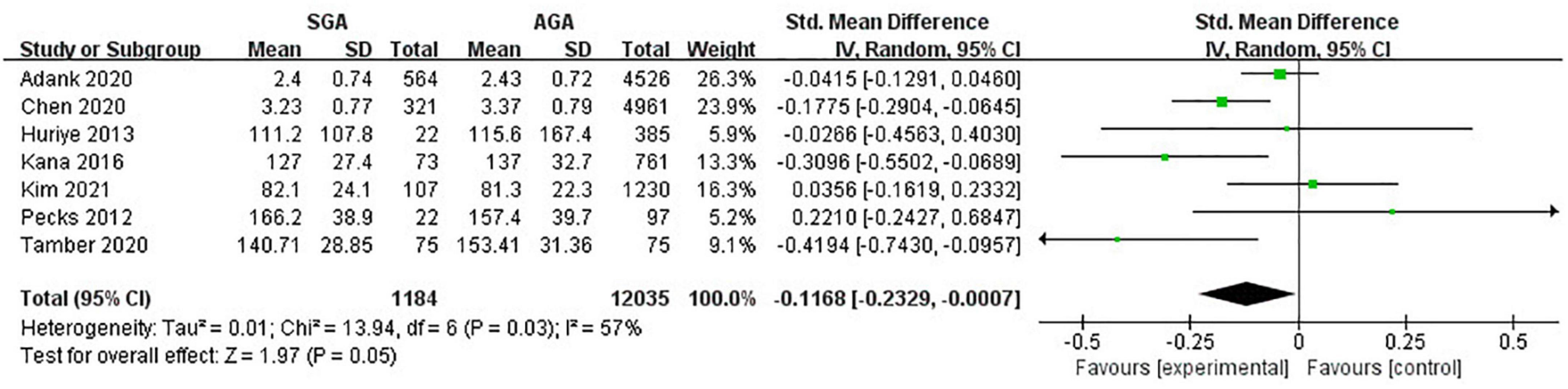
Figure 4. Forest plot exploring the association between maternal TG levels and delivery of SGA. LDL-C, low-density lipoprotein-cholesterol; SGA, small for gestational age.
Eight studies (14,213 pregnancies) explored the maternal HDL-C levels during pregnancies with SGA infants compared with AGA group. Overall, no evident relationship was observed between maternal lipid concentrations and delivery of SGA (SMD −0.08; 95% CI −0.19 to 0.02; P = 0.11) (Figure 5).
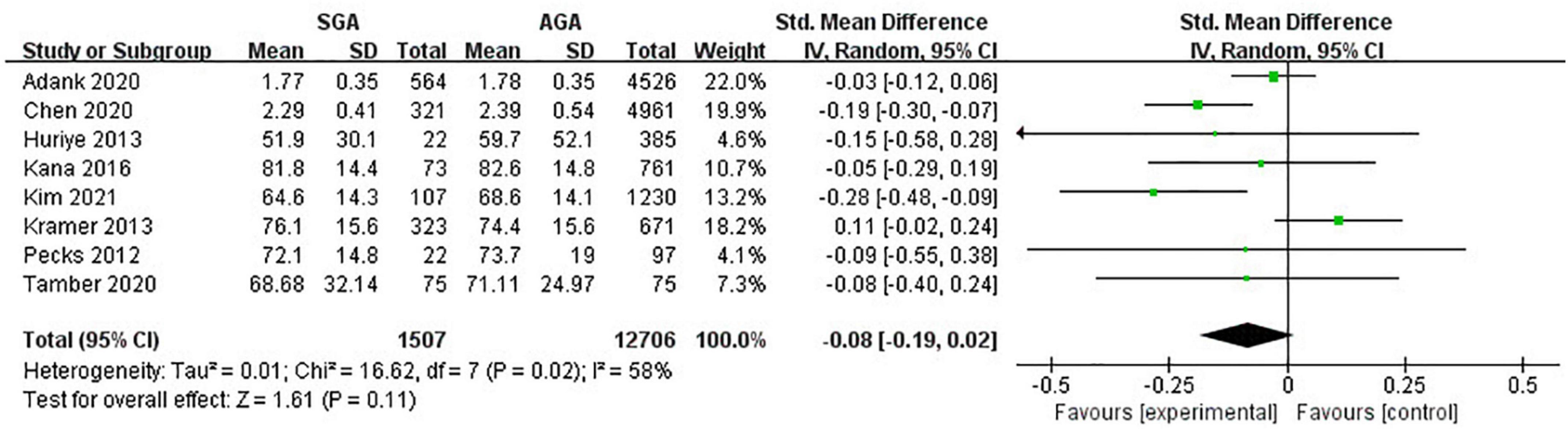
Figure 5. Forest plot exploring the association between maternal TG levels and delivery of SGA. HDL-C, high-density lipoprotein-cholesterol; SGA, small for gestational age.
This systematic review demonstrated that maternal lipid levels during pregnancy was associated with delivery of SGA. The incidence of giving birth to fetuses with SGA increased in parallel to the risk of gestational dyslipidemia, especially lower maternal TC, TG and LDL-C levels throughout pregnancy. Based on the above-mentioned findings, it is notable that maternal lipid levels during pregnancy will contribute to predict SGA infant delivery.
Gestational dyslipidemia, particularly lower levels of TC, TG and LDL-C, was a risk factor for delivery of SGA. Though the physiology and molecular mechanisms underlying this association remains unclear, which may be connected to the maternal or fetal transport mechanisms, there are some possible theories explaining this relationship.
The first assumption was about maternal and fetal cholesterol levels. During pregnancy, maternal blood lipids were transported to infants, and birthweight was related to the amount of transported lipids. Yamamoto et al. (34) observed the transport of cholesterol coming from the mother to the embryos with pregnant mice. Furthermore, they disclosed that cholesterol was transported to fetuses even after placenta formation. What’s more, studies on mice have revealed that maternal cholesterol concentrations influenced the sterol metabolism and fetal birthweight, thus the maternal lipid levels were associated with birthweight. When it comes to humans, the levels of cholesterol in maternal blood were positively correlated with those in fetuses during pregnancy, and there was also a positive correlation between the area of the fatty streak of the fetuses and the maternal cholesterol levels (35, 36). Therefore, when cholesterol levels are low in maternal blood, the fetuses are referred to have lower cholesterol concentrations. As cholesterol levels and birthweight were related positively, women with low cholesterol levels during pregnancy had a tendency to give birth to neonates with SGA. It was also speculated by Kana et al. (18) that low cholesterol intake could probably lead to abnormal lipid metabolism in infants, which would consequently result in SGA.
In addition, the LDL-C consumption suppressing the growth of infants theory might also explain the findings of the review. Morteza et al. pointed out that pregnancies complicated with SGA were associated with insulin resistance, and arrived at a conclusion that the hormonal imbalance inherent in insulin resistance complicates SGA pregnancies by decreasing the consumption of LDL-C and reducing the TG levels (37). It was also reported by Satter et al. that women with increased LDL-C concentrations during pregnancy were not diagnosed with any complications, but those whose LDL-C levels failed to sufficiently grow to the required values gave birth to fetuses with SGA (38). These two articles showed that LDL-C consumption, namely reduced LDL-C levels, brings about SGA. However, according to the study conducted by Chen et al. (13), it was doubtful whether hormonal imbalance was underlying insulin resistance, as oral glucose tolerance test for the participants showed results within normal range. Hence, this mechanism deserves to be further explored.
The fact concerning placental change was another possible theory. Cholesterol, an important component of cell membranes, played a biochemical role as metabolic precursors of transmembrane signaling, cell proliferation and steroid hormones (39, 40). But abnormalities in cholesterol mechanisms could give rise to atherosclerotic disorders such as cardiovascular and cerebrovascular diseases (41). Taking the basic effect of dyslipidemia in atherosclerosis into account, it might be possible for altered cholesterol biosynthesis and gestational dyslipidemia to lead to atherosclerotic placental change. Subsequently, Atherosclerotic placental change could result in decreased material blood flow to the infant, thereby reducing nutrient supply for the infant and interfering fetal growth (42).
Apart from the three explanations mentioned above, Maria et al. (12) hypothesized that besides genetics, lifestyle factors might play a significant role in the association of maternal lipid levels with SGA as well, which indicates a novel perspective worth further exploring.
However, our findings should be elucidated carefully because of small number and size of eligibility articles, more high quality studies are warranted to investigate the relationship between maternal lipid concentrations in pregnancy and delivery of SGA.
The major strengths of this review were a comprehensive literature search strategy, adherence to robust review methodology and inclusion of studies with good quality. However, some limitations such as small number of eligibility publications, small sample size of individual studies and different inclusion criteria for trial participants couldn’t be neglected. The reliability of the systematic review was influenced by diverse lipid measurement methods. Furthermore, the results were subject to maternal glucose concentrations during pregnancy. Though this heterogeneity could be reduced by performing subgroup analysis. Unfortunately, it was not feasible because only several articles explore the influences of maternal lipid levels on SGA infant delivery independent of glucose levels. Another main limitation of our present systematic review was that blood samples were taken during different gestational ages, lack of relevant data made it unable to report the association between maternal lipid levels and delivery of SGA during early, mid- and late pregnancy respectively. What’s more, as all included studies focused on singleton pregnancies, the findings of our study were not applicable for twin gestations. It also should be acknowledged that the primary outcome, delivery of SGA, diagnosed with a quite inexact measure, despite it has come into widespread use in clinical and research areas.
In spite of above-mentioned limitations, this review represented the up-to-date and most comprehensive findings of the effects of maternal lipid levels throughout pregnancy on giving birth to infants with SGA.
The present review suggested the relationship between maternal lipid levels throughout pregnancy and SGA infant delivery. Gestational, particularly decreased concentrations of TC, TG and LDL-C, were at significantly higher incidence of delivery of SGA. Though, according to our review, HDL-C had nothing to do with SGA infant delivery, further fundamental and clinical studies are in need to provide more firm conclusions. This finding would not only assist with predicting future SGA infant birth, but also contribute to preventing SGA infant birth. Putting the health guidance system into practice, tracking information on daily habits and diet, and working out tailored dietary guidance programs are justified, which can help reduce the rate of pregnancies complicated with SGA.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
YW and ZC contributed to planning the review, screening all titles and abstracts, reviewing full articles, and performing data extraction. FZ was a third reviewer in case where there were inconsistencies and contributed to interpreting the data and revising the manuscript. YW performed the data analysis and drafted the initial manuscript. All authors have read and approved the final version of the manuscript.
This work was funded by the Science and Technology Program of Nantong City (MS12020036 and MS12021040).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.934505/full#supplementary-material
1. Chabra S. Small for gestational age and low birthweight: distinct entities. Am J Gastroenterol. (2018) 113:441–2. doi: 10.1038/ajg.2017.499
2. Barker DJ. Adult consequences of fetal growth restriction. Clin Obstet Gynecol. (2006) 49:270–83. doi: 10.1097/00003081-200606000-00009
3. Pallotto EK, Kilbride HW. Perinatal outcome and later implications of intrauterine growth restriction. Clin Obstet Gynecol. (2006) 49:257–69.
4. Liu Q, Yang H, Sun X, Li G. Risk factors and complications of small for gestational age. Pak J Med Sci. (2019) 35:1199–203. doi: 10.12669/pjms.35.5.253
5. Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA. (2017) 317:2207–25. doi: 10.1001/jama.2017.3635
6. Lu Q, Zhang X, Wang Y, Li J, Xu Y, Song X, et al. Sleep disturbances during pregnancy and adverse maternal and fetal outcomes: a systematic review and meta-analysis. Sleep Med Rev. (2021) 58:101436. doi: 10.1016/j.smrv.2021.101436
7. Kozuki N, Katz J, Lee AC, Vogel JP, Silveira MF, Sania A, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. (2015) 145:2542–50. doi: 10.3945/jn.115.216374
8. Morris RK, Bilagi A, Devani P, Kilby MD. Association of serum PAPP-A levels in first trimester with small for gestational age and adverse pregnancy outcomes: systematic review and meta-analysis. Prenat Diagn. (2017) 37:253–65. doi: 10.1002/pd.5001
9. Oldereid NB, Wennerholm UB, Pinborg A, Loft A, Laivuori H, Petzold M, et al. The effect of paternal factors on perinatal and paediatric outcomes: a systematic review and meta-analysis. Hum Reprod Update. (2018) 24:320–89. doi: 10.1093/humupd/dmy005
10. Cohen JM, Beddaoui M, Kramer MS, Platt RW, Basso O, Kahn SR, et al. Maternal antioxidant levels in pregnancy and risk of preeclampsia and small for gestational age birth: a systematic review and meta-analysis. PLoS One. (2015) 10:e0135192. doi: 10.1371/journal.pone.0135192
11. Abdel-Hamid TA, AbdelLatif D, Ahmed E, Abdel-Rasheed M, Mageed AA. Relation between maternal and neonatal serum lipid profile and their impact on birth weight. Am J Perinatol. (2022) 39:1112–1116. doi: 10.1055/s-0040-1721690
12. Adank MC, Benschop L, Kors AW, Peterbroers KR, Smak Gregoor AM, Mulder MT, et al. Maternal lipid profile in early pregnancy is associated with foetal growth and the risk of a child born large-for-gestational age: a population-based prospective cohort study : maternal lipid profile in early pregnancy and foetal growth. BMC Med. (2020) 18:276. doi: 10.1186/s12916-020-01730-7
13. Chen Q, Chen H, Xi F, Sagnelli M, Zhao B, Chen Y, et al. Association between maternal blood lipids levels during pregnancy and risk of small-for-gestational-age infants. Sci Rep. (2020) 10:19865. doi: 10.1038/s41598-020-76845-1
14. Kim SY, Lee SM, Kwon GE, Kim BJ, Koo JN, Oh IH, et al. Maternal dyslipidemia and altered cholesterol metabolism in early pregnancy as a risk factor for small for gestational age neonates. Sci Rep. (2021) 11:21066. doi: 10.1038/s41598-021-00270-1
15. Kramer MS, Kahn SR, Dahhou M, Otvos J, Genest J, Platt RW, et al. Maternal lipids and small for gestational age birth at term. J Pediatr. (2013) 163:983–8. doi: 10.1016/j.jpeds.2013.05.014
16. Parlakgumus HA, Aytac PC, Kalayci H, Tarim E. First trimester maternal lipid levels and serum markers of small- and large-for-gestational age infants. J Matern Fetal Neonatal Med. (2014) 27:48–51. doi: 10.3109/14767058.2013.799658
17. Pecks U, Brieger M, Schiessl B, Bauerschlag DO, Piroth D, Piroth D, et al. Maternal and fetal cord blood lipids in intrauterine growth restriction. J Perinat Med. (2012) 40:287–96. doi: 10.1515/jpm.2011.135
18. Serizawa K, Ogawa K, Arata N, Ogihara A, Horikawa R, Sakamoto N, et al. Association between low maternal low-density lipoprotein cholesterol levels in the second trimester and delivery of small for gestational age infants at term: a case-control study of the national center for child health and development birth cohort. J Matern Fetal Neonatal Med. (2017) 30:1383–7. doi: 10.1080/14767058.2016.1214701
19. Bartels A, Egan N, Broadhurst DI, Khashan AS, Joyce C, Stapleton M, et al. Maternal serum cholesterol levels are elevated from the 1st trimester of pregnancy: a cross-sectional study. J Obstet Gynaecol. (2012) 32:747–52.
20. Ghio A, Bertolotto A, Resi V, Volpe L, Di Cianni G. Triglyceride metabolism in pregnancy. Adv Clin Chem. (2011) 55:133–53.
21. Husain F, Latif S, Uddin M, Nessa A. Lipid profile changes in second trimester of pregnancy. Mymensingh Med J. (2008) 17:17–21.
22. Lippi G, Albiero A, Montagnana M, Salvagno GL, Scevarolli S, Franchi M, et al. Lipid and lipoprotein profile in physiological pregnancy. Clin Lab. (2007) 53:173–7.
23. Nasioudis D, Doulaveris G, Kanninen TT. Dyslipidemia in pregnancy and maternal-fetal outcome. Minerva Ginecol. (2019) 71:155–62.
24. Iqbal J, Al Qarni A, Hawwari A, Alghanem AF, Ahmed G. Metabolic syndrome, dyslipidemia and regulation of lipoprotein metabolism. Curr Diabetes Rev. (2018) 14:427–33.
25. Liberis A, Petousis S, Tsikouras P. Lipid disorders in pregnancy. Curr Pharm Des. (2021) 27:3804–7.
26. Jiang S, Jiang J, Xu H, Wang S, Liu Z, Li M, et al. Maternal dyslipidemia during pregnancy may increase the risk of preterm birth: a meta-analysis. Taiwan J Obstet Gynecol. (2017) 56:9–15. doi: 10.1016/j.tjog.2016.07.012
27. Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. (2015) 122:643–51.
28. Zhang Y, Lan X, Cai C, Li R, Gao Y, Yang L, et al. Associations between maternal lipid profiles and pregnancy complications: a prospective population-based study. Am J Perinatol. (2021) 38:834–40.
29. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
30. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Moher D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12.
31. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5.
32. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
33. Shi J, Luo D, Weng H, Zeng XT, Lin L, Chu H, et al. Optimally estimating the sample standard deviation from the five-number summary. Res Synth Methods. (2020) 11:641–54.
34. Yamamoto S. Management of lipid nutrition for pregnant women: effects on health in the next generation. J Lipid Nutr. (2014) 23:63–9.
35. Napoli C, D’Armiento FP, Mancini FP, Postiglione A, Witztum JL, Palumbo G, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. (1997) 100:2680–90. doi: 10.1172/JCI119813
36. Napoli C, Glass CK, Witztum JL, Deutsch R, D’Armiento FP, Palinski W, et al. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. (1999) 354:1234–41. doi: 10.1016/S0140-6736(99)02131-5
37. Morteza A, Abdollahi A, Bandarian M. Serum nitric oxide syntheses and lipid profile of the mothers with IUGR pregnancies uncomplicated with preeclampsia. Does insulin resistance matter? Gynecol Endocrinol. (2012) 28:139–42. doi: 10.3109/09513590.2011.589921
38. Sattar N, Greer IA, Galloway PJ, Packard CJ, Shepherd J, Kelly T, et al. Lipid and lipoprotein concentrations in pregnancies complicated by intrauterine growth restriction. J Clin Endocrinol Metab. (1999) 84:128–30.
39. Martin MG, Pfrieger F, Dotti CG. Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep. (2014) 15:1036–52. doi: 10.15252/embr.201439225
40. Narwal V, Deswal R, Batra B, Kalra V, Hooda R, Sharma M, et al. Cholesterol biosensors: a review. Steroids. (2019) 143:6–17.
41. Khaw KT, Sharp SJ, Finikarides L, Afzal I, Lentjes M, Luben R, et al. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open. (2018) 8:e020167.
Keywords: small for gestational age, lipids, cholesterol, triglycerides, pregnancy, systematic review
Citation: Wang Y, Chen Z and Zhang F (2022) Association between maternal lipid levels during pregnancy and delivery of small for gestational age: A systematic review and meta-analysis. Front. Pediatr. 10:934505. doi: 10.3389/fped.2022.934505
Received: 02 May 2022; Accepted: 14 September 2022;
Published: 06 October 2022.
Edited by:
Sara De Carolis, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Kumar Chandan Srivastava, Al Jouf University, Saudi ArabiaCopyright © 2022 Wang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhang, emhhbmdmZW5nODIwOTA5QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.