
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 13 December 2022
Sec. Pediatric Immunology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.933266
This article is part of the Research TopicState of the Art in Immunopathologic Mechanisms Underlying Preterm Birth Pathways and Biomarkers for Prematurity PredictionView all 6 articles
 Martin Becker1,2,3,4
Martin Becker1,2,3,4 Jennifer Dai1,2,3
Jennifer Dai1,2,3 Alan L. Chang1,2,3
Alan L. Chang1,2,3 Dorien Feyaerts1
Dorien Feyaerts1 Ina A. Stelzer1
Ina A. Stelzer1 Miao Zhang1,2,3
Miao Zhang1,2,3 Eloise Berson1,2,5
Eloise Berson1,2,5 Geetha Saarunya1,2,3
Geetha Saarunya1,2,3 Davide De Francesco1,2,3
Davide De Francesco1,2,3 Camilo Espinosa1,2,3
Camilo Espinosa1,2,3 Yeasul Kim1,2,3
Yeasul Kim1,2,3 Ivana Marić1,2,3
Ivana Marić1,2,3 Samson Mataraso1,2,3
Samson Mataraso1,2,3 Seyedeh Neelufar Payrovnaziri1,2,3
Seyedeh Neelufar Payrovnaziri1,2,3 Thanaphong Phongpreecha1,3,5
Thanaphong Phongpreecha1,3,5 Neal G. Ravindra1,2,3
Neal G. Ravindra1,2,3 Sayane Shome1,2,3
Sayane Shome1,2,3 Yuqi Tan6,7
Yuqi Tan6,7 Melan Thuraiappah1,2,3
Melan Thuraiappah1,2,3 Lei Xue1,2,3
Lei Xue1,2,3 Jonathan A. Mayo2
Jonathan A. Mayo2 Cecele C. Quaintance2
Cecele C. Quaintance2 Ana Laborde2
Ana Laborde2 Lucy S. King8
Lucy S. King8 Firdaus S. Dhabhar9,10,11,12
Firdaus S. Dhabhar9,10,11,12 Ian H. Gotlib8
Ian H. Gotlib8 Ronald J. Wong3
Ronald J. Wong3 Martin S. Angst1
Martin S. Angst1 Gary M. Shaw2
Gary M. Shaw2 David K. Stevenson2
David K. Stevenson2 Brice Gaudilliere1,2
Brice Gaudilliere1,2 Nima Aghaeepour1,2,3*
Nima Aghaeepour1,2,3*
Psychosocial and stress-related factors (PSFs), defined as internal or external stimuli that induce biological changes, are potentially modifiable factors and accessible targets for interventions that are associated with adverse pregnancy outcomes (APOs). Although individual APOs have been shown to be connected to PSFs, they are biologically interconnected, relatively infrequent, and therefore challenging to model. In this context, multi-task machine learning (MML) is an ideal tool for exploring the interconnectedness of APOs on the one hand and building on joint combinatorial outcomes to increase predictive power on the other hand. Additionally, by integrating single cell immunological profiling of underlying biological processes, the effects of stress-based therapeutics may be measurable, facilitating the development of precision medicine approaches.
Objectives: The primary objectives were to jointly model multiple APOs and their connection to stress early in pregnancy, and to explore the underlying biology to guide development of accessible and measurable interventions.
Materials and Methods: In a prospective cohort study, PSFs were assessed during the first trimester with an extensive self-filled questionnaire for 200 women. We used MML to simultaneously model, and predict APOs (severe preeclampsia, superimposed preeclampsia, gestational diabetes and early gestational age) as well as several risk factors (BMI, diabetes, hypertension) for these patients based on PSFs. Strongly interrelated stressors were categorized to identify potential therapeutic targets. Furthermore, for a subset of 14 women, we modeled the connection of PSFs to the maternal immune system to APOs by building corresponding ML models based on an extensive single cell immune dataset generated by mass cytometry time of flight (CyTOF).
Results: Jointly modeling APOs in a MML setting significantly increased modeling capabilities and yielded a highly predictive integrated model of APOs underscoring their interconnectedness. Most APOs were associated with mental health, life stress, and perceived health risks. Biologically, stressors were associated with specific immune characteristics revolving around CD4/CD8 T cells. Immune characteristics predicted based on stress were in turn found to be associated with APOs.
Conclusions: Elucidating connections among stress, multiple APOs simultaneously, and immune characteristics has the potential to facilitate the implementation of ML-based, individualized, integrative models of pregnancy in clinical decision making. The modifiable nature of stressors may enable the development of accessible interventions, with success tracked through immune characteristics.
The adverse pregnancy outcomes (APOs) that we will focus on in this work - including early gestational age (GA) at delivery (a continuous surrogate for preterm birth), gestational diabetes, severe preeclampsia (severe pree), or preeclampsia superimposed on hypertension (superimposed pree) - are associated with severe short- and long-term consequences for both the mother and infant (1–4), underscoring the importance of management and prevention (5–9). Currently, however, highly efficacious therapeutic options to prevent APOs are lacking, highlighting the critical clinical need to identify actionable risk targets and prevent their development (10). In this context, epidemiological evidence has linked psychosocial and stress-related factors (PSFs) to APOs (11–16). Such PSFs are defined as internal or external stimuli that can induce biological changes (17) and are threats perceived by an individual that can result in psychopathological symptoms (18, 19). Importantly, the complexity of PSFs can be conveniently captured through questionnaires (11). In this work, we use the previously studied self-filled Dhabhar Quick-Assessment Questionnaire for Stress and Psychosocial Factors (DQAQ-SPF), that is designed to quantify commonly studied factors (perceived pregnancy risks, life stress, support) and factors not traditionally studied in the context of APOs (concerns about health, pain, and sleep) efficiently (12).
The connection between APOs and PSFs has been observed in multiple studies. For example, PSFs have been associated with spontaneous preterm birth (PTB), while protective psychological factors such as a robust support network decrease PTB risk (12, 20, 21). Similarly, hypertensive disorders of pregnancy are more likely to occur when women experience high levels of psychosocial stress, depression, and/or anxiety (11, 16, 22, 23). Moreover, recent studies have shown that chronic and financial stressors are strongly associated with gestational diabetes (24). In addition, maternal exposure to bereavement-related stress increases the risk of diabetes in a fetus and child later in life, thereby extending the adverse effects of PSFs to a child's well-being (13, 23). Given this strong connection between PSFs and APOs, PSFs are significant and accessible targets to determine risk for, clinically manage, and potentially prevent APOs.
Even though there is a strong connection between PSFs and APOs, PSFs are multifaceted, and their relations to individual APOs can be complex. This is further complicated by the difficulties of studying APOs in isolation, as they are tightly interrelated and integrated into an intricate biological environment. For instance, a common reason for preterm delivery is the development of preeclampsia early during pregnancy (21). In addition, gestational diabetes has been linked to increased risks of PTB and preeclampsia (25, 26). Furthermore, pre-pregnancy comorbidities and risk factors (RFs) create an unfavorable pathophysiological state during pregnancy that is associated with the development of APOs. For example, hypertensive disorders of pregnancy are correlated with a higher body mass index (BMI) and a history of diabetes (11, 27); similarly, gestational weight gain in women with gestational diabetes is associated with hypertensive disorders, preeclampsia, and PTB (26). The risk for PTB also increases in pregnancies with pre-existing systemic comorbidities such as diabetes or hypertension (28). This interconnectedness of APOs, as well as their relations with various risk factors, warrants the investigation of multiple APOs simultaneously on an integrated level, moving towards a systems approach for understanding the relations among pathophysiological risk factors (RFs; including diabetes, hypertension, and pre-pregnancy BMI), APOs, and PSFs.
In this work, we modeled the connection of PSFs to APOs and pathophysiological RFs (APORFs) jointly to identify key associations underlying the holistic nature of their interrelatedness. To this end, we used multitask machine learning (MML), a powerful method to model interrelated and comparably infrequent outcomes simultaneously (29–31). For this, we leveraged a dataset of 200 pregnant women with various APORFs, for which PSFs were assessed during the first trimester in an extensive questionnaire. We modeled and predicted APORFs for these patients based on PSFs using MML. For an overview of this experiment, see Figure 1, Model 1.
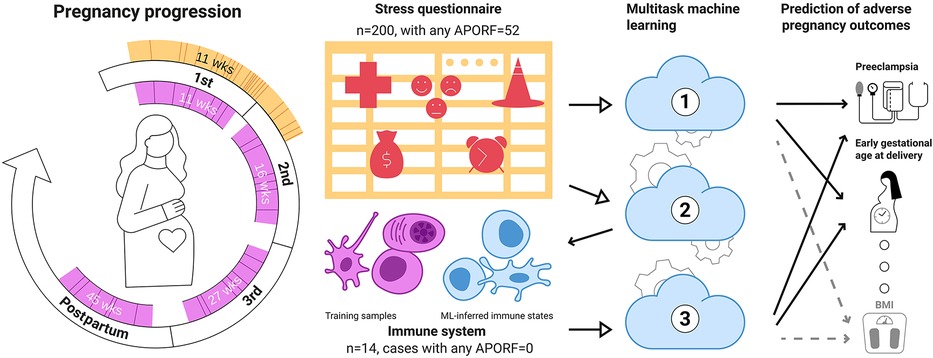
Figure 1. Multitask machine learning for the joint modeling of adverse pregnancy outcomes, stress, and biology. A stress questionnaire assessing psychosocial and stress-related factors (PSFs) related to perceived pregnancy risks, emotions and feelings, and life stress was administered during the first trimester (orange) to 200 women. 52 of these women experienced at least one adverse pregnancy outcome (solid lines) or pathophysiological risk factor (dashed lines). Distribution of gestational age at questionnaire completion. In most instances (>95%), the questionnaires were completed before 21 weeks of gestation. The median gestational age when completing the questionnaire was 11 weeks. In addition, patients were followed throughout pregnancy, and immunological data (designated in pink) were obtained during the first (median = 11 weeks), second (median = 16 weeks), and third trimester (median = 27 weeks), as well as at 6 weeks postpartum (median = 45 weeks). Model 1: Based on these data, a multitask approach was used to simultaneously model multiple adverse pregnancy outcomes (in bold) as well as risk factors (RFs; Normal font) based on PSFs, building upon their interrelatedness to improve predictions. Model 2: PSFs were used to predict immune system states (pink cells) illustrating a link between stress factors and biological functions. Finally (Model 3), connections among APOs, PSFs and the immune system were investigated by deriving immune states for our wider dataset from PSFs (blue cells) for predicting APOs. Overall, our results point towards a tight relationship between adverse pregnancy outcomes, stress and immune biology. Importantly, they suggest that several PSF categories can be jointly informative in modeling APOs pointing towards potential easily accessible stress-based interventions for APOs.
Furthermore, based on a smaller dataset, we analyzed the maternal circulating immune system (in peripheral blood) to explore the biological underpinning of identified associations with the aim of revealing potential targets for measurable and accessible interventions. In particular, for a subset of 14 women, we modeled the connection of PSFs to the maternal immune system by building corresponding machine learning (ML) models from an immune data set measured using mass cytometry time of flight (CyTOF). We used these models predicting immune characteristics from PSFs to clarify potential joint pathways underlying PSFs and immunobiology. This model was then used to generate missing immune system data for patients diagnosed with APOs and successfully predicted APORFs from these in-silico engineered immune system characteristics using MML, highlighting the links among stress, the immune system, and pregnancy. An overview of the corresponding experiments is illustrated in Figure 1 (Model 2 and 3).
Data was accumulated from the Prematurity Research Center Cohort (PRCC), established in 2011 at Stanford University Hospitals and Clinics. Women were enrolled prior to 12 weeks gestation. An extensive self-filled stress questionnaire, the Dhabhar Quick-Assessment Questionnaire for Stress and Psychosocial Factors (DQAQ-SPF) previously employed in the context of preterm birth (12), was provided during the first trimester of pregnancy to assess chronic stress, life events, emotions, personality, and sleep (Supplementary Tables S3, S4). In addition, perceived pregnancy risk was assessed with questions regarding concern for birth complications (“Some women may say that their risk for having birth complications like an early delivery, low birth weight or pregnancy complications (not birth defects) is low, average, or high. Think about yourself compared to most other pregnant women your age.”) and birth defects (similar to previous question). Health-related questions were based on the Medical Outcomes Study 36-Item Short-Form Health Survey, administered during the enrollment visit, at a median of 11 weeks gestation. Participants rated items on an eight-point scale or provided “yes/no” answers regarding specific life experiences; they were allowed to answer “not applicable” or skip questions. Demographic data collected included race/ethnicity, education, and age (also see Supplementary Table S1).
79 stressors, including binary, categorical, ordinal, and numeric variables, were selected for prediction. First, we removed all non-numeric variables. Then, we added certain text-based variables by parsing and converting them to numbers, including, for example, “preferred bedtime.” We then removed features with only one unique and/or missing values. Life stress from 41 to 50 (“How stressful was your life between the ages of 41–50?”) was dropped from our analysis, as our entire patient population was younger than 41. The initial cohort had 283 patients; however, 83 patients without preeclampsia information were removed from our dataset. Analysis was based on the remaining 200 pregnant women; 18 with hypertension, 11 with diabetes, 29 with gestational diabetes, 19 with preeclampsia (6 superimposed, 13 severe), 17 preterm births; 52 women had one or more of these APOs (Supplementary Table S1, Supplementary Figure S3). All maternal adverse pregnancy outcomes were obtained from electronic medical records; coordinators reviewed medical charts of all enrollees for the outcomes of interest.
The analysis leveraged existing immunological data collected in a prospective study, immune data was collected from pregnant women seen at the obstetrics clinics of the Lucile Packard Children's Hospital at Stanford University for prenatal care (32). Women were included if they were at least 18 years of age and in their first trimester of a singleton pregnancy. Four peripheral blood samples were collected - during the first trimester (7 to 14 weeks), second trimester (15 to 20 weeks), third trimester (24 to 32 weeks), and 6-week postpartum. Samples were analyzed using mass cytometry time of flight (CyTOF) for single-cell characterization of the immune system (32). A total of 984 immune features were extracted from each whole blood sample using a 41-parameter assay (antibody panel), including the frequencies of 24 major innate and adaptive immune cell subsets, their endogenous intracellular signaling activities (phosphorylated = activated signaling proteins of the MyD88, Jak/STAT, mTOR, and NFkB signaling pathways), and the signaling capacities of each cell subset to respond to a series of receptor-specific immune challenges [15-min stimulation with lipopolysaccharide (LPS), interferon-a (IFN-α), and a cocktail of interleukins (IL-2, IL-6)]. All timepoints of a patient were analyzed simultaneously to minimize batch effects.
The employed multitask approach builds upon the interconnectedness of APOs (Figure 2) and simultaneously models highly related risk factors (RFs) to further exploit joint combinatorial outcomes for increased power. Multitask neural networks and single task approaches were compared across the prediction of gestational diabetes, severe preeclampsia, superimposed preeclampsia, and GA at delivery, as well as diabetes, hypertension, and BMI prior to pregnancy. GA at delivery and BMI prior to pregnancy were numeric variables; the rest were binary. The only mild case of superimposed preeclampsia was removed because mild and severe preeclampsia differ in risk and management. Preeclampsia with and without underlying hypertension were treated as two binary variables due to different underlying pathology. The multitask neural network architecture consisted of a feature embedding layer followed by two fully connected hidden layers of 150 hidden units each that then fed into outcome-specific modules of 150 hidden units each that terminated in separate regression or classification heads. Batch normalization and Rectified Linear Unit (ReLU) activation were employed at each hidden layer. Deep learning models were trained for a maximum of 35 epochs with early stopping with a patience configuration of 1 epoch. The classification criterion used for loss was binary cross-entropy loss, and mean squared error was used for regression outputs (scaled such that numerical values did not overpower the binary cross-entropy loss). Deep learning models were trained using the Adam optimizer with a batch size equivalent to the length of the training set. The single task neural network had the same architecture as the multitask network except for a single regression or classification output. Both single task and multitask deep learning models were trained using repeated stratified K-Fold splitting with 5 folds to evaluate model variance. For Figure 3, the area under the receiver operator characteristic (AUROC) was used to measure model performance of binary variables, and Spearman's rank correlation coefficient (rho) was used to measure model performance of numeric variables. Model performance was evaluated on the test set predictions collected over the repeated K fold procedure. The Wilcoxon test was used to classify the level of significance of single task vs. multitask models.
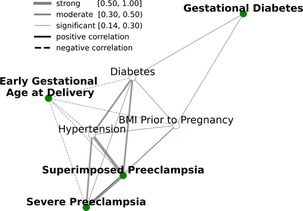
Figure 2. Relationships between APORFs during the first trimester. Green nodes represent APOs while white nodes represent RFs present before conception. Edges represent the strength of the Spearman correlation between two outcomes, with green representing strong, orange representing moderate, and grey representing weak correlations, as defined in Akoglu 2018. “Pree” is short for preeclampsia. APORFs are strongly correlated with one another, laying the foundation to jointly model these outcomes in a MML setting for improved predictive performance.
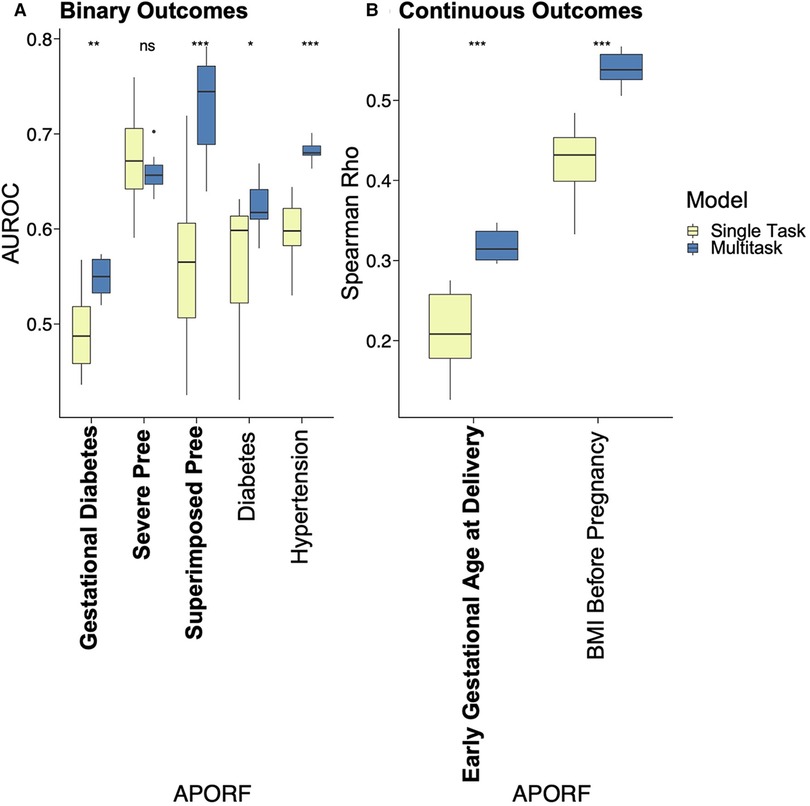
Figure 3. Performance comparison of single task vs. MML for predictive models of APOs. APOs are bolded, and pathophysiological RFs are unbolded. Results of multitask models are blue, and single task models are yellow. Asterisks represent significant differences by the Wilcoxon test between single task and multitask approaches (ns: p > 0.05, *p <= 0.05, **p <= 0.01, ***p <= 0.001, and ****p <= 0.0001). “Pree” is an abbreviation for preeclampsia. With one exception where no significant prediction differences are recorded, multitask models significantly outperformed single task models in all cases, emphasizing the interrelatedness between APORFs and successful joint modeling.
An interdependency network of PSFs was created to understand their interrelatedness (Supplementary Figure S5). For this, a correlation matrix between all these factors was calculated using Spearman correlation (omitting undefined values). Analogously to previous work (12), factors were then grouped into 15 clusters using the K-Means algorithm on the corresponding correlation vectors. A two-dimensional embedding for placing each factor was calculated using the t-distributed stochastic neighbor embedding (33) on the absolute values of the correlation vectors associated with each factor. These clusters of psychosocial and stress factors were then analyzed in the context of their impact on APOs.
For the association of APORFs with PSFs in Figure 4, the Spearman's rho was calculated to determine correlation, and the corresponding p-value (p < 0.05) was used to determine significance. Only clusters that contained significantly predictive PSFs, as determined by p-value were included in Figure 4. To determine the most promising clusters for potential targeted interventions, the number of APORFs significantly associated with each PSF cluster was visualized (Figure 4). An APORF was considered to be significantly associated with a cluster if any PSF in the cluster was significantly associated with the APORF. A heatmap that further investigates the percentage of each PSF cluster correlated with APORFs was created (Figure 4). The percentages of significant individual PSFs normalized by the total PSFs in each category were visualized as numbers within the heatmap. The number of features per cluster is depicted in parentheses following PSF x-axis labels, and the number of significant categories related to each APORF is in parentheses following APORF y-axis labels.
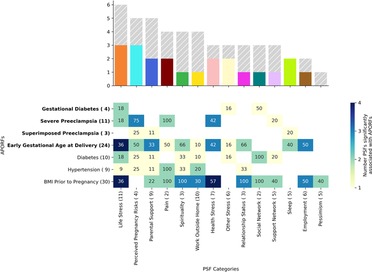
Figure 4. APORFs associated with categories of PSFs. The bar plot depicts the number of APORFs that are associated with each stress category (Supplementary Figure S4). Numbers adjacent to each APORF depict the number of categories significantly associated with each APORF. The height of the colored bar depicts the number of APOs associated with the PSF category, while the height of the gray bar depicts the number of RFs associated with the PSF category. The heatmap depicts the specific APORFs correlated with the PSF cluster, with darker colors representing a greater number of features from the cluster correlated with the outcome. The numbers in the heatmap represent the percentage of features from the cluster that was significant for the APORFs. The number of features in each cluster is stated in parentheses after each x-axis label. The life stress category was connected to the highest number of APORFs.
Next, we investigated the relationship between individual (within each cluster) PSFs and APORFs. Individual PSFs were significantly correlated with APORFs if p < 0.05 for Spearman correlations calculated. Individual PSFs, only included if they were significantly correlated with at least one APORF, were plotted based on the number of APORFs they were significantly correlated with (Figure 5). Individual PSFs, sorted based on the highest number of APORFs they were correlated with, were colored by their original clusters. Heatmaps depict the Spearman correlation value of each PSF with each APORF, with only PSFs with p < 0.05 included (Figure 5).
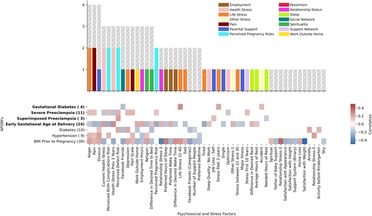
Figure 5. Number of APORFs significantly correlated with individual stress factors. The bar plot depicts PSFs, colored by cluster (cf. Supplementary Figure S4). Height of the bars represents the number of APORFs for which the PSF was significantly associated with APOs (colored bars) and RFs (gray bars). Hours of rest, why women thought they may experience birth defects (“perceived reason for defect”), and pain were correlated with the largest number of APORFs. The heatmap depicts Spearman correlation of PSFs with APORFs. Grey heatmap squares represent correlations of PSFs with APORFs that were not significant (significance was denoted by p < 0.05).
In a smaller subset of 14 patients who had participated in both studies, the same 79 features from the stress questionnaire from above were used to predict 534 single cell immune characteristics, i.e, measured single cells for each patient that were aggregated into cell frequencies and stimulus responses. A time point representation, a sequential integer from 1 through 4 representing the first, second, and third trimesters, and postpartum, was added to incorporate information about when immune cell measurements were taken. Ridge regression with grid search cross validation was used for prediction. The grid search was used to find the best alpha values (1, 0.1, 0.01, 0.001, 0.0001, 0) within each test fold. Feature values were scaled to unit variance. 10-fold cross validation was performed, for which a shuffled group K-fold was implemented to split patients for the test sets in order to keep data from the four time points for each patient within the same fold. The procedure was repeated 50 times. Spearman's rho was evaluated across all samples for each repetition, and a mean was taken for p-values and correlations for each immune feature. Benjamini-Hochberg False Discovery Rate Correction (alpha = 0.05) was then used on Spearman p-values. The results are visualized in Figure 6. Each node represents an immune feature. Their location is based on a two-dimensional embedding calculated by employing the t-distributed stochastic neighbor embedding (33) on the absolute values of the correlation vectors associated with each feature.
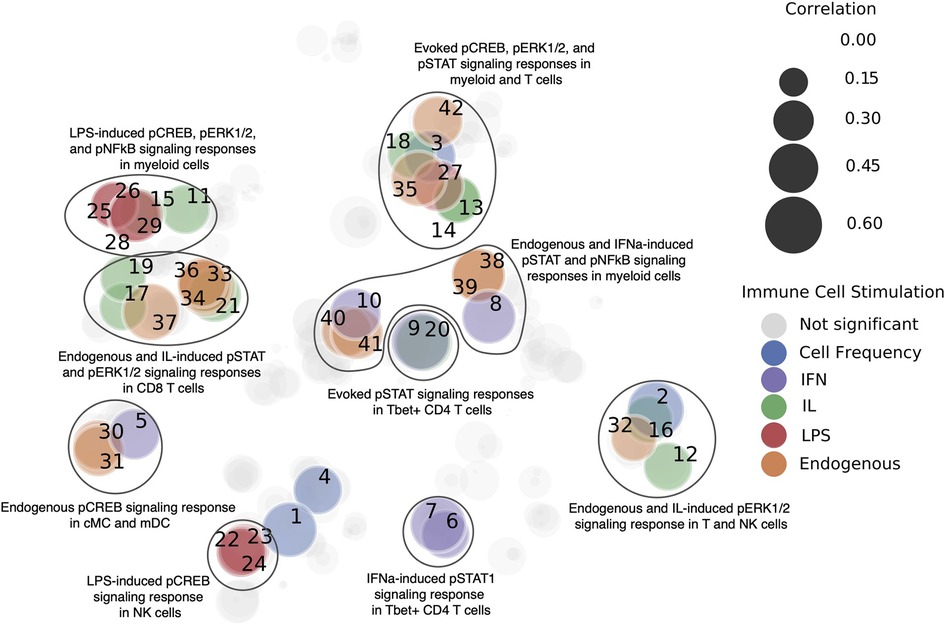
Figure 6. Immune features that are predictable from PSFs. Immune features were clustered by immune cell stimulation (designated by color), and models were trained to predict these features. Significant immune characteristics (p < 0.05 after correction via Benjamini-Hochberg false discovery rate) as predicted from the 79 original PSFs are colored. Prediction quality measured via Spearman correlation is represented by the size of the nodes. Each immune feature is labeled with a number, which maps to Supplementary Table S2. This analysis indicated that stress factors can predict components of the immune system, which paves the way for an integrative immune and stress model of pregnancy.
As only 14 patients in the cohort had data that included immune features, we generated immune features for the rest of 186 patients. These in-silico generated immune features were then used to predict APORFs. The 14 patients with immune system features were excluded from subsequent analyses to prevent bias. The immune features were generated by employing the coefficients from the ridge regression model used in the experiment for predicting immune features from PSFs. In particular, new coefficients were derived by taking the mean of the coefficients over all runs of the algorithm. Based on these coefficients a new linear model was used to predict immune cell characteristics from stress. For predicting APOs from the generated immune features, the same neural network architecture for the multitask and single task approaches in the prediction of APOs from stress was used; the generated immune features were the input into the neural network, and the output predicted was APOs.
Analyses are based on our cohort of 200 pregnant women, 52 of whom experienced at least one APO during their pregnancy. Supplementary Figures S1, S2, and Supplementary Table S1 describe the demographics of the pregnant women, the distribution of BMI, and GA at delivery. Figure 2 represents the interconnectedness of APOs and RFs in our dataset based on Spearman correlation, confirming known relationships between APORFs established in prior studies (26, 34–37). There are biological underpinnings to these correlations; for instance, prior studies found that TGF-β is a common biological factor between preeclampsia and diabetes (38, 39). Diabetes was moderately correlated with hypertension and superimposed preeclampsia (40), and significantly correlated with every other APORF. Similarly, BMI prior to pregnancy was also significantly correlated with all outcomes (41). Finally, diabetes, hypertensions, superimposed and severed preeclampsia form a tightly connected cluster. These results highlight significant connections of APOs and RFs, suggesting that joint modeling may capture plausible underlying biological processes.
We applied MML to jointly model APOs together with RFs based on psychosocial and stress-related factors (PSFs). This not only leverages the previously mentioned interrelatedness of APORFs, but also increases the number of cases from which the model can learn. The latter supports the training process as ML models perform better with more balanced numbers of training samples for cases and controls. Case counts of the individual APOs and a comparison to the joint case count in a multitask setting are illustrated in Supplementary Figure S3. Consequently, multitask learning significantly improved the joint prediction of nearly all APOs compared to single task settings, which only consider one APO or RF at a time (Figure 3). Only for preeclampsia the model performance was consistent between the single and multitask approach. This further illustrates how multitask learning depends on the interrelatedness of the predicted outcomes, as preeclampsia was only marginally related to other APOs and RFs (cf. Figure 2). On the other hand, preeclampsia superimposed on preexisting hypertension (“Superimposed Pree”), which is strongly connected to the other modeled outcomes, benefited the most from the multitask approach. The ability of MML to improve prediction of APOs confirms their interconnectedness (∼50% were moderate or strong correlations). It also motivates the following study of stress factor categories that may help to improve the prevention of several APOs simultaneously.
Our previous analysis modeled the complex connections between an extensive set of PSFs and APORFs (see Supplementary Figure S4 for a visualization of significant PSFs related to each APORF). To understand this connection in more detail, we identify specific categories of PSFs and their connections to the studied APORFs using a clustering approach. Analogously to previous work (12), PSFs were clustered according to their correlation profiles (see Supplementary Figure S5 for a visualization of the categories). Categories such as perceived pregnancy risks, mental health (“life stress”), support network, and self-rated health (“health stress”), pain, and tiredness/fatigue (“sleep”) emerged as clusters. For example, the cluster related to “life stress” included: depression, happiness, anger, sadness, perceived loneliness, and stress in the past year. The clusters related to “perceived pregnancy risks” included perceived birth defects, perceived birth complications, and perceived pregnancy risk.
To understand the general association of these PSF categories with APORFs, we investigated how each PSF category and each individual PSF are associated with the studied APORFs (see Figures 4, 5, and Supplementary Figure S4). When quantifying the number of APORFs associated with any PSF in each PSF category (Figure 4), we observed that “life stress”, and “perceived pregnancy risks” categories were associated with most APOs (three out of four; see heatmap in Figure 4). These two categories were furthermore connected to several RFs (gray bars in Figure 4), with “life stress” covering all APORFs except superimposed preeclampsia on hypertension. Figure 4 shows a more detailed analysis regarding the number of PSFs in each category connected to APORFs.
We further investigated individual PSFs and their association with APORFs. For this, we visualized the number of APORFs significantly correlated with individual stressors (Figure 5). Here, when examining the individual PSFs in each of the PSF categories and their connection to APOs (see Figure 5, colored bars), PSFs from the “life stress” category that displayed significant correlations with APOs included depression and anger (associated with severe preeclampsia) as well as sadness and tiredness (associated with early gestational age at delivery). The cluster, “health stress,” included significant associations of lack of optimism with severe preeclampsia, as well as worries about health within the last three years (“health stress prev 3 years”) and stress around current health (“current health stress”) correlated with severe preeclampsia and gestational age at delivery. Specific “perceived pregnancy risks” correlated with APOs included worries about birth complications (“perceived birth complications risk”), defects (“perceived defect risk” and “perceived reason for defect”), and the pregnancy itself (“perceived pregnancy risk”) associated with one or two APOs each, including severe and superimposed preeclampsia and GA at delivery.
When investigating the detailed relationships between APOs and individual PSFs (see Figure 5), PSFs were frequently correlated with severe preeclampsia and GA at delivery. For example, pain, perceived birth complications risk, current health stress, and perceived pregnancy risk were significantly associated with both APOs simultaneously. Also, perceived defect risk co-occurred with severe and superimposed preeclampsia. These observations may point towards interventions that target the prevention of multiple APOs at a time. Along the same lines, interventions targeting life stressors as well as other PSF categories with PSFs not associated with several APOs simultaneously (see Figures 4, 5) can be promising targets, as their interrelated nature (Supplementary Figure S5) may provide a particularly large impact in the context of a targeted therapeutic intervention. Beyond the PSFs implicated by the cluster analysis above, PSFs like pain, divorce, or employment hours exhibited high numbers of relationships to APOs and RFs. While many APOs and RFs have strong connections to PSFs, individual connections with gestational diabetes and superimposed preeclampsia were limited.
Overall, these observations point to multiple, joint as well as individual PSFs, that are deeply intertwined with APORFs. For APOs, this focuses on severe preeclampsia as well as GA at delivery, with gestational diabetes and severe preeclampsia seemingly less connected with stress factors. These results introduce the potential for stress-based interventions targeting individual PSFs as well as PSF categories and suggest shared underlying biological pathways.
Although multifactorial, the biological mechanisms underlying observed associations between stressors and APOs likely implicate immunological factors. It is known that stress affects immune function (42), while multiple studies suggest that dysfunctional maternal immune adaptations to the developing fetus are critical to the pathogenesis of APOs (43–45). To investigate the relationship of the maternal immune system to PSFs, we employed a predictive modeling ML approach (single task). For a cohort of 200 pregnant women, PSFs were assessed during the first trimester while immune changes were captured for 14 pregnant women (a subset of the initial 200 pregnant women) over the course of pregnancy using a panel of antibodies for CyTOF. Models built using the PSF data predicted several immune features, including endogenous and stimulated signaling responses in both innate and adaptive immune cells (as measured by the abundance of phosphorylated intracellular signaling proteins representative for activated MyD88, Jak/STAT, mTOR, and NFkB pathways), and the relative abundance (frequency) of innate immune cells among all immune cells (Figure 6, Supplementary Table S2). The top 10 most predictable immune cell characteristics were dominated by activated signaling responses in CD4 + and CD8 + T cell populations; endogenous pERK in γδ T cells and native CD8+ T cells, and JAK/STAT signaling response to interleukins (IL-2, IL 6) or IFNα stimulation in naive Tbet + CD4 + and CD8 + naive T cells. Interestingly, mainly JAK/STAT (pSTAT1, pSTAT3, pSTAT5), MyD88 (pERK), and pCREB signaling responses in innate and adaptive immune cells were predicted from the PSFs, while only 6 out the 42 predictable characteristics (for which the Benjamini-Hochberg corrected p-value < 0.05) were part of the mTOR (prpS6) and NFκB signaling pathway. In addition, PSFs were also able to predict the frequency of classical monocytes, CD16neg NK cells, granulocytes, and naive Tbet + CD8 T cells. CREB can be activated (phosphorylated) downstream of a signaling pathway resulting from the binding of serotonin and noradrenaline to post-synaptic G-protein coupled receptors. Dysfunction of these neurotransmitters is implicated in major depressive disorder (46). Overall, we observe distinctive connections between PSFs and immune cell characteristics, which points towards the existence of an underlying biological pathway that may be directly connected to stress. This may for example enable the quantification of the effect of stress-related interventions to target APOs.
Because the previous experiment showed a connection between stress and immune system characteristics, we further investigated whether these biological signals were connected to APOs. As immune system data were available only for 14 healthy patients, we inferred the immune system state with our trained models for all patients at each timepoint in the remainder of our dataset, including patients with APOs. Here, we used the previously trained models, which successfully predicted a subset of immune system states from the original PSFs, and input PSFs for the remaining 186 women to in-silico generate their immune system states (Figure 6). Based on these generated immune system states, we applied single task as well as MML approaches analogous to our previous setup (multitask prediction of APOs) to successfully predict APOs from immune profiles (Figure 7). This not only confirms the connection of these biological factors to APOs but also points towards a direct pathway connecting stress, the immune system, and APOs.
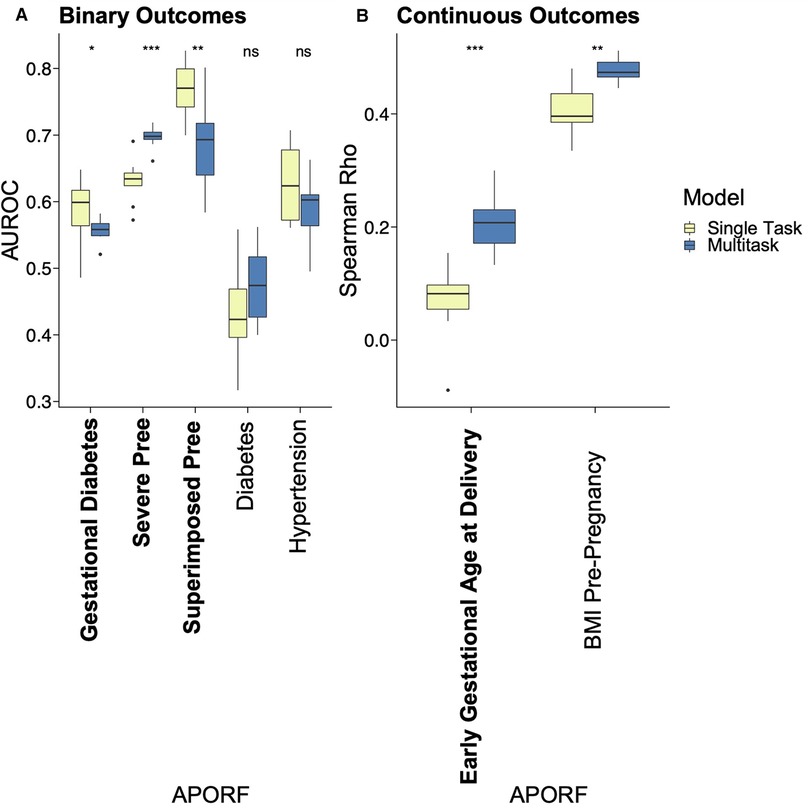
Figure 7. Multitask and single task machine learning analysis for the prediction of APOs based on generated immune features. APOs are bolded, and pathophysiological RFs are unbolded. Results of multitask models are blue, and single task models are yellow. Prediction of APOs was successful with generated immune features, confirming their biological relationship. Asterisks represent significant differences calculated by the Wilcoxon rank-sum test between single task and multitask approaches, with an increasing number of asterisks representing increased significance. Thresholds for significance include - ns: p > 0.05, *p <= 0.05, **p <= 0.01, ***p <= 0.001, and ****p <= 0.0001.
The relationship between stressors and APOs, along with the modifiable nature of stressors, introduces the possibility for actionable interventions. In prior studies, specific factors such as psychosocial stress, bereavement, and anxiety were correlated with individual APOs (11, 13, 47). Here, we aimed to simultaneously and comprehensively quantify the contribution of a multitude of stressors to understand those that contribute most to APOs. To this end, we used the extensive DQAQ-SPF questionnaire, which quantified psychosocial and stress factors and allowed individuals to rate the relative contributions of these stressors. In a previous study based on the same questionnaire (12), higher levels of deleterious factors were associated with PTB. These promising results, combined with the interrelatedness of APOs, suggested that a similar set of deleterious or protective factors could jointly affect multiple APOs simultaneously as reported in our study. The DQAQ-SPF allowed us to compare the impact of rarely studied stressors between and within categories of stressors (e.g., life stress, perceived pregnancy risks, health stress), as well as quantify their impact on each APO. Ultimately, this outlined approach could lay the foundation for developing targeted interventions based on readily accessible stress and biological parameters.
The association of life stress with GA at delivery, severe preeclampsia, and gestational diabetes, and the correlation of worries about health (“health stress”) with preeclampsia and GA at delivery, as found in the studied population, may point towards specific psychometric targets to alleviate, or prevent APOs in a joint manner. Additionally, the observed high correlation of APOs with health concerns and perceived pregnancy risks highlights the impact that negative perception can have on the health of the mother and fetus. This may enable preventive approaches such as in-depth counseling by providers in addition to pharmacological treatments to clarify and assuage these worries. Support and social networks were both correlated with GA at delivery. These results are consistent with current literature, as life stress, including feelings of guilt, loss of control, and fear, as well as pregnancy perception, body image, marital status, and work have been correlated with pregnancy complications or loss (48, 49). Support and social networks have additionally been shown as crucial to successful management of chronic disease (50). Prior studies corroborated that life stress, health stress, and perceived pregnancy risks were related to APOs, lending credence to the benefits of targeting implicated stressors.
One of the main contributions of our work is to model multiple APOs simultaneously to better capture their biological interconnectedness. Although APOs are comparably rare, and thus challenging to model, their biological relationship and frequent presence as comorbidities provide an avenue for joint modeling approaches like MML to increase predictive power. As mentioned in the introduction, hypertension during pregnancy is correlated with diabetes and BMI; gestational diabetes is connected to hypertensive disorders, preeclampsia, and PTB; and prior preeclampsia is tied to an increased risk of hypertension and diabetes (26, 27, 34). As shown by our results, MML successfully models APOs simultaneously, outperforming single task approaches. While the overall performance metrics still need to be improved for practical applications, the superior performance of MML emphasizes the interconnectedness of APOs and illustrates the potential of building on joint combinatorial outcomes to significantly increase predictive power. This motivates further research in the joint study of the corresponding biological systems in a holistic manner and may lead to an integrated and actionable understanding of APOs.
Decades of research have shown that a pregnancy success relies on finely orchestrated immunological cross-talk between the mother and the fetus, with pregnancy complications being associated with feto-maternal immune dysfunction (43–45). In addition, exposing mice to stress has been shown to suppress their immune system (51), while immune dysregulation could also be observed in humans under chronic stress exposure (42). This connection between the immune system, stress, and APOs introduces the possibility of implementing targetable and measurable stress-based interventions. In the study presented here, stressors were able to predict several immune features in a cohort of pregnant women suggesting a potential link between stress and APOs through immune system factors. Interestingly, pSTAT5 signaling responses in Tbet + CD4 T cells were predictable from stressors, and the immune model used was successfully able to predict adverse pregnancy outcomes, which corroborates prior work that has shown pSTAT5 signaling in Tbet + CD4 T cells can classify preeclamptic pregnancies from controls (43), and pSTAT5 signal in CD4 T cells can predict GA (32). Overall, the predicted features spanned innate and adaptive immune populations and their major signaling cascades, suggesting common neuroendocrine factors which affect immunity via globally expressed receptors, and thus mediate an immune-system-wide stress response. This general stress adaptation might be most efficiently therapeutically targeted by intervening with the stressors itself. Such interventions could include mindfulness-based stress reduction protocols, which have shown promise in reducing stress in pregnant (52, 53) and non-pregnant women (54), with a recent meta-analysis demonstrating their beneficial post-interventional impact on immunity-related biomarkers in non-pregnant participants (55). Pregnancy-adapted protocols continue to be subject of clinical trials (56, 57).
Moreover, to investigate the potential for connections among stress, the immune system, and APOs, stressors were used to in-silico generate immune data for a part of our patient cohort. These generated immune features successfully predicted not only GA, but other APOs as well, confirming the relationship between stressors, immunity, and APOs (Figure 7). This shows that a model for predicting immune states from stress profiles (PSFs) that is solely trained on “healthy” pregnancies can still capture immune state characteristics of abnormal pregnancies (related to APOs). This indicates that pregnancies and particularly their immune profiles are on a spectrum (or likely, multiple spectrums) in contrast to belonging to a discrete class like “healthy” and “abnormal”. Overall, this points towards a joint pathway and may enable possibilities for the assessment of stress-based interventions to improve APOs.
The predictive stressors elucidated in this study may provide the foundation for further studies to devise treatments that improve the outcome of multiple APOs simultaneously. The observed connection of life stressors and APOs may introduce possibilities for interventions that can be combined with pharmaceuticals for the best outcomes. This may have particularly strong impact on APOs that are difficult to treat, like preeclampsia (10). For example, though in some cases medications are indicated to treat symptoms of preeclampsia (10), currently the most effective treatment is delivery. However, delivery may result in PTB, which is correlated with negative health outcomes for the mother and the fetus. Therefore, treatments based on targeting stress may have the potential for effective noninvasive interventions.
The potential for noninvasive therapies that target stressors implicated in this study is tremendous. Randomized trials have shown that mind-body techniques and other nonpharmaceutical interventions including tai chi, guided imagery, progressive muscle relaxation, and yoga, have reduced mood symptoms during pregnancy (58). Noninvasive techniques such as massage during pregnancy and postpartum have also been valuable in improving mood, decreasing perception of pain during labor (59). In addition, the strong correlation of perception with APOs may point towards the possibility that altering perception could improve outcomes. As accumulation of evidence improves perception, reassurance from care teams could prove helpful (60). Additionally, further education has improved self-efficacy and decreased severity of illness perceptions in conditions such as type II diabetes, and similar techniques could be applied in women at high risk from pregnancy complications (61). Such techniques may further synergize with pharmaceuticals to target significantly implicated stressors to decrease the risk of multiple APOs.
The results in our study point towards a joint pathway connecting stressors, biology, and APOs, that may allow for preventing APOs by targeting stressors and measuring progress through biological assays. In this context, our MML model as well as the generative approach for deriving immune system measurements, compensates for relatively small numbers of APOs. However, particularly for predicting APOs from immune system features the severely limited training data (regarding sample size as well as its focus on healthy samples) significantly reduced the advantages of the MML approach. Additionally, while building models on generated data in this study, and employing a rigorous repeated cross-validation scheme, allowed for first insights into the connection between multiple APOs, the immune system, and PSFs, further research requires explicitly measured immune features to validate the observations from our analysis. Thus, in future work, additional data, especially with respect to biological measurements and more APO samples, will help strengthen our findings. In addition, self-reported data generally introduces bias, as participants may alter answers based on experiences or judgment (62) and will need further investigation in the context of our study particularly with regards to background information available to the patient; for instance, the amount of information a patient has about their medical history may vary. Extended studies with larger cohorts and specifically designed questions may enable investigating the causal structure of the observed effects and account for the confounding factors inherent to self-assessed stress related questions, particularly in the context of the complex interconnected nature of APOs and RFs.
Elucidating connections among stress, multiple APOs simultaneously, and immune characteristics using ML-based models are initial steps to facilitate the implementation of individualized, integrative models of pregnancy in clinical decision making, with a particular focus on the diagnosis and prevention of APOs. The success of the employed multitask approach confirms the interrelatedness of APOs and the potential for interventions that simultaneously target multiple APOs. Furthermore, the connection between stress and the immune system, where generated immune features were also correlated with APOs, elucidates an underlying biological link. While our study only represents a first foray into this promising area of research, the wide applicability of identifying impactful stressors with questionnaires and their modifiable nature may ultimately allow for accessible interventions, with success potentially measured biologically through immune characteristics.
Code and data for reproduction of results in this paper can be downloaded from the following link: https://nalab.stanford.edu/pregnancy-stress-multitask/. Stress related questions (except for birth “complications” and “defects”) are from the DQAQ-SPF Questionnaire, copyright (2020) Firdaus S. Dhabhar and the University of Miami, jointly with Stanford University. All rights reserved. This questionnaire may not be used fully or partially, reproduced, displayed, modified, or distributed without the express prior written permission from Dr. Dhabhar (dhabhar@gmail.com). Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board, Stanford. The patients/participants provided their written informed consent to participate in this study.
MB, ALC, NA contributed to conception and design of the study. FSD designed the Dhabhar Quick-Assessment Questionnaire for Stress and Psychosocial Factors (DQAQ-SPF) and integrated it into this project. MB, JD, ALC, JAM organized the database. MB, JD, ALC performed the statistical and machine learning analysis. MB, JD, ALC, IAS, DF, MZ wrote the first draft of the manuscript and sections of the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the NIH (R35GM138353, R01HD105256, P01HD106414, 1R01HL139844, 19PABHI34580007, R61NS114926, R01AG058417, P30AG066515), American Heart Association (19PABHI34580007), BMBF (01IS22077), Burroughs Wellcome Fund (1019816), the March of Dimes, the Robertson Foundation, the Alfred E. Man Foundation, and the Bill and Melinda Gates Foundation (INV-001734, OPP1113682, INV-003225).
We thank Anastasia Yarmolinskaya for her contribution to the illustration of Figure 1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.933266/full#supplementary-material.
APO, Adverse Pregnancy Outcome; RF, Risk Factor; APORFs, Adverse Pregnancy Outcomes and Risk Factors; ML, Machine Learning; MML, Multi-task Machine Learning.
1. Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med. (2008) 359:262–73. doi: 10.1056/NEJMoa0706475
2. Ward RM, Beachy JC. Neonatal complications following preterm birth. BJOG Int J Obstet Gynaecol. (2003) 110(Suppl 20):8–16. doi: 10.1016/s1470-0328(03)00012-0
3. Bokslag A, van Weissenbruch M, Mol BW, de Groot CJM. Preeclampsia; short and long-term consequences for mother and neonate. Early Hum Dev. (2016) 102:47–50. doi: 10.1016/j.earlhumdev.2016.09.007
4. Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. (2016) 59:1396–9. doi: 10.1007/s00125-016-3985-5
5. Grabowska K, Stapińska-Syniec A, Saletra A, Jarmużek P, Bomba-Opoń D. Labour in women with gestational diabetes mellitus. Ginekol Pol. (2017) 88:81–6. doi: 10.5603/GP.a2017.0016
6. Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. (2005) 70:134–42. doi: 10.1016/j.diabres.2005.03.022
7. Steiner JM, Lokken E, Bayley E, Pechan J, Curtin A, Buber J, et al. Cardiac and pregnancy outcomes of pregnant patients with congenital heart disease according to risk classification system. Am J Cardiol. (2021) 161:95–101. doi: 10.1016/j.amjcard.2021.08.037
8. FitzGerald TL, Cameron KL, Albesher RA, Mentiplay BF, Lee KJ, Clark RA, et al. Strength, motor skills, and physical activity in preschool-aged children born either at less than 30 weeks of gestation or at term. Phys Ther. (2021) 101:pzab037. doi: 10.1093/ptj/pzab037
9. Toijonen A, Heinonen S, Gissler M, Seikku L, Macharey G. Impact of fetal presentation on neurodevelopmental outcome in a trial of preterm vaginal delivery: a nationwide, population-based record linkage study. Arch Gynecol Obstet. (2022) 306:29–35. doi: 10.1007/s00404-021-06146-z
10. Uzan J, Carbonnel M, Piconne O, Asmar R, Ayoubi J-M. Pre-eclampsia: pathophysiology, diagnosis, and management. Vasc Health Risk Manag. (2011) 7:467–74. doi: 10.2147/VHRM.S20181
11. Caplan M, Keenan-Devlin LS, Freedman A, Grobman W, Wadhwa PD, Buss C, et al. Lifetime psychosocial stress exposure associated with hypertensive disorders of pregnancy. Am J Perinatol. (2021) 38:1412–9. doi: 10.1055/s-0040-1713368
12. Becker M, Mayo JA, Phogat NK, Quaintance CC, Laborde A, King L, et al. Deleterious and protective psychosocial and stress-related factors predict risk of spontaneous preterm birth. Am J Perinatol. (2021). doi: 10.1055/s-0041-1729162. https://www.thieme-connect.com/products/ejournals/ris/10.1055/s-0041-1729162/BIB
13. Virk J, Li J, Vestergaard M, Obel C, Kristensen JK, Olsen J. Prenatal exposure to bereavement and type-2 diabetes: a Danish longitudinal population based study. PloS One. (2012) 7:e43508. doi: 10.1371/journal.pone.0043508
14. Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci U S A. (2006) 103:3938–42. doi: 10.1073/pnas.0511183103
15. Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. (2003) 157:14–24. doi: 10.1093/aje/kwf176
16. Yu Y, Zhang S, Wang G, Hong X, Mallow EB, Walker SO, et al. The combined association of psychosocial stress and chronic hypertension with preeclampsia. Am J Obstet Gynecol. (2013) 209:438.e1–438.e12. doi: 10.1016/j.ajog.2013.07.003
17. Yaribeygi H, Panahi Y, Sahraei H, Johnston TP, Sahebkar A. The impact of stress on body function: a review. EXCLI J. ISSN 1611-2156 (2017) 16:1057. doi: 10.17179/EXCLI2017-480
18. Zahn R, Lythe KE, Gethin JA, Green S, Deakin JFW, Young AH, et al. The role of self-blame and worthlessness in the psychopathology of major depressive disorder. J Affect Disord. (2015) 186:337–41. doi: 10.1016/j.jad.2015.08.001
19. Schneider EE, Schönfelder S, Domke-Wolf M, Wessa M. Measuring stress in clinical and nonclinical subjects using a German adaptation of the perceived stress scale. Int J Clin Health Psychol. (2020) 20:173–81. doi: 10.1016/j.ijchp.2020.03.004
20. Traylor CS, Johnson JD, Kimmel MC, Manuck TA. Effects of psychological stress on adverse pregnancy outcomes and nonpharmacologic approaches for reduction: an expert review. Am J Obstet Gynecol MFM. (2020) 2:100229. doi: 10.1016/j.ajogmf.2020.100229
21. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet Lond Engl. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
22. Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. (2000) 95:487–90. doi: 10.1016/s0029-7844(99)00602-x
23. Virk J, Li J, Vestergaard M, Obel C, Lu M, Olsen J. Early life disease programming during the preconception and prenatal period: making the link between stressful life events and type-1 diabetes. PloS One. (2010) 5:e11523. doi: 10.1371/journal.pone.0011523
24. Chen L, Shi L, Chao MS, Tong X, Wang F. Stressful life events, hypertensive disorders, and high blood sugar during pregnancy. Stress Health J Int Soc Investig Stress. (2020) 36:160–5. doi: 10.1002/smi.2911
25. Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. (2017) 60:636–44. doi: 10.1007/s00125-017-4206-6
26. Shi P, Liu A, Yin X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21:508. doi: 10.1186/s12884-021-03982-4
27. Shopen N, Schiff E, Koren-Morag N, Grossman E. Factors that predict the development of hypertension in women with pregnancy-induced hypertension. Am J Hypertens. (2016) 29:141–6. doi: 10.1093/ajh/hpv073
28. Auger N, Le TUN, Park AL, Luo Z-C. Association between maternal comorbidity and preterm birth by severity and clinical subtype: retrospective cohort study. BMC Pregnancy Childbirth. (2011) 11:67. doi: 10.1186/1471-2393-11-67
29. Ramesh S, Dall’Alba D, Gonzalez C, Yu T, Mascagni P, Mutter D, et al. Multi-task temporal convolutional networks for joint recognition of surgical phases and steps in gastric bypass procedures. Int J Comput Assist Radiol Surg. (2021) 16:1111–9. doi: 10.1007/s11548-021-02388-z
30. Wu Z, Jiang D, Wang J, Hsieh C-Y, Cao D, Hou T. Mining toxicity information from large amounts of toxicity data. J Med Chem. (2021) 64:6924–36. doi: 10.1021/acs.jmedchem.1c00421
31. Goncharov M, Pisov M, Shevtsov A, Shirokikh B, Kurmukov A, Blokhin I, et al. CT-Based COVID-19 triage: deep multitask learning improves joint identification and severity quantification. Med Image Anal. (2021) 71:102054. doi: 10.1016/j.media.2021.102054
32. Aghaeepour N, Ganio EA, Mcilwain D, Tsai AS, Tingle M, Van Gassen S, et al. An immune clock of human pregnancy. Sci Immunol. (2017) 2:eaan2946. doi: 10.1126/sciimmunol.aan2946
33. van der ML, Hinton G. Visualizing data using t-SNE. J Mach Learn Res. (2008) 9:2579–605. https://www.jmlr.org/papers/v9/vandermaaten08a.html
34. Nicolini G, Degli Esposti D, Ianniello E, Moroni L, Bacchelli S, Cicero AFG, et al. Long-term consequences of previous preeclampsia and complicated pregnancy: analysis of echocardiographic aspects. J Cardiovasc Med Hagerstown Md. (2021) 22:939–45. doi: 10.2459/JCM.0000000000001229
35. Kivelä J, Sormunen-Harju H, Girchenko PV, Huvinen E, Stach-Lempinen B, Kajantie E, et al. Longitudinal metabolic profiling of maternal obesity, gestational diabetes, and hypertensive pregnancy disorders. J Clin Endocrinol Metab. (2021) 106:e4372–88. doi: 10.1210/clinem/dgab475
36. Engeland A, Bjørge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, et al. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur J Epidemiol. (2011) 26:157–63. doi: 10.1007/s10654-010-9527-4
37. Feig DS, Shah BR, Lipscombe LL, Wu CF, Ray JG, Lowe J, et al. Preeclampsia as a risk factor for diabetes: a population-based cohort study. PLoS Med. (2013) 10:e1001425. doi: 10.1371/journal.pmed.1001425
38. Pantsulaia T. Role of TGF-beta in pathogenesis of diabetic nephropathy. Georgian Med News. (2006) 131:13–8.
39. Jones RL, Stoikos C, Findlay JK, Salamonsen LA. TGF-beta superfamily expression and actions in the endometrium and placenta. Reprod Camb Engl. (2006) 132:217–32. doi: 10.1530/rep.1.01076
40. Leguizamón GF, Zeff NP, Fernández A. Hypertension and the pregnancy complicated by diabetes. Curr Diab Rep. (2006) 6:297–304. doi: 10.1007/s11892-006-0064-1
41. Giannakou K, Evangelou E, Yiallouros P, Christophi CA, Middleton N, Papatheodorou E, et al. Risk factors for gestational diabetes: an umbrella review of meta-analyses of observational studies. PloS One. (2019) 14:e0215372. doi: 10.1371/journal.pone.0215372
42. Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. (2014) 58:193–210. doi: 10.1007/s12026-014-8517-0
43. Han X, Ghaemi MS, Ando K, Peterson LS, Ganio EA, Tsai AS, et al. Differential dynamics of the maternal immune system in healthy pregnancy and preeclampsia. Front Immunol. (2019) 10:1305. doi: 10.3389/fimmu.2019.01305
44. Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, Arenas-Hernandez M. Immune cells in term and preterm labor. Cell Mol Immunol. (2014) 11:571–81. doi: 10.1038/cmi.2014.46
45. Saito S, Shiozaki A, Nakashima A, Sakai M, Sasaki Y. The role of the immune system in preeclampsia. Mol Aspects Med. (2007) 28:192–209. doi: 10.1016/j.mam.2007.02.006
46. Thor KB, Kirby M, Viktrup L. Serotonin and noradrenaline involvement in urinary incontinence, depression and pain: scientific basis for overlapping clinical efficacy from a single drug, duloxetine. Int J Clin Pract. (2007) 61:1349–55. doi: 10.1111/j.1742-1241.2007.01433.x
47. Huizink AC, Robles de Medina PG, Mulder EJH, Visser GHA, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. (2003) 44:810–8. doi: 10.1111/1469-7610.00166
48. Slot A, Krog MC, Bliddal S, Olsen LR, Nielsen HS, Kolte AM. Feelings of guilt and loss of control dominate in stress and depression inventories from women with recurrent pregnancy loss. Eur J Contracept Reprod Health Care Off J Eur Soc Contracept. (2022) 27:153–8. doi: 10.1080/13625187.2021.1943740
49. Yılmaz M, Değirmenci F, Yılmaz DV. A psychosocial examination of feelings and thoughts about pregnancy: a qualitative study. Midwifery. (2021) 103:103106. doi: 10.1016/j.midw.2021.103106
50. Reeves D, Blickem C, Vassilev I, Brooks H, Kennedy A, Richardson G, et al. The contribution of social networks to the health and self-management of patients with long-term conditions: a longitudinal study. PloS One. (2014) 9:e98340. doi: 10.1371/journal.pone.0098340
51. Li S-B, Borniger JC, Yamaguchi H, Hédou J, Gaudilliere B, de Lecea L. Hypothalamic circuitry underlying stress-induced insomnia and peripheral immunosuppression. Sci Adv. (2020) 6:eabc2590. doi: 10.1126/sciadv.abc2590
52. Vieten C, Astin J. Effects of a mindfulness-based intervention during pregnancy on prenatal stress and mood: results of a pilot study. Arch Womens Ment Health. (2008) 11:67–74. doi: 10.1007/s00737-008-0214-3
53. Thomas M, Vieten C, Adler N, Ammondson I, Coleman-Phox K, Epel E, et al. Potential for a stress reduction intervention to promote healthy gestational weight gain: focus groups with low-income pregnant women. Womens Health Issues Off Publ Jacobs Inst Womens Health. (2014) 24:e305–311. doi: 10.1016/j.whi.2014.02.004
54. Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. (2008) 22:969–81. doi: 10.1016/j.bbi.2008.01.012
55. Dunn TJ, Dimolareva M. The effect of mindfulness-based interventions on immunity-related biomarkers: a comprehensive meta-analysis of randomised controlled trials. Clin Psychol Rev. (2022) 92:102124. doi: 10.1016/j.cpr.2022.102124
56. Hulsbosch LP, Nyklíček I, Potharst ES, Meems M, Boekhorst MGBM, Pop VJM. Online mindfulness-based intervention for women with pregnancy distress: design of a randomized controlled trial. BMC Pregnancy Childbirth. (2020) 20:159. doi: 10.1186/s12884-020-2843-0
57. Skovbjerg S, Birk D, Bruggisser S, Wolf ALA, Fjorback L. Mindfulness-based stress reduction adapted to pregnant women with psychosocial vulnerabilities-a protocol for a randomized feasibility study in a danish hospital-based outpatient setting. Pilot Feasibility Stud. (2021) 7:118. doi: 10.1186/s40814-021-00860-w
58. Oyarzabal EA, Seuferling B, Babbar S, Lawton-O’Boyle S, Babbar S. Mind-Body techniques in pregnancy and postpartum. Clin Obstet Gynecol. (2021) 64:683–703. doi: 10.1097/GRF.0000000000000641
59. Pachtman Shetty SL, Fogarty S. Massage during pregnancy and postpartum. Clin Obstet Gynecol. (2021) 64:648–60. doi: 10.1097/GRF.0000000000000638
60. Pereira M, Megevand P, Tan MX, Chang W, Wang S, Rezai A, et al. Evidence accumulation relates to perceptual consciousness and monitoring. Nat Commun. (2021) 12:3261. doi: 10.1038/s41467-021-23540-y
61. Batch BC, Spratt SE, Blalock DV, Benditz C, Weiss A, Dolor RJ, et al. General behavioral engagement and changes in clinical and cognitive outcomes of patients with type 2 diabetes using the Time2Focus Mobile app for diabetes education: pilot evaluation. J Med Internet Res. (2021) 23:e17537. doi: 10.2196/17537
62. Choi BCK, Pak AWP. A catalog of biases in questionnaires. Prev Chronic Dis. (2005) 2:A13. https://www.cdc.gov/pcd/issues/2005/jan/04_0050.htm15670466
Keywords: adverse pregnancy outcomes (APO), psychosocial and stress-related factors, prediction, multitask machine learning, immune states, single-cell
Citation: Becker M, Dai J, Chang AL, Feyaerts D, Stelzer IA, Zhang M, Berson E, Saarunya G, De Francesco D, Espinosa C, Kim Y, Marić I, Mataraso S, Payrovnaziri SN, Phongpreecha T, Ravindra NG, Shome S, Tan Y, Thuraiappah M, Xue L, Mayo JA, Quaintance CC, Laborde A, King LS, Dhabhar FS, Gotlib IH, Wong RJ, Angst MS, Shaw GM, Stevenson DK, Gaudilliere B and Aghaeepour N (2022) Revealing the impact of lifestyle stressors on the risk of adverse pregnancy outcomes with multitask machine learning. Front. Pediatr. 10:933266. doi: 10.3389/fped.2022.933266
Received: 30 April 2022; Accepted: 14 November 2022;
Published: 13 December 2022.
Edited by:
Marcia Guimaraes Da Silva, São Paulo State University, BrazilReviewed by:
Nicholas L Rider, Liberty University, United States© 2022 Becker, Dai, Chang, Feyaerts, Stelzer, Zhang, Berson, Saarunya, De Francesco, Espinosa, Kim, Marić, Mataraso, Payrovnaziri, Phongpreecha, Ravindra, Shome, Tan, Thuraiappah, Xue, Mayo, Quaintance, Laborde, King, Dhabhar, Gotlib, Wong, Angst, Shaw, Stevenson, Gaudilliere and Aghaeepour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nima Aghaeepour bmFnaGFlZXBAc3RhbmZvcmQuZWR1
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.