- 1Department of Pediatrics, College of Medicine, Shaqra University, Shaqra, Saudi Arabia
- 2Department of Obstetrics and Gynaecology, Faculty of Medicine, University of Khartoum, Khartoum, Sudan
- 3Department of Pediatrics, Unaizah College of Medicine and Medical Sciences, Qassim University, Unaizah, Saudi Arabia
- 4Department of Pediatrics, College of Medicine, Qassim University, Buraydah, Saudi Arabia
- 5Department of Obstetrics and Gynecology, Unaizah College of Medicine and Medical Sciences, Qassim University, Unaizah, Saudi Arabia
Background: The World Health Organization set a Global Nutrition Target of a 30% reduction in LBW by 2025. Maternal malnutrition/undernutrition is among the most important modifiable risk factors for impaired fetal growth. This study investigates the effect of maternal undernutrition on LBW in Sudan.
Methods: A cross-sectional study was conducted at Saad Abuelela Hospital in Khartoum, Sudan, from May to October 2020. The sociodemographic and obstetric data of the women were gathered via questionnaire, and their mid-upper arm circumference (MUAC) was measured. Maternal undernutrition was defined as a MUAC of <23 cm.
Results: In total, 1,505 pairs of pregnant women and their newborns were enrolled in the study. The medians [interquartile (IQR)] of the age, parity, and gestational age were 27.0 (9.0) years, 1.0 (3.0), and 38.0 (2.0) weeks, respectively. The median (IQR) of the birth weight was 3,028.0 (690.0) g. Of the 1,505 participants, 182 (12.1%) delivered LBW infants. Multivariate logistic regression showed that MUAC [adjusted odds ratio (AOR) = 0.91, 95% confidence interval (CI) = 0.87–0.96] and gestational age (AOR = 0.79, 95% CI = 0.73–0.85) were negatively associated with LBW. The level of antenatal care <2 visits (AOR = 2.10, 95% CI = 1.30–3.57) was associated with LBW. Women with undernutrition were at a higher risk of delivering LBW infants (AOR = 1.66, 95% CI = 1.09–2.53).
Conclusion: LBW is a health problem in Sudan, and women with undernutrition were at a higher risk of delivering LBW infants.
Introduction
It has been estimated that over 20 million deliveries have resulted in LBW infants (LBW; <2,500 g) annually; the vast majority of these LBW deliveries are in low- and middle-income countries (1). Several factors, such as infections, low education level, low income, and occupation, are associated with LBW (2–4). It has been found that about 3.6 million infants die (mainly in southern Asia and sub-Saharan Africa) during the neonatal period (1). The World Health Organization (WHO) set a Global Nutrition Target of a 30% reduction in LBW by 2025 (1). Maternal nutrition affects the growth of the fetus as well as birth and neonatal outcomes (5–8). Reports indicate that more than one-third of child deaths are caused by maternal and child undernutrition (9). Maternal malnutrition/undernutrition is among the most important modifiable risk factors for impaired fetal growth (9). Nutrition plays a fundamental role in health of pregnant women and the growth of fetuses. Poor maternal nutrition can lead to an increased risk of stillbirth, an increased risk of neonatal morbidity, death, and permanent deficits in growth and neurocognitive development (10).
Maternal undernutrition is a problem in developing countries (11). The prevalence of undernutrition among pregnant African women was 23.5% (12). It has been estimated that up to 20% of African women of reproductive age are undernourished (9, 13, 14).
Investigating the association between maternal undernutrition and LBW is vital for evidence-based interventions to reduce the burden of LBW. Several studies have assessed the association between birth weight/LBW and maternal undernutrition in African countries (15–19). LBW is a health problem in Sudan (20, 21). It has been reported that 12.5% of pregnant Sudanese women in Khartoum are undernourished (22). To the best of our knowledge, there are no evidence-based publications on the association between maternal undernutrition and LBW in Sudan. As such, this study was conducted to investigate the effect of maternal undernutrition on LBW in Sudanese women.
Materials and Methods
This cross-sectional study was conducted at Saad Abuelela Hospital in Khartoum, Sudan, from May to October 2020.
Inclusion Criteria
Women with a single and alive newborn.
Exclusion Criteria
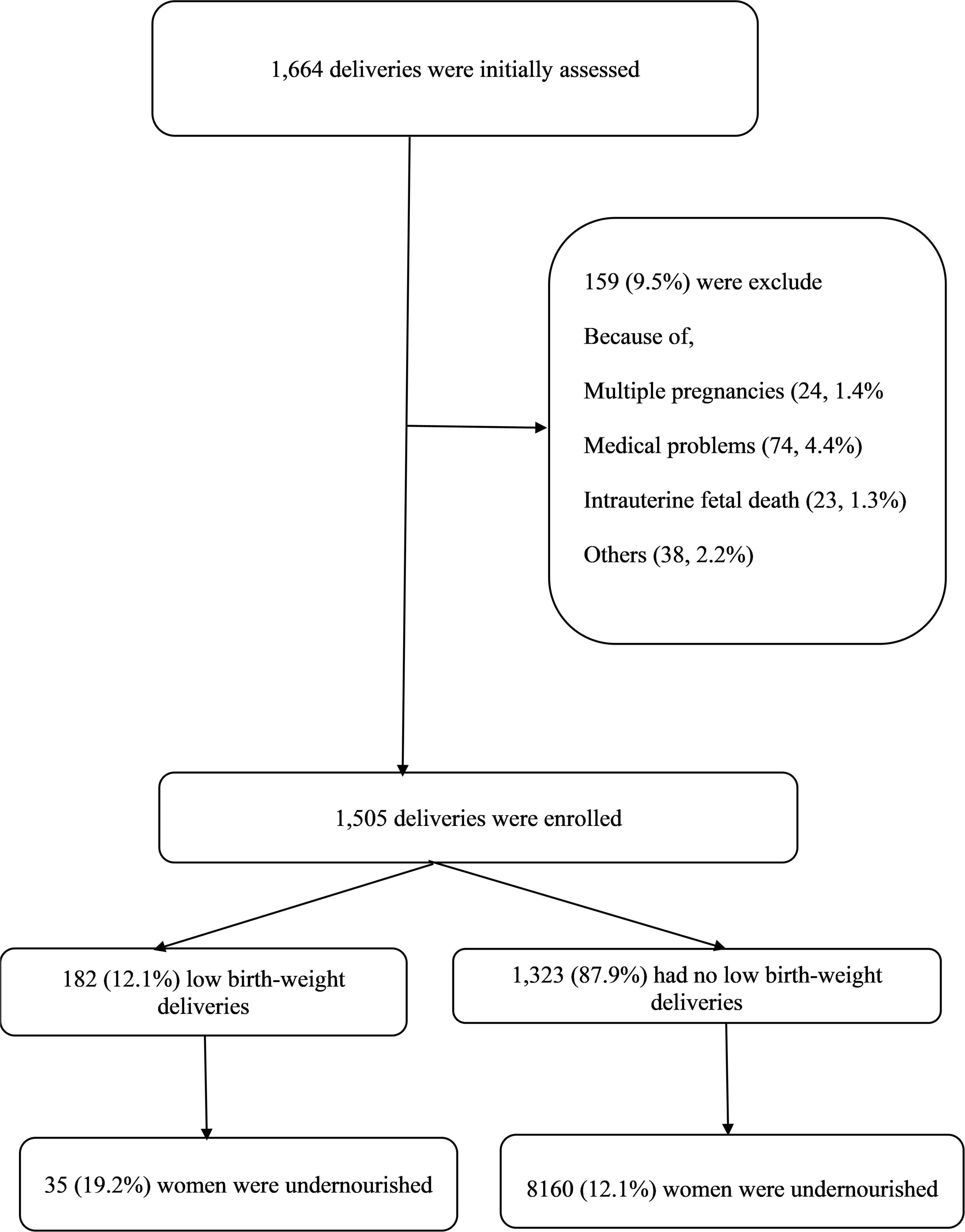
Women with multiple pregnancies, intra-uterine fetal death, delivering a baby with one or more congenital anomalies, and women with diseases known to influence the birth weight such as thyroid disease, diabetes mellitus, hypertension, antepartum hemorrhage, or any other chronic disease (Figure 1) were excluded.
After signing informed consent, data were collected by trained medical officers who graduated from medical schools. The socio-demographic and obstetric data were gathered and recorded face-to-face using a structured questionnaire about obstetric history (age, parity, gestational age, antenatal attendance, education level, miscarriage history, and employment). Gestational age was calculated using a combination of the dates of the last menstrual period and early pregnancy ultrasound.
Newborn weights were recorded within 1 h of delivery. The mother’s mid-upper arm circumference (MUAC) was measured after delivery using a flexible non-stretchable standard tape measure. The circumference was measured at the mid-point between the tip of the acromion process of the scapula and the olecranon process of the ulna. Measurements were taken on the right arm to the nearest 0.1 cm (23). Maternal undernutrition was defined as a MUAC of <23 cm. Hemoglobin was measured (before delivery) using an automated hematology analyzer according to the manufacturer’s instructions (Sysmex, KX-21, Japan). A Salter scale (which was checked for accuracy daily) was used to weigh the newborns immediately (by the staff) after birth to the nearest 10 g. The gender of each newborn was recorded.
Sample Size Determination
A sample size calculation for a cross-sectional study was applied and was estimated as 1,505 women using the recent prevalence (14.5%) of LBW in the study area (20). Thus, we assumed that the ratio of women with LBW to the women with no LBW was 1:6. Depending on our findings, we expect that 20.0% of the women who had LBW had undernutrition, and 12.5% of the women who had no LBW had undernutrition (22). This sample had a type I error of 5% and adequate power (80% power; β = 0.2).
Statistics
Data were entered into the Statistical Package for Social Sciences (SPSS) version 22 software (SPSS Inc.) for analysis. Continuous data (including MUAC) were checked for normality and were not normally disturbed. Therefore, the median [interquartile (IQR) range] was used to express their values. Categorized data were presented as frequencies and proportions. Multicollinearity (variance inflation factor, <4) was checked for but not detected. Univariate analyses were performed with LBW as the dependent variable and clinical obstetrics data [age, parity, employment, education level, antenatal care (ANC), MUAC, hemoglobin, gestational age, and newborn gender] as the independent variables. MUAC and undernutrition were entered one by one in each model. Variables with a p < 0.20 univariate analysis were entered to build the multivariable logistic regression models, and backward-stepwise regression was used for adjustment. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. A two-sided p < 0.05 was considered statistically significant.
Results
Basic Characteristics of the Participants
A total of 1,505 pairs of pregnant women and their newborns were enrolled in the study. The median (IQR) of the age, parity, and gestational age was 27.0 (9.0) years, 1.0 (3.0), and 38.0 (2.0) weeks, respectively. Half of these women were primipara (170, 50.1%). The vast majority of these women were housewives (1,331, 88.8%), and over half (836, 55.5%) had an education level ≥ secondary school. In total, 106 (7.0%) attended ≥ three antenatal visits, and 328 (21.8%) of the studied women had a history of miscarriage. Three hundred and twelve (20.7%) women had a cesarean delivery.
The median (IQR) of the birth weight was 3,028.0 (690.0) g. Of 1,505 participants, 182 (12.1%) delivered LBW infants.
The MUAC, gestational age, ANC, and education levels were significantly lower in women who delivered LBW infants. No significant difference was found in age, parity, hemoglobin levels, residence, employment, miscarriage history, and newborn gender between the two groups (Table 1).
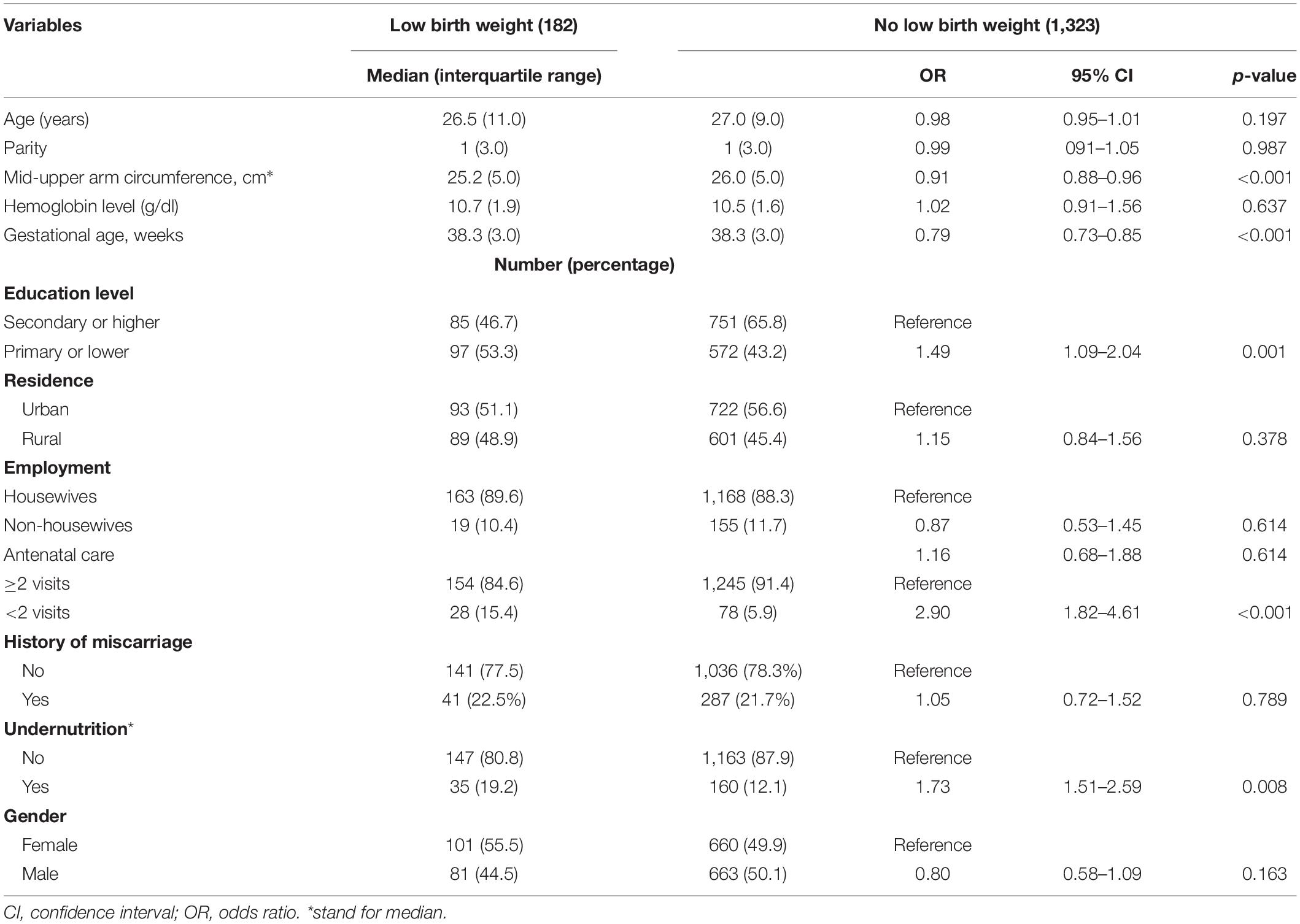
Table 1. Univariate analysis of the factors associated with low birth weight in Khartoum, Sudan, 2020.
The median (IQR) of MUAC was significantly lower in women who gave birth to LBW infants [10.0 (6.5) ng/mL vs. 18.3 (22.1) ng/mL]. In total, 35/182 (19.2%) women with LBW infant and 160/1,323 (12.1%, p = 0.010) women who did not deliver a LBW infant had undernutrition.
Multivariate logistic regression showed that MUAC [adjusted odds ratio (AOR) = 0.91, 95% CI = 0.87–0.96] and gestational age (AOR = 0.79, 95% CI = 0.73–0.85) were negatively associated with LBW. A level of antenatal care <2 visits (AOR = 2.10, 95% CI = 1.30–3.57) was associated with LBW. Women with undernutrition were at a higher risk of delivering LBW infants (AOR = 1.66, 95% CI = 1.09–2.53), Table 2.
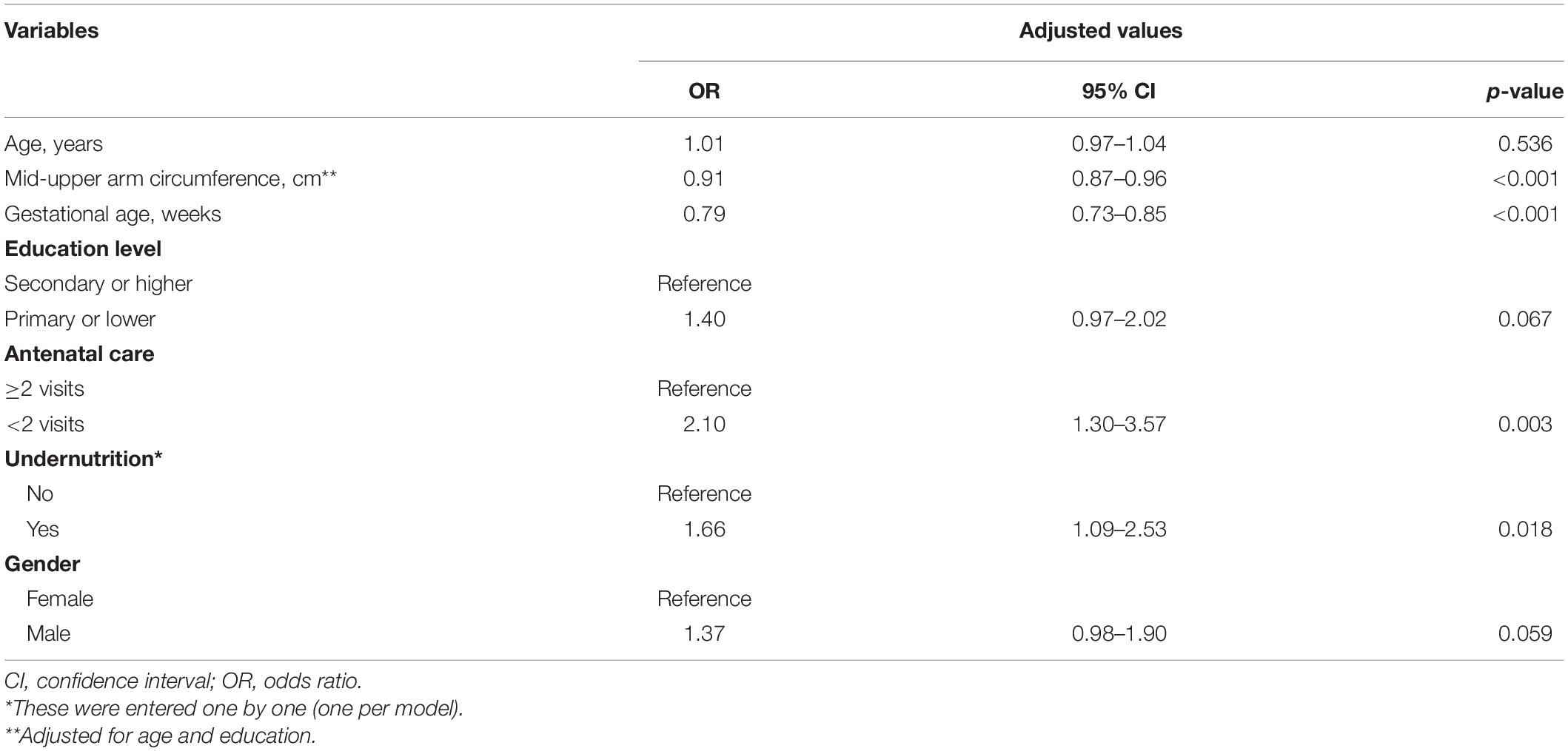
Table 2. Logistic regression analysis of the factors associated with low birth weight in Khartoum, Sudan, 2020.
Discussion
In this study, 12% of deliveries resulted in a LBW infant. The prevalence of LBW in our study is comparable with the LBW prevalence (14.3%) which was previously reported in the same hospital (20) and in different hospitals in neighboring Ethiopia (24–26). The prevalence of LBW in our study is lower than the LBW prevalence (21.6%) reported at the Debre Markos Hospital (Ethiopia) (27). However, the prevalence of LBW in the current study is much higher than the LBW prevalence in Ghana (9.7%) (28) and in Nigeria (7.3%) (29). Notably, the prevalence of LBW in our study is lower than the pooled LBW prevalence in Sub-Saharan Africa (9.76%) recently reported in a meta-analysis (30). The LBW prevalence difference between the current study and a later one can be explained by the difference in study design and the sociodemographic characteristics of different settings. It is worth mentioning that a high LBW prevalence indicates a difficulty in achieving the World Health Assembly’s (WHA’s) target of reducing the LBW prevalence to ≤10.5% by 2025 (31).
In the current study, pregnant women who had attended less than two ANC visits were at a 2.10 higher risk of delivering an LBW newborn. This is consistent with the previous studies conducted in Sudan (32), Ethiopia (33), Kenya, Zimbabwe (34), and Tanzania (35). Moreover, in their meta-analysis, Tessema et al. reported that ANC visits were associated with reduced LBW occurrence (30). This can be explained by the opportunity for ANC to access various preventive measures (nutritional counseling and health provisions, such as iron supplements) and screen for any possible problems that might lead to LBW.
In the current study, MUAC (AOR = 0.91) was negatively associated with LBW. Women with undernutrition were at a higher risk of delivering LBW (AOR = 1.66) newborns. Several previous studies have shown that maternal undernutrition is associated with LBW (15–17). In their 2017 meta-analysis of 4,633 participants from 13 studies, Cates et al. reported that the risk of delivering a baby with LBW was associated with low MUAC (relative risk = 1.60) (18). In 2011, a meta-analysis by Han et al. of 78 studies involving 1,025,794 women found that underweight women were at an increased risk of having LBW infants (19).
Our finding of an association between gestational age and LBW is in agreement with the results from neighboring Ethiopia (15).
We reported no association between maternal age, residence, newborn gender, maternal Hb and delivering LBW infants. Previous studies have shown that maternal Hb (17), maternal age, rural residence, and female gender were significantly associated with LBW (15, 33).
The other assessment tools for the micronutrient deficiencies during pregnancy are costly and technically difficult. Anthropometrics measurements, such as MUAC, are useful in assessing the malnutrition state. Unlike body-mass index, MUAC does not change during pregnancy; therefore, it seems to be the best tool for assessing the nutritional status during pregnancy (36, 37).
Strength and Limitations of the Study
The present study has some strengths including its large sample and taking newborn weight within 1 h of delivery. However, our study has limitations such as it was a hospital-based study, which might not reflect what was going on in the community. The study was conducted at a single hospital, the finding might not be generalizable to the entire birth cohort in the area. The cross-sectional nature of the study makes it difficult to draw inferences about the cause–effect relations among study variables. The number of ANC visits was taken from verbal response of respondents. There might be recall bias as respondents had to remember their ANC visits. In addition, important variables like physical activity and dietary diversity were not assessed.
Conclusion
LBW is a health problem in Sudan. Women with undernutrition are at a higher risk of delivering LBW infants.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Research and Ethical Committee of the Department of Obstetrics and Gynecology, Faculty of Medicine, University of Khartoum, Sudan (# 2020, 04). Informed consent was given by the participants. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
JB designed the research, analyzed the data, and interpreted the results. AA-N and AA designed the research and wrote the manuscript. DR conducted the research, analyzed the data, and wrote the manuscript. IA designed the research, conducted the research, and analyzed the data. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Deanship of Scientific Research, Qassim University, Saudi Arabia for funding the publication of this manuscript.
References
2. Umbers AJ, Aitken EH, Rogerson SJ. Malaria in pregnancy: small babies, big problem. Trends Parasitol. (2011) 27:168–75. doi: 10.1016/j.pt.2011.01.007
3. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, et al. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. (2010) 375:1969–87. doi: 10.1016/S0140-6736(10)60549-1
4. Black RE, Allen LH, Bhutta ZA, Caulfi LE, De Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. (2008) 371:243–60. doi: 10.1016/S0140-6736(07)61690-0
5. King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. (2000) 71:1218–25. doi: 10.1093/ajcn/71.5.1218s
6. Butte NF, Ellis KJ, Wong WW, Hopkinson JM, Smith EOB. Composition of gestational weight gain impacts maternal fat retention and infant birth weight. Am J Obstet Gynecol. (2003) 189:1423–32. doi: 10.1067/s0002-9378(03)00596-9
7. Butte NF, Wong WW, Treuth MS, Ellis KJ, Smith EOB. Energy requirements during pregnancy based on total energy expenditure and energy deposition. Am J Clin Nutr. (2004) 79:1078–87. doi: 10.1093/ajcn/79.6.1078
8. Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The preterm prediction study: association between maternal body mass index and spontaneous and indicated preterm birth. Am J Obstet Gynecol. (2005) 192:882–6. doi: 10.1016/j.ajog.2004.09.021
9. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. (2013) 382:427–51.
10. Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. (2003) 133:1592S–6. doi: 10.1093/jn/133.5.1592S
11. Zahangir MS, Hasan MM, Richardson A, Tabassum S. Malnutrition and non-communicable diseases among Bangladeshi women: an urban-rural comparison. Nutr Diabetes. (2017) 7:e250. doi: 10.1038/nutd.2017.2
12. Desyibelew HD, Dadi AF. Burden and determinants of malnutrition among pregnant women in Africa: a systematic review and meta-analysis. PLoS One. (2019) 14:e0221712. doi: 10.1371/journal.pone.0221712
13. Lartey A. Maternal and child nutrition in Sub-Saharan Africa: challenges and interventions. Proc Nutr Soc. (2008) 67:105–8. doi: 10.1017/S0029665108006083
14. Ververs MT, Antierens A, Sackl A, Staderini N, Captier V. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. (2013) 5:ecurrents.dis.54a8b618c1bc031ea140e3f293459 9c8. doi: 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8
15. Ahmed S, Hassen K, Wakayo T. A health facility based case-control study on determinants of low birth weight in Dassie town, Northeast Ethiopia: the role of nutritional factors. Nutr J. (2018) 17:103. doi: 10.1186/s12937-018-0409-z
16. Alemu B, Gashu D. Association of maternal anthropometry, hemoglobin and serum zinc concentration during pregnancy with birth weight. Early Hum Dev. (2020) 142:104949. doi: 10.1016/j.earlhumdev.2019.104949
17. Girma S, Fikadu T, Agdew E, Haftu D, Gedamu G, Dewana ZGB. Factors associated with low birthweight among newborns delivered at public health facilities of Nekemte town, West Ethiopia: a case control study. BMC Pregnancy Childbirth. (2019) 19:220. doi: 10.1186/s12884-019-2372-x
18. Cates JE, Unger HW, Briand V, Fievet N, Valea I, Tinto H, et al. Malaria, malnutrition, and birthweight: a meta-analysis using individual participant data. PLoS Med. (2017) 14:e1002373. doi: 10.1371/journal.pmed.1002373
19. Han Z, Mulla S, Beyene J, Liao G, McDonald SD. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int. J. Epidemiol. (2011) 40:65–101. doi: 10.1093/ije/dyq195
20. Abdelrahiem SK, Bilal JA, Al Nafeesah A, Al-Wutayd O, Rayis DA, Adam I. Low and high birth weight in a hospital population in Sudan: an analysis of clinical cut-off values. Int J Gynecol Obstet. (2021) 154:427–30. doi: 10.1002/ijgo.13543
21. Mustafa A, Bilal NE, Abass A-E, Elhassan EM, Adam I. The association between Helicobacter pylori seropositivity and low birthweight in a Sudanese maternity hospital. Int J Gynecol Obstet. (2018) 143:191–4.
22. Hassan B, Rayis DA, Ahmed ABA, ALhabardi N. Prevalence and associated factors of undernutrition among pregnant Sudanese women. Trans R Soc Trop Med Hyg. (2022) 116:352–8. doi: 10.1093/trstmh/trab128
23. Tang AM, Chung M, Dong K, Terrin N, Edmonds A, Assefa N, et al. Global mid-upper arm circumference cut-offs for adults: a call to action. Public Health Nutr. (2020) 23:3114–5. doi: 10.1017/S1368980020000385
24. Lake EA, Fite RO. Low birth weight and its associated factors among newborns delivered at Wolaita Sodo university teaching and referral hospital, southern Ethiopia, 2018. Int J Pediatr. (2019) 2019:4628301. doi: 10.1155/2019/4628301
25. Adane T, Dachew BA. Low birth weight and associated factors among singleton neonates born at Felege Hiwot referral hospital, North West Ethiopia. Afr Health Sci. (2018) 18:1204–13. doi: 10.4314/ahs.v18i4.42
26. Berhane M, Workneh N, Admassu B. Prevalence of low birth weight and prematurity and associated factors in neonates in Ethiopia: results from a hospital-based observational study. Ethiop J Health Sci. (2019) 29:677–88. doi: 10.4314/ejhs.v29i6.4
27. Alebel A, Wagnew F, Tesema C, Gebrie A, Ketema DB, Asmare G. Factors associated with low birth weight at Debre Markos Referral Hospital, Northwest Ethiopia: a hospital based cross - sectional study. BMC Res Notes. (2019) 12:105. doi: 10.1186/s13104-019-4143-1
28. Agbozo F, Lecturer MP, Abubakari A, Der J, Lecturer MP, Jahn A. Prevalence of low birth weight, macrosomia and stillbirth and their relationship to associated maternal risk factors in Hohoe Municipality. Ghana. Midwifery. (2016) 40:200–6. doi: 10.1016/j.midw.2016.06.016
29. Dahlui M, Azahar N, Oche OM, Aziz NA, Dahlui M, Azahar N, et al. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria Demographic and Health Survey. Glob Health Action. (2016) 9:28822. doi: 10.3402/gha.v9.28822
30. Tessema ZT, Tamirat KS, Teshale AB, Tesema GA. Prevalence of low birth weight and its associated factor at birth in Sub-Saharan Africa: a generalized linear mixed model. PLoS One. (2021) 16:e0248417. doi: 10.1371/journal.pone.0248417
31. United Nations Children’s Fund (UNICEF), World Health Organization (WHO). UNICEF-WHO Low Birthweight Estimates: Levels And Trends 2000–2015. Geneva: World Health Organization (2019).
32. Saeed OAM, Ahmed HA, Ibrahim AMF, Mahmood EAA, Abdu-Allah TOA. Risk factors of low birth weight at three hospitals in Khartoum State, Sudan. Sudan J Paediatr. (2014) 14:22–8.
33. Jember DA, Menji ZA, Yitayew YA. Low birth weight and associated factors among newborn babies in health institutions in Dessie, Amhara, Ethiopia. J Multidiscip Healthc. (2020) 13:1839–48. doi: 10.2147/JMDH.S285055
34. Endalamaw A, Engeda EH, Ekubagewargies DT, Belay GM, Tefera MA. Low birth weight and its associated factors in Ethiopia: a systematic review and meta-analysis. Ital J Pediatr. (2018) 44:141. doi: 10.1186/s13052-018-0586-6
35. Siza JE. Risk factors associated with low birth weight of neonates among pregnant women attending a referral hospital in northern Tanzania. Tanzan J Health Res. (2008) 10:1–8. doi: 10.4314/thrb.v10i1.14334
36. Bari A, Sultana N, Mehreen S, Sadaqat N, Imran I, Javed R. Patterns of maternal nutritional status based on mid upper arm circumference. Pakistan J Med Sci. (2020) 36:382–6. doi: 10.12669/pjms.36.3.1331
Keywords: undernutrition, pregnant women, low birth weight, Sudan, cross sectional study
Citation: Bilal JA, Rayis DA, AlEed A, Al-Nafeesah A and Adam I (2022) Maternal Undernutrition and Low Birth Weight in a Tertiary Hospital in Sudan: A Cross-Sectional Study. Front. Pediatr. 10:927518. doi: 10.3389/fped.2022.927518
Received: 24 April 2022; Accepted: 24 May 2022;
Published: 21 June 2022.
Edited by:
Andrew Steenhoff, Children’s Hospital of Philadelphia, United StatesReviewed by:
Carl Bose, University of North Carolina at Chapel Hill, United StatesKrysten North, Harvard Medical School, United States
Copyright © 2022 Bilal, Rayis, AlEed, Al-Nafeesah and Adam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdullah Al-Nafeesah, YS5hbG5hZmVlc2FoQHF1LmVkdS5zYQ==
 Jalal A. Bilal1
Jalal A. Bilal1 Abdullah Al-Nafeesah
Abdullah Al-Nafeesah Ishag Adam
Ishag Adam