
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 12 September 2022
Sec. Pediatric Oncology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.925538
 Chun Chen1,2,3†
Chun Chen1,2,3† Lei Hang4†
Lei Hang4† Yan Wu1,2,3
Yan Wu1,2,3 Qing Zhang1,2,3
Qing Zhang1,2,3 Yifei Zhang1,2,3
Yifei Zhang1,2,3 Jun Yang1,2,3*
Jun Yang1,2,3* Jin Xie1,2,3*
Jin Xie1,2,3* Jingrong Lu1,2,3*
Jingrong Lu1,2,3*Background: The incidence rate of children with thyroid cancer has an increasing trend. This study aimed to investigate the clinical characteristics and therapeutic approaches of differentiated thyroid cancer (DTC) in Chinese children.
Materials and methods: From January 1998 to March 2022, 52 cases undergoing surgical resection in Xinhua Hospital affiliated to Shanghai Jiao Tong University were divided by age (≤ 7 years old: n = 14 and 8–13 years old, n = 38). Treatment methods and clinical features were analyzed to evaluate prognostic factors for oncological outcomes.
Results: Among the 52 cases, the proportion of local invasion in the pre-school group was found to be higher than that in the school-age group (p = 0.01). T stage was significantly different between the two groups (p ≤ 0.05); the proportion of T1–2 was higher in the school-age group (32 cases, 84.2%), while the proportion of T4 was higher in the pre-school group (6 cases, 42.8%) relatively. The postoperative complication rate was dramatically higher in pre-school children (p ≤ 0.05). Additionally, the total thyroidectomy rate in the non-recurrent group was slightly higher than that in the recurrent group (p ≤ 0.05). Over half of the recurrent cases had low T stage and low ATA (American Thyroid Association) risk levels at initial diagnosis (78.3 and 51.4%).
Conclusion: The local invasion, tumor stage, and recurrent laryngeal nerve (RLN) injury rates of the pre-school group were higher than that of the school-age group, where young age served as a potential hazard in DTC children. Hence, surgeons should emphasize high-risk features and optimize individualized surgical procedures for DTC children.
Thyroid cancer has become the most prevalent endocrine cancer in children, and the incidence rate of differentiated thyroid cancer (DTC) has increased rapidly in the last decade (1). DTC (papillary and follicular thyroid carcinoma) originated from follicular cells is the most common histologic subtype of thyroid cancer. Previous studies have indicated that the high incidence rate of thyroid cancers in children may have an underlying link to height and body mass index (BMI) in the process of growth (2). Meanwhile, dust and radiation exposure has been reported as a potential risk leading to the increased diagnosis of earlier-stage thyroid cancers (3).
Symptoms in children are atypical at initial diagnosis, so thyroid cancer is often diagnosed in an advanced stage or after the peripheral invasion and distant metastases. Previous studies have confirmed that the range of tumor invasion is more extensive, as is often the case, the rate of distant metastases and locoregional relapse is higher in DTC children than adults (4, 5). Fortunately, mortality after thyroidectomy in well-differentiated pediatric individuals is very low (6, 7).
Compared to adolescents, the clinical manifestation of DTC in preadolescent children is more aggressive, with a higher recurrence and distant metastases rate (5). A recent study has unveiled that lymph node metastases were found in over 40% of children with DTC (1). Since another previous study has found a higher proportion of girls than boys in children with thyroid cancer, the researchers hypothesized that the difference may be induced by estrogen, which may be a latent growth factor for malignant thyroid cells (8). On the contrary, a subsequent clinical study has pointed out that transient postoperative complications after total thyroidectomy in children were frequent relatively. Children are in the osteogenic stage of development, so the inadvertent section of parathyroid gland could lead to serious consequences and prolonged impacts on the quality of life (9). However, a number of studies were concerned with the characteristics of adolescents that in very young children are not well discussed, especially those less than 14 years old (10).
This study presents a retrospective analysis focusing on 52 DTC children who underwent thyroidectomy in our department to explore the differences in general information, clinical manifestations, TNM stages, ATA risk levels, cervical lymph node metastases, therapeutic approaches, and postoperative complications. It is important to sum up experience from limited case resources to investigate the possible factors of prevention and treatment protocols in treating young pediatric DTC patients.
From January 1998 to March 2022, the clinical data involved 52 DTC aged less than or equal to 13 years old who were treated at Xinhua Hospital Affiliated to Medical School of Shanghai Jiao Tong University by the same senior head and neck surgeon team (ethics code: XHEC-WJW-2022-272). Surgical procedures were designed by best indicator regarding tumor extent, lymph nodes invasion, and general status. According to the Chinese situation, the enrolled patients were divided into the pre-school age group (≤ 7 years old) and the school-age group (8–13 years old) regarding the individual difference, organ development, and life pattern of the two groups. The clinical characteristics of pre-school age and school-age groups were compared, and the relevant factors of recurrent and non-recurrent groups were analyzed.
Data were collected from their parents and otolaryngologists in our department, including age, gender, initial symptoms, local invasion, TNM stage, tumor size, surgical procedures, ATA (American Thyroid Association) risk levels, locoregional relapse, distant metastases, postoperative complications, and treatment approaches. All cases were primary tumors with complete clinical data and follow-up results. TNM stage was assessed based on the 8th edition of American Joint Committee on Cancer (AJCC) guidelines (11). The clinical characteristics and symptoms are defined in Table 1.
Total RNA was extracted from tumor tissue and normal control paraffin sections using TRIzol reagent and reverse transcribed by the companion diagnostic sequencing kit (Takara, Tokyo, Japan). Single-nucleotide marker sites (BAT25, BAT26, MONO-27, NR21, NR24) were detected by Chaoxin Gene Biological Incorporation (Shanghai, China) (12). Every sample was examined three times. Tumor with two or more unstable sites was defined as MSI-H (microsatellite instability high); tumor with one unstable site was defined as MSI-L (microsatellite instability low); tumor without unstable sites was defined as MSS (microsatellite stable).
SPSS 23.0 was used in the statistical analysis. Measure data were described as mean ± standard deviation (SD). An independent sample T-test was used to analyze age, tumor diameter, and follow-up time of pre-school and school-age groups along with recurrent and non-recurrent groups. Data analysis by ANOVA was required to prove the homogeneity of variance from normal distribution data. If one of the above conditions was not met, non-parametric test could be used instead. Power analysis was utilized to screen out and examine the efficiency of indicators. The χ2 test was used to assess the diagnosis, TNM stage, ATA risk levels, surgical procedures, histology, postoperative complications, and other clinical conditions. The progression-free survival rate (PFS) in both age groups was evaluated by Kaplan–Meier curves and log-rank test. The significance level was set as p < 0.05.
In the 24-year time span, the 52 cases were divided into the pre-school group (≤ 7 years old, n = 14) and the school-age group (8–13 years old, n = 38) (Table 2). The histological subtype of all cases was papillary carcinoma. Univariate factor analysis of risk factors was examined by power analysis, the Kaiser–Meyer–Olkin (KMP) value was 0.78, and the p-value of the Bartlett test was significant (p ≤ 0.001), which indicated no significant difference in the degree of correlation between variables, and our data were suitable for factor analysis. Although no obvious difference existed in gender between the two groups, the proportion of female patients in the school-age group was slightly higher (63.2 versus 50.0%, p > 0.05). As shown in Table 2, there was no statistically significant difference between the two groups in the initial symptoms of foreign body sensation or cervical mass (p = 0.34, p = 0.83). However, the findings observed that pre-school children had a higher proportion of local invasions such as strap muscles, trachea, or recurrent laryngeal nerve (6 cases, 42.9%, p = 0.01). Regarding the tumor size, no significant difference was observed between pre-school and school-age children (p > 0.05). Additionally, it was found that T-stage showed a significant difference between the two groups (p = 0.04); the proportion of T1–2 stage cases was higher in the school-age group (32 cases, 84.2%). In comparison, the proportion of T4 stage cases was higher in the pre-school group (6 cases, 42.8%). There was no concordance in N and M stages between the two groups. According to the 8th AJCC guideline, no statistically significant difference existed regarding ATA risk levels between the two groups, while the proportion of high-risk cases in the pre-school group (5 cases, 35.8%) was higher than that in the school-age group (8 cases, 21.1%). Total thyroidectomy rate in the school-age group (32 cases, 84.2%) was found to be higher than that in the pre-school group (7 cases, 50.0%, p = 0.01). There was no statistical difference in lymph node dissection, hypocalcemia, and locoregional relapse rate between pre-school and school-age groups (p = 0.09, p = 0.95, p = 0.74). However, the pre-school patients showed statistically significance in increased transient RLN injury incidence rate versus school-age patients (21.4% versus 0, p = 0.02). No cases suffered from postoperative hemorrhage, permanent hypocalcemia, or permanent RLN paralysis.
Among the 52 cases, 37 patients (9.49 ± 2.63 years old) had no recurrence and 15 patients (8.53 ± 2.56 years old) recurred (Table 3). There was no significant difference in gender between the recurrence group and the non-recurrence group as well as initial symptoms and local invasion (p > 0.05). Moreover, no significant difference was present in regard to tumor size and cervical lymph node metastases between the two groups (p > 0.05). No statistically significant difference was found in the clinical stage between the recurrence group and the non-recurrence group (p > 0.05). Furthermore, there was no significant difference existed in ATA risk levels between the two groups (p > 0.05). The 1- and 3-year PFS rates of total thyroidectomy patients were 89.4 and 81.3%, respectively. The proportion of total thyroidectomy in the non-recurrence group (31 cases, 83.8%) was found to be higher than that in the recurrence group (8 cases, 53.3%, p ≤ 0.001). There was no statistical difference in the incidence rate of transient RLN injury and hypocalcemia between the two groups (p > 0.05). As to comprehensive treatments, we found no concordance between I131 radiotherapy or thyroxine tablets and recurrence. In the recurrence group, residual thyroidectomy and cervical lymph node dissection were performed in patients with lobectomy (7 cases, 46.7%), including 5 patients who underwent I131 radiotherapy following reoperation, and no one recurred with the combined strategy of thyroxine tablets.
Variables correlated with recurrence built a multivariate Cox regression model. Cervical lymph node metastases (both ipsilateral and bilateral metastases, HR 1.12; 95% CI 0.08–15.18 and HR 3.46; 95% CI 0.30–39.77) and young age (HR 1.06; 95% CI 0.28–3.09) were found to be contributing factors for recurrence (Table 4).
All children were followed up for a mean of 37.9 months after surgery, and all cases survived up to the follow-up time point, with a 5-year PFS rate of 61.86% for all cases. The 5-year PFS rate was 52.9% in the pre-school group and 64.0% in the school-age group. No deaths were observed in the two groups during the follow-up period. Kaplan–Meier curve shows no statistically significant difference in PFS between the two age groups (p > 0.05) (Figure 1).
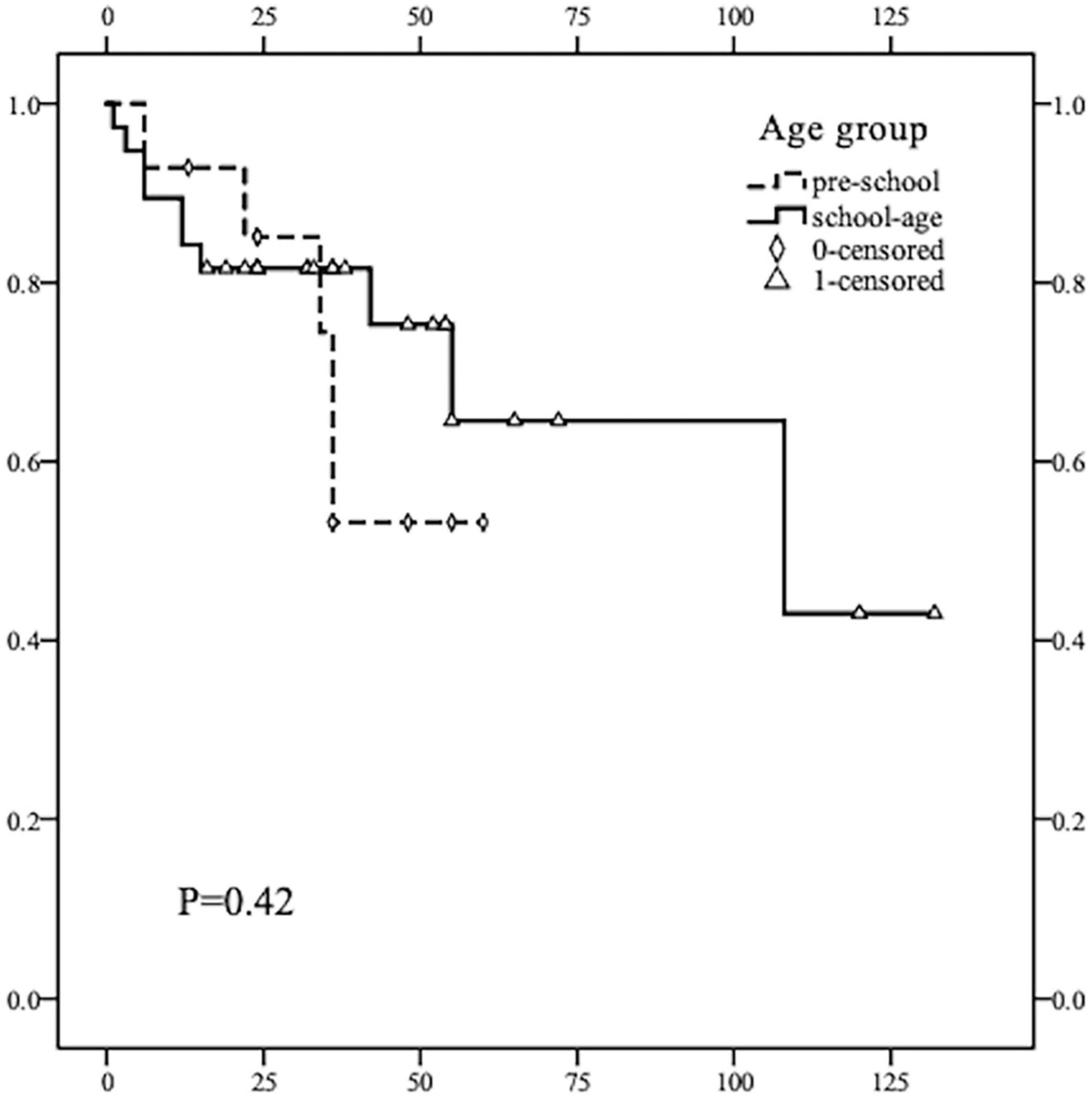
Figure 1. Kaplan–Meier survival curve comparison of PFS rate in children of different age groups (≤ 7 years old and 8–13 years old). The recurrence rate of thyroid cancer in children of the two groups was not statistically significant (p = 0.42, p > 0.05). The average and median times of recurrence DTC in the pre-school children were earlier than that in the school-age children.
Notably, two pre-school children recurred after the first surgery with cervical lymph node metastases and tracheal invasion, making salvage surgery encounter more difficulties; both cases developed postoperative pulmonary metastasis after complete resection of residual tumor tissue. Microsatellite instability has been used as an important molecular marker for the prognosis of some refractory tumors and for predicting the therapeutic effect of immunotherapy (12). Previous research has reported that patients with thyroid cancer may benefit from immunotherapy (13). To further explore the characteristics and treatment strategies of these two patients, single-nucleotide marker sites (BAT25, BAT26, MONO-27, NR21, NR24) were amplified by MSI-PCR. Not surprisingly, the examined single-nucleotide marker sites of both samples exceeded 30% (more than 2 of the 5 sites) and were defined as high microsatellite instability (MSI-H) (Figure 2).

Figure 2. Polymerase chain reaction analysis of microsatellite markers. (A) The blue curve represents normal tissue, while the red curve represents tumor tissue. In case 1, there was microsatellite instability in BAT26, BAT25, and MONO27 sites between two control tissues (p < 0.05), which indicated high microsatellite instability (MSI-H). (B) In case 2, there was microsatellite instability in BAT25, MONO27, and NR21 sites between two control tissues (p < 0.05), which indicated high microsatellite instability (MSI-H).
The incidence of DTC in children and adolescents has continuously risen in recent years, which largely attributes to the improvements in diagnostic methods. Previous studies have pointed out that DTC in children and adolescents is different from that of adults in clinical manifestations, recurrence rate, pulmonary metastasis, and prognosis (14, 15). An analysis conducted by Al-Qahtani et al. in children and adolescents with DTC under 18 years old has indicated that young age was dramatically related to the recurrence in situ (14). Domestic studies have compared specificity and difference in clinical characteristics of DTC between preadolescent children and adolescents; the incidence of distant metastases was reported to be higher in those of young age (16). This study first provides insights into the clinical features in DTC children younger than or equal to 13 years of age. Clinical characteristics of the pre-school group and the school-age group were compared and analyzed; prognosis analysis between recurrence and non-recurrence groups was conducted as well. We hope this study could provide a more comprehensive acknowledgment for the risk factors and distribution characteristics in children with DTC.
In this study, the cervical node was the most common initial clinical manifestation in pre-school children, whereas in the school-age group, the initial symptom of foreign body sensation and cervical lymph node was most common. In light of the non-typical symptoms and anatomical location, thyroid cancer in children is often diagnosed in an advanced stage, especially in pre-school age children without well-managed speech expression ability. The proportion of local invasion was higher in the pre-school group than that in the school-age group, demonstrating that compared to the school-age group, tumors in the pre-school group were more subtle and aggressive (17). In this study, a significant difference was observed in the T stage between the two age groups, with a higher proportion of T1–2 in the school-age group and a higher proportion of T4 in the pre-school group. Similarly, there was no statistically significant difference regarding ATA risk level between the two groups in this study. However, in terms of percentage, the proportion of high-risk cases appeared to be higher in the pre-school group than that in the school-age group. Therefore, young age serves as a critical risk factor in the T stage and ATA risk level of DTC in children, which has some similarities to another large retrospective cohort study by Lebbink et al. (5). Positive physical examination and ultrasonic examination should be attached of importance for the early diagnosis and treatment of DTC in pre-school children. Notably, it is acknowledged that there is overdiagnosis in children with DTC due to the overdiagnosis with ultrasonography screening, leading to lifelong side effects, medical care, and even psychological problems (18). For clinical implications, children with potential specific risk factors (such as adolescence in girls, Hashimoto thyroiditis, and family history) are feasible for active screening (19).
In terms of surgery procedures, the total thyroidectomy proportion was 50.0 and 84.2% in the pre-school group and school-age group, respectively, which may be relevant to conservative surgical methods under the aspiration of pre-school age children’s parents. As to the recurrence risks, the proportion of bilateral total thyroidectomy in the non-recurrence group was found to be significantly higher than that in the recurrence group by univariate factor analysis, while cervical lymph node metastases and young age were found to be risk factors of recurrence by the multivariate regression model. However, the surgical procedure analysis has certain limitations of the small cohort; also, this study involved children in a broad period, during which much has changed in terms of surgical instruments, equipment, and multimodal regimens over the years. It is necessary to conduct further studies with higher patient volumes and multicenter studies to support the optimum strategy. As reported, no difference was found between unilateral thyroidectomy and total thyroidectomy with respect to disease-free survival (DSS), and both procedures had a good prognosis in children (20).
There is still controversy concerning the therapeutic approach in children with DTC, regarding the resection range and the necessity of postoperative I131 radiotherapy (21). The 2015 ATA guidelines recommended less use of I131 radiotherapy for low-risk DTC patients because the impact of I131 radiotherapy on children remains unclear, which may affect the quality of life and carry an economic burden (22). It has been reported that a similar low recurrence rate between total thyroidectomy and total thyroidectomy is associated with preventive central neck dissection in adults (23). Besides, NGO et al. pointed out that lymph node metastases in the central region of papillary thyroid cancer (PTC) children were as high as 83.3% (24). A cohort study of 102 children PTC cases has suggested that for patients with unifocal T1a without clinically evident nodal disease, a unilateral thyroidectomy with VI zone dissection should be considered (25). Generally speaking, in patients with tumor size ≥ 4 cm and/or multifocal neoplasia, and/or local invasion, and/or lymph node metastases, and/or distant metastases, total thyroidectomy followed by I131 radiotherapy is suggested (26). For our statistics, surgical complications were more frequent in the pre-school group than that in the school-age group, which may be related to recurrent laryngeal nerve invasion and complex anatomical structure in younger children. The overall incidence rate of recurrence and pulmonary metastasis in this study was 28.8 and 19.2%, which echoed to the proportion reported in previous studies (27).
Compared to the non-recurrence group, those in the recurrence group were slightly younger (8.53 ± 2.56 years old), indicating that younger DTC patients should attach great importance to regular follow-ups and examinations. In this study, no statistical difference in gender was noted between non-recurrent and recurrent groups, which was consistent with the work of Demidchik et al. (28). Moreover, no statistical difference was present in the clinical stage and ATA risk level between recurrent and non-recurrent groups. This study stumbled upon a high proportion of T1a, T1b, and T2 in the recurrent group (73.4%), which indicated that more recurrent cases had a low T stage at initial diagnosis. Also, the ATA risk level is insufficient in assessing the risk of DTC recurrence in children adequately. It is reported that the overall cumulative survival rate for children with thyroid cancer was high up to 97–99% (20), which appeared to be 100% in our study after surgical resection and I131 radiotherapy with thyroxine tablets. Most of the previous studies focused on exploring the survival rate for children with thyroid cancer but pay less attention to the prognosis factors on account of the very low mortality.
The curative effects of traditional treatment in metastatic thyroid cancers are limited. Immunotherapy is expected to become a new treatment modality to improve the prognosis of these patients. MSI is important in selecting patients who may benefit from immune checkpoint inhibitors. A publication by Genutis et al. described a total of 485 thyroid cancer patients screened for MSI deficiency, which found that all MSI-H cases were follicular thyroid carcinoma (FTC) patients (28). The insights gained from the result of MSI in two refractory cases may highlight the importance of MSI examination for immunotherapy in recurrent and aggressive DTC patients. Further clinical data and trials are expected to support the application of immunotherapy in thyroid cancer. There was a potential bias of age, tumor stage, body mass index, and economic situations. Therefore, larger clinical trials should be designed to explore the feasibility and efficacy of therapeutic regimens.
In conclusion, the proportion of local invasion, tumor stage, and recurrent laryngeal nerve injury in the pre-school age group was higher than that of the school-age group. Young age may be presumed to be a risk factor in children with DTC. Nearly half of the recurrent cases were assessed as low T stage and low-risk at initial diagnosis; hence, strategies in evaluating the risks of children with DTC locoregional relapse should be further investigated. To raise awareness of the risk of DTC in young children, further studies and appropriate treatment measures should be designed.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Xinhua Hospital Affiliated with the Shanghai Jiao Tong University of Medicine (No. XHEC-WJW-2022-272, February 28, 2022). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
CC and JL contributed to the conception and design of the study and wrote the first draft of the manuscript. LH performed the statistical analysis. JX and JY participated in drafting the manuscript or revising for intellectual content. YZ and YW contributed to the manuscript revision. QZ supervised the writing and editing. JX modified and improved the English. All authors contributed to manuscript revision, read, and approved the submitted version.
This research work was supported by a grant from the National Natural Science Foundation of China (Project No. 81970876).
We appreciate LH for contributing to the registry and providing the data for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Clement SC, Lebbink CA, Hesselink M, Teepen JC, Santen H. Presentation and outcome of subsequent thyroid cancer among childhood cancer survivors compared to sporadic thyroid cancer; a matched national study. Eur J Endocrinol. (2020) 183:169–80.
2. Kitahara CM, Gamborg M, Berrington AB, Sorensen TIA, Baker JL. Childhood height and body mass index were associated with risk of adult thyroid cancer in a large cohort study. Cancer Res. (2014) 74:235–42.
3. van Gerwen M, Cerutti JM, Rapp J, Genden E, Riggins GJ, Taioli E. Post-9/11 excess risk of thyroid cancer: Surveillance or exposure? Am J Ind Med. (2021) 64:881–4. doi: 10.1002/ajim.23268
4. Liu L, Huang F, Liu B, Huang R. Detection of distant metastasis at the time of ablation in children with differentiated thyroid cancer: The value of pre-ablation stimulated thyroglobulin. J Pediatric Endocrinol Metabol. (2018) 31:751–6.
5. Lebbink CA, van den Broek MFM, Kwast ABG, Derikx JPM, Dierselhuis MP, Kruijff S, et al. Opposite incidence trends for differentiated and medullary thyroid cancer in young dutch patients over a 30-year time span. Cancers (Basel). (2021) 13:5104. doi: 10.3390/cancers13205104
6. Hay ID, Gonzalez-Losada T, Reinalda MS, Honetschlager JA, Richards ML, Thompson GB. Long-term outcome in 215 children and adolescents with papillary thyroid cancer treated during 1940 through 2008. World J Surg. (2010) 34:1192–202.
7. Stefan AI, Piciu A, Mester A, Apostu D, Badan M, Badulescu CI. Pediatric thyroid cancer in Europe: An overdiagnosed condition? A literature review. Diagnostics (Basel). (2020) 10:112. doi: 10.3390/diagnostics10020112
8. Massimino M, Evans DB, Podda M, Spinelli C, Collini P, Pizzi N, et al. Thyroid cancer in adolescents and young adults. Pediatr Blood Cancer. (2018) 65:e27025.
9. Stokhuijzen E, van der Steeg AF, Nieveen van Dijkum EJ, van Santen HM, van Trotsenburg AS. Quality of life and clinical outcome after thyroid surgery in children: A 13 years single center experience. J Pediatr Surg. (2015) 50:1701–6. doi: 10.1016/j.jpedsurg.2015.02.067
10. Markovina S, Grigsby PW, Schwarz JK, DeWees T, Moley JF, Siegel BA, et al. Treatment approach, surveillance, and outcome of well-differentiated thyroid cancer in childhood and adolescence. Thyroid. (2014) 24:1121–6.
11. Lamartina L, Grani G, Arvat E, Nervo A, Zatelli MC, Rossi R, et al. 8th edition of the AJCC/TNM staging system of thyroid cancer what to expect (ITCO#2). Endocr Relat Cancer. (2018) 25:L7–11.
12. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. (2017) 357:409–13. doi: 10.1126/science.aan6733
13. Chang L, Chang M, Chang HM, Chang F. Microsatellite instability: A predictive biomarker for cancer immunotherapy. Appl Immunohistochem Mol Morphol. (2018) 26:e15–21. doi: 10.1097/PAI.0000000000000575
14. Al-Qahtani KH, Tunio MA, Al Asiri M, Aljohani NJ, Bayoumi Y, Riaz K, et al. Clinicopathological features and treatment outcomes of differentiated thyroid cancer in Saudi children and adults. J Otolaryngol Head Neck Surg. (2015) 44:48.
15. Alzahrani AS, Alkhafaji D, Tuli M, Al-Hindi H, Sadiq BB. Comparison of differentiated thyroid cancer in children and adolescents (</=20 years) with young adults. Clin Endocrinol (Oxf). (2016) 84:571–7. doi: 10.1111/cen.12845
16. Cistaro A, Quartuccio N, Garganese MC, Villani MF, Altini C, Pizzoferro M, et al. Prognostic factors in children and adolescents with differentiated thyroid carcinoma treated with total thyroidectomy and RAI: A real-life multicentric study. Eur J Nuclear Med Mol Imaging. (2022) 49:1374–85.
17. Wada N, Sugino K, Mimura T, Nagahama M, Kitagawa W, Shibuya H, et al. Pediatric differentiated thyroid carcinoma in stage I: Risk factor analysis for disease free survival. BMC Cancer. (2009) 9:306. doi: 10.1186/1471-2407-9-306
18. Zaridze D, Maximovitch D, Smans M, Stilidi I. Thyroid cancer overdiagnosis revisited. Cancer Epidemiol. (2021) 74:102014. doi: 10.1016/j.canep.2021.102014
19. Marotta V, Sciammarella C, Chiofalo MG, Gambardella C, Bellevicine C, Grasso M, et al. Hashimoto’s thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. (2017) 24:485–93. doi: 10.1530/ERC-17-0085
20. Zhang B, Wu W, Shang X, Huang D, Liu M, Zong L. Incidence and prognosis of thyroid cancer in children: Based on the SEER database. Pediatr Surg Int. (2022) 38:445–56. doi: 10.1007/s00383-022-05069-3
21. Massimino M, Cecchetto G, Bisogno G, Chiaravalli S, Podda M, Ferrari A, et al. Papillary thyroid carcinoma in pediatric age: An example of a rare tumour managed within a cooperative comprehensive project. Curr Pediatric Rev. (2016) 12:265–71.
22. Pasqual E, Sosa JA, Chen Y, Schonfeld SJ, Berrington de Gonzalez A, Kitahara CM. Trends in the management of localized papillary thyroid carcinoma in the United States (2000-2018). Thyroid. (2022) 32:397–410. doi: 10.1089/thy.2021.0557
23. Gambardella C, Patrone R, Di Capua F, Offi C, Mauriello C, Clarizia G, et al. The role of prophylactic central compartment lymph node dissection in elderly patients with differentiated thyroid cancer: A multicentric study. BMC Surg. (2019) 18:110. doi: 10.1186/s12893-018-0433-0
24. Ngo DQ, Ngo QX, Le QV. Pediatric thyroid cancer: risk factors for central lymph node metastasis in patients with cN0 papillary carcinoma. Int J Pediatr Otorhinolaryngol. (2020) 133:3–11. doi: 10.1016/j.ijporl.2020.110000
25. Sudoko CK, Jenks CM, Bauer AJ, Isaza A, Mostoufi-Moab S, Surrey LF, et al. Thyroid lobectomy for T1 papillary thyroid carcinoma in pediatric patients. JAMA Otolaryngol Head Neck Surg. (2021) 147:943–50. doi: 10.1001/jamaoto.2021.2359
26. Spinelli C, Rallo L, Morganti R, Mazzotti V, Inserra A, Cecchetto G, et al. Surgical management of follicular thyroid carcinoma in children and adolescents: A study of 30 cases. J Pediatric Surg. (2018) 54:521–6.
27. Qu Y, Huang R, Li L. Clinical analysis of the factors that influence disease progression of differentiated thyroid carcinoma in children. J Paediatrics Child Health. (2017) 53:903–7.
28. Demidchik YE, Saenko VA, Yamashita S. Childhood thyroid cancer in Belarus, Russia, and Ukraine after Chernobyl and at present. Arq Bras Endocrinol Metabol. (2007) 51:748–62. doi: 10.1590/s0004-27302007000500012
Keywords: differentiated thyroid cancer, recurrence, complication, children differentiated thyroid cancer, children
Citation: Chen C, Hang L, Wu Y, Zhang Q, Zhang Y, Yang J, Xie J and Lu J (2022) Retrospective analysis of clinical characteristics and risk factors of differentiated thyroid cancer in children. Front. Pediatr. 10:925538. doi: 10.3389/fped.2022.925538
Received: 21 April 2022; Accepted: 18 August 2022;
Published: 12 September 2022.
Edited by:
Andrea Di Cataldo, University of Catania, ItalyReviewed by:
Maura Massimino, Fondazione Istituto Nazionale Tumori (IRCCS), ItalyCopyright © 2022 Chen, Hang, Wu, Zhang, Zhang, Yang, Xie and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Yang, eWFuZ2p1bkB4aW5odWFtZWQuY29tLmNu; Jin Xie, eGllamluQHhpbmh1YW1lZC5jb20uY24=; Jingrong Lu, bHZqaW5ncm9uZ0B4aW5odWFtZWQuY29tLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.