
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr., 23 June 2022
Sec. Pediatric Oncology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.923419
This article is part of the Research TopicThe Future of Pediatric Precision Oncology: Novel Targets, Innovative Drug Combinations and Targeted Protein DegradationView all 5 articles
With the markedly increased cure rate for children with newly diagnosed pediatric B-cell acute lymphoblastic leukemia (B-ALL), relapse and refractory B-ALL (R/R B-ALL) remain the primary cause of death worldwide due to the limitations of multidrug chemotherapy. As we now have a more profound understanding of R/R ALL, including the mechanism of recurrence and drug resistance, prognostic indicators, genotypic changes and so on, we can use newly emerging technologies to identify operational molecular targets and find sensitive drugs for individualized treatment. In addition, more promising and innovative immunotherapies and molecular targeted drugs that are expected to kill leukemic cells more effectively while maintaining low toxicity to achieve minimal residual disease (MRD) negativity and better bridge hematopoietic stem cell transplantation (HSCT) have also been widely developed. To date, the prognosis of pediatric patients with R/R B-ALL has been enhanced markedly thanks to the development of novel drugs. This article reviews the new advancements of several promising strategies for pediatric R/R B-ALL.
Acute lymphoblastic leukemia (ALL), particularly B-cell lineage ALL (B-ALL), which has been the most prevalent childhood tumor, is a malignant disease characterized by uncontrolled proliferation of immature lymphoid cells that can invade bone marrow, blood, and extramedullary (EM) sites (1), especially in the central nervous system (CNS) or testicle. Since 1960, with the application of combined chemotherapy, risk stratification, CNS prevention strategies and minimal residual disease (MRD) monitoring, the cure rate of newly diagnosed pediatric ALL has improved steadily. At present, the 5-year overall survival (OS) rate of children with ALL has reached over 90% (2–4); the cumulative recurrence rate has been reduced to less than 10% (5).
Although there have been some significant developments during the past few decades, the outcome of patients with relapsed or refractory (R/R) ALL remains static (6–8). The relapse rate has been reported to be 15–20% in developed countries over the last two decades. However, there has been no standard treatment for patients with R/R ALL, and the long-term survival rate after recurrence is approximately 30∼60% (9–14), depending on the duration of follow-up and the risk groups involved. The 10-year OS and EFS rates were 36 and 30%, respectively, for children treated in the Acute Lymphoblastic Leukemia Relapse Berlin-Frankfurt-Munster (ALL-REZ-BFM) 90 trial (9). Similarly, the 5-year OS and EFS rates of children with first-relapsing ALL treated on the United Kingdom (UK) ALL R2 protocol were 56 and 47%, respectively (15). In Nordic countries, the 5-year OS was 44.7% during 1992–2001 and 57.5% during 2002–2011 (16). With the first relapse of ALL, fewer than 50% of children survive long-term, and the prognosis is even worse for relapses two or later, with a survival rate of approximately 20%.
Of note, R/R ALL is frequently associated with treatment resistance, possibly arising from enrichment of preexisting resistant subclones and/or from mutation acquisition during chemotherapy exposure (2, 17). A comprehensive genomic characterization of the diagnosis-relapse pair observed a dynamic clonal evolution in all cases, with relapse almost exclusively originating from a subclone at diagnosis (18).
Considering that the cause of relapse is related to chemoresistance (19) and that modern chemotherapy regimens have reached the limits of their tolerance, which can no longer improve, it is vital to combine chemotherapy with accurate individualized therapy based on immunotherapy and targeted therapy to amplify the cure rates and quality of life in the future. To date, the continuous development of newly emerging targeted immunotherapies has proven to lead to superior disease-free survival and OS rates, markedly lower toxicities and better MRD clearance. This article focuses on new advancements in several promising strategies for choosing the most proper and effective treatments for R/R B-ALL.
Refractory ALL is characterized by poor sensitivity to commonly used chemotherapeutic drugs, a low clinical remission rate and a significantly shortened survival cycle. Schmid et al. (20) stated that refractory acute leukemia can be defined as having at least one of the following conditions: (1) Failure of initial induction therapy after two or more courses of treatment; (2) early recurrence less than 6 months after the first remission; (3) inefficacy to response to induction chemotherapy after recurrence; and (4) multiple relapses.
Relapse of ALL is defined as recurrence of the disease at any site after a period of complete remission, either while still on or after completion of front-line therapy (21). In the BFM group study, very early was defined as less than 18 months from diagnosis; early as more than 18 months from diagnosis and less than 6 months from treatment discontinuation; and late as more than 6 months from treatment discontinuation (22). In the Children’s Oncology Group (COG) study, early diagnosis was less than 36 months from initial diagnosis; late diagnosis was 36 months or more from initial diagnosis. In the St Jude’s Children’s Research Hospital study, early was less than 6 months from completion of frontline therapy; late was 6 months or more from completion of frontline therapy.
The current treatment protocols for relapsed ALL stratify patients according to their clinical characteristics at diagnosis and relapse and offer different therapeutic options (23).
Previous trials have revealed that the site of relapse (9) and duration of first CR indeed influence both event-free survival (EFS) and OS in childhood ALL (24, 25). In general, isolated bone marrow and early relapse (26) are associated with worse prognoses than isolated extramedullary or late relapse (12, 24, 26).
Nguyen et al. substantiated that the time to relapse, age over 10 years old, presence of CNS disease at diagnosis and male sex were significant predictors of inferior post-relapse (27). Patients suffering very early BM relapse had a particularly terrible outcome, with a survival rate of 0–20% (27, 28), while those experiencing early BM relapses had survival rates from 10 to 40% (29). For late BM relapse patients, survival rates range from 14 to 50% (30).
In addition, persistence of a negative measurable MRD at the end of induction or consolidation therapy correlates with a better chance of surviving after hematopoietic stem cell transplant (HSCT) (31–33), as a number of previous studies have proved that the risk of relapse after transplantation is higher in patients with MRD positivity before HSCT than in those without detectable MRD (34–36).
Moreover, a study conducted by Irving et al. confirmed that TP53 alterations and NR3C1/BTG1 deletions were associated with a higher risk of progression (37). Additionally, DNA methylation, which is a worthy factor, holds prognostic information in relapsed B-ALL (38). Thus, screening relapse patients for key genetic abnormalities will improve prognosis and risk stratification.
Modern R/R B-ALL therapy continues to be based on standard principles, including induction chemotherapy, stem cell transplantation and analogous supportive care (6, 39, 40), particularly the prevention and treatment of infections. Most ALL relapses occur during treatment or within the first 2 years after treatment completion (41). The traditional strategy is to administer chemotherapy drugs at the maximum tolerated dose (MTD), which aims to kill as many tumor cells as possible. However, modern protocols, such as the BFM ALL 2000 protocol, allow for lower treatment dose intensity to improve quality of life, especially for low-risk pediatric patients, while maintaining a high cure rate (42, 43). Moreover, only specific subgroups of patients could rebound from low-intensity chemotherapy (43).
However, some studies have confirmed that low-dose cytarabine and Accra-Adriamycin combined with granulocyte colony-stimulating factor cannot improve the survival prognosis of patients with R/R ALL compared to the Hyper-CVAD regimen (44). A retrospective study evaluated the safety and efficacy of a salvage regimen consisting of G-CSF, low-dose cytarabine, aclarubicin, L-asparaginase and prednisone among R/R ALL patients. The incidence of 2-year OS was approximately 30%, the disease-free survival rate was 15%, and the drug-related mortality rate was 5.6% (45).
Clofarabine, a second-generation purine nucleoside analog, has been approved by the United States Food and Drug Administration (FDA) for treating pediatric R/R ALL patients based on prior phase II studies, with an overall remission rate of 45–55% (46–48). A phase II trial of clofarabine in combination with etoposide and cyclophosphamide conducted in pediatric R/R ALL patients showed encouraging response rates and sustained remission, with an overall remission rate of 44% (39).
Due to multidrug chemotherapy, 70–98% of first-relapse patients achieve a second CR, depending on the risk stratification group (15, 24, 27, 49). Pediatric patients with second or third bone marrow relapse have even poorer outcomes, with only 44 and 27%, respectively, achieving a subsequent CR (50).
Nevertheless, according to the trials carried out by St. Jude Children’s Hospital, OS rates have been similar recently without a notable upgrade (3, 42, 51), which indicates that the existing treatment has been pushed to its limit. In addition, the prevailing view about the emergence of ALL relapse is that it might originate from a chemoresistant clone (52). These quiescent dormant cells are less sensitive to cytotoxic drugs and hide in niches where the microenvironment confers protection from chemotherapy, escaping therapeutic killing (53–55). Thus, new antileukemic agents are urgently needed. The origin of relapse and the simple mechanism of several promising drugs are displayed in Figure 1 below.
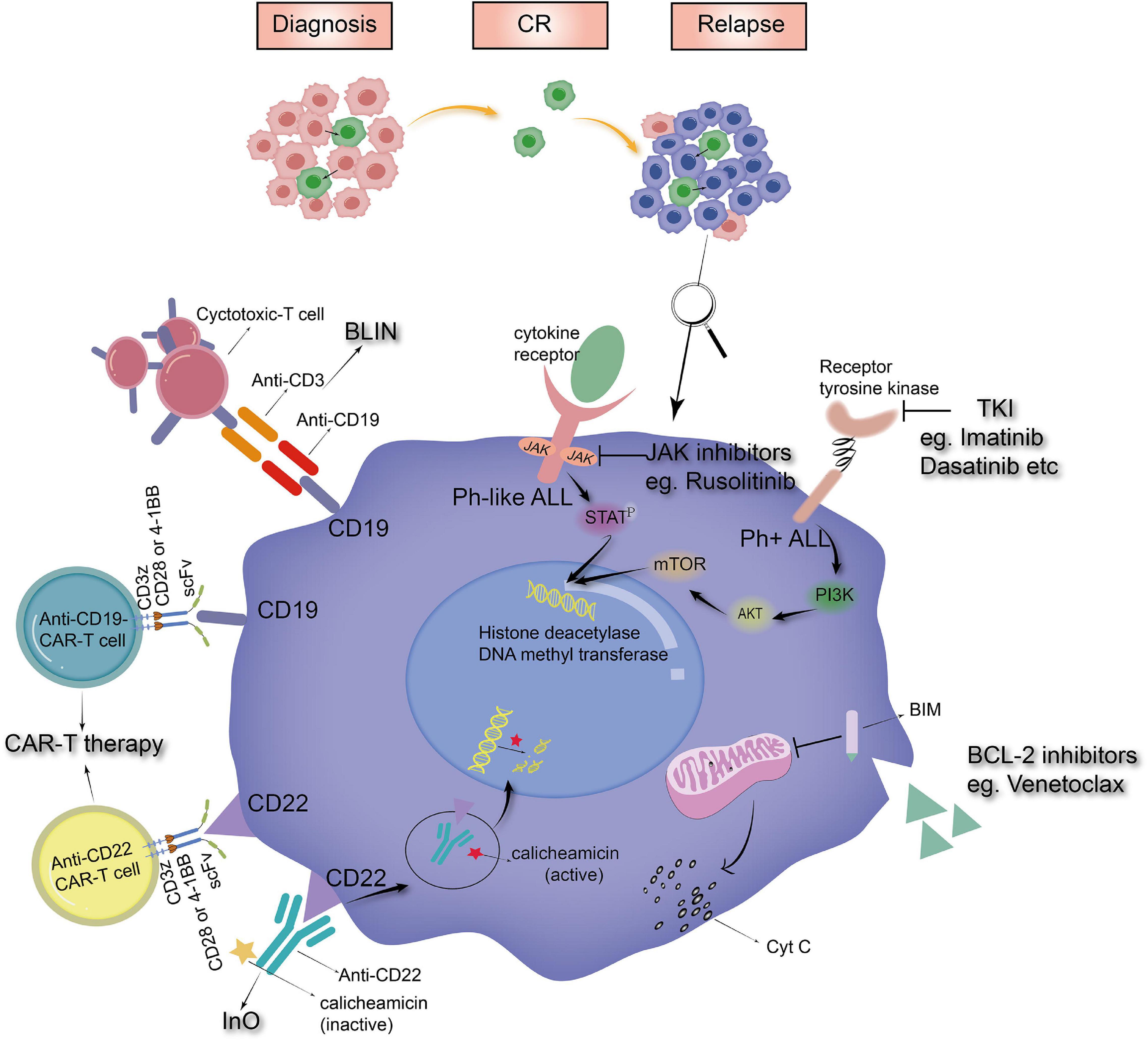
Figure 1. The origin of relapse and the mechanism of novel drugs (BLIN, blinatumomab; InO, inotuzumab ozogamicin; CAR-T therapy, chimeric antigen receptor modified T-cell therapy; TKI, tyrosine kinase inhibitors).
Blinatumomab, a bispecific monoclonal antibody construct, enables CD3-positive T cells to recognize and eliminate CD19-positive ALL cells (56, 57). It was approved for the treatment of R/R B-cell ALL by the FDA on July 11, 2017 (58). Current studies have demonstrated that blinatumomab has significant monotherapy activity, which induces superior remission among refractory and relapsed patients with markedly lower toxicities in adult patients (59, 60). The main toxicities of blinatumomab are central nervous system injury and cytokine release syndrome (CRS).
Pediatric data using blinatumomab are still quite scarce, and the results of most adult trials and many adult reviews cannot simply be transferred to the pediatric environment due to differences in biology and prognosis between adults and children. According to the existing clinical trials in children, we can obtain such results that regardless of the single-arm test or compared with the current chemotherapy regimen, blinatumomab also shows good efficacy and tolerability. The relevant clinical trials and their results are shown in Table 1.
Astonishingly, blinatumomab has particular value in fragile children, such as those with Down Syndrome, or children carrying poor-risk molecular/cytogenetic features (including TCF3-HLF-positive B-ALL) (63, 65), which has greatly encouraged us to apply it to some rare refractory ALL subtypes and as a bridge tool to HSCT.
Instead, patients treated with blinatumomab had fewer side effects of hematological toxicity and infection, whereas central nervous system events, such as encephalopathy, tremor and depression, were more frequent. Clinical trials suggest that the low reactivity of blinatumomab is related to high tumor burden (68). It seems to be a poor choice for patients with bone marrow blasts over 50%, a previous history of leukemia or concurrent extramedullary leukemia.
Of note, to solve the problem of the rapid occurrence of chemoresistance and easy relapse, an innovative treatment without chemotherapy is being studied. To date, in a phase II trial, researchers have treated patients with dasatinib plus glucocorticoids, followed by two cycles of blinatumomab without chemotherapy (69). Of the 63 patients selected, the incidences of CR and OS were 98 and 95%, respectively, with a disease-free survival rate of 88%. This new regimen is feasible, effective and safe, the treatment-related mortality rate is very low, and the overall and disease-free survival outcomes are very promising.
Inotuzumab ozogamicin (InO) is an antibody–drug conjugate (ADC) composed of a humanized anti-CD22 monoclonal antibody conjugated with calicheamicin that can induce double-stranded DNA breakage and subsequent cell death. The FDA approved InO for the treatment of R/R B-ALL in February 2017. Presently, in vitro experiments have confirmed that the drug is safe and effective against B-line ALL in children, and its main side effect is hepatotoxicity, especially sinus obstruction syndrome after transplantation (70–73).
It was reported that CD22 is expressed in the vast majority of childhood B-cell precursor ALL (BCP-ALL), but pediatric experience with InO is limited. Nevertheless, existing clinical trials have implied that InO can induce deep remission, maximize EFS and OS, and potentially minimize treatment-associated toxicity in patients with R/R ALL, along with a superior CR rate ranging from 45 to 67%, and promote subsequent HSCT or other treatments.
Although most studies have shown that patients receiving InO have better quality of life and superior disease-free survival, hepatotoxicity is more frequent in the InO arm (51% vs. 34%); the incidence of sinusoidal obstruction syndrome (SOS), drug-induced liver injuries (DILI) and venous occlusive disease after InO treatment was 1.5, 7.9, and 11%, respectively, which were seen more frequently than in the control group receiving standardized chemotherapy (1, 1, and 1%) (74). Table 2 lists several relevant InO results applied to pediatrics.
Chimeric antigen receptor modified T (CAR-T) cell therapy has recently emerged as a promising tactic for treating B-lineage malignancies. It has been developed a great deal recently. The structure of the latest generation of CAR-T cells includes the extracellular antibody domain of a single chain variant fragment (ScFv) targeting the surface markers of leukemic cells, the intracellular T-cell signal domain, including the CD3ζ domain and costimulatory domain, such as 4-1BB or CD28, as well as more functional elements, such as cytokines (78–80). It can be produced not only from autologous T cells but also from allogeneic T cells after a previous allogeneic HSCT at disease recurrence, rarely leading to graft-vs.-host disease. Approval for the treatment of R/R ALL in pediatric and young adult patients has been granted. Additionally, the FDA approved the use of Breyanzi (lisocabtagene maraleucel, liso-cel) for the treatment of R/R large B-cell lymphoma on February 5, 2021 (81). Odora Anagnostou et al. systematically reviewed and analyzed the efficacy of CD19-specific CAR-T-cell therapy, and the CR rate and cumulative recurrence rate after treatment were 81 and 36%, respectively (82). The CR rates of patients with autologous and allogeneic T-cell-derived CAR-T cells were 83 and 34%, respectively. CD22 CAR-T cells are confirmed to be an effective salvage therapy for patients who have experienced relapse after or are refractory to CD19-targeted therapies.
Notably, the control of EM relapse with CAR-T cells and their penetration of the blood–testis barrier and blood–brain barrier have been reported (83–85). In a post hoc analysis, 195 patients with relapsed or refractory CD19-positive ALL or lymphocytic lymphoma in which participants received CD19-directed CAR T-cell therapy were recruited. Among these 195 patients, the CR rate was similar between the CNS-positive group and the CNS-negative group (97% vs. 96%), with no significant difference in relapse-free survival (60% vs. 60%) or OS rate (83% vs. 71%). The study confirmed that CAR-T therapy actively clears CNS disease and maintains durable remission in children and young adults with CNS relapsed or refractory B-ALL or lymphocytic lymphoma without increasing the risk of severe neurotoxicity (86).
The most common adverse reactions are CRS (87, 88) and attention deficit or insanity, with incidences of up to 75∼100% (89, 90) and 64% (91), respectively. Other toxicities include infusion reaction, tumor lysis syndrome, allergic reaction and immunogenicity, infection and anti-infection prevention.
With the continuous research and development of CAR-T cells, different modified CAR-T cells have also been developed. The experiments of Magnani et al. suggested that CAR-T cells genetically engineered with sleeping beauty (SB) transposons and differentiated into cytokine-induced killer (CIK) cells have good anti-leukemia activity and no serious side effects (92). Dai et al. conducted a study on CD19/CD22 bispecific CAR T-cell therapy in patients with relapse or inefficacy after CD19 targeted therapy (93). It was confirmed that bispecific CAR T cells could stimulate strong antileukemic activity; the MRD-negative CR rate was 100%, and the incidence of neurotoxicity was 0.
Nevertheless, despite its many advantages over other forms of cancer therapy, including in vivo expansion and long-term persistence, treatment with CAR-T cells remains a work in progress. Therefore, the alternative platform of CAR projects and gene editing techniques (such as CRISPR–Cas9 gene editing) have also been introduced into the construction of CARs to overcome the current limitations of this therapy. New alternative platforms include γδ T cells and NK cells. Using γδ T cells from donors as the host of CAR can avoid graft host reaction because these cells lack allogeneic (94, 95). In vivo experiments have proven that it is feasible to produce γδ CAR-T effector cells with high purity and high efficiency (96). Another research hotspot is CAR-NK cells, which are cheaper and less toxic than CAR-T cells (97–102). In phase I and phase II trials, 64% of patients with R/R CD19-positive cancers achieved remission after receiving anti-CD19 CAR-NK-cell therapy, with an adverse reaction rate of 0% (103). Similarly, CRISPR/Cas9 technology is unveiling a new era for CAR-T-cell therapy (104–107). Through genome editing technology, it is possible to create the next generation of CAR T-cell products, including universal CAR T cells, by destroying endogenous T-cell receptors (TCRs) or human leukocyte antigens (HLAs), removing inhibitory regulators to produce more powerful CAR T cells, and adding suicide genes or inducible safety switches to create more controllable CAR T cells. The relevant clinical trials and their results are shown in Table 3.
With the increased understanding of genetic mutations discovered in ALL, treatments targeting genetic mutations and signaling pathways are emerging. As a new landmark therapeutic approach, targeted therapy has been described as a promising treatment for pediatric ALL with specific genetic abnormalities. The main purpose of using molecularly targeted drugs combined with chemotherapy or immunotherapy is to improve outcomes of R/R B-ALL patients (113). Herein, there are many novel molecular targeted drugs under development.
Of the chromosomal abnormalities described in ALL, the most common is a fusion gene called BCR-ABL. This disorder is classified as Philadelphia chromosome-positive (Ph +) ALL (114–116). Ph + ALL patients account for approximately 3% of pediatric ALL patients. Prior to the emergence of tyrosine kinase inhibitors (TKIs) targeting the BCR-ABL gene, the 5-year EFS of Ph + ALL patients with chemotherapy alone was dismal, at only approximately 30% (117), and the OS rate of chemotherapy combined with HSCT was approximately 45%. However, the incorporation of TKI into the chemotherapy regimen significantly increased the long-term survival rate of Ph + patients, with a long-term survival rate of nearly 60–75% in the imatinib group (118–120) and over 80% in the dasatinib group (119, 121).
The first generation of TKI is imatinib. In patients with recurrent Ph + ALL, the initial CR rate of imatinib is 20–29%, and the CR rate is more than 90% when imatinib is added to intensive chemotherapy (122). The 5-year OS rate and EFS rate of the second-generation TKI dasatinib are 86 and 60%, respectively (123, 124). However, taking into account the rapid development of drug resistance and short response (125–128), a new generation of TKIs, such as ponatinib (129), has been developed. A phase II clinical trial conducted by Cortes et al. (130) on second-generation TKI resistance or intolerance or CML and ALL carrying T315I mutations in adults showed that the 5-year OS and EFS of the patients reached 73 and 53%, respectively, confirming the third-generation BCR-ABL inhibitor ponatinib. It can overcome the problem of drug resistance of second-generation TKIs (131). Its application in adults plays a good role in predicting the application of ponatinib in children with Ph + ALL. The clinical efficacy of ponatinib in children with Ph + ALL needs further study.
Ph-like ALL has a gene expression pattern similar to Ph + ALL but lacks the BCR-ABL fusion gene (132). The activation of the JAK pathway, which is a cytoplasmic tyrosine kinase with a crucial role in signal transduction from multiple hemopoietic growth-factor (HGF) receptors, is one of the most common abnormal events in Ph-like ALL (133). As the first approved inhibitor of the JAK kinase family, ruxolitinib has particular efficacy against both JAK1 and JAK2 (134) and was approved as the first inhibitor of the JAK family by the FDA and the European Drug Administration (EMA) in 2011 and 2012, respectively (135–138). Another JAK2 inhibitor, pacritinib (139), has also shown exceptional efficacy in hematologic malignancy (140). Other inhibitors, including tofacitinib (141) and peficitinib (142), mainly inhibit JAK3 and have been approved for the treatment of rheumatoid arthritis (RA) in the United States.
Preliminary clinical studies have confirmed that roxolitinib is well tolerated in the treatment of R/R tumors (including leukemia) in children (134, 137). Notably, several Ph + ALL mouse models constructed by Appelmann and Kong et al. confirmed that dasatinib (143) or nilotinib (144) combined with ruxolitinib significantly prolonged the survival time of mice, eliminated leukemia proliferating cells (LPCs) more effectively, and decreased the activity of phosphorylated JAK2 at the molecular level, thus limiting the emergence of ABL1 mutants of dasatinib and reducing drug resistance and recurrence compared to single-agent therapy both in vitro and in humanized mice.
In addition, it has been reported that triple intrathecal injection (IT) therapy plus ruxolitinib was well tolerated by Ph-negative B-ALL patients with systemic and central nervous system recurrence and successfully eradicated highly refractory leptomeningeal ALL (132, 145). This case confirms that ruxolitinib combined with chemotherapy can eradicate chemotherapy-resistant CNS leukemia.
The BCL-2 protein family, which is critical for controlling cell survival, has been identified as one of the six hallmarks of cancer (146), containing six anti-apoptotic proteins, such as BCL-2, and several proapoptotic proteins that maintain the balance between cell death and survival. BCL-2, which can confer a survival advantage to tumor cells by preventing apoptosis (147, 148), is particularly dysregulated in multiple human cancers (149–152). Therefore, overexpression of BCL-2 might be associated with chemoresistance. Ventoclax (ABT-199), as an efficient and safe BCL-2 inhibitor, has become a novel method of targeted therapy in ALL. Navitoclax (ABT-263) has been demonstrated as a single agent or in combination with other drugs to successfully ameliorate leukemia progression while easily causing significant thrombocytopenia (153, 154). Other inhibitors include ABT-737 (155).
To date, the unexpected efficacy and potential therapeutic strategies of ventoclax observed in patient-derived xenograft tumor (PDX) models lacking BIM expression and in MLL-rearranged ALL xenografts in vivo and in vitro have been confirmed (156, 157). Ventoclax combined with other drugs can increase chemosensitivity, prevent drug resistance, and reduce the incidence of dose-dependent side effects of chemotherapy compared to the single agent use of this drug. Moreover, several clinical cases of ETP-ALL have proven that ventoclax combined with low-intensity chemotherapy (158), nelarabine (159), decitabine (160) or bortezomib (161) has a good antileukemia effect.
R/R ALL is characterized by clonal heterogeneity. Genetic and epigenetic abnormalities, which can increase the proliferation potential of leukemic cells, drive treatment resistance and eventually give rise to relapse or treatment failures (162). In the last two decades, there have been important genomic discoveries in ALL, such as RNA sequencing (RNA-seq), next-generation sequencing (NGS) and genomic-capture high-throughput sequencing (gc-HTS-seq) (163), which play an essential role in risk stratification and have therapeutic and prognostic implications (163–165).
Notably, comprehensive genomic studies have revolutionized our understanding of the molecular taxonomy of ALL by clarifying the subclassification of ALL. Li et al. performed an initial study to reanalyze and delineate the transcriptome landscape of BCP-ALL through RNA-seq, which identified six additional undescribed gene expression subgroups apart from eight previously described subgroups and revealed related prognostic stratification. In addition, gc-HTS seq also expands the spectrum of suitable MRD targets and allows for the identification of genomic fusions associated with risk and treatment stratification in childhood ALL. Similarly, a novel technique called digital multiplex ligation-dependent probe amplification (digital MLPA™) can provide fast and highly optimized aberration profiling for the decipherment of the clonal origin of relapse and yields extremely relevant information for clinical prognosis assessment (166).
The results described above strongly suggest that genomic techniques should be introduced into the clinical diagnostic workup. Recently, personalized medicine in ALL has been used to characterize patients into prognosis groups based on karyotypes to guide treatment options. The highlights of these techniques include detecting inherited predispositions of ALL, finding relevant molecularly targeted therapies through genomic-defined ALL subtypes and monitoring treatment response via pharmacogenomics and novel MRD biomarkers (167). These mutations confer chemoresistance and might have implications for therapeutic decisions. Insights into the genomics of ALL further provide a compelling biologic rationale to expand the scope of precision medicine therapies for childhood ALL. Ultimately, tailored treatment strategies for ALL-related gene damage and pathways might improve antileukemic efficacy, diminish recurrence, and reduce adverse toxicity.
However, the choice of options for follow-up personalized treatment only through abnormities in patients’ genotypes is extremely limited.
Currently, high-throughput drug sensitivity (HDS) screening has already been used for the development of personalized treatment approaches in acute myeloid leukemia (AML). It can improve the long-term survival rate of AML patients through individual molecular characteristics or drug sensitivity profiles (168). A vast number of preliminary clinical trials have exhibited outstanding and encouraging benefits in seeking optimum individualized approaches based on HDS screening (169–171).
Intriguingly, EI Andersson et al. systematically explored the diversity of drug responses in T-cell prolymphocytic leukemia (T-PLL) patient samples by using this platform and correlated the findings with somatic mutations and gene expression profiles. The trial showed that all T-PLL samples were sensitive to SNS-032, a cyclin-dependent kinase inhibitor, indicating previously unexplored targeted tactics for treating T-PLL, which has notoriously chemorefractory behavior (172).
In the absence of targetable mutations, these drug sensitivity screenings can provide treatment options rather than genetic abnormalities. However, HDS screening of anticancer drugs has not yet been generally applied in clinical practice for ALL patients, and it has great potential in personalized treatment, particularly in drug target problems for chemo-resistance groups (173). By performing HDS screening, we can analyze and determine optimal individual dosages combined with or without targeted drugs, which improves treatment efficacy while reducing or avoiding toxicity (174–176). Nevertheless, we still need larger trials to evaluate the utility of these technologies in routine clinical settings along with long turnover times and high costs.
R/R ALL is still a difficult topic among children. Existing immunotherapy and molecular targeted therapy have proven their effectiveness, feasibility and safety, and they have significantly improved the prognosis of patients since they were approved for use. The latest research results also prove the prognosis of the era without chemotherapy. Moreover, novel techniques, such as whole-genome sequencing, next-generation sequencing and HDS screening, play a vital role in selecting appropriate individualized drugs and reducing treatment failure caused by drug resistance. For the treatment of patients with R/R B-ALL, we need a comprehensive understanding of patients’ genotypes and immune indicators, combined with the course of the disease, economic status and level of medical technology in the country, to choose the appropriate treatment.
In the future, we will further study the molecular mechanism of R/R ALL, design safer and more effective precision medicine to address the chemotherapy resistance and recurrence of tumor cells, prolong life expectancy and enhance the quality of life of pediatric patients. It is believed that the dismal situation of children with R/R ALL will be overcome.
SM collected the data and wrote the manuscript. ZF and JR reviewed and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
This research was supported by the National Natural Science Foundation of China (No. 82070172).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. (2015) 373:1541–52.
3. Jeha S, Pei D, Choi J, Cheng C, Sandlund JT, Coustan-Smith E, et al. Improved CNS control of childhood acute lymphoblastic leukemia without cranial irradiation: st jude total therapy study 16. J Clin Oncol. (2019) 37:3377–91. doi: 10.1200/JCO.19.01692
4. Freyer DR, Devidas M, La M, Carroll WL, Gaynon PS, Hunger SP, et al. Postrelapse survival in childhood acute lymphoblastic leukemia is independent of initial treatment intensity: a report from the Children’s Oncology Group. Blood. (2011) 117:3010–5. doi: 10.1182/blood-2010-07-294678
5. Wyatt KD, Bram RJ. Immunotherapy in pediatric B-cell acute lymphoblastic leukemia. Hum Immunol. (2019) 80:400–8.
6. Bhojwani D, Pui C-H. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol. (2013) 14:e205–17.
7. Lew G, Chen Y, Lu X, Rheingold SR, Whitlock JA, Devidas M, et al. Outcomes after late bone marrow and very early central nervous system relapse of childhood B-acute lymphoblastic leukemia: a report from the Children’s Oncology Group phase III study AALL0433. Haematologica. (2021) 106:46–55. doi: 10.3324/haematol.2019.237230
8. Parker C, Krishnan S, Hamadeh L, Irving JAE, Kuiper RP, Révész T, et al. Outcomes of patients with childhood B-cell precursor acute lymphoblastic leukaemia with late bone marrow relapses: long-term follow-up of the ALLR3 open-label randomised trial. Lancet Haematol. (2019) 6:e204–16. doi: 10.1016/S2352-3026(19)30003-1
9. Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A, et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol. (2010) 28:2339–47. doi: 10.1200/JCO.2009.25.1983
10. Herold R, von Stackelberg A, Hartmann R, Eisenreich B, Henze G. Acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group (ALL-REZ BFM) experience: early treatment intensity makes the difference. J Clin Oncol. (2004) 22:569–70. doi: 10.1200/JCO.2004.99.153
11. Seibel NL. Survival after relapse in childhood acute lymphoblastic leukemia. Clin Adv Hematol Oncol. (2011) 9:476–8.
12. Bailey LC, Lange BJ, Rheingold SR, Bunin NJ. Bone-marrow relapse in paediatric acute lymphoblastic leukaemia. Lancet Oncol. (2008) 9:873–83. doi: 10.1016/S1470-2045(08)70229-8
13. van den Berg H, de Groot-Kruseman HA, Damen-Korbijn CM, de Bont ES, Schouten-van Meeteren AY, Hoogerbrugge PM. Outcome after first relapse in children with acute lymphoblastic leukemia: a report based on the Dutch Childhood Oncology Group (DCOG) relapse all 98 protocol. Pediatr Blood Cancer. (2011) 57:210–6. doi: 10.1002/pbc.22946
14. Hunger SP, Raetz EA. How I treat relapsed acute lymphoblastic leukemia in the pediatric population. Blood. (2020) 136:1803–12. doi: 10.1182/blood.2019004043
15. Roy A, Cargill A, Love S, Moorman AV, Stoneham S, Lim A, et al. Outcome after first relapse in childhood acute lymphoblastic leukaemia - lessons from the United Kingdom R2 trial. Br J Haematol. (2005) 130:67–75. doi: 10.1111/j.1365-2141.2005.05572.x
16. Oskarsson T, Soderhall S, Arvidson J, Forestier E, Montgomery S, Bottai M, et al. Relapsed childhood acute lymphoblastic leukemia in the Nordic countries: prognostic factors, treatment and outcome. Haematologica. (2016) 101:68–76. doi: 10.3324/haematol.2015.131680
17. Yang JJ, Cheng C, Yang W, Pei D, Cao X, Fan Y, et al. Genome-wide interrogation of germline genetic variation associated with treatment response in childhood acute lymphoblastic leukemia. JAMA. (2009) 301:393–403. doi: 10.1001/jama.2009.7
18. Antic Z, Yu J, Bornhauser BC, Lelieveld SH, van der Ham CG, van Reijmersdal SV, et al. Clonal dynamics in pediatric B-cell precursor acute lymphoblastic leukemia with very early relapse. Pediatr. Blood Cancer. (2022) 69:e29361. doi: 10.1002/pbc.29361
19. Li BS, Brady SW, Ma XT, Shen S, Zhang YC, Li YJ, et al. Therapy-induced mutations drive the genomic landscape of relapsed acute lymphoblastic leukemia. Blood. (2020) 135:41–55. doi: 10.1182/blood.2019002220
20. Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D, et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. (2006) 108:1092–9. doi: 10.1182/blood-2005-10-4165
21. Henze G, Stackelberg AV, Eckert C. ALL-REZ BFM–the consecutive trials for children with relapsed acute lymphoblastic leukemia. Klin Padiatr. (2013) 225(Suppl 1):S73–8. doi: 10.1055/s-0033-1337967
22. Brown PA, Shah B, Advani A, Aoun P, Boyer MW, Burke PW, et al. Freedman-Cass, and M. Campbell, Acute Lymphoblastic Leukemia, Version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:1079–109. doi: 10.6004/jnccn.2021.0042
23. Locatelli F, Schrappe M, Bernardo ME, Rutella S. How I treat relapsed childhood acute lymphoblastic leukemia. Blood. (2012) 120:2807–16. doi: 10.1182/blood-2012-02-265884
24. Malempati S, Gaynon PS, Sather H, La MK, Stork LC, Children’s Oncology G. Outcome after relapse among children with standard-risk acute lymphoblastic leukemia: Children’s Oncology Group study CCG-1952. J Clin Oncol. (2007) 25:5800–7. doi: 10.1200/JCO.2007.10.7508
25. Eckert C, Parker C, Moorman AV, Irving JAE, Kirschner-Schwabe R, Groeneveld-Krentz S, et al. Risk factors and outcomes in children with high-risk B-cell precursor and T-cell relapsed acute lymphoblastic leukaemia: combined analysis of ALLR3 and ALL-REZ BFM 2002 clinical trials. Eur J Cancer. (2021) 151:175–89. doi: 10.1016/j.ejca.2021.03.034
26. Einsiedel HG, von Stackelberg A, Hartmann R, Fengler R, Schrappe M, Janka-Schaub G, et al. Long-term outcome in children with relapsed ALL by risk-stratified salvage therapy: results of trial acute lymphoblastic leukemia-relapse study of the Berlin-Frankfurt-Munster Group 87. J Clin Oncol. (2005) 23:7942–50. doi: 10.1200/JCO.2005.01.1031
27. Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. (2008) 22:2142–50. doi: 10.1038/leu.2008.251
28. Wheeler K, Richards S, Bailey C, Chessells J. Comparison of bone marrow transplant and chemotherapy for relapsed childhood acute lymphoblastic leukaemia: the MRC UKALL X experience. Medical Research Council Working Party on Childhood Leukaemia. Br J Haematol. (1998) 101:94–103. doi: 10.1046/j.1365-2141.1998.00676.x
29. Gaynon PS, Qu RP, Chappell RJ, Willoughby MLN, Tubergen DG, Steinherz PG, et al. Survival after relapse in childhood acute lymphoblastic leukemia - Impact of site and time to first relapse - the Children’s Cancer Group experience. Cancer. (1998) 82:1387–95. doi: 10.1002/(sici)1097-0142(19980401)82:7<1387::aid-cncr24>3.0.co;2-1
30. Chessells JM. Relapsed lymphoblastic leukaemia in children: a continuing challenge. Br J Haematol. (1998) 102:423–38. doi: 10.1046/j.1365-2141.1998.00776.x
31. Eckert C, Henze G, Seeger K, Hagedorn N, Mann G, Panzer-Grumayer R, et al. Use of allogeneic hematopoietic stem-cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. (2013) 31:2736. doi: 10.1200/JCO.2012.48.5680
32. Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T, et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol. (2015) 168:395–404. doi: 10.1111/bjh.13142
33. Truong TH, Jinca C, Mann G, Arghirescu S, Buechner J, Merli P, et al. Allogeneic hematopoietic stem cell transplantation for children with acute lymphoblastic leukemia: shifting indications in the era of immunotherapy. Front Pediatr. (2021) 9:782785. doi: 10.3389/fped.2021.782785
34. Coustan-Smith E, Gajjar A, Hijiya N, Razzouk BI, Ribeiro RC, Rivera GK, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia after first relapse. Leukemia. (2004) 18:499–504.
35. Paganin M, Zecca M, Fabbri G, Polato K, Biondi A, Rizzari C, et al. Minimal residual disease is an important predictive factor of outcome in children with relapsed ‘high-risk’ acute lymphoblastic leukemia. Leukemia. (2008) 22:2193–200. doi: 10.1038/leu.2008.227
36. Lovisa F, Zecca M, Rossi B, Campeggio M, Magrin E, Giarin E, et al. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol. (2018) 180:680–93. doi: 10.1111/bjh.15086
37. Irving JAE, Enshaei A, Parker CA, Sutton R, Kuiper RP, Erhorn A, et al. Integration of genetic and clinical risk factors improves prognostication in relapsed childhood B-cell precursor acute lymphoblastic leukemia. Blood. (2016) 128:911–22. doi: 10.1182/blood-2016-03-704973
38. Borssen M, Nordlund J, Haider Z, Landfors M, Larsson P, Kanerva J, et al. DNA methylation holds prognostic information in relapsed precursor B-cell acute lymphoblastic leukemia. Clin Epigenet. (2018) 10:31. doi: 10.1186/s13148-018-0466-3
39. Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. (2011) 118:6043–9. doi: 10.1182/blood-2011-08-374710
40. Messinger YH, Gaynon PS, Sposto R, van der Giessen J, Eckroth E, Malvar J, et al. Bortezomib with chemotherapy is highly active in advanced B-precursor acute lymphoblastic leukemia: Therapeutic Advances in Childhood Leukemia & Lymphoma (TACL) Study. Blood. (2012) 120:285–90. doi: 10.1182/blood-2012-04-418640
41. Gaynon PS. Childhood acute lymphoblastic leukaemia and relapse. Br J Haematol. (2005) 131:579–87.
42. Pui CH. Precision medicine in acute lymphoblastic leukemia. Front Med. (2020) 14:689–700. doi: 10.1007/s11684-020-0759-8
43. Schrappe M, Bleckmann K, Zimmermann M, Biondi A, Moricke A, Locatelli F, et al. Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: results of an international randomized trial (AIEOP-BFM ALL 2000). J Clin Oncol. (2018) 36:244–53. doi: 10.1200/JCO.2017.74.4946
44. Liu L, Jiao W, Zhang Y, Qu Q, Li X, Wu D. Efficacy of low-dose cytarabine and aclarubicin in combination with granulocyte colony-stimulating factor (CAG regimen) compared to Hyper-CVAD regimen as salvage chemotherapy in relapsed/refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Leuk Res. (2015) 39:323–8. doi: 10.1016/j.leukres.2015.01.003
45. Zhou K, Song Y, Zhang Y, Wei X, Fu Y, Yu F, et al. Efficacy and safety of G-CSF, low-dose cytarabine and aclarubicin in combination with l-asparaginase, prednisone in the treatment of refractory or relapsed acute lymphoblastic leukemia. Leuk Res. (2017) 62:29–33.
46. Choi JY, Hong CR, Hong KT, Kang HJ, Kim S, Lee JW, et al. Effectiveness and safety of clofarabine monotherapy or combination treatment in relapsed/refractory childhood acute lymphoblastic leukemia: a pragmatic, non-interventional study in Korea. Cancer Res Treat. (2021) 53:1184–94. doi: 10.4143/crt.2020.289
47. Lu A, Fang Y, Du X, Li Y, Cai Z, Yu K, et al. Efficacy, safety and pharmacokinetics of clofarabine in Chinese pediatric patients with refractory or relapsed acute lymphoblastic leukemia: a phase II, multi-center study. Blood Cancer J. (2016) 6:e400. doi: 10.1038/bcj.2016.8
48. Larson RA. Three new drugs for acute lymphoblastic leukemia: nelarabine, clofarabine, and forodesine. Semin Oncol. (2007) 34:S13–20. doi: 10.1053/j.seminoncol.2007.11.002
49. Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, et al. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. (2005) 103:368–76. doi: 10.1002/cncr.20743
50. Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, et al. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. (2010) 28:648–54. doi: 10.1200/JCO.2009.22.2950
51. Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. (2009) 360:2730–41. doi: 10.1056/NEJMoa0900386
52. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. (2013) 32:135–40. doi: 10.1038/onc.2012.48
53. Ruan YS, Kim HN, Ogana H, Kim YM. Wnt signaling in leukemia and its bone marrow microenvironment. Int J Mol Sci. (2020) 21:6247.
54. Kim HN, Ruan YS, Ogana H, Kim YM. Cadherins, selectins, and integrins in CAM-DR in leukemia. Front Oncol. (2020) 10:592733. doi: 10.3389/fonc.2020.592733
55. Ford AM, Mansur MB, Furness CL, van Delft FW, Okamura J, Suzuki T, et al. Protracted dormancy of pre-leukemic stem cells. Leukemia. (2015) 29:2202–7. doi: 10.1038/leu.2015.132
56. Zhu M, Kratzer A, Johnson J, Holland C, Brandl C, Singh I, et al. Blinatumomab pharmacodynamics and exposure-response relationships in relapsed/refractory acute lymphoblastic leukemia. J Clin Pharmacol. (2018) 58:168–79. doi: 10.1002/jcph.1006
57. Weiland J, Elder A, Forster V, Heidenreich O, Koschmieder S, Vormoor J. CD19: a multifunctional immunological target molecule and its implications for Blineage acute lymphoblastic leukemia. Pediatr Blood Cancer. (2015) 62:1144–8. doi: 10.1002/pbc.25462
58. Pulte ED, Vallejo J, Przepiorka D, Nie L, Farrell AT, Goldberg KB, et al. Blinatumomab for treatment of relapsed and refractory precursor B-cell acute lymphoblastic leukemia. Oncologist. (2018) 23:1366–71.
59. Wolach O, Stone RM. Blinatumomab for the treatment of philadelphia chromosome-negative, precursor B-cell acute lymphoblastic leukemia. Clin Cancer Res. (2015) 21:4262–9. doi: 10.1158/1078-0432.CCR-15-0125
60. Rambaldi A, Ribera JM, Kantarjian HM, Dombret H, Ottmann OG, Stein AS, et al. Blinatumomab compared with standard of care for the treatment of adult patients with relapsed/refractory Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia. Cancer. (2020) 126:304–10. doi: 10.1002/cncr.32558
61. Beneduce G, De Matteo A, Stellato P, Testi AM, Bertorello N, Colombini A, et al. Blinatumomab in children and adolescents with relapsed/refractory b cell precursor acute lymphoblastic leukemia: a real-life multicenter retrospective study in seven aieop (associazione italiana di ematologia e oncologia pediatrica) centers. Cancers. (2022) 14:426. doi: 10.3390/cancers14020426
62. Locatelli F, Whitlock JA, Peters C, Chen-Santel C, Chia V, Dennis RM, et al. Blinatumomab versus historical standard therapy in pediatric patients with relapsed/refractory Ph-negative B-cell precursor acute lymphoblastic leukemia. Leukemia. (2020) 34:2473–8. doi: 10.1038/s41375-020-0770-8
63. Locatelli F, Zugmaier G, Mergen N, Bader P, Jeha S, Schlegel PG, et al. Blinatumomab in pediatric relapsed/refractory B-cell acute lymphoblastic leukemia: RIALTO expanded access study final analysis. Blood Adv. (2022) 6:1004–14. doi: 10.1182/bloodadvances.2021005579
64. Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, et al. Effect of blinatumomab vs chemotherapy on event-free survival among children with high-risk first-relapse b-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. (2021) 325:843–54. doi: 10.1001/jama.2021.0987
65. Clesham K, Rao V, Bartram J, Ancliff P, Ghorashian S, O’Connor D, et al. Blinatumomab for infant acute lymphoblastic leukemia. Blood. (2020) 135:1501–4. doi: 10.1182/blood.2019004008
66. Brown PA, Ji L, Xu X, Devidas M, Hogan LE, Borowitz MJ, et al. Effect of postreinduction therapy consolidation with blinatumomab vs chemotherapy on disease-free survival in children, adolescents, and young adults with first relapse of b-cell acute lymphoblastic leukemia: a randomized clinical trial. JAMA. (2021) 325:833–42. doi: 10.1001/jama.2021.0669
67. Gore L, Locatelli F, Zugmaier G, Handgretinger R, O’Brien MM, Bader P, et al. Survival after blinatumomab treatment in pediatric patients with relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Blood Cancer J. (2018) 8:80. doi: 10.1038/s41408-018-0117-0
68. Badar T, Szabo A, Advani A, Wadleigh M, Arslan S, Khan MA, et al. Real-world outcomes of adult B-cell acute lymphocytic leukemia patients treated with blinatumomab. Blood Adv. (2020) 4:2308–16. doi: 10.1182/bloodadvances.2019001381
69. Hoelzer D. Chemotherapy-free treatment — a new era in acute lymphoblastic leukemia? N Engl J Med. (2020) 383:1673–4. doi: 10.1056/NEJMe2027937
70. Al-Salama ZT. Inotuzumab ozogamicin: a review in relapsed/refractory B-cell acute lymphoblastic leukaemia. Target Oncol. (2018) 13:525–32. doi: 10.1007/s11523-018-0584-z
71. Bhojwani D, Sposto R, Shah NN, Rodriguez V, Yuan C, Stetler-Stevenson M, et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia. (2019) 33:884–92.
72. Brivio E, Chantrain CF, Gruber TA, Thano A, Rialland F, Contet A, et al. Inotuzumab ozogamicin in infants and young children with relapsed or refractory acute lymphoblastic leukaemia: a case series. Br J Haematol. (2021) 193:1172–7. doi: 10.1111/bjh.17333
73. Calvo C, Cabannes-Hamy A, Adjaoud D, Bruno B, Blanc L, Boissel N, et al. Inotuzumab ozogamicin compassionate use for French paediatric patients with relapsed or refractory CD22-positive B-cell acute lymphoblastic leukaemia. Br J Haematol. (2020) 190:e53–6. doi: 10.1111/bjh.16732
74. McDonald GB, Freston JW, Boyer JL, DeLeve LD. Liver complications following treatment of hematologic malignancy with anti-CD22-Calicheamicin (Inotuzumab Ozogamicin). Hepatology. (2019) 69:831–44. doi: 10.1002/hep.30222
75. O’Brien MM, Ji L, Shah NN, Rheingold SR, Bhojwani D, Yuan CM, et al. Phase II trial of inotuzumab ozogamicin in children and adolescents with relapsed or refractory B-cell acute lymphoblastic leukemia: children’s oncology group protocol AALL1621. J Clin Oncol. (2022) 40:956–967.
76. Brivio E, Locatelli F, Lopez-Yurda M, Malone A, Diaz-de-Heredia C, Bielorai B, et al. A phase 1 study of inotuzumab ozogamicin in pediatric relapsed/refractory acute lymphoblastic leukemia (ITCC-059 study). Blood. (2021) 137:1582–90. doi: 10.1182/blood.2020007848
77. Fuster JL, Molinos-Quintana A, Fuentes C, Fernandez JM, Velasco P, Pascual T, et al. Blinatumomab and inotuzumab for B cell precursor acute lymphoblastic leukaemia in children: a retrospective study from the Leukemia Working Group of the Spanish Society of Pediatric Hematology and Oncology (SEHOP). Br J Haematol. (2020) 190:764–71. doi: 10.1111/bjh.16647
78. Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. (2015) 15:1145–54. doi: 10.1517/14712598.2015.1046430
79. Moon EK, Carpenito C, Sun J, Wang LC, Kapoor V, Predina J, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. (2011) 17:4719–30. doi: 10.1158/1078-0432.CCR-11-0351
80. Guedan S, Calderon H, Posey AD Jr., Maus MV. Engineering and design of chimeric antigen receptors. Mol Ther Methods Clin Dev. (2019) 12:145–56.
81. Meng J, Wu XQ, Sun Z, Xun RD, Liu MS, Hu R, et al. Efficacy and safety of CAR-T Cell products axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel for the treatment of hematologic malignancies: a systematic review and meta-analysis. Front Oncol. (2021) 11:698607. doi: 10.3389/fonc.2021.698607
82. Anagnostou T, Riaz IB, Hashmi SK, Murad MH, Kenderian SS. Anti-CD19 chimeric antigen receptor T-cell therapy in acute lymphocytic leukaemia: a systematic review and meta-analysis. Lancet Haematol. (2020) 7:e816–26. doi: 10.1016/S2352-3026(20)30277-5
83. Fabrizio VA, Phillips CL, Lane A, Baggott C, Prabhu S, Egeler E, et al. Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: a Pediatric Real World CAR Consortium Report. Blood Adv. (2022) 6:600–10. doi: 10.1182/bloodadvances.2021005564
84. Rubinstein JD, Krupski C, Nelson AS, O’Brien MM, Davies SM, Phillips CL. Chimeric antigen receptor T cell therapy in patients with multiply relapsed or refractory extramedullary leukemia. Biol Blood Marrow Transplant. (2020) 26:e280–5. doi: 10.1016/j.bbmt.2020.07.036
85. Htun KT, Gong Q, Ma L, Wang P, Tan Y, Wu G, et al. Successful treatment of refractory and relapsed CNS acute lymphoblastic leukemia With CD-19 CAR-T immunotherapy: a case report. Front Oncol. (2021) 11:699946. doi: 10.3389/fonc.2021.699946
86. Leahy AB, Newman H, Li Y, Liu H, Myers R, DiNofia A, et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: a post-hoc analysis of pooled data from five clinical trials. Lancet Haematol. (2021) 8:e711–22.
87. Hall EM, Yin DE, Goyal RK, Ahmed AA, Mitchell GS, St Peter SD, et al. Tisagenlecleucel infusion in patients with relapsed/refractory ALL and concurrent serious infection. J Immunother Cancer. (2021) 9:e001225.
88. Gardner RA, Finney O, Annesley C, Brakke H, Summers C, Leger K, et al. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. (2017) 129:3322–31. doi: 10.1182/blood-2017-02-769208
89. Titov A, Petukhov A, Staliarova A, Motorin D, Bulatov E, Shuvalov O, et al. The biological basis and clinical symptoms of CAR-T therapy-associated toxicites. Cell Death Dis. (2018) 9:897. doi: 10.1038/s41419-018-0918-x
90. Sachdeva M, Duchateau P, Depil S, Poirot L, Valton J. Granulocyte-macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J Biol Chem. (2019) 294:5430–7. doi: 10.1074/jbc.AC119.007558
91. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. (2017) 377:2531–44.
92. Magnani CF, Gaipa G, Lussana F, Belotti D, Gritti G, Napolitano S, et al. Sleeping Beauty-engineered CAR T cells achieve antileukemic activity without severe toxicities. J Clin Invest. (2020) 130:6021–33. doi: 10.1172/JCI138473
93. Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. (2020) 13:30.
94. Rozenbaum M, Meir A, Aharony Y, Itzhaki O, Schachter J, Bank I, et al. Gamma-Delta CAR-T Cells Show CAR-directed and independent activity against leukemia. Front Immunol. (2020) 11:1347. doi: 10.3389/fimmu.2020.01347
95. Rischer M, Pscherer S, Duwe S, Vormoor J, Jurgens H, Rossig C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br J Haematol. (2004) 126:583–92. doi: 10.1111/j.1365-2141.2004.05077.x
96. Paul MK, Mukhopadhyay AK. Tyrosine kinase - Role and significance in Cancer. Int J Med Sci. (2004) 1:101–15.
97. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. (2018) 8:1083–9.
98. Chen X, Han J, Chu J, Zhang L, Zhang J, Chen C, et al. A combinational therapy of EGFR-CAR NK cells and oncolytic herpes simplex virus 1 for breast cancer brain metastases. Oncotarget. (2016) 7:27764–77. doi: 10.18632/oncotarget.8526
99. Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. (2014) 28:917–27. doi: 10.1038/leu.2013.279
100. Fang F, Xiao W, Tian Z. NK cell-based immunotherapy for cancer. Semin Immunol. (2017) 31:37–54.
101. Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology. (2014) 3:e28147. doi: 10.4161/onci.28147
102. Romanski A, Uherek C, Bug G, Seifried E, Klingemann H, Wels WS, et al. CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies. J Cell Mol Med. (2016) 20:1287–94. doi: 10.1111/jcmm.12810
103. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. (2020) 382:545–53. doi: 10.1056/NEJMoa1910607
104. Li C, Mei H, Hu Y. Applications and explorations of CRISPR/Cas9 in CAR T-cell therapy. Brief Funct Genomics. (2020) 19:175–82. doi: 10.1093/bfgp/elz042
105. Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. (2010) 11:636–46.
106. Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol. (2014) 32:347–55. doi: 10.1038/nbt.2842
107. Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. (2011) 29:143–8. doi: 10.1038/nbt.1755
108. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. (2018) 378:439–48.
109. Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. (2020) 4:5414–24.
110. Wang S, Wang X, Ye C, Cheng H, Shi M, Chen W, et al. Humanized CD19-targeted chimeric antigen receptor T (CAR-T) cells for relapsed/refractory pediatric acute lymphoblastic leukemia. Am J Hematol. (2021) 96:E162–5. doi: 10.1002/ajh.26123
111. Hu GH, Zhao XY, Zuo YX, Chang YJ, Suo P, Wu J, et al. Unmanipulated haploidentical hematopoietic stem cell transplantation is an excellent option for children and young adult relapsed/refractory Philadelphia chromosome-negative B-cell acute lymphoblastic leukemia after CAR-T-cell therapy. Leukemia. (2021) 35:3092–100. doi: 10.1038/s41375-021-01236-y
112. Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-Targeted Chimeric Antigen Receptor (CAR) T Cells in CAR-Naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. (2021) 39:3044–55. doi: 10.1200/JCO.20.03458
113. Lejman M, Kusmierczuk K, Bednarz K, Ostapinska K, Zawitkowska J. Targeted therapy in the treatment of pediatric acute lymphoblastic leukemia-therapy and toxicity mechanisms. Int J Mol Sci. (2021) 22:9827. doi: 10.3390/ijms22189827
114. Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. (2011) 117:1583–94. doi: 10.1002/cncr.25690
115. Ravandi F, Kebriaei P. Philadelphia chromosome-positive acute lymphoblastic leukemia. Hematol Oncol Clin North Am. (2009) 23:1043–63.
116. Short NJ, Kantarjian H, Jabbour E, Ravandi F. Which tyrosine kinase inhibitor should we use to treat Philadelphia chromosome-positive acute lymphoblastic leukemia? Best Pract Res Clin Haematol. (2017) 30:193–200. doi: 10.1016/j.beha.2017.05.001
117. Iacobucci I, Mullighan CG. Genetic Basis of Acute Lymphoblastic Leukemia. J Clin Oncol. (2017) 35:975–83.
118. Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. (2012) 13:936–45. doi: 10.1016/S1470-2045(12)70377-7
119. Shen SH, Chen XJ, Cai JY, Yu J, Gao J, Hu SY, et al. Effect of dasatinib vs imatinib in the treatment of pediatric philadelphia chromosome-positive acute lymphoblastic leukemia a randomized clinical trial. Jama Oncol. (2020) 6:358–66. doi: 10.1001/jamaoncol.2019.5868
120. Biondi A, Gandemer V, De Lorenzo P, Cario G, Campbell M, Castor A, et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. (2018) 5:E641–52. doi: 10.1016/S2352-3026(18)30173-X
121. Slayton WB, Schultz KR, Kairalla JA, Devidas M, Mi XL, Pulsipher MA, et al. Dasatinib plus intensive chemotherapy in children, adolescents, and young adults with philadelphia chromosome-positive acute lymphoblastic leukemia: results of children’s oncology group trial AALL0622. J Clin Oncol. (2018) 36:2306–14. doi: 10.1200/JCO.2017.76.7228
122. Ottmann OG, Druker BJ, Sawyers CL, Goldman JM, Reiffers J, Silver RT, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. (2002) 100:1965–71. doi: 10.1182/blood-2001-12-0181
123. Foa R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. (2011) 118:6521–8. doi: 10.1182/blood-2011-05-351403
124. Ravandi F, Othus M, O’Brien SM, Forman SJ, Ha CS, Wong JYC, et al. US intergroup study of chemotherapy plus dasatinib and allogeneic stem cell transplant in philadelphia chromosome positive ALL. Blood Adv. (2016) 1:250–9. doi: 10.1182/bloodadvances.2016001495
125. Braun TP, Eide CA, Druker BJ. Response and Resistance to BCR-ABL1-Targeted Therapies. Cancer Cell. (2020) 37:530–42. doi: 10.1016/j.ccell.2020.03.006
126. Iqbal Z, Aleem A, Iqbal M, Naqvi MI, Gill A, Taj AS, et al. Akhtar, sensitive detection of pre-existing BCR-ABL kinase domain mutations in cd34+cells of newly diagnosed chronic-phase chronic myeloid leukemia patients is associated with imatinib resistance: implications in the post-imatinib era. PLoS One. (2013) 8:e55717. doi: 10.1371/journal.pone.0055717
127. Johansson B, Fioretos T, Mitelman F. Cytogenetic and molecular genetic evolution of chronic myeloid leukemia. Acta Haematol-Basel. (2002) 107:76–94. doi: 10.1159/000046636
128. Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. (2006) 354:2542–51. doi: 10.1056/NEJMoa055104
129. O’Hare T, Shakespeare WC, Zhu XT, Eide CA, Rivera VM, Wang F, et al. AP24534, a Pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. (2009) 16:401–12. doi: 10.1016/j.ccr.2009.09.028
130. Cortes JE, Kim DW, Pinilla-Ibarz J, Paquette R, Chuah C, Nicolini FE, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. (2018) 132:393–404. doi: 10.1182/blood-2016-09-739086
131. Jabbour E, Short NJ, Ravandi F, Huang X, Daver N, DiNardo CD, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: long-term follow-up of a single-centre, phase 2 study. Lancet Haematol. (2018) 5:e618–27. doi: 10.1016/S2352-3026(18)30176-5
132. Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang YL, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. (2014) 371:1005–15. doi: 10.1056/NEJMoa1403088
133. Maude SL, Tasian SK, Vincent T, Hall JW, Sheen C, Roberts KG, et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. (2012) 120:3510–8. doi: 10.1182/blood-2012-03-415448
134. Verstovsek S, Kantarjian H, Mesa RA, Pardanani AD, Cortes-Franco J, Thomas DA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. (2010) 363:1117–27. doi: 10.1056/NEJMoa1002028
135. Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. (2012) 18:3008–14. doi: 10.1158/1078-0432.CCR-11-3145
136. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. (2005) 365:1054–61. doi: 10.1016/S0140-6736(05)71142-9
137. Loh ML, Tasian SK, Rabin KR, Brown P, Magoon D, Reid JM, et al. A phase 1 dosing study of ruxolitinib in children with relapsed or refractory solid tumors, leukemias, or myeloproliferative neoplasms: a Children’s Oncology Group phase 1 consortium study (ADVL1011). Pediatr Blood Cancer. (2015) 62:1717–24. doi: 10.1002/pbc.25575
138. Ajayi S, Becker H, Reinhardt H, Engelhardt M, Zeiser R, von Bubnoff N, et al. Ruxolitinib. Recent Results Cancer Res. (2018) 212:119–32.
139. William AD, Lee ACH, Blanchard S, Poulsen A, Teo EL, Nagaraj H, et al. Discovery of the Macrocycle 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)]heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (SB1518), a Potent Janus Kinase 2/Fms-Like Tyrosine Kinase-3 (JAK2/FLT3) Inhibitor for the Treatment of Myelofibrosis and Lymphoma. J Med Chem. (2011) 54:4638–58. doi: 10.1021/jm200326p
140. Verstovsek S, Odenike O, Singer JW, Granston T, Al-Fayoumi S, Deeg HJ. Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies. J Hematol Oncol. (2016) 9:137. doi: 10.1186/s13045-016-0367-x
141. Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, et al. Discovery of CP-690,550: a potent and selective janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. (2010) 53:8468–84. doi: 10.1021/jm1004286
142. Poulsen A, William A, Blanchard S, Lee A, Nagaraj H, Wang HS, et al. Structure-based design of oxygen-linked macrocyclic kinase inhibitors: discovery of SB1518 and SB1578, potent inhibitors of Janus kinase 2 (JAK2) and Fms-like tyrosine kinase-3 (FLT3). J Comput Aid Mol Des. (2012) 26:437–50. doi: 10.1007/s10822-012-9572-z
143. Appelmann I, Rillahan CD, de Stanchina E, Carbonetti G, Chen C, Lowe SW, et al. Janus kinase inhibition by ruxolitinib extends dasatinib- and dexamethasone-induced remissions in a mouse model of Ph+ ALL. Blood. (2015) 125:1444–51. doi: 10.1182/blood-2014-09-601062
144. Kong Y, Wu YL, Song Y, Shi MM, Cao XN, Zhao HY, et al. Ruxolitinib/nilotinib cotreatment inhibits leukemia-propagating cells in Philadelphia chromosome-positive ALL. J Transl Med. (2017) 15:184. doi: 10.1186/s12967-017-1286-5
145. Ebadi M, Wasko J, Weisdorf DJ, Gordon PM, Rashidi A. Ruxolitinib combined with chemotherapy can eradicate chemorefractory central nervous system acute lymphoblastic leukaemia. Br J Haematol. (2019) 187:e24–7. doi: 10.1111/bjh.16142
148. Srinivas G, Kusumakumary P, Nair MK, Panicker KR, Pillai MR. Bcl-2 protein and apoptosis in pediatric acute lymphoblastic leukemia. Int J Mol Med. (1998) 1:755–9. doi: 10.3892/ijmm.1.4.755
149. Tsujimoto Y, Croce CM. Recent progress on the human bcl-2 gene involved in follicular lymphoma: characterization of the protein products. Curr Top Microbiol Immunol. (1988) 141:337–40. doi: 10.1007/978-3-642-74006-0_45
150. Robertson LE, Plunkett W, McConnell K, Keating MJ, McDonnell TJ. Bcl-2 expression in chronic lymphocytic leukemia and its correlation with the induction of apoptosis and clinical outcome. Leukemia. (1996) 10:456–9.
151. Agarwal B, Naresh KN. Bcl-2 family of proteins in indolent B-cell non-Hodgkin’s lymphoma: study of 116 cases. Am J Hematol. (2002) 70:278–82. doi: 10.1002/ajh.10139
152. Rassidakis GZ, Jones D, Lai R, Ramalingam P, Sarris AH, McDonnell TJ, et al. BCL-2 family proteins in peripheral T-cell lymphomas: correlation with tumour apoptosis and proliferation. J Pathol. (2003) 200:240–8. doi: 10.1002/path.1346
153. Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. (2008) 68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836
154. Pullarkat VA, Lacayo NJ, Jabbour E, Rubnitz JE, Bajel A, Laetsch TW, et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. (2021) 11:1440–53. doi: 10.1158/2159-8290.CD-20-1465
155. Mohamad Anuar NN, Nor Hisam NS, Liew SL, Ugusman A. Clinical review: navitoclax as a pro-apoptotic and anti-fibrotic agent. Front Pharmacol. (2020) 11:564108. doi: 10.3389/fphar.2020.564108
156. Diaz-Flores E, Comeaux EQ, Kim KL, Melnik E, Beckman K, Davis KL, et al. Bcl-2 is a therapeutic target for hypodiploid B-lineage acute lymphoblastic leukemia. Cancer Res. (2019) 79:2339–51. doi: 10.1158/0008-5472.CAN-18-0236
157. Khaw SL, Suryani S, Evans K, Richmond J, Robbins A, Kurmasheva RT, et al. Venetoclax responses of pediatric ALL xenografts reveal sensitivity of MLL-rearranged leukemia. Blood. (2016) 128:1382–95. doi: 10.1182/blood-2016-03-707414
158. Daver N, Thomas D, Ravandi F, Cortes J, Garris R, Jabbour E, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. (2015) 100:653–61. doi: 10.3324/haematol.2014.118588
159. McEwan A, Pitiyarachchi O, Viiala N. Relapsed/Refractory ETP-ALL successfully treated with venetoclax and nelarabine as a bridge to allogeneic stem cell transplant. Hemasphere. (2020) 4:e379. doi: 10.1097/HS9.0000000000000379
160. Zappone E, Cencini E, Defina M, Sicuranza A, Gozzetti A, Ciofini S, et al. Venetoclax in association with decitabine as effective bridge to transplant in a case of relapsed early T-cell lymphoblastic leukemia. Clin Case Rep. (2020) 8:2000–2. doi: 10.1002/ccr3.3041
161. La Starza R, Cambo B, Pierini A, Bornhauser B, Montanaro A, Bourquin JP, et al. Venetoclax and bortezomib in relapsed/refractory early T-cell precursor acute lymphoblastic leukemia. JCO Precis Oncol. (2019) 3:00172. doi: 10.1200/PO.19.00172
162. Antic Z, Lelieveld SH, van der Ham CG, Sonneveld E, Hoogerbrugge PM, Kuiper RP. Unravelling the sequential interplay of mutational mechanisms during clonal evolution in relapsed pediatric acute lymphoblastic leukemia. Genes. (2021) 12:214. doi: 10.3390/genes12020214
163. zur Stadt U, Alawi M, Adao M, Indenbirken D, Escherich G, Horstmann MA. Characterization of novel, recurrent genomic rearrangements as sensitive MRD targets in childhood B-cell precursor ALL. Blood Cancer J. (2019) 9:96. doi: 10.1038/s41408-019-0257-x
164. Tran TH, Hunger SP. The genomic landscape of pediatric acute lymphoblastic leukemia and precision medicine opportunities. Semin Cancer Biol. (2020) [Online ahead of print] doi: 10.1016/j.semcancer.2020.10.013
165. Li JF, Dai YT, Lilljebjorn H, Shen SH, Cui BW, Bai L, et al. Transcriptional landscape of B cell precursor acute lymphoblastic leukemia based on an international study of 1,223 cases. Proc Natl Acad Sci U S A. (2018) 115:E11711–20. doi: 10.1073/pnas.1814397115
166. Kiss R, Gango A, Benard-Slagter A, Egyed B, Haltrich I, Hegyi L, et al. Comprehensive profiling of disease-relevant copy number aberrations for advanced clinical diagnostics of pediatric acute lymphoblastic leukemia. Mod Pathol. (2020) 33:812–24. doi: 10.1038/s41379-019-0423-5
167. Xu H, Yu H, Jin R, Wu X, Chen H. Genetic and epigenetic targeting therapy for pediatric acute lymphoblastic leukemia. Cells. (2021) 10:3349. doi: 10.3390/cells10123349
168. Albin N, Mc Leer A, Sakhri L. [Precision medicine: a major step forward in specific situations, a myth in refractory cancers?]. Bull Cancer. (2018) 105:375–96. doi: 10.1016/j.bulcan.2018.01.009
169. Yadav B, Pemovska T, Szwajda A, Kulesskiy E, Kontro M, Karjalainen R, et al. Quantitative scoring of differential drug sensitivity for individually optimized anticancer therapies. Sci Rep. (2014) 4:5193. doi: 10.1038/srep05193
170. Swords RT, Azzam D, Al-Ali H, Lohse I, Volmar CH, Watts JM, et al. Ex-vivo sensitivity profiling to guide clinical decision making in acute myeloid leukemia: a pilot study. Leuk Res. (2018) 64:34–41. doi: 10.1016/j.leukres.2017.11.008
171. Kurtz SE, Eide CA, Kaempf A, Khanna V, Savage SL, Rofelty A, et al. Molecularly targeted drug combinations demonstrate selective effectiveness for myeloid- and lymphoid-derived hematologic malignancies. Proc Natl Acad Sci USA. (2017) 114:E7554–63. doi: 10.1073/pnas.1703094114
172. Andersson EI, Putzer S, Yadav B, Dufva O, Khan S, He L, et al. Discovery of novel drug sensitivities in T-PLL by high-throughput ex vivo drug testing and mutation profiling. Leukemia. (2018) 32:774–87. doi: 10.1038/leu.2017.252
173. Chebouba L, Boughaci D, Guziolowski C. Proteomics versus clinical data and stochastic local search based feature selection for acute myeloid leukemia patients’ classification. J Med Syst. (2018) 42:129. doi: 10.1007/s10916-018-0972-z
174. Verougstraete N, Stove V, Verstraete AG, Stove C. Quantification of eight hematological tyrosine kinase inhibitors in both plasma and whole blood by a validated LC-MS/MS method. Talanta. (2021) 226:122140. doi: 10.1016/j.talanta.2021.122140
175. Mueller-Schoell A, Groenland SL, Scherf-Clavel O, van Dyk M, Huisinga W, Michelet R, et al. Therapeutic drug monitoring of oral targeted antineoplastic drugs. Eur J Clin Pharmacol. (2021) 77:441–64.
Keywords: relapsed or refractory acute lymphoblastic leukemia (R/R ALL), pediatrics, immunotherapy, targeted therapy, personalized therapy
Citation: Mengxuan S, Fen Z and Runming J (2022) Novel Treatments for Pediatric Relapsed or Refractory Acute B-Cell Lineage Lymphoblastic Leukemia: Precision Medicine Era. Front. Pediatr. 10:923419. doi: 10.3389/fped.2022.923419
Received: 19 April 2022; Accepted: 02 June 2022;
Published: 23 June 2022.
Edited by:
Kaat Durinck, Ghent University, BelgiumReviewed by:
Yongsheng Ruan, Southern Medical University, ChinaCopyright © 2022 Mengxuan, Fen and Runming. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhou Fen, ZGFpc3lfbWF5QDE2My5jb20=; Jin Runming, amlucnVubUBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.