
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 18 July 2022
Sec. Pediatric Endocrinology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.913343
 Michael M. Schündeln1*
Michael M. Schündeln1* Sebastian Fritzemeier1
Sebastian Fritzemeier1 Sarah C. Goretzki1
Sarah C. Goretzki1 Pia K. Hauffa1
Pia K. Hauffa1 Martin Munteanu2,3
Martin Munteanu2,3 Cordula Kiewert2
Cordula Kiewert2 Berthold P. Hauffa2
Berthold P. Hauffa2 Gudrun Fleischhack1
Gudrun Fleischhack1 Stephan Tippelt1
Stephan Tippelt1 Corinna Grasemann4
Corinna Grasemann4Background: Childhood primary brain tumors (CPBT) are the second largest group of childhood malignancies and associated with a high risk for endocrine late effects.
Objective: To assess endocrine late effects and their relevance for the development of osteopathologies in survivors.
Methods: This single center cross sectional study investigated data from 102 CPBT survivors with a mean age of 13.0 years and a mean age at diagnosis of 8.7 years. Clinical, biochemical, radiographic, and anamnestic data regarding endocrine and bone health were obtained at study visits. In addition, data regarding tumor stage and therapy was obtained by chart review. An expert opinion was applied to define presence of osteopathologies.
Results: Impaired bone health, defined by at least one pathological screening parameter, was present in 65% of patients. 27.5% were found to have overt osteopathologies per expert opinion. 37.8% displayed a severe vitamin D deficiency (25-OH vitamin D < 10 ng/ml) and 11% a secondary hyperparathyroidism. Patients with osteopathologies had lower 25-OH vitamin D levels compared to patients without osteopathologies. Multiple endocrine late effects were present: diabetes insipidus in 10.8%, aberrant pubertal development in 13.7%, central hypocortisolism in 14.9%, thyroid dysfunction in 23.8% and growth hormone deficiency in 21.8%. A total of 31.3% of survivors displayed any endocrinopathy. Tumors located near hypothalamic structures and patients who received irradiation had a higher likelihood of endocrine morbidity.
Conclusion: This study indicates that endocrine deficiencies are common in pediatric survivors of CPBTs. Osteopathologies are present in this cohort. A prominent effect of hormonal deficiencies on bone health was not detected, possibly because patients were sufficiently treate for their endocrine conditions or indicating resilience of the childhood bone remodeling process. Vitamin D deficiency is frequent and should be treated as recommended.
Childhood primary brain tumors (CPBTs) represent the second largest group of childhood malignancies following acute lymphoblastic leukemia (ALL; 1). CPBTs comprise of a multitude of entities with a broad spectrum of biological characteristics as reviewed by Pollack et al. (2).
As in other childhood malignancies, the treatment outcome of CPBTs has improved markedly in the past decades. The overall 5-year survival is currently reported to be over 74% for all forms of CPBTs (3, 4). However, tumor- and therapy-associated long term morbidities are becoming increasingly apparent in long term survivors (5).
In survivors of childhood malignancies, endocrine late effects, which manifest progressively over the years until well into adulthood, constitute a high burden of disease (6–11). Especially in survivors of CPBTs endocrinopathies are common late effects due to affections of the central endocrine regulating structures of the hypothalamic-pituitary region (12, 13).
Closely related to the endocrine late effects, bone health has been shown to be impaired in a significant number of children and adults surviving pediatric malignancies (14, 15). Adolescent survivors of CPBTs are prone to osteoporosis as reported by Cohen et al. (16) and Remes et al. (17).
To date the onset of osteopathologies in survivors of childhood malignancies is not yet well characterized. Recent studies in ALL indicate that impaired bone health may be observed as early as at the time of manifestation of the malignancy (18, 19) and is present in many survivors during follow-up (15).
Bone health is difficult to assess since a comprehensive set of clinical, biochemical, radiographic and life style factors needs to be analyzed and interpreted, as reviewed recently by Mostoufi-Moab and Ward (20). In the pediatric setting, this assessment is further complicated by the need to apply an age- and pubertal stage-appropriate interpretation of these parameters. Beyond low bone mineral content and alterations in bone metabolism, chronic bone pain, pathological fractures and stunted growth may also account for impaired bone health in pediatric patients with chronic disease including survivors of childhood malignancies (21).
Due to the detrimental effect of endocrine dysfunction (e.g., deficiency of sex steroids) on bone health, and based on the feed-back mechanism between bone metabolism and metabolic health (22, 23) it appears that endocrine late effects, disease comorbidity and bone health are related in survivors of CPBTs, for review see: Desentis-Desentis et al. (24).
In this study we report endocrine status and occurrence of osteopathologies in a single center cohort of 102 survivors of childhood brain malignancy.
All patients who had been diagnosed with CPBT from 1997 until 2013 and were undergoing follow-up at the oncology outpatient clinic of the tertiary care center from August 2014 until August 2016 were invited to participate in this single-center cross-sectional study.
Detailed data regarding stratification and treatment of the patients are presented in Table 1.
Clinical and anamnestic parameters were assessed as previously described in detail (25): “Briefly, a physical examination, including determination of weight, height and pubertal status was performed. Patients or parents were also asked to complete a standardized questionnaire regarding vitamin D, calcium and nutritional supplement intake, screen hours and hours of physical activity per day. In addition, the patients were asked for presence of bone pain in the form of regularly occurring (on more than half of the days in the last month), spontaneous back pain or exercise related knee pain. Pubertal development was assessed by a pediatric endocrinologist according to Tanner staging. Standard deviation scores (SDS) for pubertal development were calculated using ‘Puberty Plot Web Application’ by Stef van Buuren1 (26). The application calculates pubertal SDS for breast development, pubic hair stage and testicular volume based on the Tanner stages for breast development, testicular volume and pubic hair stage. Skeletal age was determined by an experienced pediatric radiologist according to the method of Greulich and Pyle using X-ray images of the left hand (27).”
The following parameters were obtained from the patients’ charts: The tumor localization was categorized into the following seven areas: cerebellum, diencephalon, medulla oblongata, mesencephalon, pons, spinal cord, and telencephalon. In addition, the localization was categorized according to the proximity to the pituitary. Tumors in the sella turcica, basal ganglia, thalamus, bottom of third ventricle and chiasma opticum were labeled as “hypothalamic-pituitary region.” The type of diagnosis including grading were documented. The staging was recorded according to Chang classification for medulloblastoma (M0 = localized, M1 = dissemination of tumor cells to the CSF, M2 = intracranial metastases, M3 = spinal metastases, and M4 = metastases outside of the CNS; 28). The treatment modalities were recorded separately for surgery, chemotherapy and irradiation. In addition, the status of residual tumor and the number of relapses before the study visit were documented. The cumulative dosage of chemotherapy as well as type and dosage of irradiation dosage patient “as treated.”
Upon chart review, fractures which had occurred after the diagnosis of brain tumor were recorded. Vertebral fractures or more than two subsequent fractures of long bones were categorized as pathological fractures.
Endocrinopathies were documented from the patients’ charts if a prior diagnosis of diabetes insipidus, growth hormone (GH) deficiency, precocious puberty, delayed pubertal development, hypocortisolism or a thyroid disorder (Hashimoto thyroiditis, hypothyroidism, and Graves’ disease) was documented.
The following biochemical parameters of growth, pubertal status, bone turnover and vitamin D metabolism were assessed in serum or plasma samples as part of the routine diagnostic laboratory workup in the central laboratory of the University Hospital Essen: 25-OH vitamin D (ng;ml); 1,25-(OH)2 vitamin D (pg;ml); serum phosphate (mmol/l), serum calcium (mmol/l), albumin (g/dl), total serum alkaline phosphatase, TSAP (U/l); bone-specific alkaline phosphatase, BAP (U/l); insulin-like growth factor-1, IGF-1 (ng/ml), cortisol (nmol/l), thyroid stimulating hormone, TSH (mU/l), free thyroxine, fT4 (pmol/l), PTH (pg/ml) and osteocalcin (ng/ml). LH (U/L), FSH (U/L), testosterone (nmol/L), estradiol (pg/ml), DHEAS (μg/ml), androstenendione (ng/ml), Inhibin B (ng/l), AMH (μg/l). Plasma levels of osteoprotegerin (pmol/l), receptor activator of nuclear factor kappa-B ligand (RANKL, pmol/l), and tartrate-resistant acid phosphatase 5b (TRAP5b, U/l) were measured using commercially available ELISA assays, according to the manufacturer’s instructions (Osteoprotegerin: OPG, Quidel®; RANKL: sRANKL, BIOMEDICA and TRAP 5b: TRAP5b, Quidel®; all TECOmedical, Germany).
Serum markers of LH, FSH, testosterone, estradiol, DHEAS, androstenendione, Inhibin B and AMH were measured and interpreted to assess endocrine function in participants, but will not be further discussed in the manuscript.
Additionally, the urinary calcium to creatinine ratio (mg/mg) as well as markers of bone resorption including N-terminal telopeptide, NTX (nmol bone collagen equivalent/mmol creatinine) and deoxypyridinoline, DPD (mg/g creatinine) were assessed in spot urine samples. Pediatric reference ranges were available and applied for all parameters. Serum IGF-1 levels were expressed as SDS values, according to age and sex, based on the data from Blum and Breier (29). To calculate the IGF-1 SDS, we used the software tool “SDSEasy” (Mediagnost, Reutlingen, Germany).
BMD was examined via dual-energy X-ray absorptiometry (DXA; Lunar Prodigy, GE-Healthcare, Madison, WI, United States) in a subgroup of patients. BMD was assessed at the lumbar spine (L1 – L4; anteroposterior view) and the left femoral neck. Z-scores (DXA Z) were calculated for the lumbar spine measurements based on age specific normal values (30, 31). A single investigator blinded to the clinical status of the patients was responsible for all BMD measurements. Height-adjusted Z-scores (HAZ) from DXA were calculated as described previously (32). The Bone Health Index (BHI) and its SDS (BHI-SDS) was calculated using the software BoneXpert from indices of three metacarpal bones as a parameter to approximate bone density from X-rays of the left hand (33).
The assessment process of defining the bone health status for the purpose of this study has been previously described in detail (15) The terms “osteopathology” and “impaired bone health” are being used in this manuscript to describe different levels of skeletal late effects. The term “osteopathology” refers to overt bone disease and the term “impaired bone health” refers to a condition with at least one pathological reading in selected parameters of bone health, as displayed in Table 3. Bone health and endocrine status was assessed jointly by two clinical experts (CG and BH). Both of whom are experienced pediatric endocrinologists.
All patients with a vitamin D deficiency (25-OH Vitamin D < 20 ng/ml), as defined by Holick (34) and no signs for decreased renal function or hypercalcemia were started on 1,000 IU of oral Vitamin D3 daily according to the global consensus recommendations (35). 25-OH vitamin D levels were reassessed 3 months later (if possible) and during the regular follow up visit the following year in the survivorship clinic.
Statistical analyses and local polynomial regression fitting was performed with the R base/stats package (36). PRISM for MAC 7.0 (GraphPad Software, Inc., La Jolla, CA, United States) and R base package ggplot2 (37) was used for figures. Values are expressed as the mean ± standard deviation (SD) and range unless stated otherwise. As in most of the variables normal distribution could not be assumed, associations between single variables were described by Spearman correlation coefficient. Differences in continuous variables between the groups were tested using the Mann–Whitney-U test for two-group comparisons, and the Kruskal–Wallis tests for more than two groups. When an overall difference was found, post hoc analysis was carried out by pairwise comparison of groups using the Mann–Whitney-U test, correcting for multiple testing with the Bonferroni-Holm method. Group differences in categorical variables were tested using the chi-square test statistics. For all tests, statistical significance was presumed at P < 0.05.
One hundred four patients were eligible to participate. Of those, two declined for personal reasons. Of the recruited 102 patients, 55 were male, and 47 were female. The majority of patients were diagnosed with glioma of any type (n = 57), medulloblastoma (n = 18), ependymoma (n = 12), and germinoma (n = 9). Disease in about half of all patients (n = 58) was categorized as WHO grade I and II. The patients were treated with the following modalities: Most patients (n = 84) underwent (brain-)surgery and about half of the patients (n = 52) underwent irradiation and/or chemotherapy (n = 54). The details regarding combination of various treatment modalities are displayed in Table 1.
The mean age of the patients at follow up was 13.0 ± 4.1 years (2.39–21.8). Mean age at diagnosis was 8.66 ± 4.13 years (0.0–17.5), making for a mean of 4.32 ± 3.3 years after first diagnosis of CPBT (range 0.22–15.7).
The mean height SDS was –0.53 ± 1.20 (–4.09–1.86). Mean BMI SDS was 0.72 ± 1.31 (–2.33–4.21). The Tanner stage SDS for pubic hair development and testicular volume/breast development was –0.36 ± 1.00 (–3.02–1.80) and –0.48 ± 1.20 (–3.17–2.01), respectively. In total there were 25 prepubertal (=Tanner 1) children present in this group of survivors.
The questionnaire was completed by 94 of the 102 survivors. The respondents report an average of 2.16 ± 1.53 h (0–5) of daily screen time. Of the patients, eight stated that they would spend most of the day non-ambulatory (sitting or lying down). Fourteen where physically active up to 3 h. Seventy-two were active more than 3 h per day.
More detailed descriptive statistics of the 102 patients are displayed in Table 2 and Supplementary Tables 1A,B. For the following analyses, the applicable age-, sex-, and pubertal status adjusted reference ranges were used for the assessments of the individual data and SDS were applied.
As expected, parameters of bone metabolism (BAP, Osteocalcin, DPD, TRAP5B, and Osteoprotegerin) displayed an age and puberty-associated pattern with mostly lower values in the postpubertal age group (Figure 1).
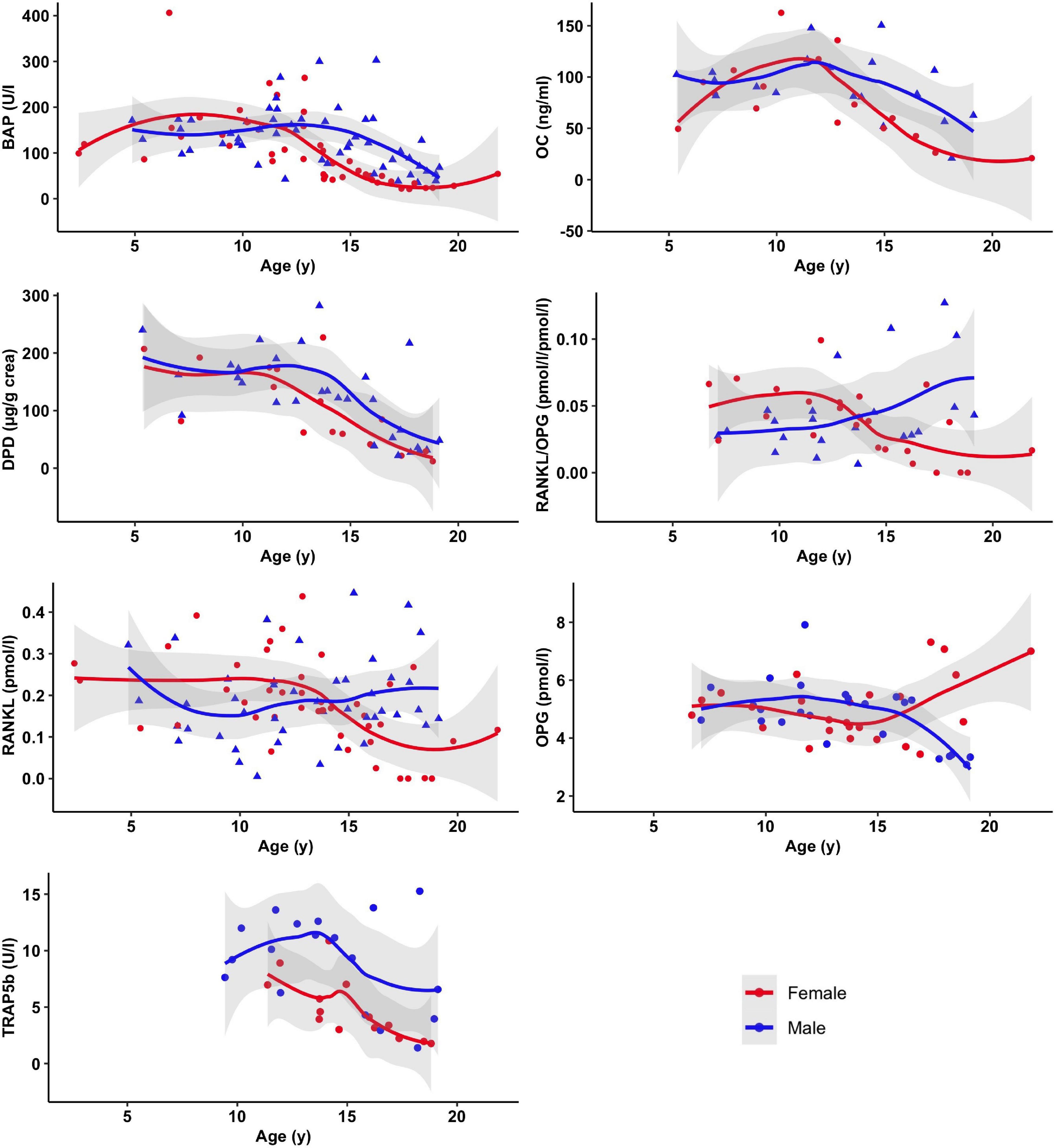
Figure 1. Selected markers of bone metabolism as a function of age and sex. Displayed are mean and 95% confidence band of SE of smoothed curves by local polynomial regression.
The frequency of aberrant biochemical and radiological findings based on normative values as described in Schündeln et al. (15) are summarized in Table 3. Overall in 65% of patients any kind of bone health impairment was detected. Non-pathological fractures and non-severe vitamin D deficiency were not counted as evidence for impaired bone health. Likewise, the levels of OPG and RANKL were not accounted for in this categorization, since no normative values in childhood and adolescents are available.
The mean DXA Z score was similar in female and male patients (–0.73 ± 1.45 vs. –0.87 ± 0.90 for both, P = 0.95). Similar results were found when comparing the height adjusted Z-scores (HAZ, 0.19 ± 1.01 vs. -0.42 ± 0.90, P = 0.15). No further difference in bone health related parameters were detectable between male and female patients for the parameters displayed in Table 2.
An expert opinion on the status of bone health was obtained for all patients. Twenty-six patients were labeled as “bone healthy” or “most likely bone healthy” and 28 patients were labeled as affected with “osteopathology” or “most likely affected with osteopathology.” In 48 patients a definite classification based on the available parameters by the experts was not possible. Of these, 32 patients were not categorizable due to unconcise or conflicting findings and data. For 16 patients, data was incomplete.
In univariate analyses, no difference regarding patients’ sex, age or age at diagnosis and the status of bone health was detected. A higher tumor grade than WHO° 1 did not significantly increase the odds ratio for osteopathologies (“osteopathology” or “most likely osteopathology”; Odds ratio = 0.65, 95% CI = 0.22–1.90, P = 0.43), nor did the presence of a higher than M0 stage (Odds ratio = 1.5, 95% CI = 0.37–6.60, P = 0.57), or the status of relapse (Odds ratio = 0.63, 95% CI = 0.11–3.12, P = 0.57) affect bone health status in the cohort. Likewise, the type of treatment was not found to have an impact on the bone health status in a multivariate analysis (irradiation: Odds ratio = 1.58, 95% CI = 0.42–6.58, P = 0.50; surgery: Odds ratio = 0.35, 95% CI = 0.04–1.89, P = 0.25; and the application of a chemotherapy: Odds ratio = 0.81, 95% CI = 0.19–3.05, P = 0.76).
However, in a small number of patients, receiving vincristine (VCR), the cumulative dosage was associated with the bone health status: Patients with osteopathologies had received significantly higher amounts of cumulative VCR than patients without osteopathologies [45.9 ± 10.3 (n = 9) vs. 20.7 ± 8.88 (n = 13) mg/m2, P < 0.001, Figure 2].
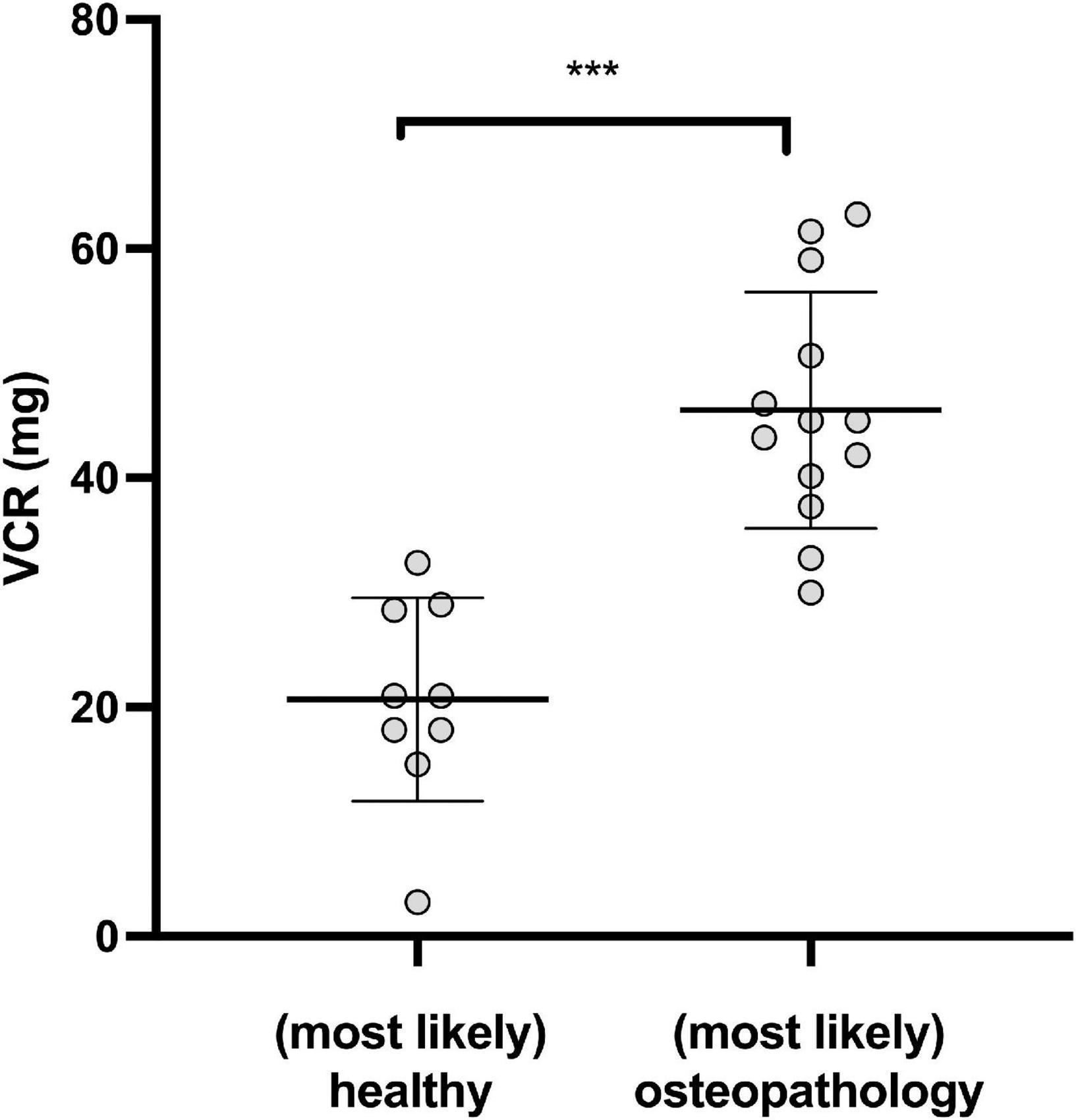
Figure 2. Osteopathologies and Vincristine (VCR). Cumulative dosage of VCR in patients with (most likely) osteopathologies and (most likely) healthy survivors. (***P < 0.001).
Vitamin D deficiency serum 25 OH vitamin D < 20 ng/ml, as defined by the Institute of Medicine (38) was found in 76.8% of the patients and a severe vitamin D deficiency [serum 25 OH vitamin D < 10 ng/ml (34)] in 37.8% of patients. Figure 3 shows a lower overall vitamin D level in patients with osteopathologies (11.3 ± 8.14 vs. 18.4 ± 9.39 mg/mg, P < 0.05). In the group of patients with sufficient vitamin D levels the calcium:creatinine ratio in urine was higher, indicating adequate calcium stores, compared to the group of patients who displayed a severe vitamin D deficiency (0.13 ± 0.09 vs. 0.06 ± 0.05 mg/mg, P = 0.01, Figure 3).
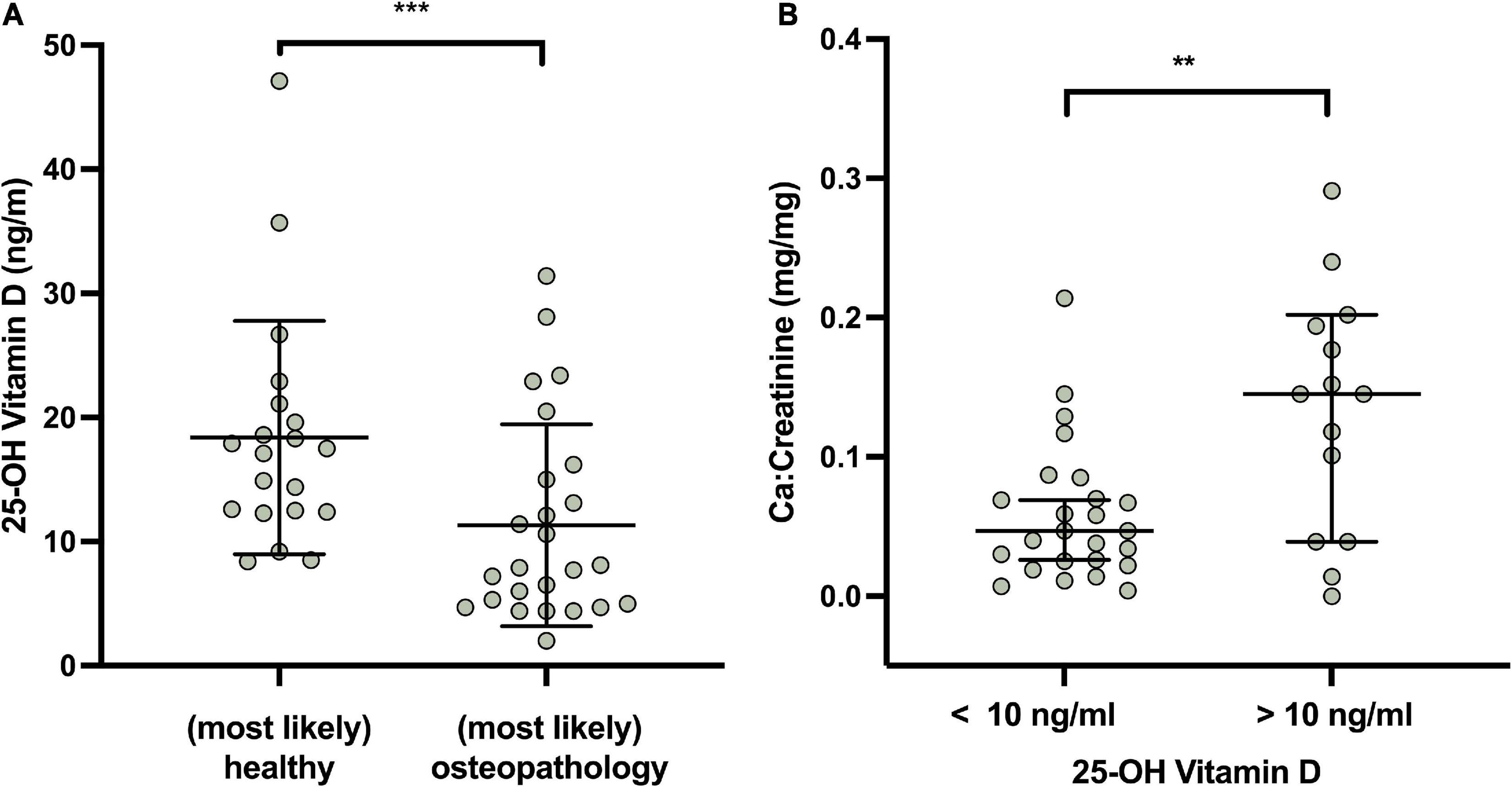
Figure 3. Vitamin D (in ng/ml), osteopathologies and calcium metabolism in survivors. (A) Expert opinion and Vitamin D levels. (B) Urinary Ca/Creatinine in patients with severe vitamin D deficiency and normal vitamin D values. Lines indicate mean and standard deviation. Statistically significant differences between the groups, determined by Mann–Whitney test, are indicated with asterisks (***P < 0.001; **P < 0.01).
Following supplementation, a significant increase in vitamin D levels 14.7 ± 9.51 ng/ml to 25.8 ± 17.6 ng/ml (P < 0.001) was observed after 3 months, however, this effect waned slightly to a mean of 21.2 ± 8.77 ng/ml after 12 months, which, nonetheless was significantly higher than the initial value (P < 0.001, Figure 4). The levels of PTH decreased accordingly, although not significantly from 42.7 ± 17.7 ng/ml to 39.2 ± 24.4 ng/ml after 3 months to 41.7 ± 25.4 ng/ml after 1 year.
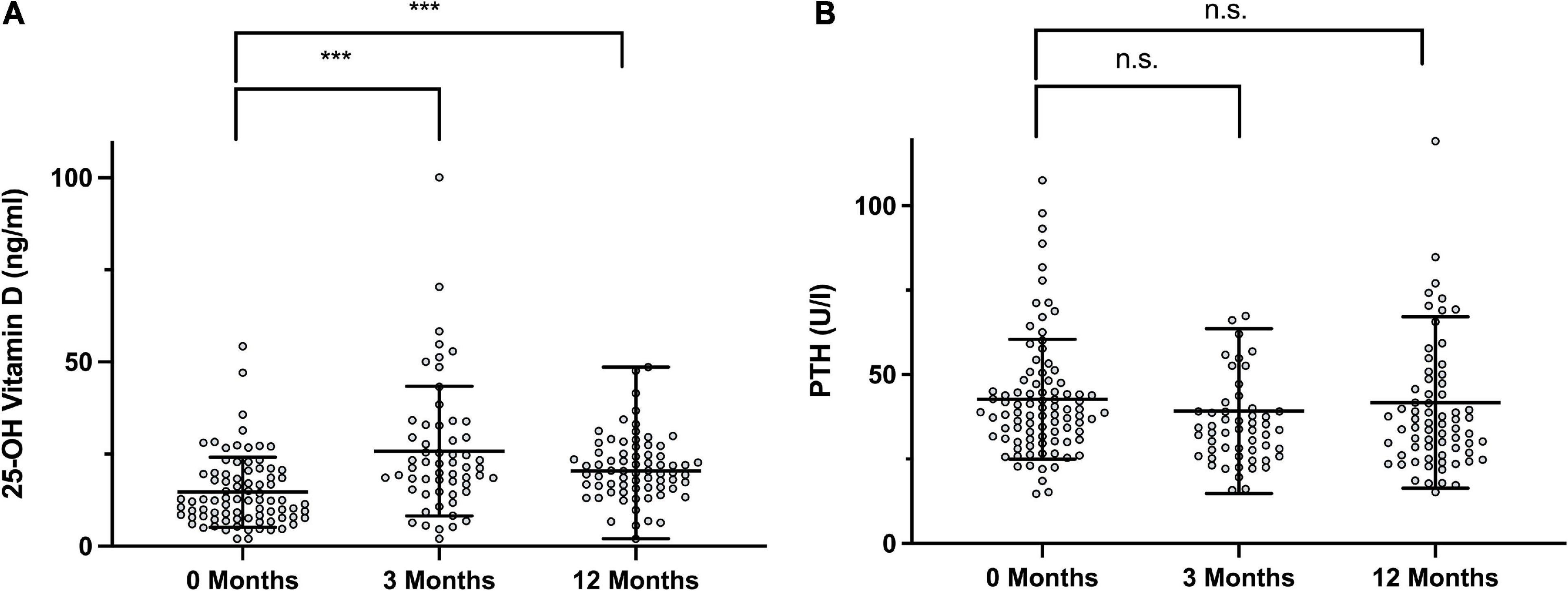
Figure 4. Longitudinal Follow Up of 25-hydroxy vitamin D and PTH levels. (A) Vitamin D at the initial visit, after 3 months and after 12 months (+12 M). (B) Parathyroid hormone (PTH) at the respective time points. Statistically significant differences between the groups, determined by Mann–Whitney test, are indicated with asterisks (***P < 0.001).
The assessment of survivors for diagnosed pathologies of the endocrine system is summarized in Table 4. In brief, 10.8% of patients had a diagnosis of diabetes insipidus centralis due to loss of anti-diuretic hormone (ADH) production, 13.7% showed aberrant pubertal development, in most cases due to a central hypogonadism with low gonadotropins. A central hypocortisolism based on low ACTH production, resulting in secondary adrenal insufficiency, was present in 14.9%. GH deficiency was diagnosed in 21.8%. A thyroid dysfunction was found in 23.8% of the survivors, again mostly based on central hypothyroidism with impaired TSH production. Each of the central deficiencies (ADH, ACTH, Gonadotropins, TSH and GH) is described as one “endocrine axis” in the following text and figures.
Patients with endocrine late effects were treated and monitored according to international standards of care for their endocrine diseases by pediatric endocrinologists of the university hospital.
There was a cumulative effect of irradiation on affected endocrine axes in the study cohort. In summary, 31.3% of survivors displayed an endocrinopathy in at least one axis. In 21.6% more than one axis was involved. The fraction of survivors with at least one axis involved was higher in irradiated patients than in non-irradiated patients (40.1 vs. 12.2%).
The localization of the tumor was significantly related with endocrine function. Patients with a tumor categorized as “located near hypothalamic structures” had a 3.7 times higher likelihood of one or more impaired axes compared to the rest of the cohort (Odds ratio = 3.69, 95% CI = 1.52–9.26, < 0.01).
In a multivariate analysis, irradiation, but not chemotherapy or surgery in a patient was associated with a higher likelihood for (at least one) impaired endocrine axis (irradiation: Odds ratio = 8.53, 95% CI = 2.80–30.6, P < 0.001; chemotherapy: Odds ratio = 1.37, 95% CI = 0.45–4.04, P = 0.42; and surgery: Odds ratio = 0.88, 95% CI = 0.26–3.06, P = 0.83).
Patients with high grade (WHO grade III or IV) CPBT had a higher number of impaired axes (P < 0.01 in WHO°I and°II CPBT (n = 0–4, median = 0) when compared to patients with WHO° III and°IV CPBT (n = 0–5, median = 0).
Patients with one or more affected axes were more prone to develop (most likely) osteopathologies (79% with osteopathologies vs. 21% without osteopathologies, P < 0.01).
In the analyzed cohort, the survivors’ height SDS was correlated inversely with the WHO° of tumors. (Figure 5A, P < 0.001, Kruskal–Wallis test). Patients with WHO° IV tumors displayed significantly lower height SDS compared to patients diagnosed with WHO° I tumors [–1.28 ± 1.07 vs –0.03 ± 1.04, (P < 0.001, Mann–Whitney-U test with Bonferroni-Holm correction for multiple testing)]. In addition, survivors who had undergone irradiation therapy, were of shorter stature than non-irradiated individuals (Figure 5B, P < 0.001). After correcting for multiple testing a significant difference was seen between between non-irradiated patients and patients with craniospinal irradiation [–1.41 ± 1.30 vs –0.14 ± 1.10, (P < 0.001)].
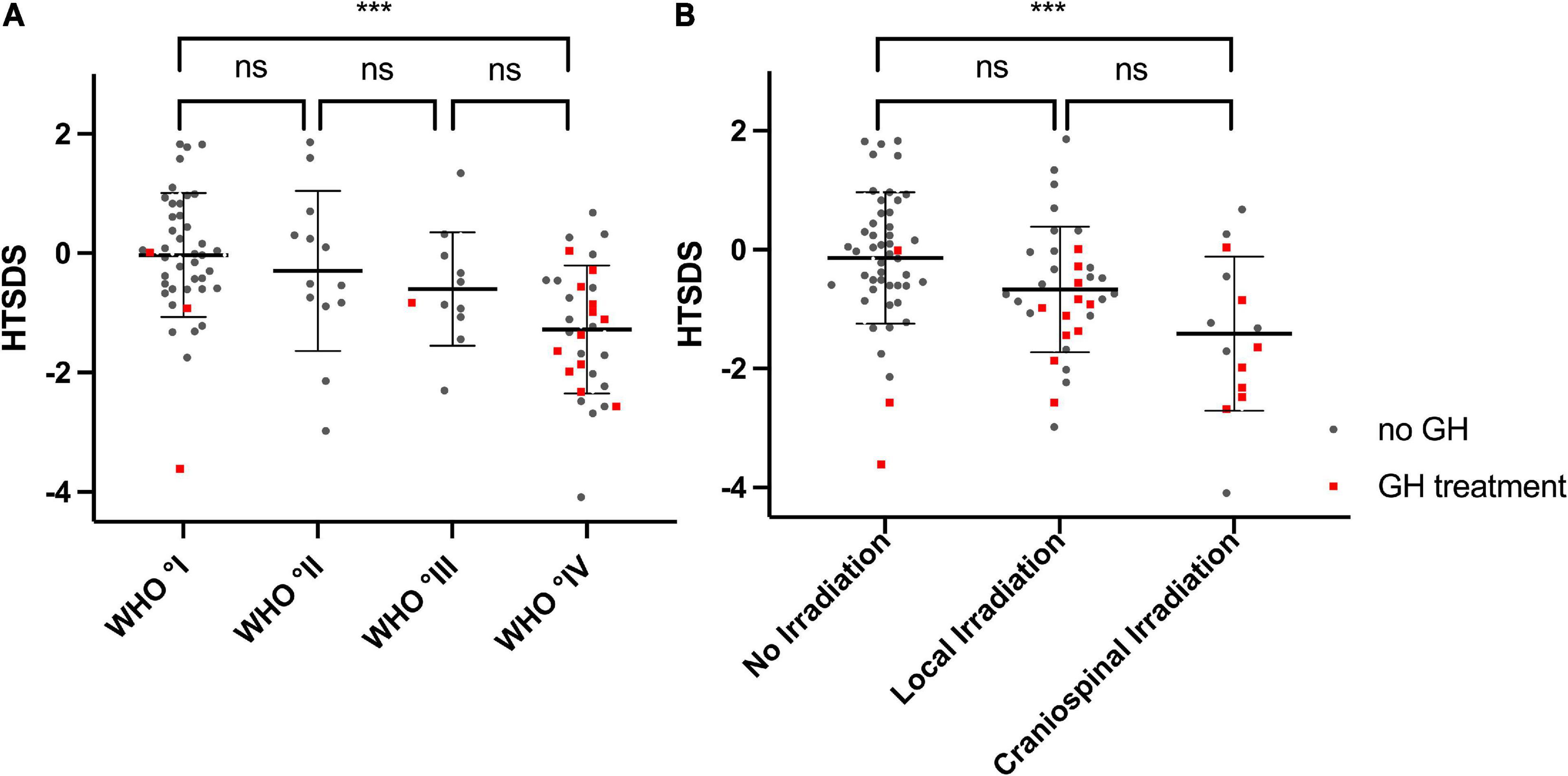
Figure 5. Height SDS and WHO Degree, Height SDS and irradiation treatment: (A) Height SDS is decreasing with increasing WHO degree of the tumors (P < 0.001 Kruskal–Wallis test). (B) Height SDS is lower in patients who received irradiation (P < 0.001 Kruskal–Wallis test), likely due to the increased percentage of patients with a GH deficiency in that group. Lines indicate mean and sd. Red dots represent individuals who receive(d) GH treatment. ***p < 0.001.
In survivors of childhood malignancies, endocrine late effects and osteopathologies constitute late effects with a high burden of disease (39). In many aspects this is especially relevant following the diagnosis of CPBT. With a focus on endocrine and bone health, this study from a large German tertiary center describes late effects of 102 adolescent survivors after diagnosis of CPBT.
In the present cohort, about two third of survivors displayed impaired bone health with at least one pathological reading of the assessed parameters. Especially (secondary) hyperparathyroidism, abnormal findings in alkaline phosphatase levels as well as in the bone resorption markers NTX and DPD were prevalent. Clinically, one third of the patients were experiencing bone pain regularly.
However, punctual pathological findings of one or some of the many parameters of bone health may not reflect overt osteopathology. We therefore attempted to further categorize the bone health status of survivors with or without osteopathologies. A categorization was only possible in about half of the cohort. In some cases, information was missing and in many cases data was unconcise. This was comparable to our experience with the data in survivors of childhood ALL (15) and reflects the complexity and difficulty of the task to identify those survivors in need for attention for bone health issues in the routine clinic [as recently reviewed by Marcucci et al. (14)].
Regarding parameters of bone health, we hypothesized that survivors suffering from endocrine late effects, and survivors with more aggressive tumors and higher intensity of treatment or irradiation would be more prone to osteopathologies. However, we were not able to pinpoint any specific factors leading to osteopathologies with respect to tumor entity, stage of tumor or treatment modality, with the exception of higher vincristine (VCR) dosages (see below). This may be a sign of robustness or resilience of the childhood bone remodeling process (40). Nonetheless, we did find a positive association of VCR dose and osteopathologies in the subgroup of patients who had been treated with VCR. A possible causal explanation could be a VCR associated neuropathy leading to decreased physical activity and thus impaired bone health. Additionally, a VCR-related disturbance in sodium regulation may lead to osteopenia (41, 42). However, other unidentified confounding factors may be present.
The hormone vitamin D plays an important role in the maintenance of calcium homeostasis and thereby bone health but may have extraskeletal effects aswell (43, 44). Severe vitamin D deficiency in the present cohort was associated with lower calcium stores, as indicated by low calcium excretion in the urine, and with osteopathologies. The fraction of survivors with severe Vitamin D deficiency was higher than reported for the general German population (45). Preventative measures, including avoidance of sun exposure and a higher rate of immobility/in-house activity may contribute to lower levels of vitamin D in these children. Additionally, in this cohort of CPBT survivors a high proportion of recent immigrants/refugees are present, a group which is known to be more prone to be vitamin D deficient in Germany (46). Supplementation of vitamin D3 did lead to a significant increase of serum levels initially. During follow-up, however, as reported by others as well (47) adherence waned off and PTH and vitamin D serum levels after 1 year were similar to the initial values. These results highlight the need for regular follow up with information of doctors, nurses and families on the importance of sufficient vitamin D stores and support of survivors. Screen time in the group of survivors was similar to that in the general pediatric population, according to a recent study by Schmidt et al. (48).
In this study, the height SDS of survivors was inversely correlated to the WHO degree of the tumor. And, like other groups (49) we could show that growth was more stunted in children who received irradiation. Irradiated patients also displayed a higher number of impaired endocrine axes, pointing toward the intertwined effects of irradiation, the resulting hormonal deficiencies and the tumor stage on growth in children and adolescents. As shown before (50, 51), patients who underwent irradiation or chemotherapy were prone to develop central endocrine dysfunction [as also reviewed by Roddy and Mueller (52)]. It proved impossible to disentangle the effects of therapy on the hormonal system (e.g., GH axis or gonadotropic-gonadal axis) from effects on bone metabolism.
In the present cohort, endocrine late effects with impairment of at least one endocrine axis was detected in one third of the survivors and impairment of more than one axis was found in one fourth of the cohort. This is a higher rate of endocrine late effects than expected and described by other authors and is likely explained by the comprehensive analyses and thorough assessment of hormonal parameters. Of note, endocrine deficiencies may be underdiagnosed in pediatric patients with brain tumors, due to subtle clinical signs with potentially multifactorial causes and the challenging biochemical workup. This conclusion is shared by other authors (12). A recommendation for regular follow-ups of survivors of CPBTs with pediatric endocrinology even in the absence of overt hormonal deficiencies seems warranted. As described previously by Shaw (53) patients with tumors in proximity to the hypothalamic-pituitary region were more likely to display hormonal deficiencies. The extend of endocrine disease, expressed as number of impaired axes, did not correlate with the presence of diagnosed osteopathologies.
The goal of this study was to thoroughly describe bone status and endocrine function of a large group of CPBT survivors. It has, however, several limitations. The cohort is heterogeneous regarding type and grade of tumor, treatment modality, etc., which makes it difficult to draw conclusions from a cross-sectional study, even though the cohort is representative of the spectrum of CPBTs typically seen in a large tertiary center. A multicentric approach with longitudinal follow-up would be desirable and may lead to more concise results. Also, with a cross sectional study such as this one, unlike a longitudinal study (12), one can only make limited assumptions regarding the time course of the described findings.
The high rate of survivors with a undeterminable bone health status is comparable to the rate in the previously described cohort of ALL patients. This – again – mirrors the aforementioned challenges to reliably describe bone health.
This study indicates that endocrine deficiencies are common and (maybe) underdiagnosed in pediatric survivors of CPBTs. Osteopathologies are present in this cohort, however, at a lower rate than in survivors of ALL. A prominent effect of hormonal deficiencies on bone health was not detected, possibly indicating that patients were either sufficiently treated for the endocrine conditions or being a sign of robustness of the childhood bone remodeling process. Vitamin D should be supplemented as recommended. Sustained supplementation and patient adherence are laborious and hard to accomplish.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Research Ethics Committee of the Medical Faculty, University of Duisburg-Essen. Written informed consent to participate in this study was provided by the participants or their legal guardian/next of kin and eligible patients.
CG, MS, and BH: study conception and design. SF, SG, PH, MS, CK, and MM: acquisition of data. BH, CG, MS, and SF: analysis and interpretation of data. MS, CG, and SF: drafting and revising the manuscript. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the survivors and families who participated in this study. We would also like to thank Jens Bauer, Department of Pediatrics, Division of Rare Diseases, St. Josef-Hospital, Ruhr-University Bochum, Bochum, Germany for his expert assistance in data-management. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.913343/full#supplementary-material
ACTH, Adrenocorticotrophic hormone; ADH, Antidiuretic hormone; 25-OH VD, Serum levels of 25-OH vitamin D; BSAP, Bone-specific alkaline phosphatase; BMI, Body mass index; Ca:crea, Urinary calcium to creatinine ratio; CPBT, Childhood primary brain tumor; DPD, Urinary deoxypyridinoline; GH, Growth Hormone; HAZ, Height-adjusted Z-scores; IGF-1, Insulin-like growth factor-1; NTX, Urinary N-telopeptide; PTH, Parathyroid hormone; RANKL, Receptor activator of nuclear factor kappa-B ligand; SD, Standard deviation; SDS, Standard deviation score; TRAP5b, Tartrate-resistant acid phosphatase 5b; TSAP, Total serum alkaline phosphatase; TSH, Thyroid-stimulating hormone; VCR, Vincristine
1. Kaatsch P, Grabow D, Spix C. German Childhood Cancer Registry-Annual Report 2017 (1980-2016). Mainz: University medicine at the Johannes Gutenberg University in Mainz (2018).
2. Pollack IF, Agnihotri S, Broniscer A. Childhood brain tumors: current management, biological insights, and future directions. J Neurosurg Pediatr. (2019) 23:261–73. doi: 10.3171/2018.10.PEDS18377
3. Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the childhood cancer survivor study. Eur J Paediatr Neurol. (2010) 14:298–303.
4. Erdmann F, Frederiksen LE, Bonaventure A, Mader L, Hasle H, Robison LL, et al. Childhood cancer: survival, treatment modalities, late effects and improvements over time. Cancer Epidemiol. (2020) 71:101733.
5. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. (2006) 355:1572–82.
6. Brignardello E, Felicetti F, Castiglione A, Chiabotto P, Corrias A, Fagioli F, et al. Endocrine health conditions in adult survivors of childhood cancer: the need for specialized adult-focused follow-up clinics. Eur J Endocrinol. (2013) 168:465–72. doi: 10.1530/EJE-12-1043
7. Chemaitilly W, Li Z, Krasin MJ, Brooke RJ, Wilson CL, Green DM, et al. Premature ovarian insufficiency in childhood cancer survivors: a report from the St. Jude lifetime cohort. J Clin Endocrinol Metab. (2017) 102:2242–50.
8. Chemaitilly W, Cohen LE, Mostoufi-Moab S, Patterson BC, Simmons JH, Meacham LR, et al. Endocrine late effects in childhood cancer survivors. J Clin Oncol. (2018) 36:2153–9.
9. Van Iersel L, Li Z, Srivastava DK, Brinkman TM, Bjornard KL, Wilson CL, et al. Hypothalamic-pituitary disorders in childhood cancer survivors: prevalence, risk factors and long-term health outcomes. J Clin Endocrinol Metab. (2019) 104:6101–15.
10. Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-Term endocrine and metabolic consequences of cancer treatment: a systematic review. Endocr Rev. (2018) 40:711–67.
11. Wang KW, De Souza RJ, Fleming A, Singh SK, Johnston DL, Zelcer SM, et al. Adiposity in childhood brain tumors: a report from the Canadian Study of determinants of endometabolic health in children (CanDECIDE Study). Sci Rep. (2017) 7:45078. doi: 10.1038/srep45078
12. Clement SC, Schouten-Van Meeteren AYN, Boot AM., Claahsen-Van Der Grinten HL., Granzen B, Sen Han K, et al. Prevalence and risk factors of early endocrine disorders in childhood brain tumor survivors: a nationwide, multicenter study. J Clin Oncol. (2016) 34:4362–70. doi: 10.1200/JCO.2016.67.5025
13. Lawson SA, Horne VE, Golekoh MC, Hornung L, Burns KC, Fouladi M, et al. Hypothalamic–pituitary function following childhood brain tumors: analysis of prospective annual endocrine screening. Pediatr Blood Cancer. (2019) 66:e27631. doi: 10.1002/pbc.27631
14. Marcucci G, Beltrami G, Tamburini A, Body JJ, Confavreux CB, Hadji P, et al. Bone health in childhood cancer: review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol. (2019) 30:908–20.
15. Schündeln MM, Hauffa PK, Munteanu M, Kiewert C, Unger N, Bauer JJ, et al. Prevalence of osteopathologies in children and adolescents after diagnosis of acute lymphoblastic leukemia. Front Pediatr. (2020) 8:509. doi: 10.3389/fped.2020.00509
16. Cohen LE, Gordon JH, Popovsky EY, Sainath NN, Feldman HA, Kieran MW, et al. Bone density in post-pubertal adolescent survivors of childhood brain tumors. Pediatr Blood Cancer. (2012) 58:959–63. doi: 10.1002/pbc.23300
17. Remes TM, Arikoski PM, Lähteenmäki PM, Arola MO, Pokka TML, Riikonen VP, et al. Bone mineral density is compromised in very long-term survivors of irradiated childhood brain tumor. Acta Oncol. (2018) 57:665–74.
18. Ward LM, Ma J, Lang B, Ho J, Alos N, Matzinger MA, et al. Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. J Bone Miner Res. (2018) 33:1435–43. doi: 10.1002/jbmr.3447
19. Ma J, Siminoski K, Wang P, Jaremko JL, Koujok K, Matzinger MA, et al. The accuracy of incident vertebral fracture detection in children using targeted case-finding approaches. J Bone Miner Res. (2021) 36:1255–68.
20. Mostoufi-Moab S, Ward LM. Skeletal morbidity in children and adolescents during and following cancer therapy. Horm Res Paediatr. (2019) 91:137–51.
21. Williams KM. Update on bone health in pediatric chronic disease. Endocrinol Metab Clin North Am. (2016) 45:433–41. doi: 10.1016/j.ecl.2016.01.009
22. Karsenty G. Convergence between bone and energy homeostases: leptin regulation of bone mass. Cell Metab. (2006) 4:341–8. doi: 10.1016/j.cmet.2006.10.008
23. Schündeln MM, Bäder L, Kiewert C, Herrmann R, Führer D, Hauffa BP, et al. Plasma concentrations of osteocalcin are associated with the timing of pubertal progress in boys. J Pediatr Endocrinol Metab. (2017) 30:141–7. doi: 10.1515/jpem-2016-0243
24. Desentis-Desentis MF, Rivas-Carrillo JD, Sánchez-Enríquez S. Protective role of osteocalcin in diabetes pathogenesis. J Bone Miner Metab. (2020) 38:765–71.
25. Schündeln MM, Goretzki SC, Hauffa PK, Wieland R, Bauer J, Baeder L, et al. Impairment of bone health in pediatric patients with hemolytic anemia. PLoS One. (2014) 9:e108400. doi: 10.1371/journal.pone.0108400
26. van Buuren S, Ooms J. Stage line diagram?: an age-conditional reference diagram for tracking development. Stat Med. (2009) 28:1569–79. doi: 10.1002/sim.3567
27. Greulich W, Pyle S. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed. Stanford, CA: Stanford University Press (1959).
28. Chang CH, Housepian EM, Herbert C. An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. (1969) 93:1351–9. doi: 10.1148/93.6.1351
30. Ellis KJ, Shypailo RJ, Hardin DS, Perez MD, Motil KJ, Wong WW, et al. Z score prediction model for assessment of bone mineral content in pediatric diseases. J Bone Miner Res. (2001) 16:1658–64.
31. GE Healthcare. Lunar enCORE Pediatrics Reference Data. (2010). Available online at: https://www.gehealthcare.com/products/bone-and-metabolic-health/pediatrics (accessed July 06, 2022).
32. Schündeln MM, Marschke L, Bauer JJ, Hauffa PK, Schweiger B, Führer-Sakel D, et al. A piece of the puzzle: the bone health index of the bonexpert software reflects cortical bone mineral density in pediatric and adolescent patients. PLoS One. (2016) 11:e0151936. doi: 10.1371/journal.pone.0151936
33. Thodberg HH, van Rijn RR, Tanaka T, Martin DD, Kreiborg S. A paediatric bone index derived by automated radiogrammetry. Osteoporos Int. (2010) 21:1391–400. doi: 10.1007/s00198-009-1085-9
34. Holick MF. High prevalence of vitamin d inadequacy and implications for health. Mayo Clin Proc. (2006) 81:353–73.
35. Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. (2016) 101:83–106.
36. R Core Team. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria (2020). Available online at: URL http://www.R-project.org/ (accessed June 06, 2022).
37. Wickham H. Tidyverse: Easily Install and Load the “Tidyverse”. R package version 1.2.1. (2017). Available online at: https://CRAN.R-project.org/package=tidyverse (accessed June 06, 2022).
38. Institute of Medicine (IOM). Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press (2011).
39. Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude lifetime cohort study (SJLIFE). Lancet. (2017) 390:2569–82.
40. Weber DR. Bone health in childhood chronic disease bisphosphonate first page preview. Endocrinol Metab Clin North Am. (2020) 49:637–50.
41. Verzicco I, Regolisti G, Quaini F, Bocchi P, Brusasco I, Ferrari M, et al. Electrolyte disorders induced by antineoplastic drugs. Front Oncol. (2020) 10:779. doi: 10.3389/fonc.2020.00779
42. Levy-Shraga Y, David D, Vered I, Kochavi B, Stein D, Modan-Moses D. Hyponatremia and decreased bone density in adolescent inpatients diagnosed with anorexia nervosa. Nutrition. (2016) 32:1097–102. doi: 10.1016/j.nut.2016.03.015
43. Saggese G, Vierucci F, Boot AM, Czech-Kowalska J, Weber G, Camargo CA, et al. Vitamin D in childhood and adolescence: an expert position statement. Eur J Pediatr. (2015) 174:565–76. doi: 10.1007/s00431-015-2524-6
45. Thierfelder W, Dortschy R, Hintzpeter B, Kahl H, Scheidt-Nave C. [Biochemical measures in the German health interview and examination survey for children and adolescents (KiGGS)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2007) 50:757–70.
46. Hintzpeter B, Scheidt-Nave C, Muller MJ, Schenk L, Mensink GBM. Higher prevalence of vitamin D deficiency is associated with immigrant background among children and adolescents in Germany. J Nutr. (2008) 138:1482–90. doi: 10.1093/jn/138.8.1482
47. Kunz C, Hower J, Knoll A, Ritzenthaler KL, Lamberti T. No improvement in vitamin D status in German infants and adolescents between 2009 and 2014 despite public recommendations to increase vitamin D intake in 2012. Eur J Nutr. (2019) 58:1711–22. doi: 10.1007/s00394-018-1717-y
48. Schmidt SCE, Anedda B, Burchartz A, Eichsteller A, Kolb S, Nigg C, et al. Physical activity and screen time of children and adolescents before and during the COVID-19 lockdown in Germany: a natural experiment. Sci Rep. (2020) 10:21780.
49. Antal Z, Balachandar S. Growth disturbances in childhood cancer survivors. Horm Res Paediatr. (2019) 91:83–92.
50. Constine LS, Woolf PD, Cann D, Mick G, McCormick K, Raubertas RF, et al. Hypothalamic-Pituitary dysfunction after radiation for brain tumors. N Engl J Med. (1993) 328:87–94.
51. Schmiegelow M, Feldt-Rasmussen U, Rasmussen AK, Poulsen HS, Müller J. A population-based study of thyroid function after radiotherapy and chemotherapy for a childhood brain tumor. J Clin Endocrinol Metab. (2003) 88:136–40. doi: 10.1210/jc.2002-020380
52. Roddy E, Mueller S. Late effects of treatment of pediatric central nervous system tumors. J Child Neurol. (2016) 31:237–54.
Keywords: childhood primary brain tumors, bone health, childhood malignancies, osteopathologies, survivorship, vitamin D
Citation: Schündeln MM, Fritzemeier S, Goretzki SC, Hauffa PK, Munteanu M, Kiewert C, Hauffa BP, Fleischhack G, Tippelt S and Grasemann C (2022) Prevalence of osteopathologies in a single center cohort of survivors of childhood primary brain tumor. Front. Pediatr. 10:913343. doi: 10.3389/fped.2022.913343
Received: 05 April 2022; Accepted: 27 June 2022;
Published: 18 July 2022.
Edited by:
Hanneke M. Van Santen, Wilhelmina Children’s Hospital, NetherlandsReviewed by:
Gerdi Tuli, Regina Margherita Hospital, ItalyCopyright © 2022 Schündeln, Fritzemeier, Goretzki, Hauffa, Munteanu, Kiewert, Hauffa, Fleischhack, Tippelt and Grasemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael M. Schündeln, bWljaGFlbC5zY2h1ZW5kZWxuQHVrLWVzc2VuLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.