
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr., 06 July 2022
Sec. Pediatric Rheumatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.910026
This article is part of the Research TopicVaccination in Children With Immune Mediated DiseasesView all 8 articles
 Marc H. Jansen1,2*
Marc H. Jansen1,2* Christien Rondaan3
Christien Rondaan3 Geertje Legger2,4
Geertje Legger2,4 Kirsten Minden5,6
Kirsten Minden5,6 Yosef Uziel7
Yosef Uziel7 Nataša Toplak2,8
Nataša Toplak2,8 Despoina Maritsi9
Despoina Maritsi9 Mirjam van den Berg10
Mirjam van den Berg10 Guy Berbers11
Guy Berbers11 Patricia Bruijning12
Patricia Bruijning12 Yona Egert13
Yona Egert13 Christophe Normand2,13
Christophe Normand2,13 Marc Bijl14
Marc Bijl14 Helen Foster15
Helen Foster15 Isabelle Kone-Paut2,16
Isabelle Kone-Paut2,16 Carine Wouters17
Carine Wouters17 Angelo Ravelli2,18
Angelo Ravelli2,18 Ori Elkayam19
Ori Elkayam19 Nicolaas M. Wulffraat1,2
Nicolaas M. Wulffraat1,2 Marloes W. Heijstek2,20
Marloes W. Heijstek2,20Background: In 2011, the first European League Against Rheumatism (EULAR) vaccination recommendations for pediatric patients with autoimmune inflammatory rheumatic diseases (pedAIIRD) were published. The past decade numerous new studies were performed to assess the safety, efficacy and immunogenicity of vaccinations in pedAIIRD. A systematic literature review (SLR) was therefore performed to serve as the basis for the updated 2021 EULAR/PRES recommendations.
Methods: An SLR was performed according to the standard operating procedures for EULAR-endorsed recommendations. Primary outcomes were efficacy, immunogenicity and safety of vaccination in pedAIIRD. The search was performed in Medline, Embase and the Cochrane Library and included studies published from November 2010 until July 2020.
Results: The SLR yielded 57 studies which were included for critical appraisal and data extraction. Only 8 studies described the occurrence of vaccine-preventable infections after vaccination (efficacy), none of these studies were powered to assess efficacy. The majority of studies assessed (humoral) immune responses as surrogate endpoint for vaccine efficacy. Studies on non-live vaccines showed that these were safe and in general immunogenic. Biologic disease-modifying antirheumatic drugs (bDMARDs) in general did not significantly reduce seroprotection rates, except for B-cell depleting therapies which severely hampered humoral responses. Four new studies on human papilloma virus vaccination showed that this vaccine was safe and immunogenic in pedAIIRD. Regarding live-attenuated vaccinations, level 1 evidence of the measles mumps rubella (MMR) booster vaccination became available which showed the safety of this booster for patients treated with methotrexate. In addition, level 3 evidence became available that suggested that the MMR and varicella zoster virus (VZV) vaccination for patients on low dose glucocorticosteroids and bDMARDs might be safe as well.
Conclusions: The past decade, knowledge on the safety and immunogenicity of (live-attenuated) vaccines in pedAIIRD significantly increased. Data on efficacy (infection prevention) remains scarce. The results from this SLR are the basis for the updated EULAR/PRES vaccination recommendations in pedAIIRD.
Vaccination is one of the greatest interventions that has been established to reduce mortality rates in children (1). Patients with autoimmune and/or inflammatory rheumatic diseases (AIIRD) have an increased risk of infection both due to their disease and more importantly to the use of immunosuppressive medication (2–4). For these patients, it is therefore even more important to prevent severe viral and bacterial infections. However, due to their immunosuppressed status, the safety of especially live-attenuated vaccines and the capacity of vaccines to induce protective immune responses is a matter of concern.
In 2011, The European League Against Rheumatism (EULAR) presented the recommendations on vaccination of pediatric patients with autoimmune/inflammatory rheumatic diseases (pedAIIRD) (5). Very few studies were available on pedAIIRD patients and data from studies performed in adult patients had to be extrapolated. Since 2011, numerous trails have been published that studied both live-attenuated and non-live vaccines and that assessed the effects of biologic disease modifying antirheumatic drugs (bDMARDS) on the outcomes of vaccination in specifically pedAIIRD.
We therefore performed a systematic literature review (SLR) on the safety, immunogenicity, and efficacy of vaccinations in pedAIIRD, to serve as the basis for the updated 2021 EULAR vaccination recommendations for pedAIIRD.
The SLR was performed according to the 2014 EULAR standard operating procedures for EULAR-endorsed recommendations (6). The original SLR performed in 2011 served as a starting point (5).
The research question was: what is the safety, efficacy or immunogenicity of vaccines in pedAIIRD, including patients treated with immunosuppressive agents? (Supplementary File 1). Safety was defined as occurrence of (severe) adverse events, effect of vaccination on underlying disease and whether live-attenuated vaccine induced infections; efficacy was defined as the capacity of vaccines to prevent vaccine-preventable infections (VPIs); immunogenicity was defined as the ability to induce humoral and cellular immune responses after vaccination. The immunogenicity of vaccines is often used as a surrogate primary endpoint for efficacy. This is a valid method when there is good correlation between pathogen-specific antibody levels and protection against infection such as for measles, rubella, hepatitis A and B. For other VPIs this correlation is less clear, for example HPV (7). This will be indicated throughout the paper.
MJ, MH and CR were in charge of the SLR. The research question was adapted into search terms according to the PICO method (patient-intervention-comparison-outcome). All available vaccines were included in the search, except for the COVID vaccine. Terminology for medication is according to the new nomenclature of DMARDs, including conventional synthetic (cs)DMARDs, targeted synthetic (ts)DMARDs and biological (b)DMARDs (8). Search terms were combined and are shown in the Supplementary File 1.
Medline (via Pubmed) and Embase were searched for literature published between November 2010 and July 2020. The list was further extended by reviewing the reference lists of identified papers to check for studies that might have been missed in the search strategy. All original studies, including case-reports were eligible for inclusion.
Exclusion criteria were studies that focussed exclusively on non-rheumatic autoimmune diseases [except for inflammatory bowel disease (IBD)] or vaccine development. Phase I trials, in vitro studies, non-English papers and abstracts presented on scientific meetings were also excluded. Papers concerning the potential role of vaccinations in inducing pedAIIRD were excluded, because these recommendations focus on the effect of vaccination on established disease. The flow chart of the search is depicted in Figure 1.
Data analysis was performed by MH, MJ and NW. Data on study design, number and type of pedAIIRD, control group, medication use and the three outcomes (safety, efficacy, immunogenicity) were extracted. The quality of the studies was critically assessed using standardized critical appraisal criteria and levels of evidence (LoE) were determined using the standards of the Oxford Centre for Evidence-Based Medicine (Supplementary Tables 1, 2). Each paper was evaluated by at least two experts. The steering committee organized a 1-day meeting of the Task Force. Prior to this meeting, all experts read and independently graded literature on methodological quality and level of evidence. Results and discrepancies were discussed, followed by the formulation and grading of the recommendations.
The final manuscript was drafted after the meeting, reviewed, revised and approved by all Task Force members, followed by final review and approval by the EULAR Executive Committee before submission to the journal.
The SLR yielded 57 studies that were included for data extraction. The studies included 2 systematic reviews, 2 randomized controlled trials (RCTs), 52 cohort studies and 1 case report. The two systematic reviews were eventually excluded from the analysis as all included studies were part of this review. Some studies investigated multiple vaccines (Table 1). The studies covered the non-live vaccines against Diphtheria Tetanus Polio (DTP, 6 studies), Hepatitis A virus (HAV, 4 studies), Hepatitis B virus (HBV, 7 studies), Human Papillomavirus (HPV, 4 studies), Influenza (13 studies), Meningococcus C (1 study) and Pneumococci (both the pneumococcal conjugate vaccination (PCV) and/or the 23-valent pneumococcal polysaccharide vaccine (PPSV-23) vaccine, 6 studies) (9–50). In addition, studies were included that reported on the live-attenuated vaccines against Measles, Mumps and Rubella (MMR, 12 studies, including 3 on the combined MMR-Varicella booster) (12–14, 51–59), Varicella Zoster Virus (VZV, 5 studies) (59–63), one study which included 1 patient with oral polio vaccine (59) and one case report on the Bacillus Calmette-Guérin (BCG) vaccine (64).
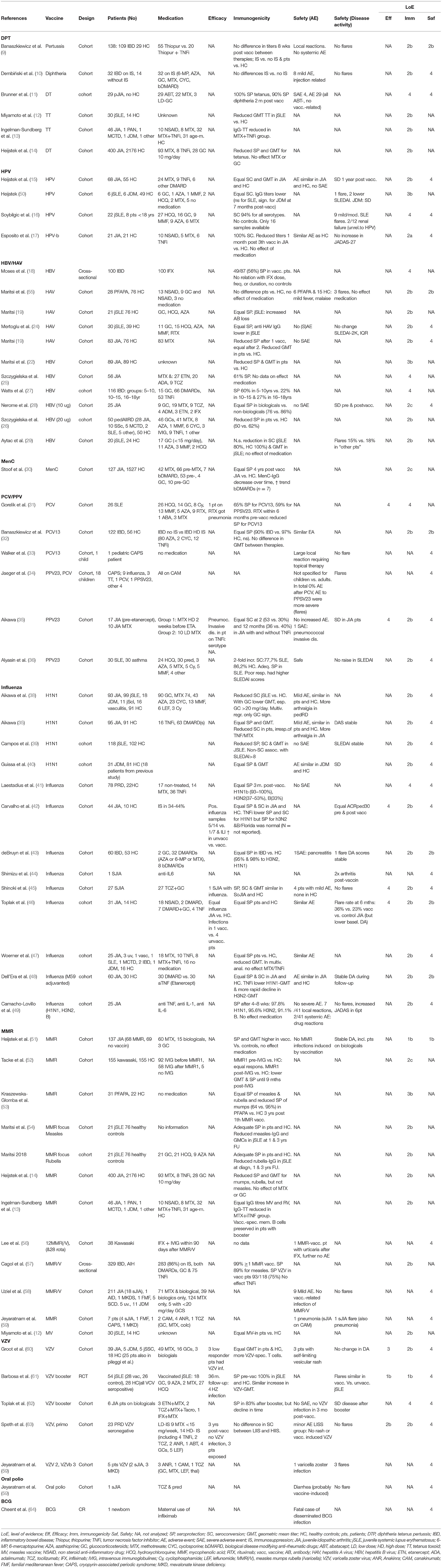
Table 1. Critical appraisal of the literature on the efficacy, immunogenicity and safety of vaccinations in pedAIIRD.
Since the 2011 recommendations, six cohort studies including in total 650 pedAIIRD patients and 2219 healthy controls assessed the DTP (3 diphtheria, 1 pertussis, 4 tetanus) vaccination, with a maximum level of evidence of 2B for immunogenicity and safety (adverse events) (9–14). Efficacy was not evaluated; all studies assessed immunogenicity. Twenty-nine patients with polyarticular juvenile idiopathic arthritis (pJIA) on abatacept mounted high levels of seroprotection rates (90–100%), whereas 30 patients with juvenile onset systemic lupus erythematosus (jSLE) showed lower antibody concentrations against tetanus toxoid than 14 healthy controls (11, 12).
With regard to safety, no severe vaccine-related adverse events were described and no disease flares were detected in vaccinated patients.
No differences were found in antibody titers or adverse events between IBD patients with various csDMARDS (6-mercaptopurine, azathioprine, methotrexate), glucocorticosteroids and bDMARDS (TNFα-inhibitors) compared to IBD patients without these drugs (9). Also in JIA patients, medication did not influence the seroprotection titers (14).
Based on these data, the national immunisation program (NIP) can be followed for pedAIIRD patients.
Since the 2011 recommendations, 11 cohort studies including in total 618 pedAIIRD patients and 278 healthy controls assessed the HAV or HBV vaccination, with a maximum level of evidence of 2B for immunogenicity and safety (18–22, 24–29).
Efficacy was not evaluated; all studies assessed immunogenicity. There is a good correlation between antibody concentration and level of protection against infection (65). In comparison to healthy controls, seroprotection rates were equal (19, 24) or reduced (26, 29), with lower antibody concentrations found in JIA and jSLE patients (19, 22, 24, 29). With regard to safety, no serious adverse events were reported and no effect on JIA and jSLE disease activity was observed (24, 29). Three of 28 periodic fever adenitis pharyngitis aphtosis (PFAPA) patients vaccinated with the HAV vaccine had a flare post-vaccination (20). No non-vaccinated PFAPA control group was available.
The lower antibody concentrations found in JIA and sJLE patients were independent of medication use, including TNFα-inhibitors, but patient numbers were small (28, 29).
Based on these data, the NIP can be followed for pedAIIRD patients.
One new study was available that assessed the antibody persistence over time after vaccination against meningococcal type C (30). The seroprotection rates 4 years after vaccination were similar in 127 JIA patients compared to 1527 juvenile controls, with a trend toward faster waning of immunity in patients on bDMARDS (n = 7). Efficacy and safety were not assessed.
Based on these data, no specific recommendations were formulated for meningococcal vaccination, and the NIP can be followed.
Since 2011, 4 cohort studies were performed including 109 patients (89 JIA, 14 jSLE, 6 JDM) and 125 healthy controls that assessed the HPV vaccine (15–17, 50). Efficacy was not assessed, all studies evaluated antibody titers as surrogate endpoint. Although the HPV vaccine is 98–100% effective against cervical intraepithelial neoplasia (CIN) caused by HPV16/18 in healthy women (66), the exact correlation between antibody levels and protection against cervical carcinoma is unknown.
Seropositivity rates were generally similar in patients and controls. Antibody concentrations were similar in 68 JIA patients including 9 on TNFα-inhibitors compared to controls after the third vaccination in one study (15). However, lower antibody concentrations were found in another study with 21 JIA patients including 6 on TNFα-inhibitors, in 6 jSLE patients and 6 JDM patients (17, 50).
Regarding safety, adverse events were similar in patients and healthy controls. JIA disease activity was similar before and after vaccination. In total 10 SLE flares were described but no conclusions can be drawn as this included the adult population and no unvaccinated control group was available (16).
Groups were too small for definite conclusions, no differences were described in antibody concentration or JIA disease activity in 6 JIA patients on TNFα-inhibitors compared to 15 JIA patients without TNFα-inhibitors (17).
Given the high risk of chronic HPV infection and HPV-associated carcinoma in situ in SLE patients (67), the high seropositivity rates after vaccination in patients with jSLE and other pedAIIRD, and the mild adverse events after vaccination, a specific recommendation was formulated for jSLE patients (3). For these patients in particular, the HPV vaccination should be strongly considered when jSLE patients have not (yet) been vaccinated.
Six cohort studies assessed vaccination against pneumococci with either the PCV (10/13) or PPSV23 vaccine in pedAIIRD patients. These studies included 224 patients (56 jSLE, 27 JIA, 122 IBD, 19 CAPS patients), 56 healthy controls and 30 asthma patients as controls. None of the studies was powered to assess efficacy, but one patient on rituximab and one on a TNFα-inhibitor got pneumonia/pneumococcal invasive disease despite vaccination (31, 35). Regarding immunogenicity, the correlation between antibody levels and protection against infections has been previously shown in RA patients after the PCV7 vaccine (68). The humoral immunogenicity of the PCV10, PCV13 vaccine and PPSV23 vaccine was shown in patients with SLE (31, 36), IBD (32) and JIA (35), despite reduced antibody titers in some studies compared to controls.
Both the PCV10, PCV13 and PPSV23 vaccine were tolerated well without severe adverse events or disease flares (35, 36). In contrast, in CAPS patients, (severe) systemic reactions were described in 12 of the 15 PPSV23 vaccinations (34). This study included 1 pediatric patient receiving the PPSV23 vaccine; two pediatric CAPS patients receiving the PCV vaccine did not experience severe systemic reactions (33, 34).
Rituximab reduced the seropositivity rates in 9 SLE patients (31), whereas antibody concentrations did not differ between patients (JIA n = 17, IBD n = 12) with and without TNFα-inhibitors (32, 35). The effect of csDMARDs was not assessed.
Based on the fact that pneumococcal conjugate vaccine is included in the NIP for all children, the high rates of immunogenicity among pedAIIRD patients and the favourable safety profile of the vaccine, the PCV10/13 vaccine is recommended for all non-vaccinated pedAIIRD patients.
Since 2011, 9 studies on the seasonal influenza vaccine and five on the pandemic H1N1 influenza strain vaccine were performed including 841 pedAIIRD patients and 457 healthy controls, although several patients seem to be included in more than one study considering the H1N1 vaccine (37, 39–49, 69).
Studies were underpowered to assess efficacy, but 3 studies described infection rates (42, 45, 46). In vaccinated patients, influenza like illness occurred less frequently than in unvaccinated patients whereas influenza rates were similar in vaccinated patients and vaccinated healthy controls (42, 46). Most studies assess immunogenicity, mainly defined as a protective level of antibodies measured by the haemagglutination inhibition assay. Most of the studies demonstrated similar high rates of immunogenicity among pedAIIRD patients and healthy controls after the seasonal influenza vaccine (41, 42, 45–49) and the H1N1 vaccine (37, 40). Two studies in a similar cohort of 118 jSLE patients showed reduced seroprotection and seroconversion rates in jSLE patients compared to controls, especially in patients on high dose glucocorticosteroids (>20 mg/day) (38, 40).
With regard to safety, influenza vaccination did not influence disease activity of the underlying disease in the majority of studies with patients with JIA and jSLE (37, 39, 42, 45, 48, 49). One study included unvaccinated patients as a control cohort and reported disease worsening in 35% of the vaccinated JIA patients vs. 23% of the unvaccinated JIA patients, however it should be mentioned that the unvaccinated patients had lower baseline disease activity (46).
No influence of methotrexate on influenza immunogenicity was found in two studies (37, 47). Controversial results were found on the effect of TNFα-inhibitors on immunogenicity of the influenza vaccines. No effect of the TNFα-inhibitors was found on immunogenicity in 59 patients in 3 prospective cohort studies, were as lower protection rates (37, 47, 49) whereas lower seroprotection rates in patients on TNFα-inhibitors and lower H1N1-specific antibodies in 30 JIA patients were found in two prospective cohorts (42, 48). High dose glucocorticosteroids (>20 mg/day) impaired the immunogenicity of the H1N1 vaccine (38). Data on other csDMARDS and bDMARDS were scarce. Unfortunately no studies are yet available on timing of immunosuppressive drugs in pedAIIRD and the influenza vaccine.
Based on data retrieved from adult AIIRD patients showing increased susceptibility for severe influenza infections in immunosuppressed AIIRD patients (3), the fact that seasonal influenza vaccination is not incorporated in the NIP, the high rates of immunogenicity among pedAIIRD patients and the favourable safety profile of the vaccines, the task force again concludes that non-live seasonal influenza vaccination should be strongly considered for pedAIIRD patients treated with glucocorticosteroids or DMARDS.
Twelve studies were performed since 2011 including 1433 pedAIIRD patients that received the MMR or MMR/V (varicella) booster vaccine (12–14, 51–59). None of the studies evaluated efficacy, but there is a high correlation between antibody levels and protection against infection (65).
Immunogenicity of the MMR vaccine was similar to reduced compared to healthy controls (13, 51, 52, 57). During long-term follow-up (>1 year), several studies showed reduced seroprotection rates or antibody concentrations toward mumps (14, 53), measles (54) or rubella (14, 70).
Regarding safety, JIA disease activity was similar in patients randomized to be vaccinated compared to JIA controls (51). The MMR vaccine is a live-attenuated virus vaccine, we therefore focussed on vaccine-induced infections with the attenuated virus. No MMR-induced infections were detected after the booster MMR vaccine (58). This also included 132 patients on bDMARDs (51, 56, 58, 59).
No effect of methotrexate or bDMARDS on immunogenicity of the MMR vaccine and on waning of MMR-specific antibody concentrations was detected in 132 patients using bDMARDS [TNFα-inhibitors (n = 123), anti-IL1 (n = 26) and anti-IL6 (n = 6)] (13, 14, 51, 57). The MMR (booster) vaccine did not induce severe adverse events or vaccine-induced infections. These data are of major importance in the new EULAR recommendations stating that the MMR booster can be considered in patients treated with bDMARDS, with most evidence currently available for TNFα-inhibitors. No data are available for the primary MMR vaccine as children are vaccinated shortly after the age of 1 year and pedAIIRD patients in these age groups are rare.
In addition, the lack of severe adverse events in pedAIIRD patients using methotrexate and the high levels of seroprotection in these patients have led to the recommendation that the MMR booster vaccination can be administered to patients using methotrexate.
Five additional studies were available on the varicella zoster virus vaccine including 137 patients with pedAIIRD and 46 healthy controls, of which four studies evaluated primo varicella vaccination in naïve patients (59–63). Twenty-five patients from one study had been previously described in a study by Pileggi et al. (71). Additionally, 3 studies evaluated the MMR/V vaccine in 602 patients with pedAIIRD (56, 57, 72). For clarity, we distinguish between the studies on primo varicella vaccination in VZV naïve patents and the varicella booster vaccine.
One study was a randomized controlled trial in which efficacy (herpes zoster infections) and safety (disease activity) was compared between 28 vaccinated jSLE patients and 26 unvaccinated jSLE patients after one booster VZV vaccination (level of evidence 1B). This study also included 28 age matched healthy controls who also received VZV vaccination consisting of ≥1,000 plaque-forming units of virus/0.5 mL (61). Immunogenicity: all patients had protective VZV antibodies pre-vaccination and after the booster VZV vaccination (61).
Regarding efficacy, the study of Barbosa on the VZV booster vaccination showed 4 herpes zoster cases in unvaccinated jSLE patients compared to no cases in vaccinated patients and controls. Both vaccinated SLE patients and controls had a significant increase in antibody levels between days 0 and 30. Regarding safety: The frequency of flares and the SLEDAI score were similar among the vaccinated and unvaccinated patients. None of the vaccinated patients experienced disseminated varicella rash or herpes zoster.
Efficacy: Regarding the occurrence of varicella after primo vaccination, 3 varicella cases were described in uncontrolled studies in low responders whereas in another study no varicella infections were described during 3 years follow-up (60, 62, 63). Overall, studies were underpowered and not designed to assess efficacy.
Immunogenicity data showed a similar (increase in) VZV-specific geometric mean titers (GMTs) in patients and controls (60, 61). A seroconversion rate of approximately 80% was described after the primo vaccination (including patients who received both 1 and 2 vaccinations (57, 62). Patients who received two vaccines had significantly higher antibody concentrations than patients (p = 0.016) and HC (p < 0.001) who received only one vaccine (60). Antibody levels decreased over time but waning was not compared to healthy controls (57, 62).
Regarding safety of the VZV vaccine, primo vaccination induced varicella infections were described in 3 patients (1 using leflunomide, 1 using cyclosporine, 1 without medications) with self-limiting vesicular rash and in 1 sJIA patient using multiple immunosuppressive drugs (methotrexate, thalidomide, leflunomide, anakinra) who developed generalized vesicles after the booster vaccination and was admitted to the hospital and treated with acyclovir (58, 59, 62, 63). Ten other patients on anti-IL1 therapy or anti-IL6 therapy did not develop VZV-vaccine induced infections (59, 62, 63). Disease activity was similar in vaccinated patients and unvaccinated jSLE patients. Most studies did not describe disease flares, except for disease flares in an uncontrolled case series after VZV vaccination in 3 MKD patients (59). Overall, these data suggest that the VZV vaccine is in general well tolerated, but one should remain vigilant for VZV-induced infection.
The effect of immunosuppressive treatment on efficacy and immunogenicity of the VZV vaccine was not systematically assessed. One study showed no effect of TNFα-inhibitors on the persistence of antibody levels (57) and no differences were found in seroconversion rates between 9 patients on low dose MTX vs. 14 patients with higher degrees of immunosuppressive drugs (63).
Regarding safety, vaccine-induced VZV infections, although rare and mainly self-limiting, were described in 3 patients who predominantly used immunosuppressive drugs or bDMARDS.
The VZV vaccination is included in the NIP of several European countries, and the question whether pedAIIRD patients can be effectively and safely vaccinated often rises. Based on the evidence described above with humoral immune responses comparable to healthy controls and lack of complicated or disseminated vaccine- induced varicella infections after VZV primo vaccination, and the risk of severe disseminated VZV infection in immunosuppressed hosts, the task force concluded that the VZV should be strongly considered in all varicella naïve patients on MTX and can even be considered under specific conditions in patients receiving TNFα-inhibitors, anti-IL1 and anti-IL6 therapy.
No studies were performed to assess the BCG vaccine in pedAIIRD patients. However, there was one case report that described a fatal case of disseminated BCG infection in a newborn whose mother used infliximab (64). This casualty led to the safety warning in the updated recommendations.
No studies were available to assess the outcomes of vaccination against yellow fever in pedAIIRD patients; data on this vaccine had to be extrapolated from studies performed in adult patients (3). There is limited data and mostly on the yellow fever booster vaccine. PedAIIRD patients usually require a primary vaccination dose. As fatal outcomes of YFV vaccination has been described in adult RA and SLE patients, and because adverse events tend to be more severe in patients with chronic inflammatory diseases, the task force recommended to withhold this vaccine from immunosuppressed pedAIIRD patients (73–75).
Since 2011, 39 studies including 811 patients with pedAIIRD focussed on the effect of bDMARDS on the outcome of vaccination (Table 2). Efficacy data are too scarce and often uncontrolled for definite conclusions. Most data are available for TNFα-inhibitors with 28 studies including 647 patients. In patients using TNFα-inhibitors more influenza like illness occurred in unvaccinated patients vs. vaccinated patients. Another study described one patient with invasive pneumococcal infection despite vaccination (37). Overall, vaccinations are able to induce an adequate (seroprotective) humoral immune response for most of the vaccines studied. There is a trend toward lower antibody concentrations and accelerated waning of humoral immunity in the studies assessing long term immunity (30, 42, 47, 48).
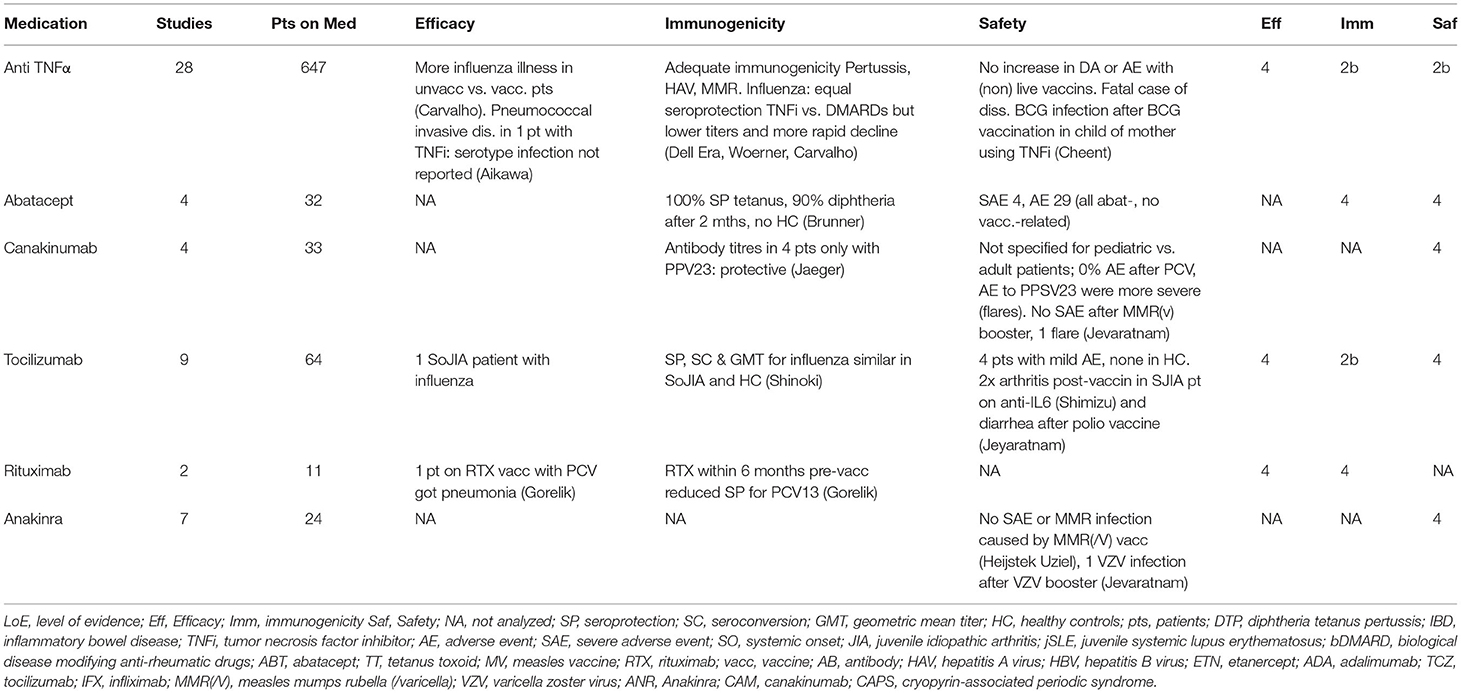
Table 2. The effect of bDMARDs on the efficacy, immunogenicity and safety of vaccinations in pedAIIRD.
Regarding safety, non-live vaccines are well tolerated without induction of disease flares. An increasing amount of pedAIIRD patients received a live-attenuated MMR booster vaccine without vaccine induced infection (51, 58). The VZV (booster and primo) vaccine was well tolerated and no VZV vaccine-induced infections were noted in all vaccinated pedAIIRD patients on TNF-blocking agents. In contrast, the BCG vaccination caused fatal disseminated BCG infections in a newborn whose mother used infliximab (64).
Overall these data indicate that, in patients using TNFα-inhibitors, vaccines are largely immunogenic and safe. Primary live-attenuated BCG and YFV vaccines remain contra-indicated, but accumulating evidence supports the safety of the MMR booster and VZV vaccination in pedAIIRD patients using TNFα-inhibitors.
The effect of IL-1 blocking agents (anakinra, canakinumab) on outcome of vaccination was studied in 11 studies with 57 pedAIIRD patients, mostly with sJIA or CAPS. Efficacy data were not available and immunogenicity was not studied systematically, the studies focussed on safety of vaccination. In 10 patients who received the MMR booster and the MMR/V vaccination 1 case of varicella zoster was reported, but there were no severe vaccination-induced MMR infections (59, 72). In patients with periodic fever syndromes on IL-1 blocking agents, vaccinations (tetanus, influenza, PCV) were well tolerated, accept for the PPSV23 vaccine that caused severe adverse events in patients with CAPS. However, these data were also based on adult patients (34). Hence, we conclude that non-live vaccines are considered safe in pedAIIRD patients receiving IL-1 blocking agents, and that the live-attenuated MMR booster or varicella vaccines can be considered for these patients on a case-by-case basis.
In total 64 patients receiving vaccinations whilst treated with anti-IL-6 were described (Table 2). Patients with sJIA treated with anti-IL-6 inhibitors showed equal seroprotection rates and antibody responses after influenza vaccination compared to healthy controls (45). Patients on IL-6 who received live-attenuated booster vaccines also had no severe adverse events (58). In addition, 32 patients on abatacept were described. They had adequate seroprotection rates after DTP vaccination and no vaccine related adverse events (11).
B-cell depleting agents are mainly studied in adult AIIRD patients and per study in pedAIIRD patients the number of patients on B-cell depleting agents is low (3). In the study of Gorelik et al. 9 patients on rituximab showed reduced seropositivity rates after PCV vaccination compared to patients without rituximab (31). These data are in line with the data in adult patients (76). Since there is no evidence on efficacy or immunogenicity of tetanus vaccination in patients receiving B-cell depleting agents in the preceding 6 months, it is the experts' opinion that passive immunization with tetanus immunoglobulins should be considered in case of an event with high risk for a tetanus infection.
In this systematic literature review 57 new studies on the safety, immunogenicity, and efficacy of vaccinations in pedAIIRD were critically appraised and summarized to serve as the basis for the updated 2021 EULAR vaccination recommendations for pedAIIRD. For the 2011 recommendations, only 27 studies were available (5). Three major outcomes were evaluated: efficacy (the capacity to prevent infection), immunogenicity (the capacity to induce immune responses) and safety (defined as severe adverse events and effect on disease activity). On the one hand, the outcomes of vaccinations included in the NIPs should be assessed in the pedAIIRD population; on the other hand the need for additional booster vaccinations or additional other vaccinations should be evaluated.
The efficacy of vaccinations included in the NIPs has been shown for healthy children and adolescents. There were no studies that were powered to assess efficacy of vaccinations in pedAIIRD. These kind of studies are difficult to perform as they require large scale studies in pedAIIRD patients, especially since the risk of infection is low due to high herd immunity. For the influenza vaccine, not included in the NIP, data on influenza infection rates had to be extrapolated from adult AIIRD patients [which show an increased risk for (complicated) influenza infections] and efficacy data for influenza vaccination are lacking (77). Therefore, immunogenicity had to be assessed as a surrogate endpoint for efficacy.
The immunogenicity of vaccines depend on the type of vaccination, disease type and medication use. In general, vaccinations were immunogenic in pedAIIRD patients, including patients using (predominantly low dose) glucocorticosteroids, methotrexate or TNF-blocking agents. During long-term follow-up, humoral immunity may wane faster in patients depending on the pathogen. In patients on high dose immunosuppressive drugs, especially prednisolone and B cell depleting therapies, measuring antibody concentrations should be considered (38).
Regarding safety, vaccinations do not increase the disease activity. This was shown by two randomized controlled trials in JIA and jSLE patients (51, 61). Safety concerns remain based on case reports of CAPS flares after the PPSV23 vaccination, but controlled studies are lacking (34). Besides disease activity, safety is an important issue in patients on high dose immunosuppressive drugs or biologicals who require a live-attenuated vaccine. Evidence has accumulated since 2011 that the live-attenuated MMR booster vaccine and the VZV vaccine do not cause complicated or disseminated vaccine-induced infections in patients on methotrexate, TNF-blocking agents and small numbers of patients on anti-IL1 or anti-IL6 treatment (51, 58–61, 63). Varicella skin vesicles can occur after primo vaccination and should be monitored (58–60). In contrast, evidence has also accumulated on severe adverse events after the YFV vaccine (in adult patients) and the BCG vaccine, reinforcing the previous recommendation that these vaccines should be withheld in immunosuppressed patients (64, 73–75).
In conclusion, evidence has grown that non-live vaccines included in the NIPs are immunogenic and safe in pedAIIRD patients. Also, evidence show that the MMR booster is safe in patients on MTX and that the MMR booster and VZV primo vaccination can be considered in patients using MTX and even TNF-blocking agents and anti-IL1 and anti-IL6 treatment. In addition to the NIP, the seasonal influenza vaccine can be considered in immunocompromised pedAIIRD patients based on the favorable immunogenicity and safety data. The current SLR also shows that individualized vaccination strategies are necessary in immunocompromised pedAIIRD patients, that take into account the actual risk of infections, the long-term persistence of immunity after vaccination, the safety of vaccinations in relation to specific pedAIIRD and the influence of (new) treatments on vaccination outcome. The current recommendations based on this SLR may add to improved vaccination strategies in this vulnerable patient population.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
MJ, MH, and CR performed the SLR. MJ and MH primarily wrote the manuscript. All authors were involved in the Delphi meeting and voting and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by European League against Rheumatism (EULAR).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.910026/full#supplementary-material
1. Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Heal. (2018) 6:e744–57. doi: 10.1016/S2214-109X(18)30247-X
2. Zink A, Manger B, Kaufmann J, Eisterhues C, Krause A, Listing J, et al. Evaluationof the RABBIT risk score for serious infections. Ann Rheum Dis. (2014) 73:1673–6. doi: 10.1136/annrheumdis-2013-203341
3. Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, Van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. (2020) 79:39–52. doi: 10.1136/annrheumdis-2019-215882
4. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. (2002) 46:2287–93. doi: 10.1002/art.10524
5. Heijstek MW, Ott De Bruin LM, Bijl M, Borrow R, Van Der Klis F, Koné-Paut I, et al. EULAR recommendations for vaccination in paediatric patients with rheumatic diseases. Ann Rheum Dis. (2011) 70:1704–12. doi: 10.1136/ard.2011.150193
6. Van Der Heijde D, Aletaha D, Carmona L, Edwards CJ, Kvien TK, Kouloumas M, et al. 2014 Update of the EULAR standardised operating procedures for EULAR-endorsed recommendations. Ann Rheum Dis. (2015) 74:8–13. doi: 10.1136/annrheumdis-2014-206350
7. Turner TB, Huh WK. HPV vaccines: translating immunogenicity into efficacy. Hum Vaccines Immunother. (2016) 12:1403–5 doi: 10.1080/21645515.2015.1103936
8. Smolen JS, Van Der Heijde D, MacHold KP, Aletaha D, Landewé R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis. (2014) 73:3–5. doi: 10.1136/annrheumdis-2013-204317
9. Banaszkiewicz A, Gawronska A, Klincewicz B, Kofla-Dłubacz A, Grzybowska-Chlebowczyk U, et al. Immunogenicity of pertussis booster vaccination in children and adolescents with inflammatory bowel disease: a controlled study. Inflamm Bowel Dis. (2017) 23:847–52. doi: 10.1097/MIB.0000000000001076
10. Dembiński Ł, Dziekiewicz M, Banaszkiewicz A. Immune response to vaccination in children and young people with inflammatory bowel disease: a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr. (2020) 71:423–32. doi: 10.1097/MPG.0000000000002810
11. Brunner HI, Tzaribachev N, Cornejo GV, Joos R, Gervais E, Cimaz R, et al. Maintenance of antibody response to diphtheria/tetanus vaccine in patients aged 2-5 years with polyarticular-course juvenile idiopathic arthritis receiving subcutaneous abatacept. Pediatr Rheumatol. (2020) 18:19. doi: 10.1186/s12969-020-0410-x
12. Miyamoto M, Ono E, Barbosa C, Terreri M, Hilário M, Salomão R, et al. Vaccine antibodies and T- and B-cell interaction in juvenile systemic lupus erythematosus. Lupus. (2011) 20:736–44. doi: 10.1177/0961203310397409
13. Ingelman-Sundberg HM, Laestadius Å, Chrapkowska C, Mördrup K, Magnusson B, Sundberg E, et al. Diverse effects on vaccine-specific serum IgG titres and memory B cells upon methotrexate and anti-TNF-α therapy in children with rheumatic diseases: A cross-sectional study. Vaccine. (2016) 34:1304–11. doi: 10.1016/j.vaccine.2016.01.027
14. Heijstek MW, Van Gageldonk PGM, Berbers GAM, Wulffraat NM. Differences in persistence of measles, mumps, rubella, diphtheriaand tetanus antibodies between children with rheumatic disease and healthy controls: a retrospective cross-sectional study. Ann Rheum Dis. (2012) 71:948–54. doi: 10.1136/annrheumdis-2011-200637
15. Heijstek MW, Scherpenisse M, Groot N, Tacke C, Schepp RM, Buisman AM, et al. Immunogenicity and safety of the bivalent HPV vaccine in female patients with juvenile idiopathic arthritis: a prospective controlled observational cohort study. Ann Rheum Dis. (2014) 73:1500–7. doi: 10.1136/annrheumdis-2013-203429
16. Soybilgic A, Onel KB, Utset T, Alexander K, Wagner-Weiner L. Safety and immunogenicity of the quadrivalent HPV vaccine in female Systemic Lupus Erythematosus patients aged 12 to 26 years. Pediatr Rheumatol. (2013) 11:29. doi: 10.1186/1546-0096-11-29
17. Esposito S, Corona F, Barzon L, Cuoco F, Squarzon L, Marcati G, et al. Immunogenicity, safety and tolerability of a bivalent human papillomavirus vaccine in adolescents with juvenile idiopathic arthritis. Expert Rev Vaccines. (2014) 13:1387–93. doi: 10.1586/14760584.2014.943195
18. Moses J, Alkhouri N, Shannon A, Raig K, Lopez R, Danziger-Isakov L, et al. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol. (2012) 107:133–8. doi: 10.1038/ajg.2011.295
19. Maritsi DN, Eleftheriou D, Onoufriou M, Vartzelis G. Decreased antibodies against hepatitis A in previously vaccinated treatment naïve juvenile SLE patients: a prospective case control study. Clin Exp Rheumatol. (2017) 35:544–5.
20. Maritsi D, Vartzelis G, Spyridis N, Garoufi A, Diamantopoulos S. SAT0500 The Immune Response to Hepatitis a Vaccine in Children with Pfapa Syndrome. Ann Rheum Dis. (2015) 74:841. doi: 10.1136/annrheumdis-2015-eular.3519
21. Maritsi DN, Coffin SE, Argyri I, Vartzelis G, Spyridis N, Tsolia MN. Immunogenicity and safety of the inactivated hepatitis A vaccine in children with juvenile idiopathic arthritis on methotrexate treatment: a matched case-control study. Clin Exp Rheumatol. (2017) 35:711–5.
22. Maritsi D, Vartzelis G, Soldatou A, Spyridis N. AB1131 Hepatitis B immunity in children with juvenile idiopathic arthritis at disease onset. Ann Rheum Dis. (2013) 71:702. doi: 10.1136/annrheumdis-2012-eular.1129
23. Maritsi D, Vougiouka O, Vartzelis G, Benetatou K, Diamantopoulos S, Tsolia M, et al. The response to hepatitis a vaccine in children with JIA on immunosuppresive treatment. Pediatr Rheumatol. (2014) 12:P41. doi: 10.1186/1546-0096-12-S1-P41
24. Mertoglu S, Sahin S, Beser OF, Adrovic A, Barut K, Yuksel P, et al. Hepatitis A virus vaccination in childhood-onset systemic lupus erythematosus. Lupus. (2019) 28:234–40. doi: 10.1177/0961203318819827
25. Szczygielska I, Hernik E, Gazda A, Kołodziejczyk B, Gietka P. Assessment of anti-HBs antibody concentration in children with juvenile idiopathic arthritis treated with biological drugs, vaccinated against viral type B hepatitis in infancy. Reumatologia. (2020) 58:15–20. doi: 10.5114/reum.2020.93508
26. Szczygielska I, Hernik E, Kwiatkowska M, Rutkowska-Sak L, Kolodziejczyk B, Gazda A. Assessment of the level of vaccine-induced anti-HBs antibodies in children with inflammatory systemic connective tissue diseases treated with immunosuppression. Reumatologia. (2015) 53:56–60. doi: 10.5114/reum.2015.51503
27. Watts A, Bennett WE, Molleston JP, Gupta SK, Croffie JM, Waseem S, et al. Incidence of low seroimmunity to hepatitis b virus in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2017) 65:551–4. doi: 10.1097/MPG.0000000000001580
28. Nerome Y, Akaike H, Nonaka Y, Takezaki T, Kubota T, Yamato T, et al. The safety and effectiveness of HBV vaccination in patients with juvenile idiopathic arthritis controlled by treatment. Mod Rheumatol. (2016) 26:368–71. doi: 10.3109/14397595.2015.1085608
29. Aytac MB, Kasapcopur O, Asian M, Erener-Ercan T, Cullu-Cokugras F, Arisoy N. Hepatitis B vaccination in juvenile systemic lupus erythematosus. Clin Exp Rheumatol. (2011) 29:882–6.
30. Stoof SP, Heijstek MW, Sijssens KM, Van Der Klis F, Sanders EAM, Teunis PFM, et al. Kinetics of the long-term antibody response after meningococcal C vaccination in patients with juvenile idiopathic arthritis: a retrospective cohort study. Ann Rheum Dis. (2014) 73:728–34. doi: 10.1136/annrheumdis-2012-202561
31. Gorelik M, Elizalde A, Wong Williams K, Gonzalez E, Cole JL. Immunogenicity of sequential 13-valent conjugated and 23-valent unconjugated pneumococcal vaccines in a population of children with lupus. Lupus. (2018) 27:2228–35. doi: 10.1177/0961203318808589
32. Banaszkiewicz A, Targońska B, Kowalska-Duplaga K, Karolewska-Bochenek K, Sieczkowska A, Gawrońska A, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. (2015) 21:1607–14. doi: 10.1097/MIB.0000000000000406
33. Walker UA, Hoffman HM, Williams R, Kuemmerle-Deschner J, Hawkins PN. Brief report: severe inflammation following vaccination against streptococcus pneumoniae in patients with cryopyrin-associated periodic syndromes. Arthritis Rheumatol. (2016) 68:516–20. doi: 10.1002/art.39482
34. Jaeger VK, Hoffman HM, van der Poll T, Tilson H, Seibert J, Speziale A, et al. Safety of vaccinations in patients with cryopyrinassociated periodic syndromes: a prospective registry based study. Rheumatol (United Kingdom). (2017) 56:1484–91. doi: 10.1093/rheumatology/kex185
35. Aikawa NE, França ILA, Ribeiro AC, Sallum AME, Bonfa E, Silva CA. Short and long-term immunogenicity and safety following the 23-valent polysaccharide pneumococcal vaccine in juvenile idiopathic arthritis patients under conventional DMARDs with or without anti-TNF therapy. Vaccine. (2015) 33:604–9. doi: 10.1016/j.vaccine.2014.12.030
36. Alyasin S, Adab M, Hosseinpour A, Amin R, Babaei M. Immunogenicity of 23-valent pneumococcal vaccine in children with systemic lupus erythematosus. Iran J Immunol. (2016) 13:204–19.
37. Aikawa NE, Campos LMA, Goldenstein-Schainberg C, Saad CGS, Ribeiro AC, Bueno C, et al. Effective seroconversion and safety following the pandemic influenza vaccination (anti-H1N1) in patients with juvenile idiopathic arthritis. Scand J Rheumatol. (2013) 42:34–40. doi: 10.3109/03009742.2012.709272
38. Aikawa NE, Campos LMA, Silva CA, Carvalho JF, Saad CGS, Trudes G, et al. Glucocorticoid: Major factor for reduced immunogenicity of 2009 influenza A (H1N1) vaccine in patients with juvenile autoimmune rheumatic disease. J Rheumatol. (2012) 39:167–73. doi: 10.3899/jrheum.110721
39. Campos LMA, Silva CA, Aikawa NE, Jesus AA, Moraes JCB, Miraglia J, et al. High disease activity: An independent factor for reduced immunogenicity of the pandemic influenza a vaccine in patients with juvenile systemic lupus erythematosus. Arthritis Care Res. (2013) 65:1121–7. doi: 10.1002/acr.21948
40. Guissa VR, Pereira RMR, Sallum AME, Aikawa NE, Campos LMA, Silva CA, et al. Influenza A H1N1/2009 vaccine in juvenile dermatomyositis: reduced immunogenicity in patients under immunosuppressive therapy. Clin Exp Rheumatol. (2012) 30:583–8.
41. Laestadius Å, Ingelman-Sundberg HM, Myrberg IH, Verme A, Sundberg E, Schweiger B, et al. Altered proportions of circulating CXCR5+ helper T cells do not dampen influenza vaccine responses in children with rheumatic disease. Vaccine. (2019) 37:3685–93. doi: 10.1016/j.vaccine.2019.05.037
42. Carvalho LM, de Paula FE, Silvestre R VD, Roberti LR, Arruda E, Mello WA, et al. Prospective surveillance study of acute respiratory infections, influenza-like illness and seasonal influenza vaccine in a cohort of juvenile idiopathic arthritis patients. Pediatr Rheumatol. (2013) 11:10. doi: 10.1186/1546-0096-11-10
43. Debruyn JCC, Hilsden R, Fonseca K, Russell ML, Kaplan GG, Vanderkooi O, et al. Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease. Inflamm Bowel Dis. (2012) 18:25–33. doi: 10.1002/ibd.21706
44. Shimizu M, Ueno K, Yachie A. Relapse of systemic juvenile idiopathic arthritis after influenza vaccination in a patient receiving tocilizumab. Clin Vaccine Immunol. (2012) 19:1700–2. doi: 10.1128/CVI.00309-12
45. Shinoki T, Hara R, Kaneko U, Miyamae T, Imagawa T, Mori M, et al. Safety and response to influenza vaccine in patients with systemic-onset juvenile idiopathic arthritis receiving tocilizumab. Mod Rheumatol. (2012) 22:871–6. doi: 10.1007/s10165-012-0595-z
46. Toplak N, Šubelj V, Kveder T, Cucnik S, Prosenc K, Trampuš-Bakija A, Todorovski L, Avcin T. Safety and efficacy of influenza vaccination in a prospective longitudinal study of 31 children with juvenile idiopathic arthritis. Clin Exp Rheumatol. (2012) 30:436–44.
47. Woerner A, Sauvain MJ, Aebi C, Otth M, Bolt IB. Immune response to influenza vaccination in children treated with methotrexate or/and tumor necrosis factor-alpha inhibitors. Hum Vaccin. (2011) 7:1293–8. doi: 10.4161/hv.7.12.17981
48. Dell'Era L, Corona F, Daleno C, Scala A, Principi N, Esposito S. Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. Vaccine. (2012) 30:936–40. doi: 10.1016/j.vaccine.2011.11.083
49. Camacho-Lovillo MS, Bulnes-Ramos A, Goycochea-Valdivia W, Fernández-Silveira L, Núñez-Cuadros E, Neth O, et al. Immunogenicity and safety of influenza vaccination in patients with juvenile idiopathic arthritis on biological therapy using the microneutralization assay. Pediatr Rheumatol. (2017) 15:62. doi: 10.1186/s12969-017-0190-0
50. Heijstek M, Groot N, Scherpenisse M, Tacke C, Berbers G, van der Klis F, et al. Safety and immunogenicity of human papillomavirus vaccination in juvenile patients with rheumatic diseases. Pediatr Rheumatol Online J. (2011) 9:O41. doi: 10.1186/1546-0096-9-S1-O41
51. Heijstek MW, Kamphuis S, Armbrust W, Swart J, Gorter S, De Vries LD, et al. Effects of the live attenuated measles-mumps-rubella booster vaccination on disease activity in patients with juvenile idiopathic arthritis: a randomized trial. JAMA. (2013) 309:2449–56. doi: 10.1001/jama.2013.6768
52. Tacke CE, Smits GP, Van Der Klis FRM, Kuipers IM, Zaaijer HL, Kuijpers TW. Reduced serologic response to mumps, measles, and rubella vaccination in patients treated with intravenous immunoglobulin for Kawasaki disease. J Allergy Clin Immunol. (2013) 131:1701–3. doi: 10.1016/j.jaci.2013.01.045
53. Kraszewska-Głomba B, Matkowska-Kocjan A, Miśkiewicz K, Szymańska-Toczek Z, Wójcik M, Banyś D, et al. Mumps, measles and rubella vaccination in children with PFAPA syndrome. Vaccine. (2016) 34:5903–6. doi: 10.1016/j.vaccine.2016.10.035
54. Maritsi DN, Vartzelis G, Kopsidas J, Spyridis N, Tsolia MN. Antibody status against measles in previously vaccinated childhood systemic lupus erythematosus patients: a prospective case-control study. Rheumatol (United Kingdom). (2018) 57:1491–3. doi: 10.1093/rheumatology/key142
55. Maritsi DN, Kopsidas I, Vartzelis G, Spyridis N, Tsolia MN. Long-term preservation of measles and rubella specific-IgG antibodies in children with enthesitis related arthritis on anti-TNFα treatment: a prospective controlled study. Rheumatol (United Kingdom). (2019) 58:1686–8. doi: 10.1093/rheumatology/kez096
56. Lee AM, Burns JC, Tremoulet AH. Safety of infliximab following live virus vaccination in kawasaki disease patients. Pediatr Infect Dis J. (2017) 36:435–7. doi: 10.1097/INF.0000000000001447
57. Cagol L, Seitel T, Ehrenberg S, Frivolt K, Krahl A, Lainka E, et al. Vaccination rate and immunity of children and adolescents with inflammatory bowel disease or autoimmune hepatitis in Germany. Vaccine. (2020) 38:1810–7. doi: 10.1016/j.vaccine.2019.12.024
58. Uziel Y, Moshe V, Onozo B, Kulcsár A, Tróbert-Sipos D, Akikusa JD, et al. Live attenuated MMR/V booster vaccines in children with rheumatic diseases on immunosuppressive therapy are safe: multicenter, retrospective data collection. Vaccine. (2020) 38:2198–201. doi: 10.1016/j.vaccine.2020.01.037
59. Jeyaratnam J, ter Haar NM, Lachmann HJ, Kasapcopur O, Ombrello AK, Rigante D, et al. The safety of live-attenuated vaccines in patients using IL-1 or IL-6 blockade: an international survey. Pediatr Rheumatol. (2018) 16:19. doi: 10.1186/s12969-018-0235-z
60. Groot N, Pileggi G, Sandoval CB, Grein I, Berbers G, Ferriani VPL, et al. Varicella vaccination elicits a humoral and cellular response in children with rheumatic diseases using immune suppressive treatment. Vaccine. (2017) 35:2818–22. doi: 10.1016/j.vaccine.2017.04.015
61. Barbosa CMPL, Terreri MTRA, Rosário PO, De Moraes-Pinto MI, Silva CAA, Hilário MOE. Immune response and tolerability of varicella vaccine in children and adolescents with systemic lupus erythematosus previously exposed to varicella-zoster virus. Clin Exp Rheumatol. (2012) 30:791–8.
62. Toplak N, Avcin T. Safety and efficacy of varicella vaccination in children with JIA treated with biologic therapy. Pediatr Rheumatol. (2014) 12:O20. doi: 10.1186/1546-0096-12-S1-O20
63. Speth F, Hinze CH, Andel S, Mertens T, Haas JP. Varicella-zoster-virus vaccination in immunosuppressed children with rheumatic diseases using a pre-vaccination check list. Pediatr Rheumatol Online J. (2018) 16:15. doi: 10.1186/s12969-018-0231-3
64. Cheent K, Nolan J, Shariq S, Kiho L, Pal A, Arnold J. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn's Disease. J Crohn's Colitis. (2010) 4:603–5. doi: 10.1016/j.crohns.2010.05.001
65. Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. (2010) 17:1055–65. doi: 10.1128/CVI.00131-10
66. Harper DM, DeMars LR. HPV vaccines – a review of the first decade. Gynecol Oncol. (2017) 146:196–204. doi: 10.1016/j.ygyno.2017.04.004
67. Kim SC, Glynn RJ, Giovannucci E, Hernández-Díaz S, Liu J, Feldman S, et al. Risk of high-grade cervical dysplasia and cervical cancer in women with systemic inflammatory diseases: a population-based cohort study. Ann Rheum Dis. (2015) 74:1360–7. doi: 10.1136/annrheumdis-2013-204993
68. Nagel J, Geborek P, Saxne T, Jönsson G, Englund M, Petersson IF, et al. The association between antibody levels before and after 7-valent pneumococcal conjugate vaccine immunization and subsequent pneumococcal infection in chronic arthritis patients. Arthritis Res Ther. (2015) 17:124. doi: 10.1186/s13075-015-0636-z
69. Aikawa N, Goldenstein-Schainberg C, Vendramini M, Campos L, Saad C, Moraes J, et al. PReS-FINAL-2177: Safety and lack of autoantibody production following influenza H1N1 vaccination in patients with juvenile idiopathic arthritis (JIA). Pediatr Rheumatol Online J. (2013) 11(Suppl 2):O12. doi: 10.1186/1546-0096-11-S2-O12
70. Maritsi DN, Coffin S, Onoufriou M, Spyridis N, Tsolia MN. Decreased antibodies against rubella in previously vaccinated treatment naive-JSLE patients: a prospective case control study. Scand J Rheumatol. (2017) 48:74–6. doi: 10.1080/03009742.2018.1446100
71. Pileggi GS, de Souza CBS, Ferriani VPL. FRI0343 Safety and efficacy of varicella vaccine in patients with juvenile rheumatic diseases: a five years experience. Ann Rheum Dis. (2013) 71:430. doi: 10.1136/annrheumdis-2012-eular.2800
72. Jansen M, Rondaan C, Legger E, Minden K, Uziel Y, Toplak N, et al. Page: EULAR/PRES Recommendations for Vaccination of Paediatric Patients With Autoimmune Inflammatory Rheumatic Diseases: update 2021. Manuscript under review.
73. Kernéis S, Launay O, Ancelle T, Iordache L, Naneix-Laroche V, Méchaï F. Safety and immunogenicity of yellow fever 17D vaccine in adults receiving systemic corticosteroid therapy: an observational cohort study. Arthritis Care Res. (2013) 65:1522–8. doi: 10.1002/acr.22021
74. Scheinberg M, Guedes-Barbosa LS, Mangueira C, Rosseto EA, Mota L, Oliveira AC, et al. Yellow fever revaccination during infliximab therapy. Arthritis Care Res (Hoboken). (2010) 62:896–8. doi: 10.1002/acr.20045
75. Whittembury A, Ramirez G, Hernández H, Ropero AM, Waterman S, Ticona M, et al. Viscerotropic disease following yellow fever vaccination in Peru. Vaccine. (2009) 27:5974–81. doi: 10.1016/j.vaccine.2009.07.082
76. Van Der Kolk LE, Baars JW, Prins MH, Van Oers MHJ. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood. (2002) 100:2257–9. doi: 10.1182/blood.V100.6.2257.h81802002257_2257_2259
Keywords: vaccination, pediatric rheumatic disease, pediatric immunology, immunosuppressants, vaccination responses, safety
Citation: Jansen MH, Rondaan C, Legger G, Minden K, Uziel Y, Toplak N, Maritsi D, van den Berg M, Berbers G, Bruijning P, Egert Y, Normand C, Bijl M, Foster H, Kone-Paut I, Wouters C, Ravelli A, Elkayam O, Wulffraat NM and Heijstek MW (2022) Efficacy, Immunogenicity and Safety of Vaccination in Pediatric Patients With Autoimmune Inflammatory Rheumatic Diseases (pedAIIRD): A Systematic Literature Review for the 2021 Update of the EULAR/PRES Recommendations. Front. Pediatr. 10:910026. doi: 10.3389/fped.2022.910026
Received: 31 March 2022; Accepted: 25 May 2022;
Published: 06 July 2022.
Edited by:
Deborah Levy, University of Toronto, CanadaReviewed by:
Roberta Audrey Berard, Western University, CanadaCopyright © 2022 Jansen, Rondaan, Legger, Minden, Uziel, Toplak, Maritsi, van den Berg, Berbers, Bruijning, Egert, Normand, Bijl, Foster, Kone-Paut, Wouters, Ravelli, Elkayam, Wulffraat and Heijstek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marc H. Jansen, bS5oLmEuamFuc2VuQHVtY3V0cmVjaHQubmw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.