- 1Department of Children Center, Pediatric and Adolescent Medicine, Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, United States
- 2Center for Digestive Health and Nutrition, Arnold Palmer Hospital for Children, Orlando, FL, United States
- 3Pediatric Gastroenterology & Nutrition of Tampa Bay, Tampa Bay, FL, United States
The exocrine pancreas plays an important role in digestion. Understanding of the physiology and regulation of exocrine function provides insight into disease processes and basis of functional testing. Specifically, exocrine pancreatic insufficiency (EPI) can cause maldigestion and thus a proper assessment of exocrine pancreatic function is important. There are indirect and direct methods for evaluating pancreatic function. Indirect methods are varied and include stool, serum, urine, and breath tests. Fecal elastase is a commonly used indirect test today. Direct methods involve stimulated release of pancreatic fluid that is collected from the duodenum and analyzed for enzyme activity. The most used direct test today is the endoscopic pancreatic function test. Indirect pancreatic function testing is limited in identifying cases of mild to moderate EPI, and as such in these cases, direct testing has higher sensitivity and specificity in diagnosing EPI. This review provides a comprehensive guide to indirect and direct pancreatic function tests as well as an in-depth look at exocrine pancreatic function including anatomy, physiology, and regulatory mechanisms.
Introduction
The exocrine pancreas plays a crucial role in digestion and as such, its function is crucial in pediatric population where growth and development are reliant upon adequate nutrition. The objective of this article is to provide a comprehensive review of the exocrine pancreas and discuss options to evaluate its function.
Anatomy of the pancreas
The pancreas consists of 5 different parts, the head, uncinate process, neck, body, and tail. The head and uncinate process located near the portal vein, superior mesenteric vein, and superior mesenteric artery. This may be a possible explanation why severe acute pancreatitis can be seen with systemic inflammatory reactions.
The pancreas has both exocrine and endocrine functions. The exocrine pancreas encompasses roughly 85% of the pancreatic mass where 10% of the gland is accounted for by extracellular matrix, 4% by blood vessels and the major ducts, and only 2% of the gland is comprised of endocrine tissue (1). The exocrine and endocrine functions are coordinated to allow a regulatory feedback system for digestive enzyme and hormone secretion. Specifically, the blood flow from the endocrine pancreas enters the capillaries of the exocrine tissue before entering the general circulation, and in the exocrine tissue, there are insulin receptors that are involved in regulation of digestive enzyme synthesis (2–4).
The exocrine pancreas is composed of acinus (a collection of about 40 acinar cells) and its draining ducts (5). The centro-acinar cell functions as an extension of the ductal epithelium into the acinus and provides progenitor cells important for pancreatic regeneration (6, 7). The acinar cells synthesize digestive enzymes (lipases, amylase, and proteases) to be stored in zymogen granules and then secreted (enzyme-containing zymogen granules fuse with the apical cell membrane surface) (8). The ductules drain into interlobular (intercalated) ducts and then into the main pancreatic ductal system.
Thanks to its highly developed endoplasmic reticulum (ER) system, the acinar cell of the exocrine pancreas has one of the highest protein synthesis rates among mammalian organ (9, 10). The ER is also a major storage site for intracellular calcium, which, when released into the cytoplasm, is a mediator for secretion of stored digestive enzymes into the pancreatic ductal system (11).
Another cell type known for its role in pathologic states is the pancreatic stellate cell that has a role in pancreatic fibrosis (12, 13). They are found around the acinar and ductular structures as well as the islets of Langerhans. In chronic pancreatitis the stellate cell is transformed into a proliferating myofibroblast cell type that synthesizes and secretes extracellular matrix proteins, proinflammatory cytokines and growth factors (14).
Physiology and regulation of pancreatic secretion
The adult pancreas secretes up to 2500 ml of colorless, odorless, alkaline, isosmotic pancreatic fluid. The flow and concentration of this fluid is highly regulated. The flow rate increases from an average rate of 0.2 or 0.3 ml/min in the resting (interdigestive) state to 4.0 ml/min during postprandial stimulation (15). The ratio of the different enzymes released is adjusted to the composition of digested food. For example, a carbohydrate-rich diet results in an increase in synthesis of amylase and a decrease in chymotrypsinogen (16), while a lipid-rich diet enhances lipase synthesis (17).
Electrolyte secretion
The principal compounds secreted by the exocrine pancreas are water, sodium, potassium, chloride, and bicarbonate. The osmolality of pancreatic juice is independent of flow rate.
Secretin is the main stimulant of electrolyte secretion from ductal and centroacinar cells. Secretin was the first hormone ever discovered at the beginning of 20th century (18). Secretin is released from enteroendocrine S cells in the duodenal mucosa when the pH of the lumen is less than 4.5 (19). Binding of secretin to its receptor activates adenylate cyclase, resulting in the generation of cyclic adenosine monophosphate (cAMP), which acts as the intracellular messenger. The duct cells and centroacinar cells contain carbonic anhydrase, which is important for their ability to secrete bicarbonate (20).
Presence of bicarbonate secretion in the proximal pancreatic ducts is largely mediated by a chloride and bicarbonate exchange transporter. In distal ducts, the luminal bicarbonate concentration is already high, and thus the bicarbonate secretion is mediated by bicarbonate conductance via the cystic fibrosis transmembrane conductance regulator (CFTR) (21). The secreted bicarbonate acts to buffer the acidic fluid entering the duodenum from the stomach and brings this fluid pH to the optimal level for pancreatic enzyme function.
The concentration of bicarbonate secreted can vary based upon the secretory rate of the pancreas. In resting state, the chloride concentration is high in the pancreatic fluid. Alternatively in an active state following secretin stimulation, the bicarbonate concentration is significantly increased. Bicarbonate concentration thus serves as a great marker for pancreatic function, and in testing discussed in detail later, a bicarbonate level lower than 80 mEq/L it is considered abnormal (22, 23).
Pancreatic enzyme secretion
The acinar cells release pancreatic enzymes from their zymogen granules into the lumen of the acinus, and these proteins combine with the water and bicarbonate secretions of the centroacinar and duct cells.
The exocrine secretion has significant reserve capacity. DiMagno et al. (24) investigated this by plotting lipase output and fecal fat excretion in patients with EPI. They reported that fecal fat excretion was increased when the lipase output fell below 10%. Later they found that maldigestion and malabsorption do not occur until the digestive enzyme secretion (when stimulated by CCK) is reduced to 5% to 10% of normal values (25).
Stimulation of pancreatic enzyme secretion
Pancreatic enzyme secretion is stimulated both by neural and humoral mechanisms.
Neural mechanisms
Direct vagal and regional reflexes stimulate pancreatic enzyme secretion. The vagal stimulation activates the cholinergic, muscarinic receptors (M3) with resultant generation of intracellular cyclic guanosine monophosphase (cGMP). The vagus-mediated cephalic phase of pancreatic secretion in humans and experimental animals results in pancreatic fluid that is low-volume with high enzyme concentration.
Distention at the gastric antrum elicits pancreatic enzyme secretion by activation of a vago-vagal reflex called the antro-pancreatic reflex (26). The antro-pancreatic reflex is an important component of the gastric phase of pancreatic secretion (27).
Humoral mechanisms
Cholecystokinin (CCK) is the major humoral mediator of enzyme secretion during the intestinal phase. Specifically, the presence of fat and protein products in the intestine will trigger release of CCK-releasing peptide that then act on CCK containing cells (I-cells) to release CCK (28).
In addition to CCK, other peptide hormones (e.g., secretin, neurotensin) and neurocrine agents (e.g., GRP, PACAP) can stimulate enzyme secretion (29). However, as mentioned above, secretin has cental role in stimulating electrolyte and bicarbonate secretion.
The effect of CCK is mediated via a specific receptor (CCK-A receptor) that can be found on acinar cells, intrapancreatic neurons, and cholinergic afferent neurons. In humans, pancreatic enzyme secretion in response to CCK stimulation or food is inhibited by atropine and somatostatin (30–32). This suggest that CCK's action on the pancreas is dependent on cholinergic mechanism.
Several other peptides including PACAP, GRP, and neurotensin can also act to stimulate pancreatic enzyme secretion (29). However, the extent to which these peptides play a role pancreatic enzyme secretion in humans is not well known.
Enzyme secretion products
Amylase
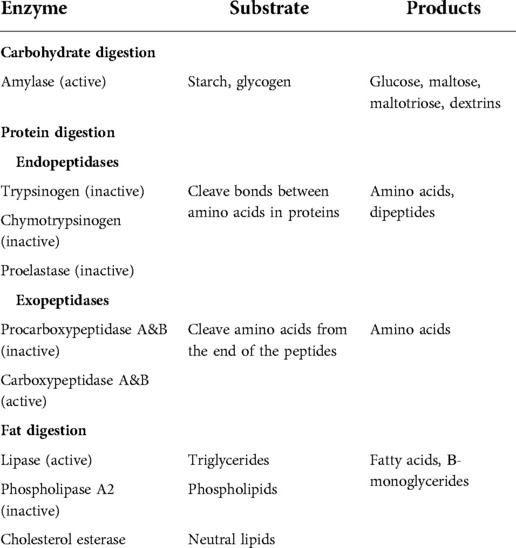
Pancreatic amylase is secreted in its active form. Amylase acts to break down starch and glycogen to glucose, maltose, maltotriose, and dextrins. The 2–9 glucose units are further breaking down by the small intestinal brush border enzymes. These simple sugars are then absorbed via the active transport mechanisms along the brush border of the intestinal epithelial cells.
Proteases
Proteins are first hydrolyzed into peptides in the stomach. These peptides then go on to the intestine and stimulate release of CCK-releasing peptide, CCK, and secretin, which then stimulate the pancreas to secrete enzymes and bicarbonate into the intestine.
The proteolytic enzymes include trypsinogen, elastase, and carboxypeptidase A and B. They are secreted as proenzymes that require activation. Trypsinogen is converted to its active form trypsin, by another enzyme, enterokinase, which is produced by the duodenal mucosal cells (33). Trypsin, in turn, activates the other proteolytic enzymes. Together, these enzymes cleave bonds between amino acids, so that they can be actively transported into the intestinal epithelial cells for absorption.
To prevent activation of these enzymes while in the pancreas, the acinar cells produce a trypsinogen inhibitor. A failure to express this trypsinogen inhibitor, pancreatic secretory trypsin inhibitor (PSTI), also known as serine protease inhibitor Kazal type 1 (SPINK1), is a known cause of familial pancreatitis.
Pancreatic lipases
The pancreatic lipase acts to break down triglycerides. Unlike the proteases discussed above, lipase is secreted in an active form. Colipase is also secreted by the pancreas and acts to enhance activity of lipase by binding to it and changing its molecular configuration.
Phospholipase A2 is secreted by the pancreas as a proenzyme and requires activation by trypsin. Phospholipase A2 hydrolyzes phospholipids.
Carboxylic ester hydrolase and cholesterol esterase act to break down lipid substrates, such as esters of cholesterol, fat-soluble vitamins, and triglycerides. These can then be then packaged into micelles for transport into the intestinal epithelial cells.
The diminished or absent lipase secretion leads to steatorrhea, one of the main clinical symptoms of exocrine pancreatic insufficiency. In our diet, fats are mainly long-chain triglycerides that are broken down into two fatty acids and one beta monoglyceride by the pancreatic lipase.
Pancreatic lipase is degraded when the luminal pH drops <4, therefore diseases that result in acidic intraluminal environments (pancreatic duct cell dysfunction, excessive gastric acid section, etc.) can inhibit fat digestion. This is the main reason that pancreatic enzyme replacement preparations have enteric coated granules.
Gastric lipase is a non-pancreatic lipase that acts to hydrolyze fats; however, it cannot fully compensate for the absence of pancreatic lipase. Infants rely upon other enzymes secreted from the pancreas (pancreatic triglyceride lipase (PTL)-related protein 2, and bile salt-stimulated lipase (BSSL)) that act in conjunction with gastric lipase to achieve efficient fat absorption (34). Interestingly, BSSL is also present in human milk, which facilitates fat absorption and growth in breast-fed infants. Table 1 lists the enzymes and their substrates and products.
Inhibition of pancreatic secretion
Inhibition of exocrine pancreatic secretion occurs through several mechanisms. Somatostatin, pancreatic polypeptide (PP), peptide YY (PYY), neuropeptide Y pancreastatin, and glucagon are all peptides that inhibit secretion indirectly through the activation of inhibitory intrapancreatic neurons. Somatostatin is produced by the delta cells in islets of Langerhans and it exerts an inhibitory effect on amino acid uptake as well as enzyme and bicarbonate secretion (32, 35).
Feedback regulation
Feedback regulation was studied in human and animals by first noting that when pancreatic fluid was diverted from the intestine an increase in pancreatic fluid secretion occurred (36). This augmented enzyme secretion occurred secondary to a rise in circulating CCK (37).
Alternatively, the increase in CCK and pancreatic fluid secretion into the intestine is inhibited by presence of trypsin in the intestine as well as other digestive enzymes (38). This feedback is accomplished via the CCK-releasing peptide in such that with the absence of peptides, CCK-releasing factor will be inactivated by trypsin and thus CCK secretion is decreased (28).
Phases of exocrine secretion
Interdigestive secretion
There is fluid secretion in the fasting (interdigestive) stage that is cyclic and follows the pattern of the migrating myoelectric complex (MMC) (39, 40). This pattern occurs every 60 to 120 min, with bursts of enzyme and bicarbonate secretions being released. Also, there is bile secreted from the gallbladder following partial gallbladder contraction during phases of the MMC. This provides a housekeeping function by cleaning the debris from the small intestine. This process involves the cholinergic nervous system and the hormones motilin and pancreatic polypeptide (39, 40).
Digestive secretion
Cephalic phase
Cephalic phase is mediated by the vagus nerve. In humans, the cephalic phase was identified in studies utilizing a sham feeding method by which the participant would chew food and spit it out. One study (41) indicated that this sham feeding stimulated pancreatic enzyme secretion that rose to about 90% at its maximum, and bicarbonate was also secreted. Atropine suppressed basal trypsin output and essentially abolished the response to sham feeding (42). This suggests that acetylcholine is a major neurotransmitter involved in mediating cephalic phase of pancreatic secretion (39). Among the hormones, gastrin-releasing peptide (GRP) is released from the pancreas upon vagal stimulation and may mediate enzyme secretion (43).
Gastric phase
Gastric phase is initiated by gastric distention by meals. This phase results in secretion of pancreatic enzymes with little effect on the secretion of water and bicarbonate. In studies mimicking gastric distention (fundus or antrum) with a balloon, a resultant low-volume enzyme-rich secretion was obtained through a gastropancreatic vago-vagal reflex (44). Output of gastric contents into the duodenum (gastric chyme with peptides and fatty acids) also act as stimulus at the level of the intestinal mucosa and begins the intestinal phase of pancreatic secretion through neural and hormonal mechanisms. Thus, the rate of gastric emptying can play an important role in pancreatic secretion. As such surgery that alters emptying can often lead to augmented signaling and mixing of gastric and pancreatic fluids.
Intestinal phase
Intestinal phase is mediated by entero-pancreatic vago-vagal reflexes and various hormones. This phase starts when chyme enters the small intestine from the stomach. Specifically, the chyme consists of hydrogen ions, fatty acids, amino acids, and peptides, and these have roles in the intestinal phase of pancreatic section (45). Of the amino acids, phenylalanine, valine, methionine, and tryptophan are known to cause a more robust pancreatic secretory response (46).
Ductal secretion is initiated by hydrogen ions, creating a low pH environment (pH below 4.5) that triggers secretin release from enteroendocrine S cells (19).
The magnitude of stimulation of the pancreas varies not only by the type of nutrients but also by the site of delivery of the nutrients (47). Elemental diet causes less pancreatic enzyme secretion compared to a standard meal, and delivery of nutrients to the jejunum causes less pancreatic secretion than delivery to the duodenum (47).
Vago-vagal reflexes were found to play a role in pancreatic enzyme and bicarbonate secretion. Particularly studies with vagotomy led to low intestinal loads of amino acids and fatty acids, and studies with atropine led to lower physiologic concentrations of CCK (48, 49).
Assessment of exocrine function
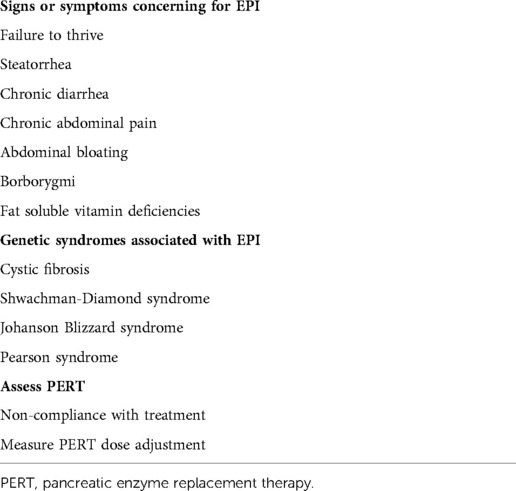
Since the 1940s there have been many tests developed to assess the exocrine pancreatic function. They Include tests that can assess the function of a single enzyme from the stool, serum, urine, or by breath test (indirect tests) and ones that assess the activity of several digestive enzymes from stimulated pancreatic fluid (direct functional tests). The main indications of the exocrine function assessments are listed in Table 2.
Development of enzyme secretions
It is important to understand the intrauterine and postnatal development of enzyme secretion for the accurate interpretation of the functional test results.
Intrauterine development of amylase, lipase, and trypsinogen secretion does not occur at the same time (50, 51). Trypsinogen and chymotrypsinogen were found to be present around 14 to 16 weeks, followed by lipase first appearing by 21 weeks of gestation. Lipase is uniformly present by postnatal age of 15 days (50). Amylase detection is postnatally and occurs much later than all other enzymes. Lebenthal and Lee et al. reported that infants at 30 days old have no detectable amylase activity in duodenal fluid; however, children at around 2 years of age had normal adult level of amylase activity (52, 53).
This postnatal appearance of amylase and lipase in infants may not cause symptoms in breast-fed infants as breast milk has significant amylase (54) and bile salt–dependent lipase in breast milk (contributes to lipid digestion in infants) (55).
There are case reports of isolated lipase/colipase deficiency, detected by duodenal fluid aspiration in children with clinical presentation of greasy stools (56–62). Additionally, isolated amylase deficiency was identified in a large retrospective pediatric database of endoscopic pancreatic function testing (ePFT) (63). An error in mRNA processing or protein secretion was suggested by Mehta et al. in a reported pediatric case with isolated amylase deficiency diagnosed after repeated ePFTs (20 and 33 months of age), despite detecting normal pancreatic amylase messenger RNA by reverse-transcriptase polymerase chain reaction in the duodenal fluid (64). Understanding of isolated pancreatic enzyme deficiencies as pathologic or physiologic is overall limited and represents area for future research.
Indirect exocrine function tests
Indirect function tests are based upon the function of a single enzyme. They measure individual pancreatic enzymes or their substrate byproducts from stool, serum, or breath samples. The examples of these tests are fecal fat, steatocrit, fecal elastase (FE-1), stool chymotrypsin, serum markers, and the 13C-mixed triglyceride breath test. Each indirect test has its own inherent limitations; however, they all share a common limitation of poor sensitivity and specificity in detecting mild to moderate EPI.
Stool based tests
Fecal elastase test
Fecal elastase (FE-1) is the most widely used indirect screening test for EPI. The basis of this tests that the elastase is resistant to hydrolysis by bacterial proteases and it remains stable in room temperature (65). A small stool sample is adequate for the test. The other advantage is that pancreatic enzyme replacement therapy (PERT) does not interfere with the result. Therefore, discontinuation of PERT is not necessary when performing the FE-1 test (66).
FE-1 has been well studied in pancreatic exocrine dysfunction associated with chronic pancreatitis, cystic fibrosis, diabetes, and celiac disease (67–70). The normal result is >200 mg/g of dry stool. A level <200 mg/g indicates EPI, and <100 mg/g correlates well with steatorrhea (71). Khan et al. proposed a method of staging EPI (into mild, moderate, and severe) based upon value of FE-1 combined with presence of symptoms and fat soluble vitamin deficiency (72).
It is important to note that large volume liquid stool can dilute the fecal elastase and provide inaccurate results, therefore for the correct analysis the stool sample should be lyophilized, and dry weight should be uses for calculations (73).
Diet is not suggested to have an large impact on FE-1 testing, however Walkowiak et al. reported that in pancreatic sufficient patients with normal range FE-1, a short term vegan diet did lower their FE-1 suggesting possible adaptation of pancreatic proteases to low protein high fiber diet (74).
The sensitivity of the FE-1 in children with CF is between 86% and 100% (71, 75, 76). In a meta-analysis, FE-1 of <200 mgc/g was found to have an overall pooled sensitivity of 77% and specificity of 88% in detecting EPI (77). As expected, the accuracy of FE-1 increases in cases of severe EPI (sensitivity of 97%) and alternatively decreases in cases of isolated deficiency or mild EPI (sensitivity 49%).
Although the FE-1 can only detect EPI reliably in the severe range, it remains more sensitive than fecal fat testing. Isolated enzyme deficiencies are not detected by FE-1, for example, steatorrhea secondary to isolated lipase or colipase deficiency (78).
Stool fat content measurement
Assessment of fecal fat is a standard method to detect fat malabsorption. The causes of fat malabsorption are varied, and as such, a positive test is neither specific nor sensitive to exocrine pancreatic dysfunction. It is an indirect assessment of lipase activities of the pancreas. This test measures the fraction of fat in the stool after initiating a standard fat containing diet. However, this procedure is not specific for lipase activity as there are other non-pancreatic etiologies of the abnormal fecal fat detection. These include gut mucosal injury (e.g., celiac disease), small bowel bacterial overgrowth, short bowel syndrome, Crohn disease, and even liver disease with cholestasis (79, 80). In patients with cystic fibrosis (CF) without pancreatic insufficiency, fat malabsorption can occur due to gastric hypersecretion or an abnormal gastrointestinal motility (81). In a study in patients with Shwachman-Diamond syndrome (SDS) and CF, steatorrhea developed when lipase fell below 2% or colipase fell below 1% (82) and as such that are clear cases here fecal fat testing would likely be positive.
Fecal fat testing is a cumbersome test for the patient and laboratory to perform; thus, has fallen out of favor as first line testing in many clinical settings. The test includes all stool collection for 72 h while the total fat intake (100 g/day) is standardized starting 3 days before and during the full 3 days of collection (83). Then the ratio of stool fat content compared to total fat intake is calculated. Typically, a level of >7 g/day is defined as malabsorption (79). It is a time-consuming test, although there is a report that 24-hour collections are adequate (84). It is important to store the collected fat in a refrigerator, otherwise the bacteria in stool will start fermenting the fat and fat content may decrease.
It is well known that the fat absorption ratio is age dependent. In children that are younger than 6 months of age, the reference values are >85%, and above that age, reference values are >93% to 95% (85, 86).
The classical method for stool fat analysis was quantitative testing via the Van de Kamer method. However, the near-infrared reflectance analysis simplified the quantification, and this correlates well with the classical Van de Kamer method (87, 88). The qualitative stool fat test is based on the use of Sudan stain of the stool and microscopic analysis of fat droplets and results are reported in a graded fashion (1 + normal; 2 + slight increase; and 3 + definite increase) (89). Qualitative analysis is lacking in its ability to separate normal from mild or inconsequential cases of steatorrhea.
The coefficient of fat absorption is another measure obtained with fecal fat testing. A value <90% is defined as insufficient, the calculation is: (fat ingestion − fat excretion)/fat ingestion) × 100(%). However, Erchinger et al. reported that for the diagnosis of fat malabsorption, the additional evaluation to calculate the ratio of fat absorption did not provide additional information compared to fecal fat content (90).
Steatocrit
Steatocrit is a fast and easily performed screening test for fat malabsorption. Recall that fat malabsorption has pancreatic and non-pancreatic etiologies, thus a positive steatocrit is not specific to pancreatic insufficiency. This test includes the collection of stools that is then homogenized and an aliquot of sample transferred to hematocrit tube and centrifuged at 12,000 rpm for 15 min. The ratio of the fat layer to the total sample length is assessed. After the test introduction in infants in 1981 (91) this simple, cheap and rapid test became popular. However, it has poor sensitivity and specificity compared with the 72-hour stool fat collection. Tran et al. reported that the sensitivity of the test can be improved via acidification of the stool sample prior to centrifugation (acid steatocrit test) (92, 93).
Stool chymotrypsin
Chymotrypsin in stool is detected by a photometric assay test (93). Unlike elastase, chymotrypsin is prone to proteolytic degradation and can limit the availability and handling of the test. Another limitation is that the test cannot differentiate human chymotrypsin from the chymotrypsin found in PERT (94). Thus, PERT must be stopped at least 3 days before the test. When compared to 13C mixed triglyceride breath testing and FE-1, fecal chymotrypsin had the lowest sensitivity and specificity at 56% and 82%, respectively (95). The test's main advantage can be that it allows assessing compliance to PERT.
Urine based test
Pancreolauryl test
The substrate for the pancreaolauryl test is dilaurate (lauric acid, a 12-carbon atom chain fatty acid, and a component of triglycerides that comprises about half of the fatty-acid content in coconut milk) combined with fluorescein. Pancreatic lipase releases the fluorescein that is then absorbed and can be measured in the urine (96) and blood (97). Later the test was modified by adding mannitol to correct for changes in intestinal permeability that could affect absorption and skew the test results (98). The results are reported as a fluorescein/mannitol ratio. However, when compared with FE-1 test, the pancreolauryl test was less accurate (99).
Serum tests
Serum testing for EPI has fallen out of favor for reasons discussed below, however understanding of these tests in relation to other pancreatic diseases and in monitoring secondary effects of EPI are important.
Amylase and lipase, are present in the blood stream due in part to physiologic release or leaking of these from the acinar cells into the systemic circulation. Thus, pancreatic disease states with inflammation can lead to elevation of these. Alternatively, atrophy or significant loss of pancreatic tissue can cause a decrease in amylase and lipase.
In the 1980s, serum IRT was found to have sensitivity and specificity in diagnosing severe cases of EPI, in which a result of less than 20 ng/ml was consistent with pancreatic steatorrhea, compared with levels higher than 20 ng/ml in those without steatorrhea (100). Interestingly around that time, IRT was recognized in dried blood spots in neonates found to have cystic fibrosis and was later adopted into the newborn screening. Adoption of serum IRT for EPI fell out of favor due to the significant limitations in age reported by Durie et al. (101) and the advent of other more specific pancreatic function tests. Thus, outside of neonatal screening, IRT is no longer used clinically for assessment of exocrine pancreatic function.
Other serum tests associated with downstream effects of EPI include decreased serum levels of fat-soluble vitamins, apolipoproteins, total cholesterol, magnesium, retinol-binding protein, calcium, zinc, selenium, and carotene (102). It was reported that patients with EPI are at risk for vitamin E deficiency (103, 104), that can lead to neurological symptoms, highlighting the importance of these adjunctive serum tests in detecting complications in EPI. Additional tests may include hemoglobin, albumin, prealbumin, and HbA1c, as well as diminished bone density, all of which can be abnormal in the setting of untreated EPI (105).
Breath test
13C mixed triglyceride breath test
The 13C is a natural nonradioactive form of the carbon. The test measures 13C–CO2, which is one of the breakdown products of digested triglycerides (106). This test is based on the function of lipase, however, like the fecal fat assay, the 13C-mixed triglyceride breath test is a test of fat maldigestion and is not specific to EPI.
The 13C mixed triglyceride breath test was first described by Vantrappen et al. in 1989 (107). The test utilizes a 13C-labelled mixed triglyceride [1,3-distearyl,2 (carboxyl-13C) octanoyl glycerol] substrate that is consumed with a meal, typically butter (or similar fat) on toast. This fat is then hydrolyzed by the pancreatic lipase (and/or other non-pancreatic fat digestion processes) and the 13C-labelled octanoate, an 8-carbon medium-chain fatty acid, is absorbed in the blood and metabolized by the liver and the 13C-labelled CO2 appears in the expired air of the patient. The13CO2 is detected in breath samples at various time points throughout a 5–6-hour study. The result of the test is expressed as percentage of 13C cumulative recovery over the testing period, with values in normal subjects being between 20%–40% of cumulative recovery (106). The 13CO2 is measured by mass spectrometry or near-infrared analysis.
The amount of 13C-labelled CO2 is an indirect measure of pancreatic lipase activity, although as mentioned above, there may be other non-pancreatic diseases influencing the result. The main advantage of the 13C-mixed triglyceride breath test is in its ability to assess the efficacy of PERT. The limitations of the test are that there is a wide variability in the amount of expired 13C-labelled CO2, and these values can fluctuate with activity level, gastric emptying rate, liver disease, intestinal diseases that affect absorption, lung disease, and endogenous CO2 production (108–110). The breath test is also difficult to perform in infants and young children.
The 13C-mixed triglyceride breath test is widely published (111–114), however, currently it is only available in a few countries in Europe and in Australia.
Direct (stimulatory) exocrine function tests
Direct pancreatic function tests measure enzyme activity in pancreatic secretions. They are stimulated tests with either secretagogues (Secretin/CCK) or meal (Lundh test). They allow to assess the activity all the main pancreatic enzymes and provide option for other analyses of the collected fluids.
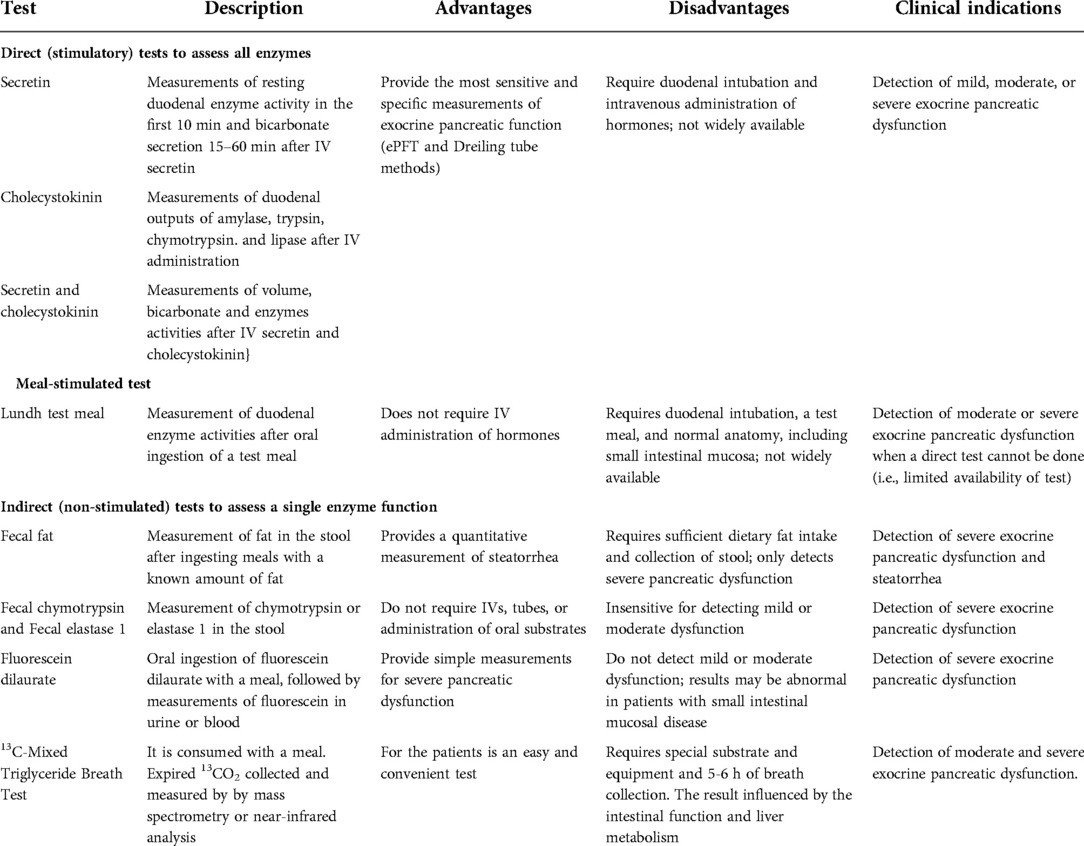
Direct pancreatic function test with secretagogue (secretin, cholecystokinin [CCK] administration is considered the gold standard to assess exocrine pancreatic function. In 1948, the first direct pancreatic function test was published (115). It used a specific double lumen tube to collect fluid samples from the duodenum (Dreiling tube) following simulation with secretagogue. Later a meal-based stimulation “Lundh meal test” was developed. This was then followed by the development of the endoscopic stimulation test in the 20th century. The advantages and disadvantages and clinical utility of the different tests are summarized in Table 3.
Dreiling tube test
The Dreiling tube method (115) was considered a gold standard for the assessment of exocrine pancreatic function. Although the test is considered highly sensitive and specific (22, 116–122), the Dreiling tube collection method has inherent limitations.
The process of collection via the Dreiling tube starts with placement of an oro-duodenal tube (guided by fluoroscopy), baseline fluid is collected, then sequential administration of secretin and CCK and collection of the outcoming pancreatic fluid via aspiration of duodenal contents at varying time points. The volume of aspirate, pH, bicarbonate concentration, total protein concentration, and pancreatic enzyme activity are recorded. Amylase, trypsin, chymotrypsin, and lipase all can all be assayed and are reported as total enzyme output determined by the volume of fluid collected.
Multiple factors can influence the results of this test including mixing of gastric acid with intestinal fluid, inaccurate measure of “total volume” as the duodenal tube cannot reliably aspirate all secreted fluid, and dislocation of the tube (123).
The Dreiling tube collection method is invasive, impractical, difficult for patients to complete, and radiation exposure associated with verification of tube positioning, and can be time consuming to perform. Protocols for specimen collection in the publications are variable and the duration of the tests vary from 45 min to 150 min (124–127). In children specifically, this method of collection has never gained favor. Instead, many turn to non-invasive indirect testing such as fecal elastase.
Lundh meal test
Another measurement of pancreatic function is the meal-based Lundh test (126). In this test, patients are asked to ingest a 300-mL liquid meal composed of dried milk, vegetable oil and dextrose (6% fat, 5% protein and 15% carbohydrate). This is then followed with the aspiration of fluid from the duodenum via a nasoduodenal tube, and measurement of enzyme activities. This is a physiological test that utilizes different phases of the meal (cephalic, gastric and intestinal), the effect of the meal on small intestinal sensory process, release of the secretin and CCK and the whole neurohumoral systems (vagal effects) and the pancreas responses to the neurohumoral system. Jensen et al. found significant correlation in lipase and bicarbonate concentrations between endoscopic secretin stimulation test and the Lundh test in 23 healthy volunteers (128).
Endoscopic pancreatic function test (ePFT)
Method of fluid collection and analysis
The test is performed during a standard pediatric upper gastrointestinal endoscopy. Before endoscopic intubation, secretin or CCK is administered intravenously. For accurate collection of pancreatic fluid, the endoscope is positioned close to the ampulla of Vater and an aspiration catheter inserted through the biopsy channel (Figure 1) and with light suction is utilized. Pancreatic fluid secretion typically starts 3 to 4 min after the secretin administration, and the optimal collection time is within 10 min from the time of secretin injection.
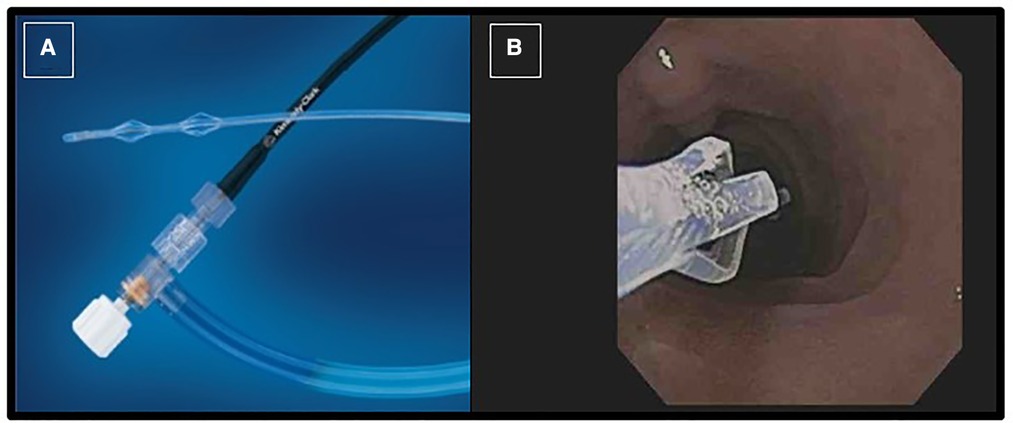
Figure 1. Picture of the collection catheter passed through endoscope working channel (A); the tip of catheter is seen in the duodenum close to the ampulla of vater (B).
There is a known dilutional effect of enzyme activities by ductal cell secretions if the fluid is collected beyond 10 min (129). Interpretation of the test results should be based on the sample with the highest (peak) enzyme activities (129, 130). However, the fluid secreted after 10 min reflects the effect of the secretin on the ductal cell function that is measured by bicarbonate concentration. In healthy subjects an increase in bicarbonate >80 mmol/L indicates normal function (123).
The fluid collected is measured for pH, protein content, and enzyme activity (amylase, lipase, trypsin, chymotrypsin, and elastase). The pH in the protein content of the fluid is utilized to assess the quality of the sample, in which a pH of less than 7 suggests possible contamination of gastric fluid and a low-protein would indicate dilution with duodenal fluid (131). Table 5 lists factors that can affect the result of the ePFT.

Table 5. Factors that have an effect on the results of ePFT (modified from (125).
History and rationale of the ePFT
The first endoscopic fluid collection was reported in 1979 (130, 132). The first pediatric study comparing the Dreiling test with ePFT was reported by Madrazo et al. (133). Since then, multiple adult and a few pediatric papers (129, 133–135) have been published. In adults this test is most used to assess bicarbonate secretion that is an indicator of the ductal cell damage in chronic pancreatitis (CP). In contrast, in children the main role of this test is to determine the acinar cell enzyme secretion. The ePFT is more practical and efficient option for direct testing then the Dreiling and the Lundh tests to assess both the ductal and acinar cell functions.
The basis to assess acinar secretion is that secretin washes out the enzyme concentrated fluid that is present in the ducts prior to stimulation with secretagogue (interdigestive fluid). The interdigestive fluid in the pancreatic ducts has significant enzyme activity. This was found in several studies evaluating basal enzyme secretion was roughly 20% of the total pancreatic enzyme capacity, indicating that this basal secretion is adequate to prevent malabsorption and steatorrhea seen when pancreatic enzyme activity is <10% (136–138). Hence any dysfunction in enzyme secretion can be detected regardless of whether it is generalized insufficiency or an isolated enzyme deficiency.
Comparison of ePFT and dreiling tube method
The fluid collected in ePFT is analyzed and reported as peak enzyme activity in unit/ml/min (139). Alternatively, the Dreiling tube method reports the test results as total enzyme output by multiplying the enzyme activity and the volume of fluid collected.
The first pediatric study comparing the Dreiling tube and ePFT reported comparable results (133). Conwell et al. also compared the two collection methods in healthy adults and in patients with chronic pancreatitis using CCK infusion and reported that the ePFT was equivalent to the Dreiling tube collection (123). They also analyzed the safety and cost of the two tests which found that ePFT was safer, shorter in duration, and less costly ($1,890 vs. $2,659). The smaller fluid volume collected by ePFT reproduced the classic acinar and duct cell secretory profiles after hormonal stimulation (123, 137, 140). Based on these studies, ePFT was found to be a useful method for the assessment of pancreatic duct cell function (141, 142).
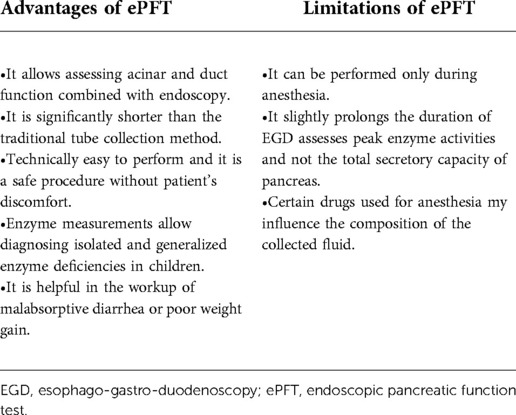
In conclusion, ePFT is comparable to the Dreiling tube method but offers several advantages over the Dreiling method. ePFT is less time consuming, does not result in patient's discomfort as performed during sedation, and eliminates need for radiation exposure. The limitations of ePFT include lack of uniformly accepted protocol, and requirement of anesthesia to perform (143). See list of advantages and limitations to ePFT in Table 4.
ePFT for acinar function in children
Following the first pediatric study in 1991 (133), Del Rosario et al. conducted a study in that one group of children received IV bolus of both CCK and secretin, while the second group received placebo after the administration of secretin and found no statistical difference in mean lipase level (129). The other important message of this study was that the peak enzyme values at the 5- and 10-minutes collections were similar in both groups, but the 15-minute specimens had significantly less enzyme activities due to dilution effect, and as such the optimal timing of collection was identified (129).
Another ePFT study in children, reported during the time of secretin shortage, compared secretin and CCK alone and in combination. It found that CCK was acceptable to be used alone for pancreatic enzyme measurements in the absence of commercially available secretin (135).
In 2016 a larger study with 508 ePFTs in children reported peak enzyme activities at 5 min that was then followed by a decrease activity over time (144). Additionally, they found discordance between ePFT and FE-1 testing in 165 children (144).
Up to now, the largest pediatric study included 1913 children and young adults summarized the experience with ePFT (secretin stimulated, collection time between 4 and 10 min) and determined that the test had high reproducibility, repeatability, and clinical validity (145). Additionally, by adding ePFT to standard upper gastrointestinal endoscopy when there was a suspicion of malabsorption, the diagnostic yield increased by 36.9% (145).
ePFT for ductal function test in children (single center data)
A method used at Arnold Palmer Hospital for Children, includes performing longer duration pancreatic fluid collection (45 min) after IV secretin administration in children where duct dysfunction was suspected. Those who had abnormal test result had genetic tests ordered. Figure 2 shows three cases with normal function and three abnormal test results with the genetic tests results added (125).

Figure 2. Ductal function assessment with bicarbonate concentration from prolonged ePFT with fluid collection up to 45 min. (A) Normal tests with the bicarbonate concentration is above 80 mmol/L. (B) Abnormal tests in patients with genetic abnormalities in three patients when the bicarbonate never reached the 80 mmol/L [adapted from Horvath, K. et al. (125)].
ePFT for ductal function in adults
After IV secretin administration, high bicarbonate secretion continues for a longer duration. Many adult studies used a 60 min collection time to assess ductal function and this subsequently led to longer anesthesia time.
A prospective ePFT study in patients (>16 years) with cystic fibrosis and healthy normal subjects administered the secretin 25 min before the endoscope insertion and collected the pancreatic fluid between 30 and 45 min (134) that significantly shorter than the 60 min test. The ePFT differentiated pancreatic-sufficient and insufficient patients with a sensitivity of 100% and specificity of 88%. Based on this study the 15 min collection was found to be sufficient to diagnose duct cell dysfunction. When CCK administration was added to secretin during ePFT it did not improve the accuracy of diagnosing EPI in adults with chronic pancreatitis (146). A similar conclusion was reported in pediatrics (135).
Figure 3 illustrates when the ePFT can be used for acinar and duct cell function assessment by using IV secretin administration.

Figure 3. This flowchart shows the optimal time of fluid collection for acinar and ductal function after IV push administration of secretin [modified from Engjom, T. et al. (134)].
Imaging modalities to assess pancreatic exocrine function
Imaging studies are important in evaluation of anatomy of the pancreas as it relates to its function and thus should be utilized in assessing causes of exocrine pancreatic dysfunction. Of all imaging studies available, the secretin enhanced MRI (s-MRI) is the only one that can highlight functionality of the exocrine pancreas by evaluating fluid secretion.
Imaging studies can detect chronic pancreatitis typically when >50% of the gland is fibrotic (147). Thus, when it comes to assessing early stages of chronic pancreatitis in children with negative imaging studies, the combination of ePFT and endoscopic ultrasound should be considered (148). Additionally, identifying early stages of chronic pancreatitis utilizing these methods may also lead to improved outcomes with total pancreatectomy with autologous islet cell transplant (149).
Ultrasound
Usually, ultrasound is the initial imaging modality in any suspected pancreatic disease as it can assess the size of pancreas, presence of peripancreatic fluid, the size and irregularity of the main duct and the presence of calcifications. Its sensitivity is 50% to 80% in adults (150).
Magnetic resonance cholangio-pancreatograpy (MRCP)
It is the test of choice as it is more sensitive, does not require radiation and can image ducts as small as 1 mm (151) and enables to detect biliary stones and anatomical variants, such as pancreas divisum. Visualization of the pancreatic ducts are enhanced by the administration of secretin, which induces fluid secretion (152), this is the secretin-MRI (s-MRI).
The s-MRI is potentially a useful method to assess exocrine function by measuring the volume of the secreted fluid. Madzak et al. evaluated s-MRI in patients with CF and healthy patients (mean age 21 years) and found that CF patients with EPI had lower diffusion coefficient before secretin in the pancreatic head and lower secreted bowel fluid volumes (P = 0.035) (153). The s-MRI was also studied in pediatric population by Trout et al. who measured the secreted fluid in 50 healthy children and reported an association between the secreted volume and body surface area. They concluded that a secreted volume <43 ml or a secretion rate <2.3 ml/min (5th percentile values) can be considered abnormal in children (154).
Endoscopic ultrasonography (EUS)
Endoscopic ultrasound involves the use of a specialized endoscopic device with ultrasound capability. Given that it is performed during endoscopy, it is considered an invasive technique and it is highly operator dependent (155). It provides highly accurate images of pancreatic ducts and parenchyma. When utilized in children, EUS can be both diagnostic and therapeutic. For example, imaging of the pancreas with EUS followed by an EUS-guided fine needle aspiration or biopsy can be useful in the diagnosis of idiopathic fibrosing pancreatitis or autoimmune pancreatitis (156). Additionally, microlithiasis can be identified by EUS as a possible contributor to acute recurrent pancreatitis in children (156). As a therapeutic modality, it can be used for the internal drainage of pancreatic pseudocysts as a complication of acute pancreatitis.
The role of EUS in evaluating the exocrine function of the pancreas was studied prospectively in 128 adult patients with EUS criteria of chronic pancreatitis and it was compared with the 13C-Mixed triglyceride breath test. They found that diagnosis of EPI increased linearly with the number of EUS criteria, and that the presence of intraductal calcifications, hyperechogenic foci with shadowing, and dilation of the main pancreatic duct were significantly and independently associated with EPI (157).
Conclusions and future directions
In conclusion, accurate assessment of pancreatic function is essential in children with clinical concerns for maldigestion and malabsorption. EPI can be caused by several etiologies including developmental delays in enzyme maturation, isolated deficiencies, genetic disorders, and chronic pancreatitis. These can be easily missed as symptoms of EPI are often non-specific. Therefore, early diagnosis and treatment are important for improved outcomes in children.
Many studies showed that indirect measures of pancreatic function are unable to detect mild and moderate exocrine dysfunctions. Among the indirect non-stimulatory tests, FE-1 is the mostly used and most convenient test but its sensitivity and specificity is low compared with the direct function tests.
Although the Dreiling tube test was considered “the gold standard” for direct pancreatic function testing in the past, it is an unacceptable means of studying pancreatic exocrine function in children. ePFT is now the preferred method as it is technically easy to perform during upper gastrointestinal endoscopy, shorter in duration, and has comparable value with the Dreiling tube method.
The ePFT can be performed when routine endoscopy is obtained for investigation in children who are suspected of having malnutrition secondary to pancreatic exocrine dysfunction. It can detect both isolated and generalized deficiencies even if they are mild or moderate degree deficiencies. Like the “gold standard” Dreiling tube test collection, there is no uniformly accepted protocol for the ePFT. Although based on the pancreatic physiology fluid collection between 4 and 10 min reliable to assess the acinar cell function.
A multicenter study is needed for the standardization of ePFT in large number of children undergoing ePFT utilizing a single and uniform protocol.
Author contributions
All authors equally contributed to the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fisher WE, Andersen DK, Windsor JA, Saluja AK, Brunicardi FC. Pancreas. In: Brunicard FC, chief editor. Schwartz principles of surgery, 10th ed. New York: McGraw Hill Education (2015). Chapter 33.
2. Ballian N, Brunicardi FC. Islet vasculature as a regulator of endocrine pancreas function. World J Surg. (2007) 31(4):705–14. doi: 10.1007/s00268-006-0719-8
3. Korc M, Iwamoto Y, Sankaran H, Williams JA, Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. I. Stimulation of protein synthesis. Am J Physiol. (1981) 240(1):G56–62. doi: 10.1152/ajpcell.1981.240.1.C56
4. Sankaran H, Iwamoto Y, Korc M, Williams JA, Goldfine ID. Insulin action in pancreatic acini from streptozotocin-treated rats. II. Binding of 125I-insulin to receptors. Am J Physiol. (1981) 240(1):G63–8. doi: 10.1152/ajpgi.1981.240.1.G63
5. Motta PM, Macchiarelli G, Nottola SA, Correr S. Histology of the exocrine pancreas. Microsc Res Tech. (1997) 37(5-6):384–98. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6%3C384::AID-JEMT3%3E3.0.CO;2-E
6. Beer RL, Parsons MJ, Rovira M. Centroacinar cells: at the center of pancreas regeneration. Dev Biol. (2016) 413(1):8–15. doi: 10.1016/j.ydbio.2016.02.027
7. Delaspre F, Beer RL, Rovira M, Huang W, Wang G, Gee S, et al. Centroacinar cells are progenitors that contribute to endocrine pancreas regeneration. Diabetes. (2015) 64(10):3499–509. doi: 10.2337/db15-0153
8. O'Konski MS, Pandol SJ. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest. (1990) 86(5):1649–57. doi: 10.1172/JCI114887
9. Konturek JW, Gabryelewicz A, Kulesza E, Konturek SJ, Domschke W. Cholecystokinin (CCK) in the amino acid uptake and enzyme protein secretion by the pancreas in humans. Int J Pancreatol. (1995) 17(1):55–61. doi: 10.1007/BF02788359
10. Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc. (1978) 53(2):211–354. doi: 10.1111/j.1469-185X.1978.tb01437.x
11. Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. (2008) 70:273–99. doi: 10.1146/annurev.physiol.70.113006.100618
12. Cyriac J, Mahadevan P, Augustine P, Ramesh H, Koshy A. Stellate cell activation in tropical calcific pancreatitis compared to alcoholic pancreatitis, adenocarcinoma of pancreas and Normal pancreas. JOP. (2012) 13(4):376–86. doi: 10.6092/1590-8577/552
13. Li J, Chen B, Fellows GF, Goodyer CG, Wang R. Activation of pancreatic stellate cells is beneficial for exocrine but not endocrine cell differentiation in the developing human pancreas. Front Cell Dev Biol. (2021) 9:694276. doi: 10.3389/fcell.2021.694276
14. Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. (2007) 117(1):50–9. doi: 10.1172/JCI30082
15. Pandol SJ. The exocrine pancreas. San Rafael (CA): Morgan & Claypool Life Sciences (2010). Colloquium series on integrated systems physiology: from molecule to function to disease Morgan & Claypool Life Sciences; #14. Available from: https://www.ncbi.nlm.nih.gov/books/NBK54128/ doi: 10.4199/C00026ED1V01Y201102ISP014
16. Brannon PM. Adaptation of the exocrine pancreas to diet. Annu Rev Nutr. (1990) 10:85–105. doi: 10.1146/annurev.nu.10.070190.000505
17. Birk RZ, Brannon PM. Regulation of pancreatic lipase by dietary medium chain triglycerides in the weanling rat. Pediatr Res. (2004) 55(6):921–6. doi: 10.1203/01.PDR.0000127430.04127.4F
18. Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. (1902) 28(5):325–53. doi: 10.1113/jphysiol.1902.sp000920
19. Chey WY, Konturek SJ. Plasma secretion and pancreatic secretion in response to liver extract meal with varied pH and exogenous secretin in the dog. J Physiol. (1982) 324:263–72. doi: 10.1113/jphysiol.1982.sp014111
20. Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. (2005) 67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247
21. Ishiguro H, Yamamoto A, Nakakuki M, Yi L, Ishiguro M, Yamaguchi M, et al. Physiology and pathophysiology of bicarbonate secretion by pancreatic duct epithelium. Nagoya J Med Sci. (2012) 74(1-2):1–18. PMID: 22515107; PMCID: PMC4831246
22. Stevens T, Parsi MA. Update on endoscopic pancreatic function testing. World J Gastroenterol. (2011) 17(35):3957–61. doi: 10.3748/wjg.v17.i35.3957
23. Wu B, Conwell DL. The endoscopic pancreatic function test. Am J Gastroenterol. (2009) 104(10):2381–3. doi: 10.1038/ajg.2008.181
24. DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. (1973) 288(16):813–5. doi: 10.1056/NEJM197304192881603
25. DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. (2011) 27(5):452–9. doi: 10.1097/MOG.0b013e328349e333
26. Johnson CD, Devaux MA, Treffot MJ, Sarles H. The cephalogastric phase of the pancreatic response to food in the dog. Pancreas. (1991) 6(2):190–6. doi: 10.1097/00006676-199103000-00010
27. Furukawa N, Okada H. Effects of antral distension on pancreatic exocrine secretion in dogs: evidence for a short reflex. Jpn J Physiol. (1987) 37(4):671–85. doi: 10.2170/jjphysiol.37.671
28. Spannagel AW, Green GM, Guan D, Liddle RA, Faull K, Reeve JR Jr. Purification and characterization of a luminal cholecystokinin-releasing factor from rat intestinal secretion. Proc Natl Acad Sci U S A. (1996) 93(9):4415–20. doi: 10.1073/pnas.93.9.4415
29. Wheeler S, Eardley JE, McNulty KF, Sutcliffe CP, Morrison JD. An investigation into the relative merits of pituitary adenylate cyclase-activating polypeptide (PACAP-27) and vasoactive intestinal polypeptide as vagal neuro-transmitters in exocrine pancreas of rats. Exp Physiol. (1997) 82(4):729–47. doi: 10.1113/expphysiol.1997.sp004061
30. Chey WY, Chang TM. Neural control of the release and action of secretin. J Physiol Pharmacol. (2003) 54(Suppl 4):105–12. PMID: 15075453
31. Gullo L, Biliotti G, Pezzilli R, Di Stefano M, Ancona D. Effect of octreotide (SMS 201-995) on meal-stimulated pancreatic secretion in three patients with external pancreatic fistula. Am J Gastroenterol. (1991) 86(7):892–4. PMID: 2058634
32. Gullo L, Pezzilli R, Barbara L. Effect of somatostatin on plasma amino acid uptake by human pancreas. Gastroenterology. (1989) 97(3):732–6. doi: 10.1016/0016-5085(89)90645-8
33. Hermon-Taylor J, Perrin J, Grant DA, Appleyard A, Bubel M, Magee AI. Immunofluorescent localisation of enterokinase in human small intestine. Gut. (1977) 18(4):259–65. doi: 10.1136/gut.18.4.259
34. Lindquist S, Hernell O. Lipid digestion and absorption in early life: an update. Curr Opin Clin Nutr Metab Care. (2010) 13(3):314–20. doi: 10.1097/MCO.0b013e328337bbf0
35. Gullo L. The effect of neurotensin on pure pancreatic secretion in man. Scand J Gastroenterol. (1987) 22(3):343–8. doi: 10.3109/00365528709078602
36. Green GM, Lyman RL. Feedback regulation of pancreatic enzyme secretion as a mechanism for trypsin inhibitor-induced hypersecretion in rats. Proc Soc Exp Biol Med. (1972) 140(1):6–12. doi: 10.3181/00379727-140-36384
37. Louie DS, May D, Miller P, Owyang C. Cholecystokinin mediates feedback regulation of pancreatic enzyme secretion in rats. Am J Physiol. (1986) 250(2 Pt 1):G252–9. doi: 10.1152/ajpgi.1986.250.2.G252
38. Walkowiak J, Witmanowski H, Strzykala K, Bychowiec B, Songin T, Borski K, et al. Inhibition of endogenous pancreatic enzyme secretion by oral pancreatic enzyme treatment. Eur J Clin Invest. (2003) 33(1):65–9. doi: 10.1046/j.1365-2362.2003.01077.x
39. Dominguez-Munoz JE, Bregulla M, Nelson DK, Glasbrenner B, Sauerbruch T, Malfertheiner P. Independent cycles of exocrine pancreatic secretion, hormones and gastroduodenal motility in healthy fasting humans: reassessment of a complex partnership. Neurogastroenterol Motil. (1998) 10(1):27–34. doi: 10.1046/j.1365-2982.1998.00084.x
40. Zimmerman DW, Sarr MG, Smith CD, Nicholson CP, Dalton RR, Barr D, et al. Cyclic interdigestive pancreatic exocrine secretion: is it mediated by neural or hormonal mechanisms? Gastroenterology. (1992) 102(4 Pt 1):1378–84. doi: 10.1016/0016-5085(92)90779-X
41. Anagnostides A, Chadwick VS, Selden AC, Maton PN. Sham feeding and pancreatic secretion. Evidence for direct vagal stimulation of enzyme output. Gastroenterology. (1984) 87(1):109–14. doi: 10.1016/0016-5085(84)90132-X
42. Katschinski M, Steinicke C, Reinshagen M, Dahmen G, Beglinger C, Arnold R, et al. Gastrointestinal motor and secretory responses to cholinergic stimulation in humans. Differential modulation by muscarinic and cholecystokinin receptor blockade. Eur J Clin Invest. (1995) 25(2):113–22. doi: 10.1111/j.1365-2362.1995.tb01535.x
43. Holst JJ, Knuhtsen S, Nielsen OV. Role of gastrin-releasing peptide in neural control of pancreatic exocrine secretion. Pancreas. (1989) 4(5):581–6. doi: 10.1097/00006676-198910000-00009
44. Kreiss C, Schwizer W, Erlacher U, Borovicka J, Lochner-Kuery C, Muller R, et al. Role of antrum in regulation of pancreaticobiliary secretion in humans. Am J Physiol. (1996) 270(5 Pt 1):G844–51. doi: 10.1152/ajpgi.1996.270.5.G844
45. Liddle RA, Goldfine ID, Rosen MS, Taplitz RA, Williams JA. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. (1985) 75(4):1144–52. doi: 10.1172/JCI111809
46. Go VL, Hofmann AF, Summerskill WH. Pancreozymin bioassay in man based on pancreatic enzyme secretion: potency of specific amino acids and other digestive products. J Clin Invest. (1970) 49(8):1558–64. doi: 10.1172/JCI106373
47. Kaushik N, Pietraszewski M, Holst JJ, O'Keefe SJ. Enteral feeding without pancreatic stimulation. Pancreas. (2005) 31(4):353–9. doi: 10.1097/01.mpa.0000183374.11919.e5
48. Adler G, Reinshagen M, Koop I, Goke B, Schafmayer A, Rovati LC, et al. Differential effects of atropine and a cholecystokinin receptor antagonist on pancreatic secretion. Gastroenterology. (1989) 96(4):1158–64. doi: 10.1016/0016-5085(89)91636-3
49. Bozkurt T, Adler G, Koop I, Arnold R. Effect of atropine on intestinal phase of pancreatic secretion in man. Digestion. (1988) 41(2):108–15. doi: 10.1159/000199739
50. Carrere J, Figarella-Branger D, Senegas-Balas F, Figarella C, Guy-Crotte O. Immunohistochemical study of secretory proteins in the developing human exocrine pancreas. Differentiation. (1992) 51(1):55–60. doi: 10.1111/j.1432-0436.1992.tb00680.x
51. Fukayama M, Ogawa M, Hayashi Y, Koike M. Development of human pancreas. Immunohistochemical study of fetal pancreatic secretory proteins. Differentiation. (1986) 31(2):127–33. doi: 10.1111/j.1432-0436.1986.tb00393.x
52. Lebenthal E, Lee PC. Development of functional responses in human exocrine pancreas. Pediatrics. (1980) 66(4):556–60. doi: 10.1542/peds.66.4.556
53. Hadorn B, Zoppi G, Shmerling DH, Prader A. Quantitative assessment of exocrine pancreatic function in infants and children. Bibl Paediatr. (1967) 86:255–8. PMID: 6054636
54. Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. (2003) 77(6):1537S–43S. doi: 10.1093/ajcn/77.6.1537S
55. Freed LM, York CM, Hamosh P, Mehta NR, Hamosh M. Bile salt-stimulated lipase of human milk: characteristics of the enzyme in the milk of mothers of premature and full-term infants. J Pediatr Gastroenterol Nutr. (1987) 6(4):598–604. doi: 10.1097/00005176-198707000-00019
56. Figarella C, Negri GA, Sarles H. Presence of colipase in a congenital pancreatic lipase deficiency. Biochim Biophys Acta. (1972) 280(1):205–11. doi: 10.1016/0005-2760(72)90227-5
57. Figarella C, De Caro A, Leupold D, Poley JR. Congenital pancreatic lipase deficiency. J Pediatr. (1980) 96(3 Pt 1):412–6. doi: 10.1016/S0022-3476(80)80683-4
58. Hildebrand H, Borgstrom B, Bekassy A, Erlanson-Albertsson C, Helin I. Isolated co-lipase deficiency in two brothers. Gut. (1982) 23(3):243–6. doi: 10.1136/gut.23.3.243
59. Behar DM, Basel-Vanagaite L, Glaser F, Kaplan M, Tzur S, Magal N, et al. Identification of a novel mutation in the PNLIP gene in two brothers with congenital pancreatic lipase deficiency. J Lipid Res. (2014) 55(2):307–12. doi: 10.1194/jlr.P041103
60. Ligumsky M, Granot E, Branski D, Stankiewicz H, Goldstein R. Isolated lipase and colipase deficiency in two brothers. Gut. (1990) 31(12):1416–8. doi: 10.1136/gut.31.12.1416
61. Muller DP, McCollum JP, Trompeter RS, Harries JT. Proceedings: studies on the mechanism of fat absorption in congenital isolated lipase deficiency. Gut. (1975) 16(10):838. PMID: 1205319
62. Sheldon W. Congenital pancreatic lipase deficiency. Arch Dis Child. (1964) 39:268–71. doi: 10.1136/adc.39.205.268
63. Hopson P, Patel S, Bornstein J, Mehta D, Horvath K. Isolated amylase deficiency in children and its clinical implication. J Pediatr Gastroenterol Nutr. (2019) 68(6):854–60. doi: 10.1097/MPG.0000000000002317
64. Mehta DI, Wang HH, Akins RE, Wang L, Proujansky R. Isolated pancreatic amylase deficiency: probable error in maturation. J Pediatr. (2000) 136(6):844–6. doi: 10.1016/S0022-3476(00)96936-1
65. Loser C, Mollgaard A, Folsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. (1996) 39(4):580–6. doi: 10.1136/gut.39.4.580
66. Sziegoleit A, Krause E, Klor HU, Kanacher L, Linder D. Elastase 1 and chymotrypsin B in pancreatic juice and feces. Clin Biochem. (1989) 22(2):85–9. doi: 10.1016/S0009-9120(89)80003-7
67. Katschinski M, Schirra J, Bross A, Goke B, Arnold R. Duodenal secretion and fecal excretion of pancreatic elastase-1 in healthy humans and patients with chronic pancreatitis. Pancreas. (1997) 15(2):191–200. doi: 10.1097/00006676-199708000-00012
68. Walkowiak J, Lisowska A, Przyslawski J, Grzymislawski M, Krawczynski M, Herzig KH. Faecal elastase-1 test is superior to faecal lipase test in the assessment of exocrine pancreatic function in cystic fibrosis. Acta Paediatr. (2004) 93(8):1042–5. doi: 10.1111/j.1651-2227.2004.tb02715.x
69. Philippe MF, Benabadji S, Barbot-Trystram L, Vadrot D, Boitard C, Larger E. Pancreatic volume and endocrine and exocrine functions in patients with diabetes. Pancreas. (2011) 40(3):359–63. doi: 10.1097/MPA.0b013e3182072032
70. Carroccio A, Verghi F, Santini B, Lucidi V, Iacono G, Cavataio F, et al. Diagnostic accuracy of fecal elastase 1 assay in patients with pancreatic maldigestion or intestinal malabsorption: a collaborative study of the Italian society of pediatric gastroenterology and hepatology. Dig Dis Sci. (2001) 46(6):1335–42. doi: 10.1023/A:1010687918252
71. Walkowiak J, Sands D, Nowakowska A, Piotrowski R, Zybert K, Herzig KH, et al. Early decline of pancreatic function in cystic fibrosis patients with class 1 or 2 CFTR mutations. J Pediatr Gastroenterol Nutr. (2005) 40(2):199–201. doi: 10.1097/00005176-200502000-00022
72. Khan A, Vege SS, Dudeja V, Chari ST. Staging exocrine pancreatic dysfunction. Pancreatology. (2022) 22(1):168–72. doi: 10.1016/j.pan.2021.11.005
73. Fischer B, Hoh S, Wehler M, Hahn EG, Schneider HT. Faecal elastase-1: lyophilization of stool samples prevents false low results in diarrhoea. Scand J Gastroenterol. (2001) 36(7):771–4. doi: 10.1080/003655201300192058
74. Walkowiak J, Madry E, Lisowska A, Szaflarska-Poplawska A, Grzymislawski M, Stankowiak-Kulpa H, et al. Adaptive changes of pancreatic protease secretion to a short-term vegan diet: influence of reduced intake and modification of protein. Br J Nutr. (2012) 107(2):272–6. doi: 10.1017/S0007114511002923
75. Phillips IJ, Rowe DJ, Dewar P, Connett GJ. Faecal elastase 1: a marker of exocrine pancreatic insufficiency in cystic fibrosis. Ann Clin Biochem. (1999) 36(Pt 6):739–42. doi: 10.1177/000456329903600606
76. Walkowiak J, Cichy WK, Herzig KH. Comparison of fecal elastase-1 determination with the secretin-cholecystokinin test in patients with cystic fibrosis. Scand J Gastroenterol. (1999) 34(2):202–7. doi: 10.1080/00365529950173104
77. Vanga RR, Tansel A, Sidiq S, El-Serag HB, Othman MO. Diagnostic performance of measurement of fecal elastase-1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2018) 16(8):1220–8.e4. doi: 10.1016/j.cgh.2018.01.027
78. Wali PD, Loveridge-Lenza B, He Z, Horvath K. Comparison of fecal elastase-1 and pancreatic function testing in children. J Pediatr Gastroenterol Nutr. (2012) 54(2):277–80. doi: 10.1097/MPG.0b013e31820b0227
79. Fine KD, Fordtran JS. The effect of diarrhea on fecal fat excretion. Gastroenterology. (1992) 102(6):1936–9. doi: 10.1016/0016-5085(92)90316-Q
80. Glasgow JF, Hamilton JR, Sass-Kortsak A. Fat absorption in congenital obstructive liver disease. Arch Dis Child. (1973) 48(8):601–7. doi: 10.1136/adc.48.8.601
81. Littlewood JM, Wolfe SP. Control of malabsorption in cystic fibrosis. Paediatr Drugs. (2000) 2(3):205–22. doi: 10.2165/00128072-200002030-00005
82. Gaskin KJ, Durie PR, Lee L, Hill R, Forstner GG. Colipase and lipase secretion in childhood-onset pancreatic insufficiency. Delineation of patients with steatorrhea secondary to relative colipase deficiency. Gastroenterology. (1984) 86(1):1–7. doi: 10.1016/0016-5085(84)90582-1
83. Thompson JB, Su CK, Ringrose RE, Welsh JD. Fecal triglycerides. II. Digestive versus absorptive steatorrhea. J Lab Clin Med. (1969) 73(3):521–30. PMID: 5766197
84. Caras S, Boyd D, Zipfel L, Sander-Struckmeier S. Evaluation of stool collections to measure efficacy of PERT in subjects with exocrine pancreatic insufficiency. J Pediatr Gastroenterol Nutr. (2011) 53(6):634–40. doi: 10.1097/MPG.0b013e3182281c38
85. Fomon SJ, Ziegler EE, Thomas LN, Jensen RL, Filer LJ Jr. Excretion of fat by Normal full-term infants fed various milks and formulas. Am J Clin Nutr. (1970) 23(10):1299–313. doi: 10.1093/ajcn/23.10.1299
86. Rings EH, Minich DM, Vonk RJ, Stellaard F, Fetter WP, Verkade HJ. Functional development of fat absorption in term and preterm neonates strongly correlates with ability to absorb long-chain fatty acids from intestinal lumen. Pediatr Res. (2002) 51(1):57–63. doi: 10.1203/00006450-200201000-00011
87. Koumantakis G, Radcliff FJ. Estimating fat in feces by near-infrared reflectance spectroscopy. Clin Chem. (1987) 33(4):502–6. doi: 10.1093/clinchem/33.4.502
88. Stein J, Purschian B, Zeuzem S, Lembcke B, Caspary WF. Quantification of fecal carbohydrates by near-infrared reflectance analysis. Clin Chem. (1996) 42(2):309–12. doi: 10.1093/clinchem/42.2.309
89. Drummey GD, Benson JA Jr, Jones CM. Microscopical examination of the stool for steatorrhea. N Engl J Med. (1961) 264:85–7. doi: 10.1056/NEJM196101122640207
90. Erchinger F, Engjom T, Jurmy P, Tjora E, Gilja OH, Dimcevski G. Fecal fat analyses in chronic pancreatitis importance of fat ingestion before stool collection. PLoS One. (2017) 12(1):e0169993. doi: 10.1371/journal.pone.0169993
91. Phuapradit P, Narang A, Mendonca P, Harris DA, Baum JD. The steatocrit: a simple method for estimating stool fat content in newborn infants. Arch Dis Child. (1981) 56(9):725–7. doi: 10.1136/adc.56.9.725
92. Tran M, Forget P, Van den Neucker A, Strik J, van Kreel B, Kuijten R. The acid steatocrit: a much improved method. J Pediatr Gastroenterol Nutr. (1994) 19(3):299–303. doi: 10.1097/00005176-199410000-00007
93. Kaspar P, Moller G, Wahlefeld A. New photometric assay for chymotrypsin in stool. Clin Chem. (1984) 30(11):1753–7. doi: 10.1093/clinchem/30.11.1753
94. Stein J, Jung M, Sziegoleit A, Zeuzem S, Caspary WF, Lembcke B. Immunoreactive elastase I: clinical evaluation of a new noninvasive test of pancreatic function. Clin Chem. (1996) 42(2):222–6. doi: 10.1093/clinchem/42.2.222
95. Loser C, Brauer C, Aygen S, Hennemann O, Folsch UR. Comparative clinical evaluation of the 13C-mixed triglyceride breath test as an indirect pancreatic function test. Scand J Gastroenterol. (1998) 33(3):327–34. doi: 10.1080/00365529850170946
96. Boyd EJ, Cumming JG, Cuschieri A, Wood RA, Wormsley KG. Prospective comparison of the fluorescein-dilaurate test with the secretin-cholecystokinin test for pancreatic exocrine function. J Clin Pathol. (1982) 35(11):1240–3. doi: 10.1136/jcp.35.11.1240
97. Malfertheiner P, Buchler M, Muller A, Ditschuneit H. The fluorescein dilaurate serum test following metoclopramide and secretin stimulation for evaluating pancreatic function. Contribution to the diagnosis of chronic pancreatitis. Z Gastroenterol. (1987) 25(4):225–32. PMID: 3590900
98. Green MR, Austin S, Weaver LT. Dual marker one day pancreolauryl test. Arch Dis Child. (1993) 68(5):649–52. doi: 10.1136/adc.68.5.649
99. Elphick DA, Kapur K. Comparing the urinary pancreolauryl ratio and faecal elastase-1 as indicators of pancreatic insufficiency in clinical practice. Pancreatology. (2005) 5(2-3):196–200. doi: 10.1159/000085271
100. Moore DJ, Forstner GG, Largman C, Cleghorn GJ, Wong SS, Durie PR. Serum immunoreactive cationic trypsinogen: a useful indicator of severe exocrine dysfunction in the paediatric patient without cystic fibrosis. Gut. (1986) 27(11):1362–8. doi: 10.1136/gut.27.11.1362
101. Durie PR, Forstner GG, Gaskin KJ, Moore DJ, Cleghorn GJ, Wong SS, et al. Age-related alterations of immunoreactive pancreatic cationic trypsinogen in sera from cystic fibrosis patients with and without pancreatic insufficiency. Pediatr Res. (1986) 20(3):209–13. doi: 10.1203/00006450-198603000-00002
102. Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol. (2013) 19(42):7258–66. doi: 10.3748/wjg.v19.i42.7258
103. Nakamura T, Takebe K, Imamura K, Tando Y, Yamada N, Arai Y, et al. Fat-soluble vitamins in patients with chronic pancreatitis (pancreatic insufficiency). Acta Gastroenterol Belg. (1996) 59(1):10–4. PMID: 8686411
104. Dutta SK, Bustin MP, Russell RM, Costa BS. Deficiency of fat-soluble vitamins in treated patients with pancreatic insufficiency. Ann Intern Med. (1982) 97(4):549–52. doi: 10.7326/0003-4819-97-4-549
105. Lindkvist B, Dominguez-Munoz JE, Luaces-Regueira M, Castineiras-Alvarino M, Nieto-Garcia L, Iglesias-Garcia J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology. (2012) 12(4):305–10. doi: 10.1016/j.pan.2012.04.006
106. Weaver LT, Amarri S, Swart GR. 13C Mixed triglyceride breath test. Gut. (1998) 43(Suppl 3):S13–9. doi: 10.1136/gut.43.2008.S13
107. Vantrappen GR, Rutgeerts PJ, Ghoos YF, Hiele MI. Mixed triglyceride breath test: a noninvasive test of pancreatic lipase activity in the duodenum. Gastroenterology. (1989) 96(4):1126–34. doi: 10.1016/0016-5085(89)91632-6
108. Kalivianakis M, Verkade HJ, Stellaard F, van der Were M, Elzinga H, Vonk RJ. The 13C-mixed triglyceride breath test in healthy adults: determinants of the 13CO2 response. Eur J Clin Invest. (1997) 27(5):434–42. doi: 10.1046/j.1365-2362.1997.1310678.x
109. Kalivianakis M, Minich DM, Bijleveld CM, van Aalderen WM, Stellaard F, Laseur M, et al. Fat malabsorption in cystic fibrosis patients receiving enzyme replacement therapy is due to impaired intestinal uptake of long-chain fatty acids. Am J Clin Nutr. (1999) 69(1):127–34. doi: 10.1093/ajcn/69.1.127
110. Kalivianakis M, Elstrodt J, Havinga R, Kuipers F, Stellaard F, Sauer PJ, et al. Validation in an animal model of the carbon 13-labeled mixed triglyceride breath test for the detection of intestinal fat malabsorption. J Pediatr. (1999) 135(4):444–50. doi: 10.1016/S0022-3476(99)70166-6
111. Dominguez-Munoz JE. Pancreatic exocrine insufficiency: diagnosis and treatment. J Gastroenterol Hepatol. (2011) 26(Suppl 2):12–6. doi: 10.1111/j.1440-1746.2010.06600.x
112. Sikkens EC, Cahen DL, Kuipers EJ, Bruno MJ. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. (2010) 24(3):337–47. doi: 10.1016/j.bpg.2010.03.006
113. van Dijk-van Aalst K, Van Den Driessche M, van Der Schoor S, Schiffelers S, van't Westeinde T, Ghoos Y, et al. 13C Mixed triglyceride breath test: a noninvasive method to assess lipase activity in children. J Pediatr Gastroenterol Nutr. (2001) 32(5):579–85. doi: 10.1097/00005176-200105000-00017
114. Amarri S, Harding M, Coward WA, Evans TJ, Weaver LT. 13Carbon Mixed triglyceride breath test and pancreatic enzyme supplementation in cystic fibrosis. Arch Dis Child. (1997) 76(4):349–51. doi: 10.1136/adc.76.4.349
115. Dreiling DA, Hollander F. Studies in pancreatic function; preliminary series of clinical studies with the secretin test. Gastroenterology. (1948) 11(5):714–29. PMID: 18100241
116. Bornman PC, Botha JF, Ramos JM, Smith MD, Van der Merwe S, Watermeyer GA, et al. Guideline for the diagnosis and treatment of chronic pancreatitis. S Afr Med J. (2010) 100(12 Pt 2):845–60. doi: 10.7196/SAMJ.4530
117. Conwell DL, Banks PA. Chronic pancreatitis. Curr Opin Gastroenterol. (2008) 24(5):586–90. doi: 10.1097/MOG.0b013e32830b10fb
118. Dominguez Munoz JE. Diagnosis of chronic pancreatitis: functional testing. Best Pract Res Clin Gastroenterol. (2010) 24(3):233–41. doi: 10.1016/j.bpg.2010.03.008
119. Keller J, Aghdassi AA, Lerch MM, Mayerle JV, Layer P. Tests of pancreatic exocrine function - clinical significance in pancreatic and non-pancreatic disorders. Best Pract Res Clin Gastroenterol. (2009) 23(3):425–39. doi: 10.1016/j.bpg.2009.02.013
120. Lankisch PG. Function tests in the diagnosis of chronic pancreatitis. Critical evaluation. Int J Pancreatol. (1993) 14(1):9–20. doi: 10.1007/BF02795225
121. Lieb JG 2nd, Draganov PV. Pancreatic function testing: here to stay for the 21st century. World J Gastroenterol. (2008) 14(20):3149–58. doi: 10.3748/wjg.14.3149
122. Sternby B, Nilsson A, Melin T, Borgstrom B. Pancreatic lipolytic enzymes in human duodenal contents. Radioimmunoassay compared with enzyme activity. Scand J Gastroenterol. (1991) 26(8):859–66. doi: 10.3109/00365529109037023
123. Conwell DL, Zuccaro G Jr., Vargo JJ, Morrow JB, Obuchowski N, Dumot JA, et al. An endoscopic pancreatic function test with cholecystokinin-octapeptide for the diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. (2003) 1(3):189–94. doi: 10.1016/S1542-3565(03)70035-4
124. Carroccio A, Iacono G, Montalto G, Cavataio F, Di Marco C, Balsamo V, et al. Exocrine pancreatic function in children with coeliac disease before and after a gluten free diet. Gut. (1991) 32(7):796–9. doi: 10.1136/gut.32.7.796
125. Horvath K, Mehta DI, Hill ID. Assessment of exocrine pancreatic function during endoscopy in children. J Pediatr Gastroenterol Nutr. (2019) 68(6):768–76. doi: 10.1097/MPG.0000000000002230
126. Lundh G. Pancreatic exocrine function in neoplastic and inflammatory disease; a simple and reliable new test. Gastroenterology. (1962) 42:275–80. doi: 10.1016/S0016-5085(62)80025-0
127. Barbezat GO, Hansen JD. The exocrine pancreas and protein-calorie malnutrition. Pediatrics. (1968) 42(1):77–92. doi: 10.1542/peds.42.1.77
128. Jensen NM, Larsen S. A rapid, endoscopic exocrine pancreatic function test and the lundh test: a comparative study. Pancreatology. (2008) 8(6):617–24. doi: 10.1159/000161013
129. Del Rosario MA, Fitzgerald JF, Gupta SK, Croffie JM. Direct measurement of pancreatic enzymes after stimulation with secretin versus secretin plus cholecystokinin. J Pediatr Gastroenterol Nutr. (2000) 31(1):28–32. doi: 10.1097/00005176-200007000-00008
130. Gabert VM, Jensen MS. A comparison of two methods to measure amylase, lipase, trypsin, and chymotrypsin activity and the effect of freezing and thawing on enzyme activities in pancreatic juice. Pancreas. (1997) 15(2):183–90. doi: 10.1097/00006676-199708000-00011
131. Xiao Z, Lopez R, Parsi MA, Dodig M, Stevens T. Comparison of autoanalyzer and back titration for measurement of bicarbonate concentration in endoscopically collected pancreatic fluid. Pancreas. (2011) 40(2):237–41. doi: 10.1097/MPA.0b013e3181f82aa3
132. Denyer ME, Cotton PB. Pure pancreatic juice studies in Normal subjects and patients with chronic pancreatitis. Gut. (1979) 20(2):89–97. doi: 10.1136/gut.20.2.89
133. Madrazo-de la Garza JA, Gotthold M, Lu RB, Hill ID, Lebenthal E. A new direct pancreatic function test in pediatrics. J Pediatr Gastroenterol Nutr. (1991) 12(3):356–60. doi: 10.1097/00005176-199104000-00012
134. Engjom T, Erchinger F, Laerum BN, Tjora E, Aksnes L, Gilja OH, et al. Diagnostic accuracy of a short endoscopic secretin test in patients with cystic fibrosis. Pancreas. (2015) 44(8):1266–72. doi: 10.1097/MPA.0000000000000425
135. Pfefferkorn MD, Fitzgerald JF, Croffie JM, Gupta SK, Caffrey HM. Direct measurement of pancreatic enzymes: a comparison of secretagogues. Dig Dis Sci. (2002) 47(10):2211–6. doi: 10.1023/A:1020127025489
136. Regan PT, Go VL, DiMagno EP. Comparison of the effects of cholecystokinin and cholecystokinin octapeptide on pancreatic secretion, gallbladder contraction, and plasma pancreatic polypeptide in man. J Lab Clin Med. (1980) 96(4):743–8. PMID: 7419959
137. Conwell DL, Zuccaro G, Morrow JB, Van Lente F, O'Laughlin C, Vargo JJ, et al. Analysis of duodenal drainage fluid after cholecystokinin (CCK) stimulation in healthy volunteers. Pancreas. (2002) 25(4):350–4. doi: 10.1097/00006676-200211000-00005
138. Liddle RA. Regulation of pancreatic secretion. In: Johnson LR, editors. Physiology of the gastrointestinal tract. 4th ed. Elsevier Academic Press (2006). Chapter 55. p. 1397–436.
139. Schibli S, Corey M, Gaskin KJ, Ellis L, Durie PR. Towards the ideal quantitative pancreatic function test: analysis of test variables that influence validity. Clin Gastroenterol Hepatol. (2006) 4(1):90–7. doi: 10.1016/S1542-3565(05)00852-9
140. Stevens T, Conwell DL, Zuccaro G, Van Lente F, Khandwala F, Purich E, et al. Electrolyte composition of endoscopically collected duodenal drainage fluid after synthetic porcine secretin stimulation in healthy subjects. Gastrointest Endosc. (2004) 60(3):351–5. doi: 10.1016/S0016-5107(04)01809-7
141. Forsmark CE. The early diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. (2008) 6(12):1291–3. doi: 10.1016/j.cgh.2008.08.008
142. Pollack BJ, Grendell JH. Where have all the dreiling tubes gone? Am J Gastroenterol. (2006) 101(2):356–9. doi: 10.1111/j.1572-0241.2006.00398.x
143. Patel N, Sellers ZM, Grover A, Liu QY, Maqbool A, Morinville VD, et al. Endoscopic pancreatic function testing (ePFT) in children: a position paper from the NASPGHAN pancreas committee. J Pediatr Gastroenterol Nutr. (2021) 72(1):144–50. doi: 10.1097/MPG.0000000000002931
144. Alfaro Cruz L, Parniczky A, Mayhew A, Hornung LN, Lin TK, Palermo JJ, et al. Utility of direct pancreatic function testing in children. Pancreas. (2017) 46(2):177–82. doi: 10.1097/MPA.0000000000000724
145. Mehta DI, He Z, Bornstein J, Molle-Rios Z, Conwell DL, Horvath K. Report on the short endoscopic exocrine pancreatic function test in children and young adults. Pancreas. (2020) 49(5):642–9. doi: 10.1097/MPA.0000000000001540
146. Law R, Lopez R, Costanzo A, Parsi MA, Stevens T. Endoscopic pancreatic function test using combined secretin and cholecystokinin stimulation for the evaluation of chronic pancreatitis. Gastrointest Endosc. (2012) 75(4):764–8. doi: 10.1016/j.gie.2011.11.011
147. Taylor CJ, Chen K, Horvath K, Hughes D, Lowe ME, Mehta D, et al. ESPGHAN And NASPGHAN report on the assessment of exocrine pancreatic function and pancreatitis in children. J Pediatr Gastroenterol Nutr. (2015) 61(1):144–53. doi: 10.1097/MPG.0000000000000830
148. Pelley JR, Gordon SR, Gardner TB. Abnormal duodenal [HCO3-] following secretin stimulation develops sooner than endocrine insufficiency in minimal change chronic pancreatitis. Pancreas. (2012) 41(3):481–4. doi: 10.1097/MPA.0b013e31823a4c33
149. Chinnakotla S, Bellin MD, Schwarzenberg SJ, Radosevich DM, Cook M, Dunn TB, et al. Total pancreatectomy and islet autotransplantation in children for chronic pancreatitis: indication, surgical techniques, postoperative management, and long-term outcomes. Ann Surg. (2014) 260(1):56–64. doi: 10.1097/SLA.0000000000000569
150. Nydegger A, Couper RT, Oliver MR. Childhood pancreatitis. J Gastroenterol Hepatol. (2006) 21(3):499–509. doi: 10.1111/j.1440-1746.2006.04246.x
151. Tipnis NA, Werlin SL. The use of magnetic resonance cholangiopancreatography in children. Curr Gastroenterol Rep. (2007) 9(3):225–9. doi: 10.1007/s11894-007-0023-2
152. Manfredi R, Lucidi V, Gui B, Brizi MG, Vecchioli A, Maresca G, et al. Idiopathic chronic pancreatitis in children: mR cholangiopancreatography after secretin administration. Radiology. (2002) 224(3):675–82. doi: 10.1148/radiol.2243011085
153. Madzak A, Engjom T, Wathle GK, Olesen SS, Tjora E, Njolstad PR, et al. Secretin-stimulated MRI assessment of exocrine pancreatic function in patients with cystic fibrosis and healthy controls. Abdom Radiol (NY. (2017) 42(3):890–9. doi: 10.1007/s00261-016-0972-8
154. Trout AT, Serai SD, Fei L, Sun Q, Abu-El-Haija M. Prospective assessment of Normal pancreatic secretory function measured by MRI in a cohort of healthy children. Am J Gastroenterol. (2018) 113(9):1385. doi: 10.1038/s41395-018-0190-9
155. Darge K, Anupindi S. Pancreatitis and the role of US, MRCP and ERCP. Pediatr Radiol. (2009) 39(Suppl 2):S153–7. doi: 10.1007/s00247-009-1145-5
156. Neff LP, Mishra G, Fortunato JE, Laudadio J, Petty JK. Microlithiasis, endoscopic ultrasound, and children: not just little gallstones in little adults. J Pediatr Surg. (2011) 46(3):462–6. doi: 10.1016/j.jpedsurg.2010.09.007
Keywords: pancreas, exocrine function, acinar cells, duct cells, indirect pancreatic function test, direct pancreatic function test
Citation: Hopson P, Smadi Y, Mehta V, Patel S, Mehta D and Horvath K (2022) Assessment of exocrine pancreatic function in children and adolescents with direct and indirect testing. Front. Pediatr. 10:908542. doi: 10.3389/fped.2022.908542
Received: 30 March 2022; Accepted: 19 October 2022;
Published: 14 November 2022.
Edited by:
Veronique Morinville, McGill University, CanadaReviewed by:
Prateek Wali, Upstate Medical University, United StatesTanja Gonska, University of Toronto, Canada
© 2022 Hopson, Smadi, Mehta, Patel, Mehta and Horvath. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Puanani Hopson aG9wc29uLnB1YW5hbmlAbWF5by5lZHU=
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Gastroenterology, Hepatology and Nutrition, a section of the journal Frontiers in Pediatrics
 Puanani Hopson
Puanani Hopson Yamen Smadi2,†
Yamen Smadi2,† Vijay Mehta
Vijay Mehta Samit Patel
Samit Patel Devendra Mehta
Devendra Mehta