- 1State Key Laboratory of Maternal and Fetal Medicine of Chongqing Municipality, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 3Birmingham Women's and Children's Foundation Trust, Fetal Medicine Centre, Birmingham, United Kingdom
- 4Institute of Metabolism and Systems Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham, United Kingdom
- 5Molecular Immunity, Murdoch Children's Research Institute, Royal Children's Hospital, Melbourne, VIC, Australia
- 6Department of Pediatrics, University of Melbourne, Parkville, VIC, Australia
- 7College of Life Sciences, University of Leicester, Leicester, United Kingdom
- 8Chongqing Women and Children's Health Center, Chongqing, China
Objectives: To investigate the impact of gestational weight gain (GWG) on the body mass index-for-age z score (BAZ) and obesity risk among twin offspring.
Methods: This study included 263 women who were pregnant with twins and their offspring. Maternal GWG was measured in each trimester, and infant weight and length were measured at 6, 12, and 24 months.
Results: Total GWG was positively correlated with offspring birthweight and BAZ at 6, 12 and 24 months [adjusted β 0.013 (95% CI: 0.008–0.019), 0.028 (95% CI: 0.005–0.050), 0.033 (95% CI: 0.010–0.056) and 0.025 (95% CI: 0.004–0.047), respectively]. Excessive total GWG was related to an increased relative risk (RR) of large for gestational age (LGA) and overweight at 6 and 12 months. Only the second trimester gestational weight gain rate (GWGR) was positively correlated with birthweight (adjusted β 0.380, 95% CI: 0.256–0.504), and RRs of 6.818 (95% CI: 1.568–29.642) and 2.852 (95% CI: 1.466–5.548) were found for LGA and overweight at 12 months, respectively.
Conclusions: Total GWG and the second trimester GWGR were correlated with BAZ and overweight/obesity risk in twin offspring; the impact was obvious in the first year of life and gradually disappeared over time.
Clinical trial registration: ChiCTR-OOC-16008203, Registered on 1 April 2016 at the Chinese Clinical Trial Registry.
Introduction
Overweight and obesity in children have become increasingly common in recent years (1) and are serious public health issues. In the United States, 23.6% of children and teenagers aged 2–19 years were obese in 2013–2016 (2). According to the latest survey, the prevalence of overweight or obesity was 10.4% among children under 6 years old and 19% among children and adolescents aged 6–17 years in China in 2015–2019 (3). In 2019, ~38 million (6%) of children under 5 years old were overweight or obese globally (4). Childhood obesity is linked to a high risk of a number of health problems later in life, such as predispositions to diabetes mellitus (5), hypertension (6), dyslipidemia (7), and other metabolic diseases (8).
According to the “Developmental Origins of Health and Diseases” theory, intrauterine environmental factors and early life nutrition conditions impact the long-term health of offspring (9). As reported previously, maternal gestational weight gain (GWG), maternal prepregnancy body mass index (pBMI) (10), smoking during pregnancy (11), gestational diabetes mellitus (GDM) (12), milk feeding patterns (13) and adverse childhood experiences (14) have an impact on childhood obesity. Among these factors, GWG, which is a key component of health care during pregnancy, is a significant indicator of the nutritional status in pregnant women. Previous studies have shown that both excessive and inadequate GWG are related to adverse maternal and neonatal outcomes and that excessive GWG is related to childhood overweight in singleton pregnancies (15–19). However, there have been few studies on the correlations between GWG in twin-pregnant women and childhood obesity in twin offspring.
Twin pregnancies have become more prevalent in many countries, partly owing to the increasing maternal age at conception and advances in assisted reproductive technology (ART) (20). Twin fetuses share genetic and intrauterine environments, which makes twin pregnancies a desirable model for evaluating the complex interaction between genetics and the environment (21). In addition, twin offspring are likely to have a preterm birth and low birth weight, which are risk factors for postnatal catch-up growth and excessive weight gain. Therefore, further studies on maternal GWG and offspring growth in twin pregnancies are necessary. In the present study, we investigated the correlations of GWG in each trimester with offspring body mass index (BMI) and early-childhood overweight risk in twin pregnancies from birth to 2 years of age using a longitudinal twin birth cohort.
Materials and methods
Recruitment of subjects
This current study was embedded in the Longitudinal Twin Study (LoTiS), which was established in Chongqing, China, in January 2016 (Trial registration Number: ChiCTR-OOC-16008203) (22). Pregnant women at 11–16 weeks of gestation with twins were recruited for the LoTiS at the First Affiliated Hospital of Chongqing Medical University or the Chongqing Health Center for Women and Children. Subsequent follow-ups were conducted in the second and third trimesters and at delivery, and twin offspring were examined at 6 weeks and at 3, 6, 12, 18, 24, 30, and 36 months of age. The detailed protocol was described previously (23). The Ethics Committee of the First Affiliated Hospital of Chongqing Medical University examined and approved this study (No. 201530). Informed written consent was collected from all participants. A total of 333 LoTiS mother-twin pairs were initially enrolled in the current study. After excluding subjects complicated with severe fetal complications, such as selective intrauterine growth restriction, twin-to-twin transfusion syndrome (n = 45), severe congenital malformations of one or both fetuses (n = 5), and incomplete GWG records for each trimester (n = 20), the final analytic sample included 263 mother-twin pairs. The overall follow-up flow chart is illustrated in Figure 1.
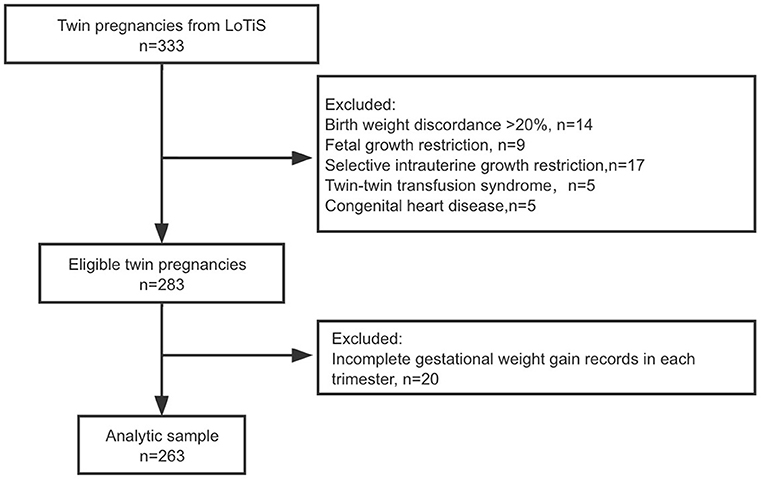
Figure 1. Flowchart showing the selection of twin pairs included in the final analysis of child growth trajectories.
Data collection
At recruitment, a face-to-face questionnaire interview was used to obtain maternal sociodemographic data, including age, prepregnancy weight, height, educational level, occupation, obstetric history, lifestyle behaviors (smoking, alcohol use), and mode of conception (ART or spontaneous). Maternal pBMI was calculated by prepregnancy weight and height. Chorionicity of a twin pregnancy was identified in the first trimester and confirmed after delivery by placental examination. DNA extracted from umbilical cord blood was used to identify the zygosity of a twin pair by short tandem repeat polymerase chain reaction.
Perinatal outcomes, including pregnancy complications, gestational age at delivery, delivery mode, infant sex, birth weight and length, were derived from medical records. Large for gestational age (LGA) was defined as weight over the 90th percentile for gestational age and sex, according to a Chinese twin population standard (24).
Information about milk feeding patterns in the first 6 months was obtained by interviewing caregivers at the children's follow-up at 6 months.
Maternal GWG and specific trimester GWGRs
For women who are pregnant with twins, prenatal examination is recommended every 2 or 3 weeks, so we were able to obtain detailed information about GWG. Prepregnancy weight was self-reported by the women at recruitment, and weight was measured by obstetric staff at each prenatal examination until delivery. We collected data on maternal weight at 12 and 28 gestational weeks and prior to delivery from medical records. If a woman had no available weight measurement at 12 or 28 gestational weeks, we adopted linear interpolation between two neighboring weight timepoints to assess the weight for that week. The maternal weight prior to delivery minus the prepregnancy weight was used to calculate total gestational weight gain (TGWG). Specific trimester GWG was obtained as follows: for the first trimester, weight at 12 gestational weeks minus prepregnancy weight; for the second trimester, weight at 28 gestational weeks minus weight at 12 gestational weeks; and for the third trimester, weight before delivery minus weight at 28 gestational weeks. The specific trimester gestational weight gain rate (GWGR) (kilograms per week) was calculated as the specific trimester GWG divided by the corresponding gestational week duration.
The GWG guideline for twin pregnancies implemented in this study was that of the US Institute of Medicine guidelines revised in 2009 (25), whereas there was no definition of specific trimester GWG. Maternal GWG was discovered to be minimal in the first trimester; thus, we classified a GWGR of <0, 0–0.083, and >0.083 kg/wk in the first trimester of pregnancy as inadequate, adequate and excessive, respectively, based on the GWGR distribution in our study population without considering the pBMI. The 75th percentile of the GWGR in the first trimester of pregnancy was 0.083 kg/wk in our study. Moreover, we presumed that the term length for a twin pregnancy was 37 gestational weeks; therefore, for each BMI category, the second- and third-trimester GWGRs were calculated as follows: (IOM recommended total GWG-1)/(37 weeks-12 weeks) (26). Based on China's BMI standard and the IOM guidelines, which have no recommendation for women who are pregnant with twins and were underweight before pregnancy, we classified GWG as inadequate, adequate, and excessive if it was below, within, or above the following recommendations: 16.8–24.5 kg (if pBMI < 24 kg/m2), 14.1–22.7 kg (if 24 ≤ pBMI < 28 kg/m2) and 11.4–19.1 kg (if pBMI ≥ 28 kg/m2). In addition, we classified the GWGR in the second and third trimesters of pregnancy as inadequate, adequate, and excessive if it was below, within, or above the following recommendations: 0.63–0.94 kg/wk (pBMI < 24 kg/m2), 0.52–0.87 kg/wk (24 kg/m2 ≤ pBMI < 28 kg/m2) and 0.42–0.72 kg/wk (pBMI ≥ 28 kg/m2).
Offspring anthropometric assessments
The birth weight and length were measured for all newborns within 1 h of delivery by uniformly trained nurses, and we acquired this information from medical records. At 6 weeks and at 3, 6, and 12 months of corrected age and 18, 24, 30, and 36 months of chronological age, infant weight and length were measured. The corrected age was defined as follows: chronological age (weeks)—(40 weeks- gestational age at birth). Anthropometric data surrounding the indicated time (±7 days) were obtained by professional nurses at the Department of Child Health Care of Chongqing Health Center for Women and Children. The infants were measured with a standing measuring meter or using a digital measuring bed while wearing light clothes and without shoes; the weight and length/height measurements were accurate to within 0.1 kg and 0.1 cm, respectively. Based on the World Health Organization (WHO) Child Growth Standards software (https://www.who.int/childgrowth/software/en/), the BMI of the offspring at each time point was calculated and transformed to z scores for sex and age. A BMI-for-age z score (BAZ) ≥1.035 was used to classify children with overweight/obesity (27). In the current study, anthropometric data at 6, 12, and 24 months were used.
Statistical analysis
Demographic characteristics and trimester GWGR information were compared according to maternal pBMI status. Continuous data are presented as the means (standard deviations) and were assessed with one-way analysis of variance. Categorical data are presented as frequencies (percentages) and were assessed with the chi-square test or Fisher's exact test. We estimated the linear effect between total GWG or trimester GWGRs and age-specific BAZ by using a generalized estimation equation (GEE) model and the non-linear effect by using a restricted cubic spline (RCS) linear regression model. The GEE model is a regression method that extends the generalized linear model to adjust for the correlation between a set of twins, which is commonly used in twin studies (28–30). The 5, 35, 65, and 95th percentiles of the distribution of specific trimester GWGRs were used in the RCS model. Moreover, we categorized the total GWG and trimester GWGRs into inadequate, adequate, and excessive, and the correlations between the total GWG and specific trimester GWGR categories (with the adequate group as the reference) and the incidence of LGA and childhood overweight were analyzed by using the GEE model. In addition, a subgroup analysis was conducted to observed the modification effect of maternal pBMI on the associations between GWGR and birthweight, age-specific BAZ. The adjusted potential confounders included maternal age, pBMI, education level, chorionicity, mode of conception, parity, smoking before pregnancy, GDM, gestational age at delivery, neonatal sex, birthweight, and milk feeding patterns within 6 months. The effects of all GEE models are presented as beta coefficients or relative risks (RRs), along with their 95% confidence intervals (CIs).
All statistical analyses were conducted in Stata 15.0 (StataCorp, College Station, TX, USA).
Results
Basic characteristics
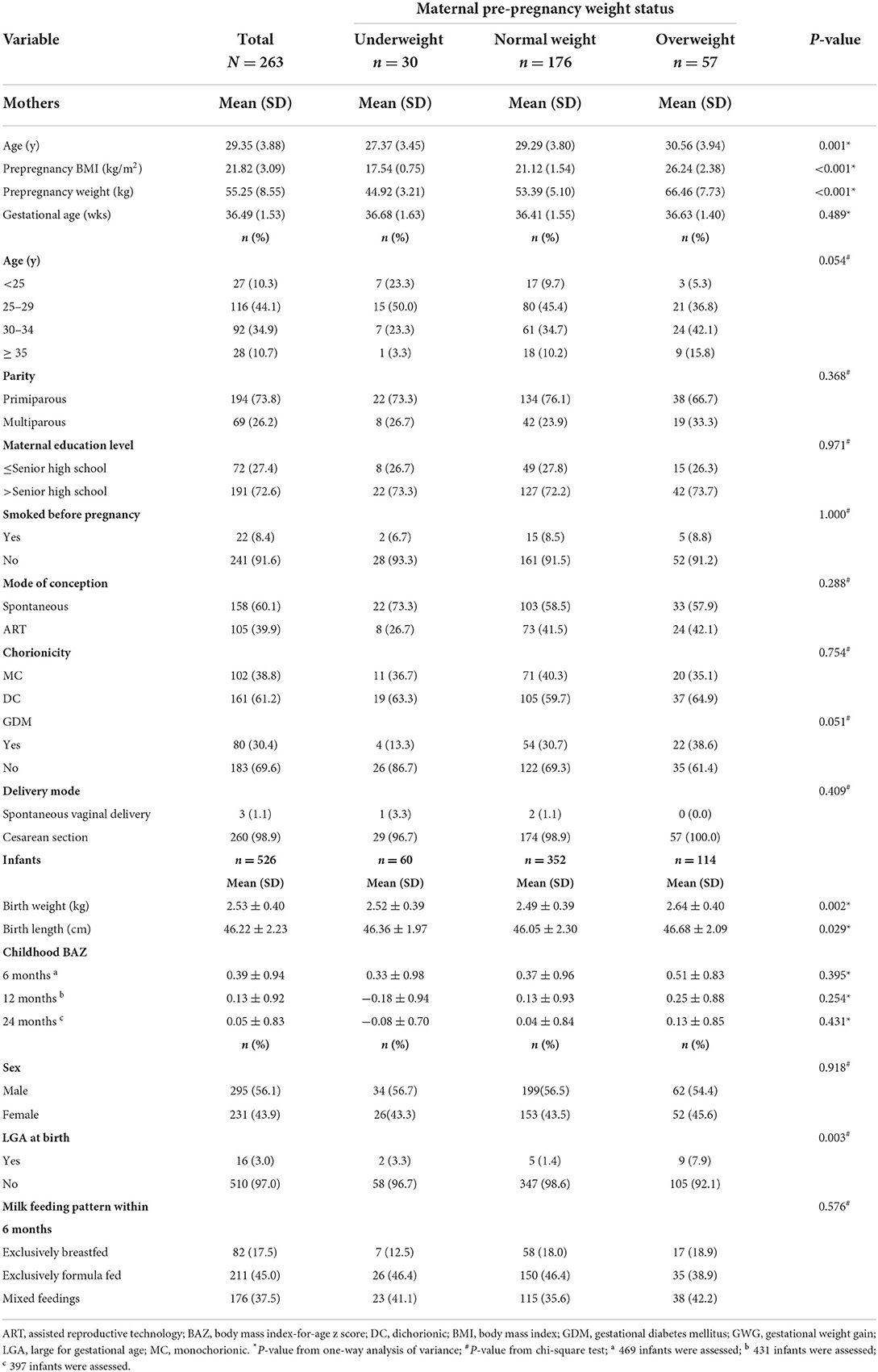
The basic characteristics of the twin-pregnant women and their offspring are shown in Table 1. In our study population, the majority of the participants were mothers who were primiparous, were highly educated, had spontaneous conception, and had a cesarean section (73.8, 72.6, 60.1, and 98.9%, respectively). Over half of the mothers had dichorionic twin pregnancies. At delivery, the average gestational age was 36.49 weeks. Based on their pBMI, 30 (11.4%) women were classified as mothers who were underweight, 176 (66.9%) were classified as mothers who were normal weight, and 57 (21.7%) were classified as mothers who were overweight. The average maternal age tended to be younger in women who were underweight and older in women who were overweight (P = 0.001). Although there was no notable difference (P = 0.051), the incidence of GDM was lowest in women who were underweight and highest in mothers who were overweight. Other baseline information showed no differences among the three groups. Regarding twin offspring, 56.1% of the newborns were boys, the average birthweight was 2.53 kg, and the average birth length was 46.22 cm. The average birth weight, birth length and incidence of LGA were highest among the neonates born to overweight mothers (all P < 0.05). There were no differences between the groups in the BAZ and milk feeding patterns within 6 months. Due to the unavoidable loss to follow-up, the follow-up numbers of infants were 469 at 6 months, 431 at 12 months and 397 at 24 months.
We compared the demographic and clinical characteristics between the included participants and initially enrolled participants, and the results showed that the characteristics were not different (Supplementary Table 1).
GWGR characteristics
The details of the total GWG and the specific trimester GWGR are depicted in Supplementary Table 2. Mothers who were underweight had the highest average total GWG, whereas those who were overweight had the lowest. In the first, second, and third trimesters, the average GWGR was 0.08, 0.69, and 0.70 kg/wk, respectively.
In the first trimester, according to the GWGR distribution, 40.7% of the pregnant women had an inadequate GWGR (≤ 0 kg/wk), 45.3% had an adequate GWGR (0–0.25 kg/wk), and 14.1% had an excessive GWGR (≥0.25 kg/wk). In the second trimester, 41.4, 48.3, and 10.3% of the study group had inadequate, adequate, and excessive GWGRs, respectively. No differences were found between the groups in the distribution of the first two trimester GWGRs. In the third trimester, 35.7, 42.2, and 22.1% of the study group had inadequate, adequate, and excessive GWGRs, respectively. The percentage of inadequate GWGRs was lowest in women who were overweight in the third trimester (P = 0.007).
GWG and offspring birthweight and age-specific BAZ
The univariate and multiple regression analyses of the relationship between total GWG and offspring birthweight and BAZ at 6, 12, and 24 months are summarized in Table 2. In an unadjusted model, total GWG was positively correlated with offspring birthweight and BAZ at 6, 12, and 24 months. After adjusting for maternal age, pBMI, parity, education level, smoking before pregnancy, GDM, gestational age, mode of conception, chorionicity and milk feeding patterns within 6 months, the positive associations were still significant between total GWG and birthweight and BAZ at 6, 12, and 24 months [adjusted β 0.013 (95% CI: 0.008–0.019), 0.028 (95% CI: 0.005–0.050), 0.033 (95% CI: 0.010–0.056) and 0.025 (95% CI: 0.004–0.047), respectively).
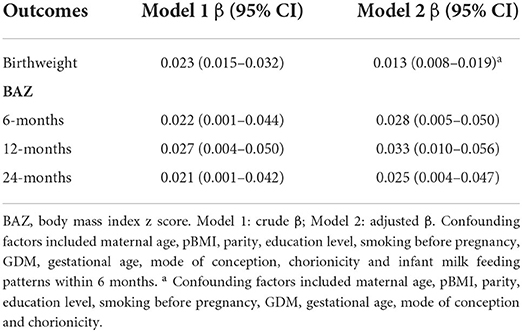
Table 2. Unadjusted and adjusted correlations between total gestational weight gain and birthweight and age-specific BAZ.
GWG and risk of LGA and early childhood overweight
Before we analyzed the impact of GWG on childhood overweight, we assessed the concordance rate of the growth patterns in twin offspring. The concordance rate of both infants being normal or overweight was higher in monozygotic (MZ) twins than in dizygotic (DZ) twins, and the concordance rate increased with age in both MZ and DZ twins (Supplementary Table 3).
The univariate and multiple logistic regression analyses of the correlation of total GWG categories with the risk of LGA and childhood overweight are shown in Table 3. After adjusting for possible confounders, excessive GWG was correlated with increased RRs of 5.947 [95% confidence interval (CI): 1.820, 25.678], 2.069 (95% CI: 1.290, 3.318) and 3.964 (95% CI: 1.949–15.190) for LGA at birth and overweight at 6 and 12 months, respectively, when compared to the reference group.
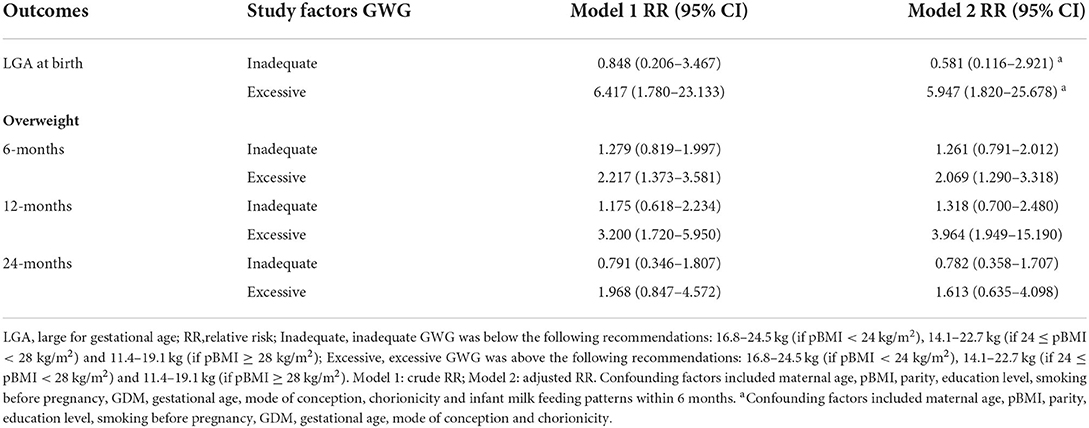
Table 3. Unadjusted and adjusted correlations between total gestational weight gain and LGA and age-specific overweight.
GWGR and offspring birthweight/BAZ
To investigate which GWG period had a key impact on offspring growth, correlations between trimester GWGRs and birthweight and childhood BAZ were determined. The results indicated that only the second trimester GWGR was positively correlated with offspring birthweight (Table 4). The mean birthweight increased by 0.38 kg when the second trimester GWGR increased by 1 kg/wk (95% CI: 0.256–0.504) after controlling for potential confounders. In addition, we detected whether non-linear effects existed between specific trimester GWGRs and offspring birthweight or childhood BAZ. Data pooling showed estimations and 95% CIs for the GWGR in different trimesters with birthweight and BAZ at 6, 12, and 24 months by RCS linear regression models among the total population; potential non-linear correlations of the second trimester GWGR with offspring BAZ at 6 and 12 months; and potential non-linear correlations of the third trimester GWGR with offspring birthweight (Supplementary Figures 1–3).
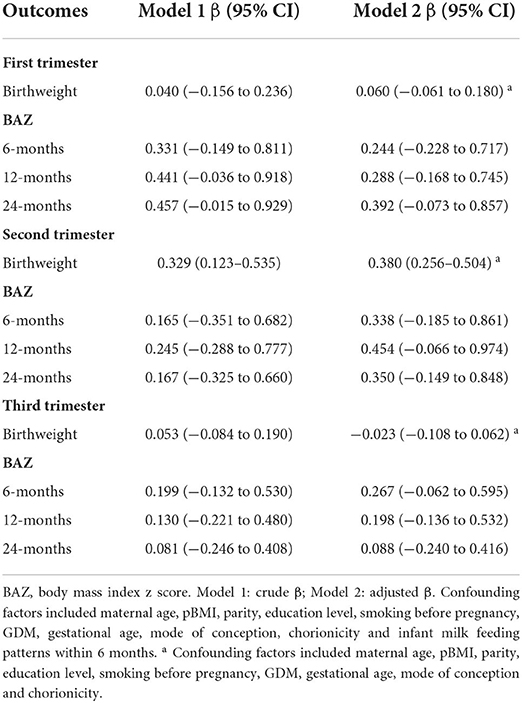
Table 4. Unadjusted and adjusted correlations between trimester GWGRs and birthweight and age-specific BAZ.
In the subgroup analyses, this positive correlation remained evident in mothers who were underweight (β 0.723, 95% CI: 0.335–1.111), normal weight (β 0.256, 95% CI: 0.102–0.409) and overweight (β 0.550, 95% CI: 0.334–0.766) (Supplementary Figure 4). In addition, the third trimester GWGR was found to be positively correlated with BAZ at 6 months (β 0.767, 95% CI: 0.154–1.381), 12 months (β 0.946, 95% CI: 0.231–1.660) and 24 months (β 0.893, 95% CI: 0.118–1.668) only among overweight mothers.
GWGR and risk of LGA and early childhood overweight
The correlations of unadjusted and adjusted RRs for LGA and overweight at 6, 12, and 24 months with excessive or inadequate trimester GWGRs are depicted in Table 5. In the general population, excessive GWGR only in the second trimester was correlated with an increased RR of 6.818 (95% CI: 1.568–29.642) and 2.852 (95% CI: 1.466–5.548) for LGA and overweight at 12 months, respectively, after controlling for confounding factors. There were no differences between inadequate trimester GWGRs and LGA or overweight at 6, 12, and 24 months.
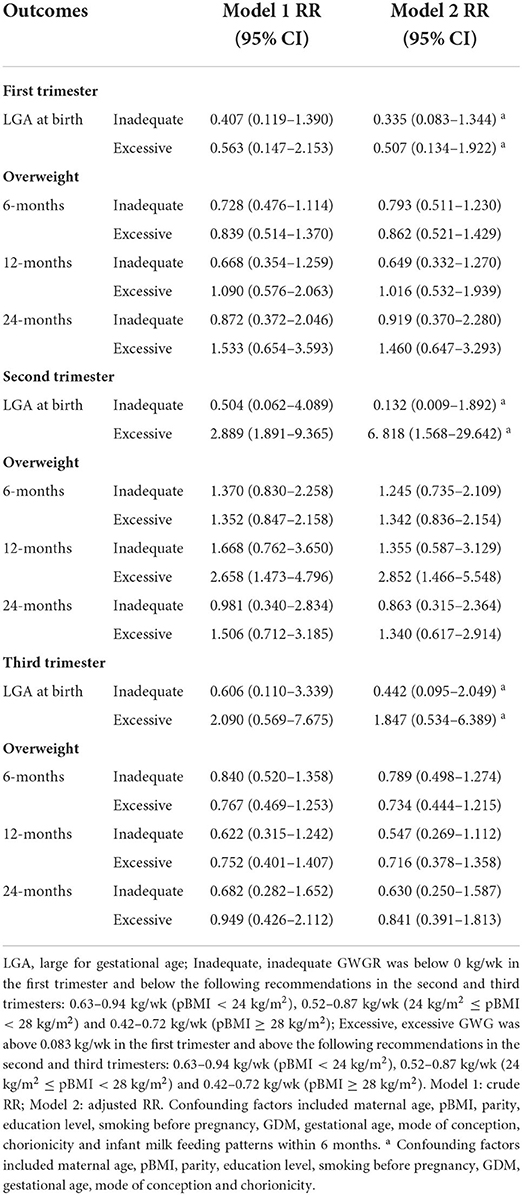
Table 5. Unadjusted and adjusted correlations between excessive or inadequate trimester GWGR and LGA and age-specific overweight.
Discussion
In this study, we examined the relationship between total GWG and offspring growth in twin pregnancies, and the results demonstrated that increased total GWG was related to higher offspring birthweight and BAZ in the first 2 years. Excessive total GWG was correlated with a higher risk of overweight at 6 and 12 months. Second, we investigated the impact of the GWGR in each trimester on twin offspring growth, and the results illustrated that only the second trimester GWGR was positively associated with offspring birthweight. Moreover, an excessive GWGR only in the second trimester was correlated with a higher risk of overweight at 12 months.
Previous twin studies have reported that genetic factors have a significant influence on BMI at all ages based on a twin model (31), but early life factors may have a stronger effect than genetic factors on childhood obesity (32). The environmental factors shared by cotwins had a noticeable effect in infancy and early childhood, but this effect was less pronounced after mid-childhood and disappeared by adulthood (33), which may mirror the growing independence of children from their parents and family environment, leading to more individualistic exercise and eating behaviors (34). In this study, we found that the concordance rate of the growth pattern in twins was higher with increasing age, suggesting that early life factors played an important role in childhood growth and development.
Recent studies have indicated that maternal GWG is correlated with offspring growth. Xie et al. discovered a significantly positive relationship between GWG and high neonatal birthweight among a Southwest Chinese population (35). A positive relationship between maternal total GWG and childhood BMI was found among different populations at different ages (10, 36). Zhang et al. reported that excessive GWG, only in the first trimester, was related to a higher risk of overweight or obesity in offspring at 12 months (37). Likewise, Diesel et al. found that excessive total GWG was related to more than double the risk of childhood obesity at 3 years old compared with no overall excessive GWG in the U.S. (38). Bodnar et al. found that higher gestational weight gain in twin pregnancies was positively associated with the risk of excess postpartum weight increase and childhood overweight/obesity in a nationally representative U.S. birth cohort (39). Our findings are in accordance with previous studies that suggested an association of higher total GWG with increased offspring BAZ at 6, 12, and 24 months and with increased RR for LGA and overweight at 6 and 12 months. A possible hypothesis is that higher GWG indicates excess nutrients in utero, thus exposing the fetus to excessive levels of fatty acids and glucose, which may increase fetal insulin production and consequently result in increased adiposity (40), although it might be beneficial for age-dependent decline brain health (41).
Since maternal weight gain is not uniform throughout gestation, therefore, some researchers have concentrated on specific trimester GWG and offspring growth. Previous studies of the effect of trimester GWG and offspring growth have been inconsistent. In singletons, a study by Xiong et al. suggested that an excessive GWGR in each trimester was positively related to offspring BAZ and the risk of overweight or obesity in early childhood (19). Hivert et al. concluded that a higher GWGR in each trimester of pregnancy was related to higher birthweight, whereas only a higher GWGR in the first two trimesters was related to increased BAZ and risk of obesity in mid-childhood, and the findings were inconsistent among mothers in different BMI categories (17). Pugh et al. found that an excessive GWGR in the second and third trimesters increased the risk of LGA in offspring (42). A previous study in twin pregnancies reported that only the second trimester GWG was related to fetal growth, driven by a correlation with abdominal circumference in the early second trimester and long bones in the late second trimester (43). Our findings suggested that, overall, only the second trimester GWGR was positively related to infant birthweight and was correlated with a higher risk of LGA and overweight at 12 months in twins, which was consistent with our previous research reporting that second trimester GWG was positively related to offspring birthweight among dichorionic twin pregnancies (44). This finding could be comforting to women who have a sufficient prepregnancy nutritional status but restricted weight gain in the first trimester because of increased vomiting and nausea symptoms, which are common in twin pregnancies (45). A biological explanation for this phenomenon is that the second trimester is the most critical stage for organogenesis, including the growth of the heart, liver, muscles and bones (17). The development of fetal adipose tissue also begins around the same time, with the first “fat lobules” appearing between 14 and 24 weeks of gestation, when nutritional, hormonal, and epigenetic signals can be affected by the mother and may lead to permanent alterations in the adipose tissue of offspring (46). Thus, the second trimester GWG may promote the growth of both organs and adipose tissue, supporting our observations related to offspring BAZ and obesity. In the third trimester, a number of complicating factors, such as intrauterine environment or placental insufficiency, may limit fetal growth in twin pregnancies, attenuating the correlation of GWG with fetal growth (47).
Since women who are pregnant with twins and have inadequate GWG are prone to preterm delivery, their offspring are prone to low birthweight and are more likely to undergo catch-up growth after birth, which can also lead to overweight later in life (48). In the present study, the proportion of preterm infants was high, and the birthweight of the infants was low. Given that infants with either low or high birthweight can be overweight in childhood, the long-term impacts of birthweight exhibit a U-shaped curve (49). Thus, we conducted an association analysis between inadequate GWG and childhood overweight and found no associations, indicating that overweight or obesity in preterm infants may be caused by other factors and warrants further study.
The strengths of this study warrant discussion. To our knowledge, this was the first study on the correlations of trimester GWGRs with offspring growth from birth to 2 years of age by using a longitudinal twin pregnancy birth cohort. Additionally, a stratified analysis was used to assess the relationship between GWG/GWGRs and birthweight/BAZ at different pBMI levels, making the study more reliable, accurate and comprehensive. Furthermore, our study converted weight, height and BMI into z scores based on the WHO growth criteria, which is a commonly used reference standard in China.
However, several limitations need to be noted. First, the participants were asked to recall their prepregnancy height and weight, which could lead to recall bias, although previous studies have revealed that the error has a minor effect on the reported associations and conclusions (50, 51). Second, there may be residual confounders, which we did not consider in this analysis. Finally, we only followed up the offspring growth to 2 years of age, and the long-term impacts of GWG are unknown. Therefore, LoTiS will continue to monitor the growth and development of twins and further elucidate the impact of GWG on growth in twins.
Conclusion
In conclusion, the impact of total GWG on BMI and the risk of obesity for offspring was obvious in the first year of life and gradually disappeared as the twin infants grew older. Additionally, only the second trimester GWGR was correlated with offspring birthweight and the risk of early-childhood obesity in twins. The findings may motivate further study to investigate the guidelines of GWG/GWGRs for twin-pregnant women of diverse ethnicities as well as the development of intervention strategies aimed at controlling the rapidly rising prevalence of childhood obesity.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the First affiliated hospital of Chongqing Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
CT and HQ designed and conceived the study. SL, LW, and YQ collected and analyzed the data. RS, MK, and PB interpreted the results. CT, LW, and HQ provided the funding resources. SL and LW wrote the draft. CT, MK, and PB edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (U21A20346, 81520108013, 82001580, and 82171662), Chongqing Science and Technology Commission (cstc2021ycjh-bgzxm0192), Chongqing Health Committee (2019GDRC012), and Chongqing Education Commission (KJZD-K202100404).
Acknowledgments
We are grateful to the families who participated in the Chongqing LoTiS.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.906086/full#supplementary-material
Abbreviations
pBMI, prepregnancy body mass index; GWG, gestational weight gain; GDM, gestational diabetes mellitus; ART, assisted reproductive technology; BMI, body mass index; LoTiS, longitudinal twin cohort study; LGA, large for gestational age; TGWG, total gestational weight gain; GWGR, gestational weight gain rate; WHO, World Health Organization; BAZ, body mass index-for-age z score; GEE, generalized estimation equation; RCS, restricted cubic spline; MZ, monozygotic; DZ, dizygotic; RR, relative risk; CI, confidence interval; DC, dichorionic; MC, monochorionic.
References
1. NCD-RisC. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. (2017) 390:2627–42. doi: 10.1016/s0140-6736(17)32129-3
2. Afshin A, Forouzanfar M, Reitsma M, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. doi: 10.1056/NEJMoa1614362
3. Pan X, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diab Endocrinol. (2021) 9:373–92. doi: 10.1016/s2213-8587(21)00045-0
4. Hunter K, Johnson B, Askie L, Golley R, Baur L, Marschner I, et al. Transforming obesity prevention for children (Topchild) collaboration: protocol for a systematic review with individual participant data meta-analysis of behavioural interventions for the prevention of early childhood obesity. BMJ open. (2022) 12:e048166. doi: 10.1136/bmjopen-2020-048166
5. Piché M, Tchernof A, Després J. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. doi: 10.1161/circresaha.120.316101
6. Reinehr T, Wunsch R. Relationships between cardiovascular risk profile, ultrasonographic measurement of intra-abdominal adipose tissue, and waist circumference in obese children. Clin Nutr. (2010) 29:24–30. doi: 10.1016/j.clnu.2009.06.004
7. Kumar S, Kelly A. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. (2017) 92:251–65. doi: 10.1016/j.mayocp.2016.09.017
8. Battineni G, Sagaro G, Chintalapudi N, Amenta F, Tomassoni D, Tayebati S. Impact of obesity-induced inflammation on cardiovascular diseases (Cvd). Int J Mol Sci. (2021) 22:4798. doi: 10.3390/ijms22094798
9. Hanson M, Gluckman P. Early developmental conditioning of later health and disease: physiology or pathophysiology? Physiol Rev. (2014) 94:1027–76. doi: 10.1152/physrev.00029.2013
10. Hu Z, Tylavsky F, Han J, Kocak M, Fowke J, Davis R, et al. Maternal metabolic factors during pregnancy predict early childhood growth trajectories and obesity risk: the candle study. Int J Obes. (2019) 43:1914–22. doi: 10.1038/s41366-019-0326-z
11. Ogden C, Carroll M, Kit B, Flegal K. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. (2014) 311:806–14. doi: 10.1001/jama.2014.732
12. Wu Y, Yu J, Liu X, Wang W, Chen Z, Qiao J, et al. Gestational diabetes mellitus-associated changes in the breast milk metabolome alters the neonatal growth trajectory. Clin Nutr. (2021) 40:4043–54. doi: 10.1016/j.clnu.2021.02.014
13. Jin H, Shan T, Li S, Fan X, Zhao Y, Li J, et al. Association of infant feeding patterns with body fat mass and obesity in the first 2 years of life: a longitudinal twin birth cohort study. Int J Food Sci Nutr. (2022) 73:263–73. doi: 10.1080/09637486.2021.1968357
14. Schroeder K, Schuler B, Kobulsky J, Sarwer D. The association between adverse childhood experiences and childhood obesity: a systematic review. Obesity Rev. (2021) 22:e13204. doi: 10.1111/obr.13204
15. Lu W, Zhang X, Wu J, Mao X, Shen X, Chen Q, et al. Association between trimester-specific gestational weight gain and childhood obesity at 5 years of age: results from shanghai obesity cohort. BMC Pediatr. (2019) 19:139. doi: 10.1186/s12887-019-1517-4
16. Fraser A, Tilling K, Macdonald-Wallis C, Sattar N, Brion M, Benfield L, et al. Association of maternal weight gain in pregnancy with offspring obesity and metabolic and vascular traits in childhood. Circulation. (2010) 121:2557–64. doi: 10.1161/circulationaha.109.906081
17. Hivert M, Rifas-Shiman S, Gillman M, Oken E. Greater early and mid-pregnancy gestational weight gains are associated with excess adiposity in mid-childhood. Obesity. (2016) 24:1546–53. doi: 10.1002/oby.21511
18. Laitinen J, Jääskeläinen A, Hartikainen A, Sovio U, Vääräsmäki M, Pouta A, et al. Maternal weight gain during the first half of pregnancy and offspring obesity at 16 years: a prospective cohort study. BJOG. (2012) 119:716–23. doi: 10.1111/j.1471-0528.2012.03319.x
19. Xiong X, Xia W, Li Y, Xu S, Zhang Y. Associations of gestational weight gain rate during different trimesters with early-childhood body mass index and risk of obesity. Obesity. (2020) 28:1941–50. doi: 10.1002/oby.22963
20. Deng C, Dai L, Yi L, Li X, Deng K, Mu Y, et al. Temporal trends in the birth rates and perinatal mortality of twins: a population-based study in China. PLoS ONE. (2019) 14:e0209962. doi: 10.1371/journal.pone.0209962
21. Talens R, Christensen K, Putter H, Willemsen G, Christiansen L, Kremer D, et al. Epigenetic variation during the adult lifespan: cross-sectional and longitudinal data on monozygotic twin pairs. Aging Cell. (2012) 11:694–703. doi: 10.1111/j.1474-9726.2012.00835.x
22. Tong C, Wen L, Wang L, Fan X, Zhao Y, Liu Y, et al. Cohort profile: the chongqing longitudinal twin study (Lotis). Int J Epidemiol. (2022) dyab264. doi: 10.1093/ije/dyab264. [Epub ahead of print].
23. Tong C, Wen L, Xia Y, Leong P, Wang L, Fan X, et al. Protocol for a longitudinal twin birth cohort study to unravel the complex interplay between early-life environmental and genetic risk factors in health and disease: the chongqing longitudinal twin study (Lotis). BMJ Open. (2018) 8:e017889. doi: 10.1136/bmjopen-2017-017889
24. Zhang B, Cao Z, Zhang Y, Yao C, Xiong C, Zhang Y, et al. Birthweight percentiles for twin birth neonates by gestational age in China. Sci Rep. (2016) 6:31290. doi: 10.1038/srep31290
25. Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Collection: Reports Funded by National Institutes of Health (2009).
26. Lutsiv O, Hulman A, Woolcott C, Beyene J, Giglia L, Armson B, et al. Examining the provisional guidelines for weight gain in twin pregnancies: a retrospective cohort study. BMC Pregn Childbirth. (2017) 17:330. doi: 10.1186/s12884-017-1530-2
27. Li N, Liu E, Sun S, Guo J, Pan L, Wang P, et al. Birth weight and overweight or obesity risk in children under 3 years in China. Am J Human Biol. (2014) 26:331–6. doi: 10.1002/ajhb.22506
28. Xi Y, Gao W, Zheng K, Lv J, Yu C, Wang S, et al. The roles of genetic and early-life environmental factors in the association between overweight or obesity and hypertension: a population-based twin study. Front Endocrinol. (2021) 12:743962. doi: 10.3389/fendo.2021.743962
29. Yang W, Li X, Pan K, Yang R, Song R, Qi X, et al. Association of life-course depression with the risk of dementia in late life: a nationwide twin study. Alzheimers Dement. (2021) 17:1383–90. doi: 10.1002/alz.12303
30. Konijnenberg E, Tomassen J, den Braber A, Ten Kate M, Yaqub M, Mulder S, et al. Onset of preclinical Alzheimer disease in monozygotic twins. Ann Neurol. (2021) 89:987–1000. doi: 10.1002/ana.26048
31. Silventoinen K, Jelenkovic A, Sund R, Hur Y, Yokoyama Y, Honda C, et al. Genetic and environmental effects on body mass index from infancy to the onset of adulthood: an individual-based pooled analysis of 45 twin cohorts participating in the collaborative project of development of anthropometrical measures in twins (Codatwins) study. Am J Clin Nutr. (2016) 104:371–9. doi: 10.3945/ajcn.116.130252
32. Kelsey M, Zaepfel A, Bjornstad P, Nadeau K. Age-related consequences of childhood obesity. Gerontology. (2014) 60:222–8. doi: 10.1159/000356023
33. Reynolds C, Tan Q, Munoz E, Jylhävä J, Hjelmborg J, Christiansen L, et al. A decade of epigenetic change in aging twins: genetic and environmental contributions to longitudinal DNA methylation. Aging Cell. (2020) 19:e13197. doi: 10.1111/acel.13197
34. Dubois L, Ohm Kyvik K, Girard M, Tatone-Tokuda F, Pérusse D, Hjelmborg J, et al. Genetic and environmental contributions to weight, height, and bmi from birth to 19 years of age: an international study of over 12,000 twin pairs. PLoS ONE. (2012) 7:e30153. doi: 10.1371/journal.pone.0030153
35. Xie Y, Peng R, Han L, Zhou X, Xiong Z, Zhang Y, et al. Associations of neonatal high birth weight with maternal pre-pregnancy body mass index and gestational weight gain: a case-control study in women from Chongqing, China. BMJ Open. (2016) 6:e010935. doi: 10.1136/bmjopen-2015-010935
36. Voerman E, Santos S, Inskip H, Amiano P, Barros H, Charles M, et al. Association of gestational weight gain with adverse maternal and infant outcomes. JAMA. (2019) 321:1702–15. doi: 10.1001/jama.2019.3820
37. Zhang W, Niu F, Ren X. Association of maternal pre-pregnancy body mass index and gestational weight gain with chinese infant growth. J Paediatr Child Health. (2019) 55:673–9. doi: 10.1111/jpc.14274
38. Diesel J, Eckhardt C, Day N, Brooks M, Arslanian S, Bodnar L. Is gestational weight gain associated with offspring obesity at 36 months? Pediatr Obes. (2015) 10:305–10. doi: 10.1111/ijpo.262
39. Bodnar L, Cartus A, Parisi S, Abrams B, Himes K, Eckhardt C, et al. Pregnancy weight gain in twin gestations and maternal and child health outcomes at 5 years. Int J Obes. (2021) 45:1382–91. doi: 10.1038/s41366-021-00792-8
40. Muhlhausler B, Ong Z. The fetal origins of obesity: early origins of altered food intake. Endocr Metab Immune Disord Drug Targets. (2011) 11:189–97. doi: 10.2174/187153011796429835
41. Di Meco A, Praticò D. Early-life exposure to high-fat diet influences brain health in aging mice. Aging Cell. (2019) 18:e13040. doi: 10.1111/acel.13040
42. Pugh S, Albert P, Kim S, Grobman W, Hinkle S, Newman R, et al. Patterns of gestational weight gain and birthweight outcomes in the eunice kennedy shriver national institute of child health and human development fetal growth studies-singletons: a prospective study. Am J Obstet Gynecol. (2017) 217:346.e1–e11. doi: 10.1016/j.ajog.2017.05.013
43. Hinkle S, Hediger M, Kim S, Albert P, Grobman W, Newman R, et al. Maternal weight gain and associations with longitudinal fetal growth in dichorionic twin pregnancies: a prospective cohort study. Am J Clin Nutr. (2017) 106:1449–55. doi: 10.3945/ajcn.117.158873
44. Wen L, Liu X, Wang L, Zheng Y, Li J, Tong C, et al. Correlation between second trimester weight gain and perinatal outcomes in dichorionic twin pregnancies: the lotis cohort study. Eur J Obstet Gynecol Reprod Biol. (2019) 233:64–9. doi: 10.1016/j.ejogrb.2018.12.014
45. Chan R, Olshan A, Savitz D, Herring A, Daniels J, Peterson H, et al. Maternal influences on nausea and vomiting in early pregnancy. Mat Child Health J. (2011) 15:122–7. doi: 10.1007/s10995-009-0548-0
46. Moreno-Mendez E, Quintero-Fabian S, Fernandez-Mejia C, Lazo-de-la-Vega-Monroy M. Early-life programming of adipose tissue. Nutr Res Rev. (2020) 33:244–59. doi: 10.1017/s0954422420000037
47. Gluckman P, Hanson M. Maternal constraint of fetal growth and its consequences. Semin Fetal Neonatal Med. (2004) 9:419–25. doi: 10.1016/j.siny.2004.03.001
48. Oestreich A, Moley K. Developmental and transmittable origins of obesity-associated health disorders. Trends Genet. (2017) 33:399–407. doi: 10.1016/j.tig.2017.03.008
49. Hoffman D, Powell T, Barrett E, Hardy D. Developmental origins of metabolic diseases. Physiol Rev. (2021) 101:739–95. doi: 10.1152/physrev.00002.2020
50. Lederman S, Paxton A. Maternal reporting of prepregnancy weight and birth outcome: consistency and completeness compared with the clinical record. Mat Child Health J. (1998) 2:123–6. doi: 10.1023/a:1022996924094
Keywords: childhood obesity, infantile body mass index, gestational weight gain, specific trimester, twin pregnancies
Citation: Li S, Qiu Y, Yuan X, Zhang Q, Kilby MD, Saffery R, Baker PN, Wen L, Tong C and Qi H (2022) Impact of maternal gestational weight gain in twin pregnancies on early childhood obesity risk: A longitudinal birth cohort study. Front. Pediatr. 10:906086. doi: 10.3389/fped.2022.906086
Received: 28 March 2022; Accepted: 13 July 2022;
Published: 02 August 2022.
Edited by:
Li Ming Wen, The University of Sydney, AustraliaReviewed by:
Alison Macchi, Florida International University, United StatesHuilan Xu, Sydney Local Health District, Australia
Copyright © 2022 Li, Qiu, Yuan, Zhang, Kilby, Saffery, Baker, Wen, Tong and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Wen, Y3FtdXdlbmxpQDE2My5jb20=; Chao Tong, Y2hhb3RvbmdjcW11QDE2My5jb20=; Hongbo Qi, cWlob25nYm83MjhAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Sisi Li
Sisi Li Yuhan Qiu
Yuhan Qiu Xi Yuan
Xi Yuan Qin Zhang
Qin Zhang Mark D. Kilby
Mark D. Kilby Richard Saffery
Richard Saffery Philip N. Baker7
Philip N. Baker7 Li Wen
Li Wen Chao Tong
Chao Tong Hongbo Qi
Hongbo Qi