
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 26 July 2022
Sec. Pediatric Critical Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.904846
This article is part of the Research TopicSimulation in Pediatric Critical Care Medicine Training and EducationView all 5 articles
 Ebor Jacob G. James1,2*
Ebor Jacob G. James1,2* Siva Vyasam1
Siva Vyasam1 Shakthi Venkatachalam3
Shakthi Venkatachalam3 Elizabeth Sanseau4
Elizabeth Sanseau4 Kyle Cassidy5
Kyle Cassidy5 Geethanjali Ramachandra2,6
Geethanjali Ramachandra2,6 Grace Rebekah7
Grace Rebekah7 Debasis D. Adhikari1
Debasis D. Adhikari1 Ellen Deutsch3,8
Ellen Deutsch3,8 Akira Nishisaki3,8
Akira Nishisaki3,8 Vinay M. Nadkarni3,8
Vinay M. Nadkarni3,8Introduction: Pediatric shock, especially septic shock, is a significant healthcare burden in low-income countries. Early recognition and management of shock in children improves patient outcome. Simulation-based education (SBE) for shock recognition and prompt management prepares interdisciplinary pediatric emergency teams in crisis management. COVID-19 pandemic restrictions on in-person simulation led us to the development of telesimulation for shock. We hypothesized that telesimulation training would improve pediatric shock recognition, process of care, and patient outcomes in both simulated and real patient settings.
Materials and Methods: We conducted a prospective quasi-experimental interrupted time series cohort study over 9 months. We conducted 40 telesimulation sessions for 76 participants in teams of 3 or 4, utilizing the video telecommunication platform (Zoom©). Trained observers recorded time-critical interventions on real patients for the pediatric emergency teams composed of residents, fellows, and nurses. Data were collected on 332 pediatric patients in shock (72% of whom were in septic shock) before, during, and after the intervention. The data included the first hour time-critical intervention checklist, patient hemodynamic status at the end of the first hour, time for the resolution of shock, and team leadership skills in the emergency room.
Results: There was a significant improvement in the percent completion of tasks by the pediatric emergency team in simulated scenarios (69% in scenario 1 vs. 93% in scenario 2; p < 0.001). In real patients, completion of tasks as per time-critical steps reached 100% during and after intervention compared to the pre-intervention phase (87.5%), p < 0.05. There was a significant improvement in the first hour hemodynamic parameters of shock patients: pre (71%), during (79%), and post (87%) intervention (p < 0.007 pre vs. post). Shock reversal time reduced from 24 h pre-intervention to 6 h intervention and to 4.5 h post intervention (p < 0.002). There was also a significant improvement in leadership performance assessed by modified Concise Assessment of Leader Management (CALM) instrument during the simulated (p < 0.001) and real patient care in post intervention (p < 0.05).
Conclusion: Telesimulation training is feasible and improved the process of care, time-critical interventions, leadership in both simulated and real patients and resolution of shock in real patients. To the best of our knowledge, this is one of the first studies where telesimulation has shown improvement in real patient outcomes.
The leading cause of pediatric global morbidity and mortality is severe sepsis, with disparities in outcomes linked to lack of access to training and resources based on geography (1–5). The concurrent application of in situ simulation for training and systems analysis offers an effective strategy to improve adherence to guidelines, resulting in early diagnosis, time-critical intervention, and overall better outcomes (6, 7). Geographic and resource barriers and infection-related restrictions to in situ simulations can be effectively overcome by remotely facilitated simulation-based activities. Distance or remote or telesimulation is defined as “simulation performed with either the facilitator, learners, or both in an offsite location separate from other members to complete educational or assessment activities.” Facilitation and assessment can be performed either synchronously or asynchronously using video conferencing tools (8–16). The concept of telesimulation using prerecorded video was adapted from the ACEP SimBox, a free and openly accessible web-based simulation platform demonstrated to be easy to use, feasible, and recommended by healthcare team users (17–21). While the SimBox utilizes a single-track video that can be paused, rewound, or fast-forward, Annenberg hotkeys provides a random-access interface that allows a facilitator to play prerecorded videos in any order at any time, permitting a video narrative to be created on-the-fly based on input from users. This allows for simulations that can arrive at a conclusion via a number of different paths or with multiple interventions and outcomes.
It is known that prompt recognition and time-critical management of shock in the “golden hour” correlates with improved patient morbidity and mortality (22). Given the burden of disease of sepsis and restrictions to in-person education activities during the COVID-19 pandemic at the teaching hospital, Christian Medical College (CMC), Vellore, India, we created a low-cost telesimulation curriculum. Our aim was to train pediatric emergency teams (PET) composed of pediatric residents, fellows, and nurses in the recognition and first-hour management of shock.
The purpose of this study was to assess the impact of a telesimulation training on the PET’s first-hour management of pediatric shock. The primary objective of this study was to assess the completion of time-critical tasks (process of care) by the PET and the hemodynamic stability of real patients at the end of the first-hour management. The secondary objectives of this study were to assess time for prospectively defined shock reversal, the development of new Multiple Organ Dysfunction Syndrome (MODS), and assessment of team leadership skills in real-patient scenarios. We also assessed the completion of time-critical tasks and leadership skills during simulated scenarios. We hypothesized that our novel telesimulation training would improve the time-critical processes of care and team leadership in simulation and that this would translate to improved time-critical processes of care, team leadership, and meaningful improvement in clinical outcomes of real patients.
The PET participated in the standard didactic series in addition to the novel telesimulation intervention described in this study. The standard didactics consist of regular lectures, case presentations, the pediatric advanced life support (PALS) course, and orientation classes on the recognition of a sick child and on the management of common pediatric emergencies, including all types of shock. The telesimulation was a novel intervention in addition to the standard curriculum. The authors wrote two scenarios, one on septic shock and another one on cardiogenic shock, with learning objectives designed to focus on the recognition, differentiation, and first-hour management of shock in pediatric patients.
For our intervention, we created a custom module with multiple patient videos for the Annenberg hotkeys distance learning platform. Annenberg hotkeys were created in the first months of 2020 in response to the COVID-19 pandemic by technologists and filmmakers at the University of Pennsylvania’s Annenberg School for Communication in consultation with physicians from The Children’s Hospital of Philadelphia and the Perelman School of Medicine at the University of Pennsylvania. Annenberg hotkeys (23) was designed to virtualize standardized patient experiences and allow interaction between medical trainees, instructors, and prerecorded patients by allowing a facilitator to rapidly display a number of video responses based on input from learners. The Annenberg hotkeys provides a more adaptive “Choose Your Own Adventure” form of videomaking in which a facilitator can essentially edit a movie on-the-fly from a series of prerecorded clips. This gives the facilitator the ability to let learners solve problems in many ways rather than requiring them to push along a linear path. The lead author (EJ) created videos of real Indian patients using his cellphone camera, with appropriate informed consent. To protect the patient’s privacy, patient videos were off-loaded from the personal cell phone to his official computer in the department and secured. These videos were superimposed on prerecorded video clips of vital sign monitors. Annenberg hotkeys maps keys on a computer keyboard to locally stored video files. These videos will play when a key is pushed. This function works through the remote meeting platforms via a share-screen option. For the telesimulation activities, three facilitators and groups of three to four participants connected via Zoom© (Figure 1). As the learners suggested interventions, the facilitator switched between videos to display the results of those interventions. For example, when a participant saw a video of an ill patient on the screen and asked the team to perform interventions such as monitor placement, vital signs, venous access, labs, and fluid and antibiotic administration, the facilitator responded directly to each of their requests by clicking the hotkey (23) linked to the specific premade video portraying the task (Figures 2, 3).
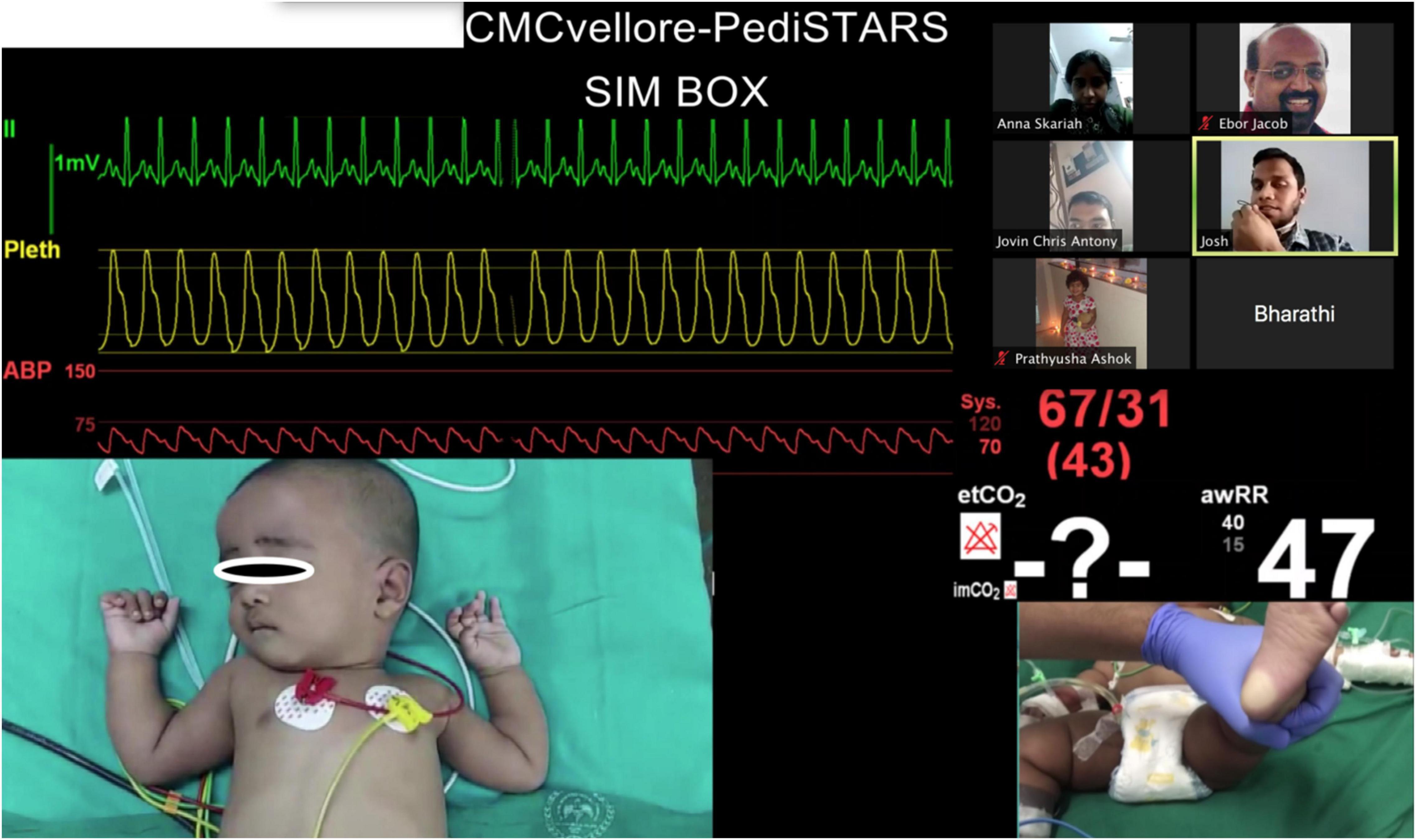
Figure 2. Telesimulation session showing pictures of real patient video clips with participants and facilitators meeting on the video conferencing platform Zoom©.
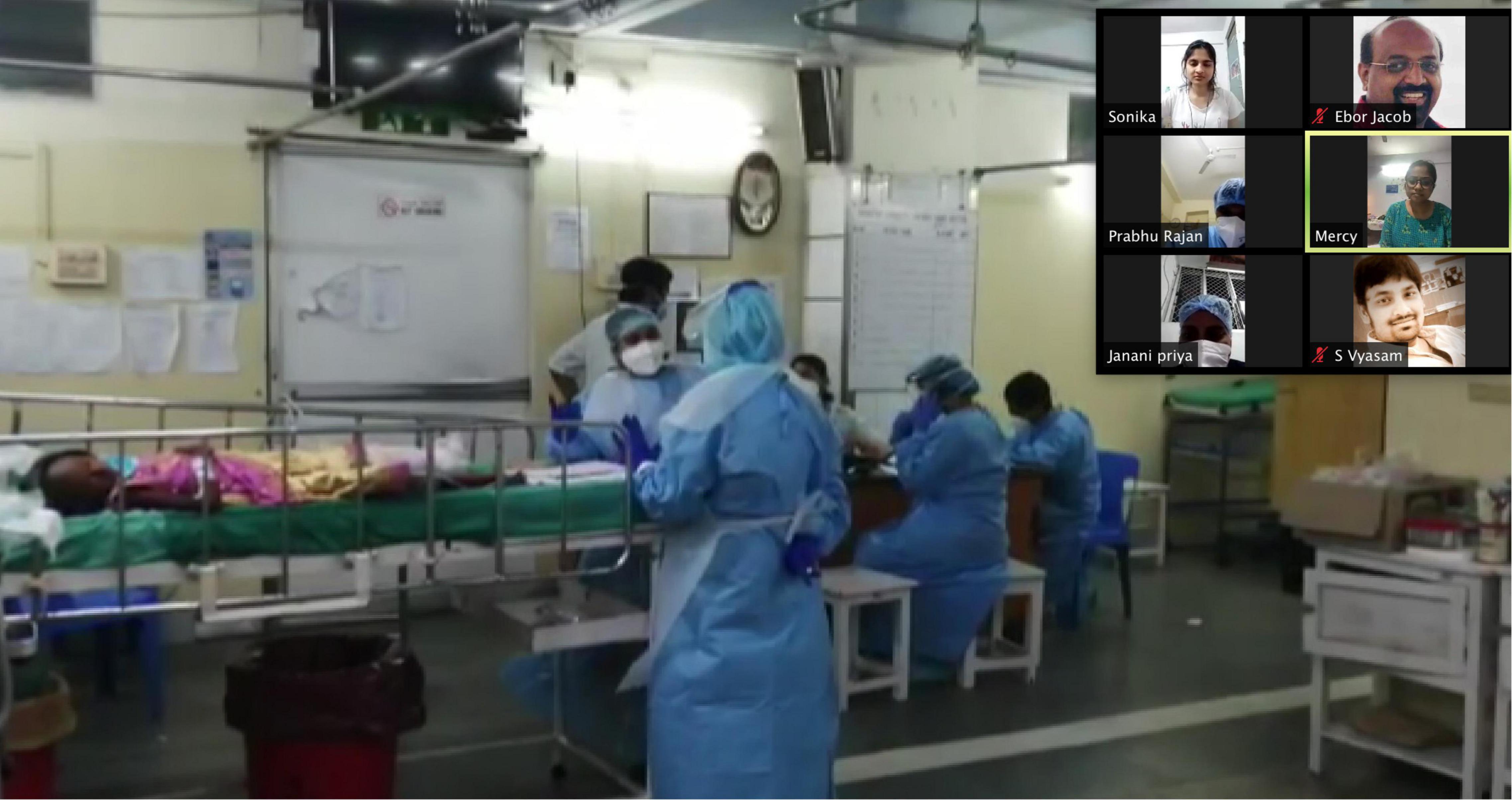
Figure 3. Telesimulation session showing video of a real healthcare teams in the emergency room responding to a patient with shock.
The PET participants included general pediatric and emergency medicine residents, fellows, and nurses working in the emergency room (ER) during the study period. Patients were eligible for the study if they were between the ages of 28 days and 15 years, as per the age cutoff for the pediatric ER in the hospital, and were presenting with clinical features of shock. Healthcare provider participants, patients, or families who did not give informed consent were excluded from the study.
We used a quasi-experimental interrupted time series model for our study (Figure 4). The study was approved by the Institutional Review Board of Christian Medical College (CMC), Vellore, India.
A total of 76 participants were recruited via convenience sampling at the local hospital, CMC, Vellore. The 9-month study period (August 2020–April 2021) was divided into three phases (Figure 4).
• Pre-intervention phase (14 weeks)—August 2020 to November 2020.
• Intervention phase (14 weeks)—November 2020 to February 2021.
• Post-intervention phase (11 weeks)—February 2021 to April 2021.
We used the standard PALS criteria to identify and classify shock. We identified patients in both compensated and hypotensive phases of shock. Patients were categorized to have compensated shock if they met the following criteria and maintained systolic blood pressure more than the age-specific 5th percentile using an automated electronic non-invasive blood pressure monitor (oscillometry) with age-appropriate cuffs.
(1) Heart rate higher than the upper limit of the age-specific normal (Supplementary Table 1), measured by an electronic monitor with adhesive electrodes.
(2) Capillary refill time >2 s, tested by applying moderate pressure to the palm, sole or finger for 5 s and measuring the time taken to return color in seconds.
(3) Cold peripheral extremities.
We defined hypotensive shock as a systolic blood pressure less than the age-specific 5th percentile. We also defined altered sensorium as a Glasgow Coma Scale (GCS) <13. Shock patients were categorized into hypovolemic, distributive, cardiogenic, and obstructive types by the PET, which was later confirmed by the investigators based on the initial clinical history, signs, and symptoms by reviewing the medical records.
Hemodynamic stabilization of the patient at the end of the first-hour management was prospectively defined if all of the following were achieved: (1) a decrease in heart rate by 5%, (2) age-specific normalization of systolic blood pressure, (3) normalization of the capillary refill time, and (4) normalization or improvement of GCS by >2 compared to baseline.
We defined multi-organ dysfunction in accordance with the international pediatric sepsis consensus conference in 2005 (24).
To assess the participant’s performance in the study, we devised a critical intervention checklist consisting of the tasks that were required to be completed by the medical team, including 8 time-critical steps (refer to Supplementary Table 4). We analyzed leadership skills and team performance using the modified CALM tool with a minimum score of 13 (worst) and a maximum score of 53 (best) (25) (Supplementary Figure 1).
During the intervention phase, 40 telesimulation sessions were conducted. Sessions were conducted via the secured video telecommunication platform Zoom© (Zoom Video Communications, San Jose, CA, United States), with all participants and facilitators remotely connecting (no two people were together in the same room for the training). Three trained facilitators (two physicians and one nurse) conducted telesimulation sessions. The lead investigator (EJ) trained the other two facilitators in a standardized way to facilitate the telesimulation. Facilitation rehearsal was conducted with meticulous planning to ensure psychological safety and to maximize learners’ interaction during debriefing (26, 27). One investigator (SV) completed observer checklists during the simulation in real time, with secondary confirmation by review of recorded sessions by the second investigator (EJ). Both of the investigators used the same checklists for the time-critical steps in the management of shock and modified CALM leadership tool.
All 76 participants participated in the telesimulation sessions two times. Each shock scenario session lasted an hour, comprised of a 10-min pre-brief, 20-min scenario, and 30-min debrief. To encourage active participation, each learner was given a role which they normally perform in real patient care. The chat box was used by other learners when one of them was talking on the Zoom© to avoid noise from too many voices. The debrief was designed to promote active reflection by the learners by encouraging plus delta by the participants followed by advocacy inquiry by the facilitators to close the gaps in performance (26). Any deficit in skills was addressed by sharing training videos on Zoom©, such as intraosseous line insertion and push–pull technique for fluid resuscitation. At the end of the session, learning points were summarized and each participant was asked to share thoughts on changes they would incorporate while caring for real patients.
As telesimulation is new to the participants, the lead investigator (EJ) conducted a 30-min virtual orientation in large groups to the participants to familiarize with the Zoom© platform and the telesimulation methodology just before the intervention phase. Simulation training consisted of small group sessions of 3–4 participants, which included pediatric residents (year 1–3), ER residents (year 1), ER fellow, and nurses working exclusively in the resuscitation bay of the ER. During the intervention period, every ER resident, general pediatric resident, ER fellows, and all nurses in the pediatric resuscitation bay participated in two telesimulation sessions on pediatric shock. Learners remained in the same teams for both sessions. The first-year pediatric and ER residents received a standard didactic educational orientation on common pediatric emergencies, including shock, during their first month of the academic year as a part of their orientation. In addition, they participated in regular lectures and bedside case discussions throughout the year.
Data were collected during all three phases of the study period. The pediatric intensive care unit (PICU) research nurse was alerted via pager by the pediatric emergency triage nurses when shock was diagnosed in a child according to the operational definitions and diagnostic criteria (Figure 5). These PICU research nurses work in shifts in the ER. They were trained in a standardized fashion to observe the performance of the team during the first-hour management and leadership skills using the modified CALM tool. Research nurses documented the team’s performance metrics using the critical intervention checklist in real time. They assessed the hemodynamic stability of children with shock during the first-hour management by collecting hemodynamic parameters in patients at regular intervals (0, 15, 30, and 60 min) during the study period. Inter-rater reliability testing was not done for these trained observers. They collected data using the same standardized checklist used for the simulated sessions (Supplementary Table 4). Later, the medical records of study patients were reviewed to note the time of shock resolution and development of MODS. The patient data, including the clinical features of shock, were recorded hourly in the ER medical records. Those children who had persistent features of shock and/or required vasoactive agents were admitted to the high dependency unit (HDU) or PICU where vital parameters were monitored and recorded hourly.
We analyzed critical intervention checklists completed by the research nurses for patients diagnosed with all types of shock during the 9 months study period (pre, during, and post intervention).
In simulation assessments, the primary outcome of interest was the percent completion of tasks by the PET. The secondary outcome of interest was team leadership skills using the modified CALM tool. Both were analyzed for each team and compared between the two simulation sessions and plotted on a graph using the box and whisker plot (Figures 6, 7).
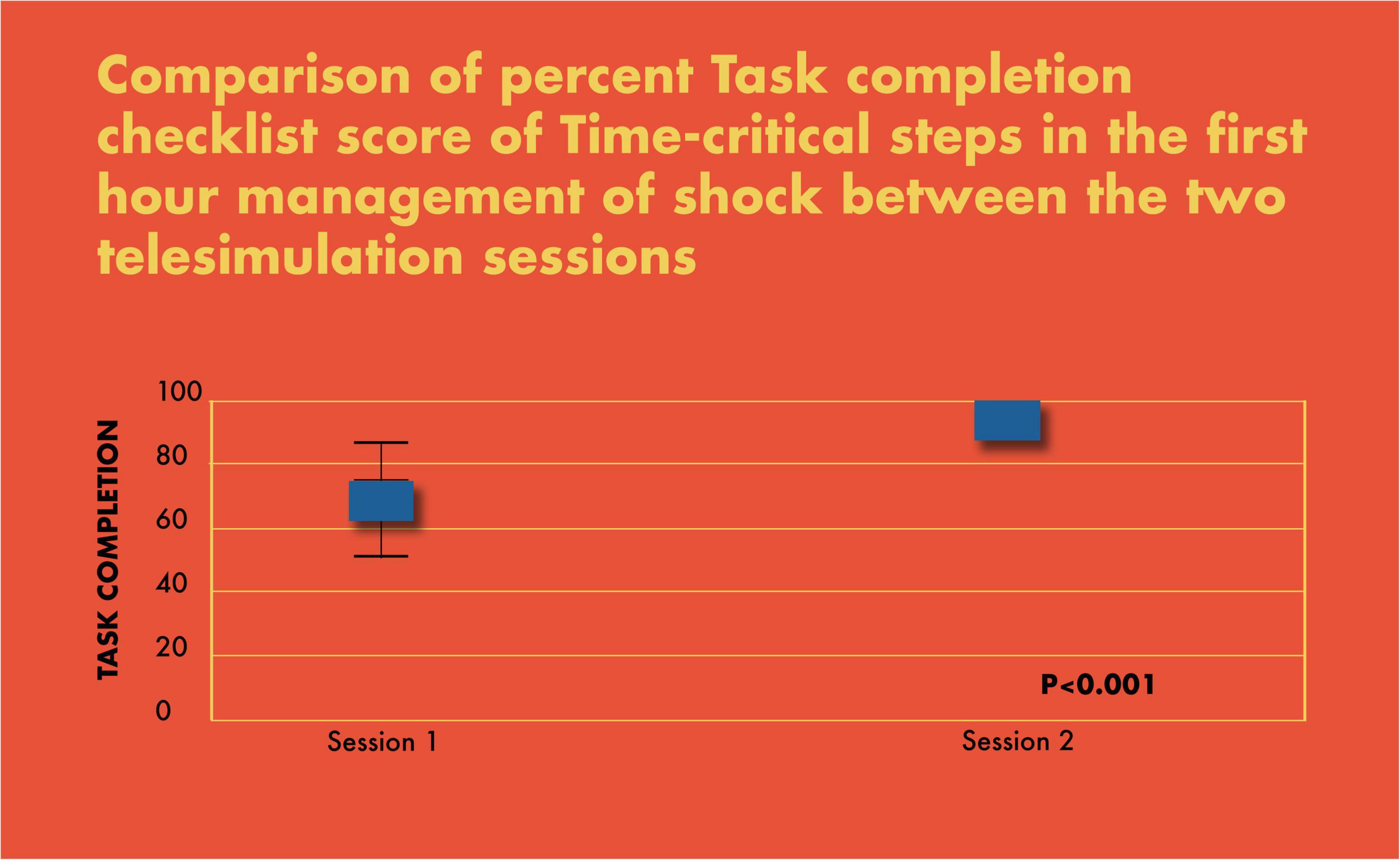
Figure 6. Comparison of percent task completion checklist score of time-critical steps in the first-hour management of shock between the two telesimulation sessions.
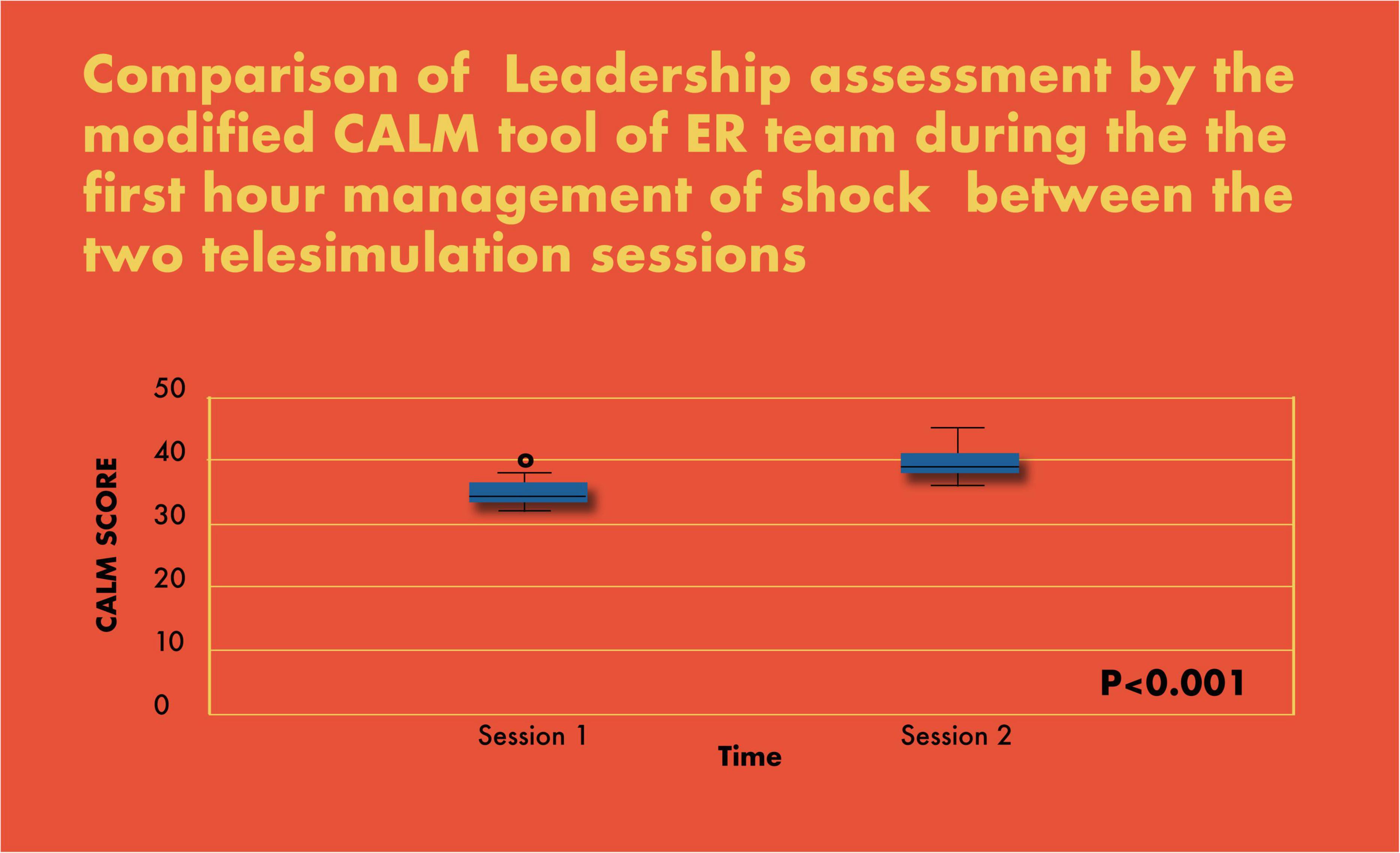
Figure 7. Comparison of leadership assessment by the modified CALM tool of ER team during the first-hour management of shock between the two telesimulation sessions.
In real patient events, the primary outcome of interest was percent completion of tasks and the prospectively defined hemodynamic stability of the patient at the end of the first hour of management. Secondary outcomes included time to reversal of shock, development of MODS, and team leadership skills. Descriptive statistics for continuous variables were reported using mean ± standard deviation (SD). The Shapiro–Wilk and Kolmogorov–Simonov tests were done to see the distribution of variables. Skewed variables were reported using the median and interquartile range (IQR). Categorical variables were reported using frequency and percentage. Association was reported using chi-square or Fisher’s exact test. A comparison of means was reported using one-way ANOVA. A comparison of pattern/trend over time with respect to the baseline was reported using generalized estimating equations (GEE).
All the 76 members of the PET which included 56 physicians comprising of all general pediatric residents (n = 40, year 1–3), ER residents (n = 10, year 1), ER fellows (n = 6), and all nurses (n = 20) participated in the study (Table 1).
During the study period, 332 patients under 15 years of age with shock presented to the ER (Table 2). There were 190 out of 332 (57%) boys and 142 out of 332 (43%) girls. Of these patients with shock, 238 out of 332 (72%) were diagnosed with septic shock, 64 out of 332 (19%) with hypovolemic shock, 28 out of 332 (8%) with cardiogenic shock, and 2 out of 332 (1%) with anaphylactic shock.
There was a significant improvement in the completion of tasks by the PET in the simulated scenarios (69% in scenario 1 vs. 93% in scenario 2; p < 0.001) (Figure 6). Similarly, there was a statistically significant increase in CALM leadership scores between the first and second simulation scenarios, p < 0.001 (Figure 7).
There was a significant improvement in the first-hour hemodynamic parameters of shock patients: pre (71%), during (79%), and post (87%) intervention (p < 0.007 pre vs. post) (Figure 8).
In real patient cases, completion of tasks as per time-critical steps reached 100% both during and post intervention compared to the pre-intervention phase (87.5%), p < 0.05 (Figure 9). The median-modified CALM score for leadership assessment increased after the intervention period: premedian 38, IQR (34–40); during median 38, IQR (36–39); postmedian 40, IQR (39–40); p < 0.05 for pre vs. post (Figure 10). Shock reversal time was reduced from 24 h (pre) to 6 h (during), and to 4.5 h (post), p < 0.002 for pre vs. post (Figure 11). These findings of clinical outcomes in real patients with shock are summarized in Table 3. There was a statistically significant decrease in the development of MODS in patients; 34% (pre), 21% (during), and 20% (post), p = 0.025 for pre vs. post (Figure 12). The survival to discharge was not different among three study phases, p = 0.71, pre vs. post.
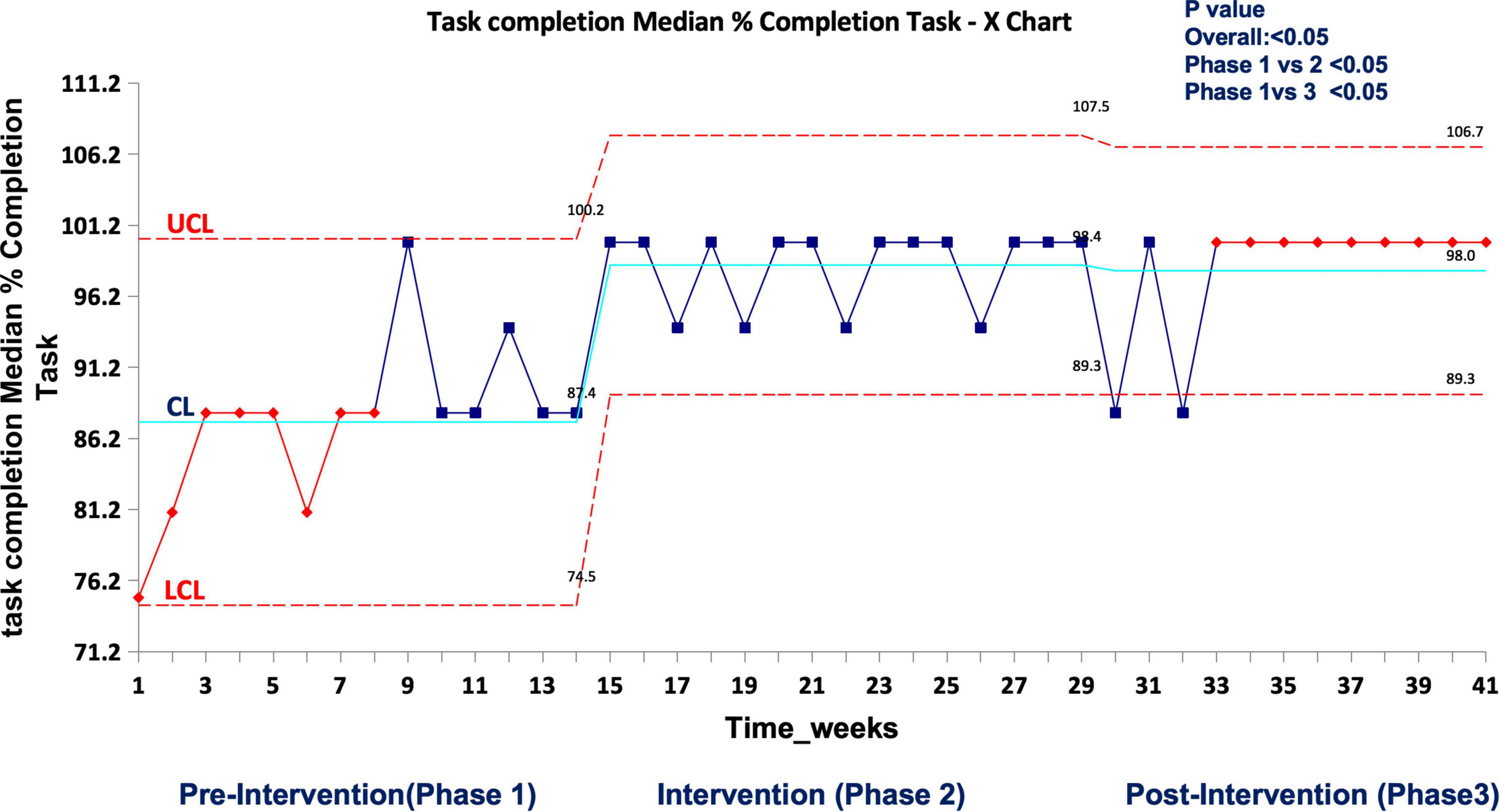
Figure 9. Median percent completion of task as per checklist during the first-hour management of shock in real patient events in the emergency room. UCL, upper control limit; CL, center line; LCL, lower control limit.
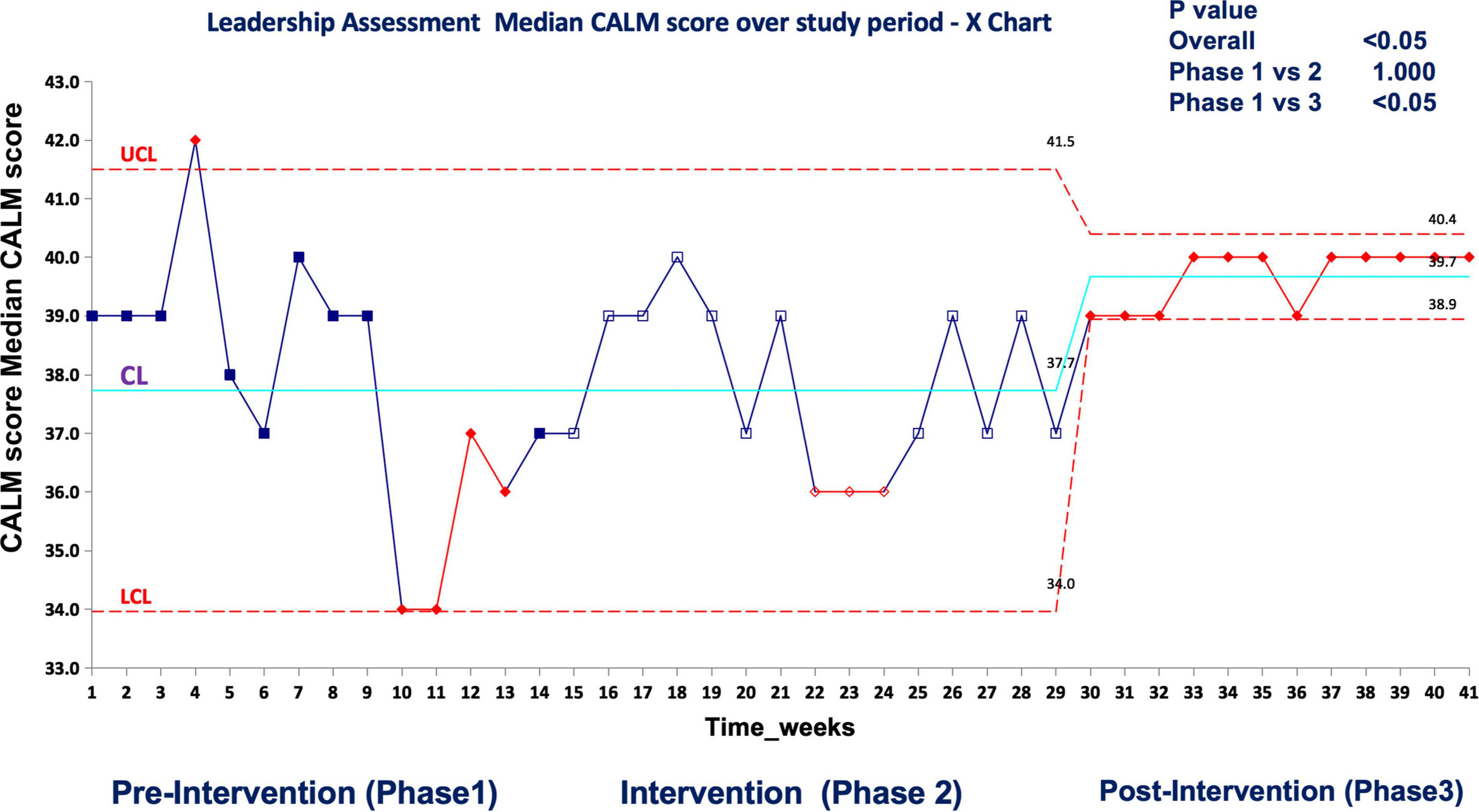
Figure 10. Median CALM score (leadership assessment) during the first-hour management of shock in real patient events in the emergency room UCL, upper control limit; CL, center line; LCL, lower control limit.
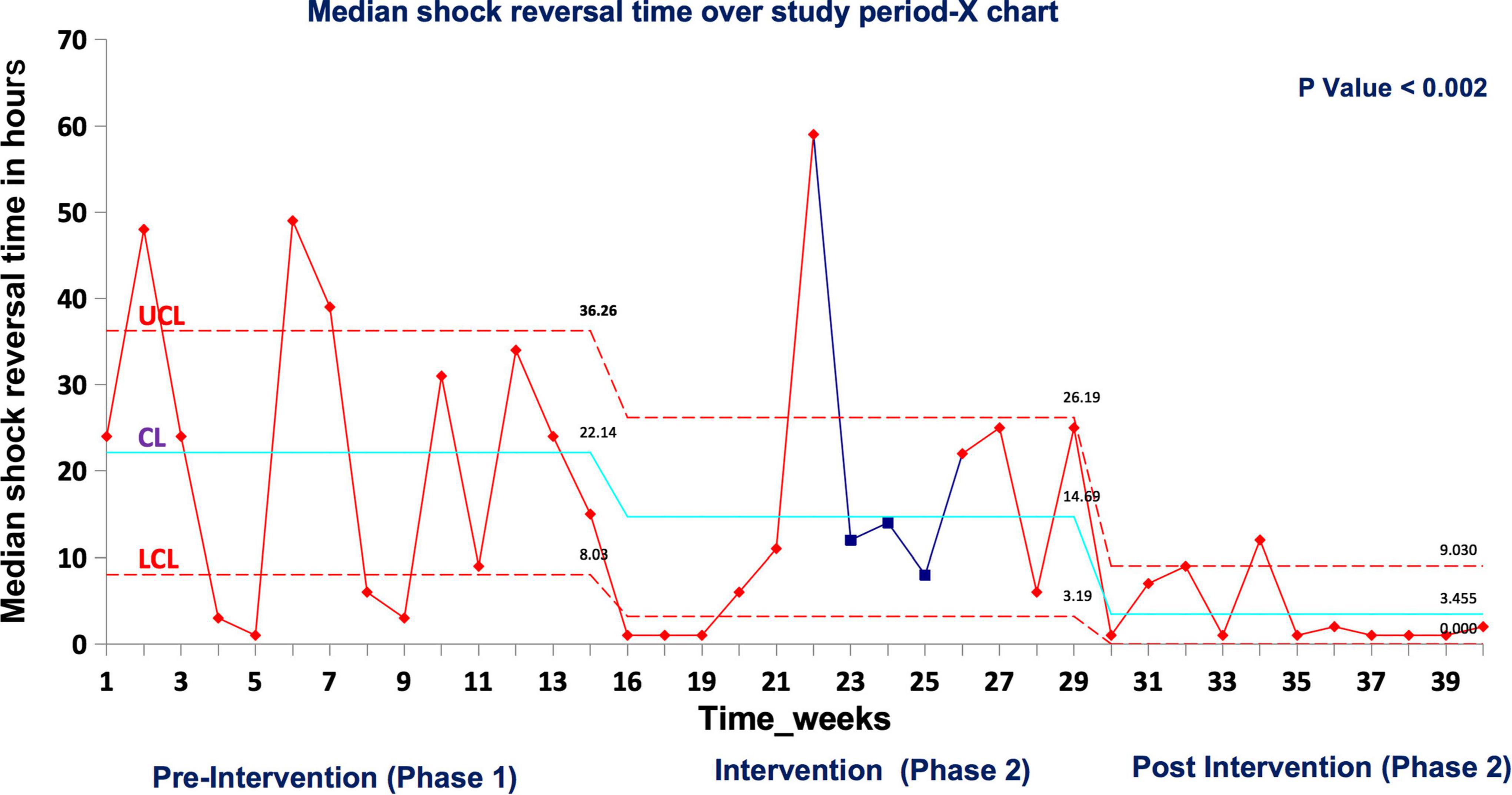
Figure 11. Median shock reversal time in real patient events UCL, upper control limit; CL, center line; LCL, lower control limit.
Our study assessed the impact of telesimulation-based training of PET on time-critical management of the first hour of shock, team leadership in both simulated and real-world contexts, and real patient outcomes in the ER. We showed a statistically significant improvement in the completion of the critical tasks by the team in the first hour during the intervention and post-intervention phases when compared to the pre-intervention phase, in both simulated and clinical environments. Most importantly, we demonstrated remarkable improvement in the hemodynamic stability, faster shock reversal time, decreasing MODS, and improved team leadership in real patients over the course of our study period. There are many barriers to quality simulation-based medical education, including resources, time, and geography. We propose that this low-cost telesimulation approach has the potential to successfully train healthcare teams, including in low- and middle-income countries, and ultimately improve real patient outcomes.
For decades, on-site simulation-based education (SBE), where all learners and facilitators are together, has demonstrated positive outcomes in healthcare (28, 29). However, few studies have been conducted to assess the effectiveness of telesimulation in patient care. In addition, access to on-site simulation can be difficult and costly in low- and middle-income countries, especially in rural and remote areas. The use of varying telesimulation methods has grown exponentially to meet demand during the COVID-19 pandemic. Studies have proven that telesimulation is feasible to do, and learners and facilitators perceive it to be an effective learning experience (30–34). However, to the best of our knowledge, no studies have assessed the impact of telesimulation on patient outcomes (Kirkpatrick levels 3 and 4) (35). Our study not only improved time-critical management during simulation but also improved the management and outcome of patients. This is very encouraging because similar low-cost telesimulation projects targeting other clinical interventions could be widely implemented in resource limited settings to improve patient care.
The importance of the first-hour management of shock, including recognition and treatment, has been proven to decrease mortality and influence patient outcomes (36, 37). We had planned our on-site simulation training before the COVID-19 pandemic. We adapted to pandemic restrictions by seeking international collaboration to develop an alternative approach to conduct simulation. Despite the challenge, our pandemic-proof intervention leveraging telesimulation with new Annenberg hotkey technology turned out to be highly effective in improving processes of care and outcomes for children in the ER.
It was difficult to identify patients in shock to videotape with parental consent. Despite this, we were able to find several patients and families willing to participate. During the intervention phase, it required tremendous coordination effort to ensure that the participating team consisted of multidisciplinary learners and the same team returned to the second scenario. Training the PICU research nurses for data collection on clinical shock cases and ensuring their availability around the clock needed a high level of collaboration with the medical and nursing leadership team.
The findings of this study must be interpreted with consideration of certain limitations. This study was conducted over 9 months with data collected pre, during, and immediately post-simulation intervention. During the 14 weeks of the intervention period, a total of 40 telesimulations were conducted. We cannot make an inference about what dose and frequency of training is necessary and whether it is feasible to create long-lasting, sustainable results. We had to choose a pragmatic dose and frequency of training for this study. Additionally, trained research nurses recorded the clinical interventions in real time. They were trained in data collection, especially to observe the preselected time-critical steps, leadership skills (modified CALM tool), and record hemodynamic parameters of the real patients. We did not assess their inter-rater reliability, which may have an impact on the consistency of data collection. Although these research nurses were present in the resuscitation bay during all emergencies, it is possible that the presence of the research nurse triggered the PET to remember shock and improve their clinically relevant reaction times (i.e., Hawthorne effect). Therefore, it is hard to infer if the same teams would have been this quick to react in the absence of an active observer. Nevertheless, the research team’s presence in our ER is not new. The research nurses have regularly collected data on several ongoing studies for many years, and the PET are used to their presence. We do expect that there may be a chance of some improvement in the CALM score with the ongoing clinical experience of the team members.
This is a single-center study in a tertiary pediatric academic hospital in India, conducted by investigators who are experienced simulation educators. It is yet to be explored if our results would be reproducible if conducted in different resource-limited settings.
Future work will include expanding our telesimulation training to reach providers working in the ten remote mission hospitals, which are part of the CMC hospital referral network across India. We are in the process of conducting a needs assessment to create new emergency scenarios commonly seen at primary care hospitals. More work is needed to demonstrate dose, frequency, and type of education on different levels of learners in different clinical settings. Our first step is to identify local champions at the remote mission hospitals to train as telesimulation facilitators and conduct their own drills for their healthcare teams. This would be the next step to evaluate if novice and newly trained facilitators can achieve similar results with both simulation and real patient outcomes in remote settings.
Our study has demonstrated that telesimulation using prerecorded video clips with innovative hotkey functions through multicenter collaboration is an innovative, low-cost solution in response to pandemic restrictions in resource-limited settings. Telesimulation training was feasible and improved time-critical interventions, leadership in both simulated and real patients, and clinical outcomes in real patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Institutional Review Board, Christian Medical College Hospital, Vellore, India. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
EJ, SVy, ES, GRa, and VN contributed to curriculum development, faculty rehearsal, and ongoing support for training. KC contributed to creating hotkeys. EJ created and edited real patient videos to make customized telesimulation scenarios. EJ and SVy conducted all telesimulation sessions. GRe supported with statistical analysis. DDA supported with the logistics of the Pediatric Emergency Team and in organizing simulation sessions. SVe contributed to the first draft of the manuscript. ED and KC helped with graphics and figures. All authors contributed and provided critical revisions, and approved the current version.
The article publishing fee was supported by the University of Pennsylvania Institute for the Advanced Study of India (“UPIASI”) through the CSR grant from Infinite Computer Solutions and the Children’s Hospital of Philadelphia Endowed chair. Fluid Research Grant by Institutional Review Board, CMC, Vellore supported fees for professional video editor and Zoom© platform.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We are grateful to Marc Auerbach MD, FAAP, M.Sc. from Yale School of Medicine, United States, for his guidance to use concept from ACEP TeleSimBox, Lilly Prasad, Nurse Manager, CMC, Vellore, for her support and co-facilitating some of the telesimulation sessions, Leni G. Mathew MD, DCH, MRCPCH, Professor and Head of the Department of Pediatrics, Sathish Kumar T MD, DCH, Professor and Head, Pediatrics Unit-2, and Sneha Varkki DNB (peds.), DCH, Professor and Head, Pediatrics Unit-3 for permitting pediatric residents to participate in the study and to attend the telesimulation training sessions. The Nursing Superintendent, CMC, Vellore for permitting the nurses to take part in the study. We acknowledge the efforts of PICU research team, CMC, Vellore for meticulously collecting the data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.904846/full#supplementary-material
1. Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in the epidemiology of pediatric severe sepsis. Pediatr Crit Care Med. (2013) 14:686–93. doi: 10.1097/PCC.0b013e3182917fad
2. Ruth A, McCracken CE, Fortenberry JD, Hall M, Simon HK, Hebbar KB. Pediatric severe sepsis: current trends and outcomes from the pediatric health information systems database. Pediatr Crit Care Med. (2014) 15:828–38. doi: 10.1097/PCC.0000000000000254
3. Institute of Medicine. Emergency Care for Children: Growing Pains. Washington, DC: The National Academies Press (2007).
4. Remick K, Gausche-Hill M, Joseph MM, Brown K, Snow SK, Wright JL, et al. Pediatric readiness in the emergency department. Pediatrics. (2018) 142:e20182459.
5. Viana ME, Valete CO, Sgorlon G, Vieira JA, Currais JC, Martins MP, et al. An international perspective on the treatment of pediatric shock: the Brazilian experience. New Horiz. (1998) 6:226–34.
6. Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. (2020) 21:e52–106.
7. Kessler DO, Walsh B, Whitfill T, Dudas RA, Gangadharan S, Gawel M, et al. Disparities in adherence to pediatric sepsis guidelines across a spectrum of emergency departments: a multicenter, cross-sectional observational in situ simulation study. J Emerg Med. (2016) 50:403.e–15.e. doi: 10.1016/j.jemermed.2015.08.004
8. Manhas DS, Anderson JM. Educational perspectives: telesimulation in neonatal resuscitation. NeoReviews. (2014) 15:e514–7.
9. Ohta K, Kurosawa H, Shiima Y, Ikeyama T, Scott J, Hayes S, et al. The effectiveness of remote facilitation in simulation-based pediatric resuscitation training for medical students. Pediatr Emerg Care. (2017) 33:564–9. doi: 10.1097/PEC.0000000000000752
10. McCoy CE, Sayegh J, Alrabah R, Yarris LM. Telesimulation: an innovative tool for health professions education. AEM Educ Train. (2017) 1:132–6. doi: 10.1002/aet2.10015
11. Henao O, Escallón J, Green J, Farcas M, Sierra JM, Sánchez W, et al. Fundamentals of laparoscopic surgery in Colombia using telesimulation: an effective educational tool for distance learning. Biomedica. (2013) 33:107–14. doi: 10.1590/S0120-41572013000100013
12. Burckett-St Laurent DA, Cunningham MS, Abbas S, Chan VW, Okrainec A, Niazi AU. Teaching ultrasound-guided regional anesthesia remotely: a feasibility study. Acta Anaesthesiol Scand. (2016) 60:995–1002. doi: 10.1111/aas.12695
13. von Lubitz DK, Carrasco B, Gabbrielli F, Ludwig T, Levine H, Patricelli F, et al. Transatlantic medical education: preliminary data on distance-based high-fidelity human patient simulation training. Stud Health Technol Inform. (2003) 94:379–85.
14. Laurent DAB, Niazi AU, Cunningham MS, Jaeger M, Abbas S, McVicar J, et al. A valid and reliable assessment tool for remote simulation-based ultrasound-guided regional anesthesia. Reg Anesth Pain Med. (2014) 39:496–501. doi: 10.1097/AAP.0000000000000165
15. Shao M, Kashyap R, Niven A, Barwise A, Garcia-Arguello L, Suzuki R, et al. Feasibility of an international remote simulation training program in critical care delivery: a pilot study. Mayo Clin Proc Innov Qual Outcomes. (2018) 2:229–33. doi: 10.1016/j.mayocpiqo.2018.06.008
16. The Terminology and Concepts Working Group. Healthcare Simulation Dictionary – AHRQ Publication No. 20-0019. 2nd ed. Rockville, MD: Agency for Healthcare Research and Quality (2020).
17. Auerbach M. ACEP SimBox. (2022). Available online at: https://www.acepsim.com/ (accessed April 15, 2022).
18. Sanseau E, Lavoie M, Tay K, Good G, Tsao S, Burns R, et al. TeleSimBox: a perceived effective alternative for experiential learning for medical student education with social distancing requirements. AEM Educ Train. (2021) 5:e10590. doi: 10.1002/aet2.10590
19. Sanseau E, Sooby RC, Kou M, Auerbach M, Tay K. How to use TeleSimBox “off the shelf” to connect remote content experts with in-person simulation participants. Cureus. (2021) 13:e16317. doi: 10.7759/cureus.16317
20. Montgomery EE, Thomas A, Abulebda K, Sanseau E, Pearson K, Chipman M, et al. Development and implementation of a pediatric telesimulation intervention for nurses in community emergency departments. J Emerg Nurs. (2021) 47:818.e–23.e. doi: 10.1016/j.jen.2021.01.013
21. Vora S, Li J, Kou M, Ng V, Price A, Claudius I, et al. ACEP SimBox: a pediatric simulation-based training innovation. Ann Emerg Med. (2021) 78:346–54. doi: 10.1016/j.annemergmed.2021.03.040
22. Schlapbach LJ, Straney L, Alexander J, MacLaren G, Festa M, Schibler A, et al. Mortality related to invasive infections, sepsis, and septic shock in critically ill children in Australia and New Zealand, 2002–13: a multicenter retrospective cohort study. Lancet Infect Dis. (2015) 15:46–54. doi: 10.1016/S1473-3099(14)71003-5
23. hotkey. In: Merriam-Webster. (n.d.). Available online at: https://www.merriam-webster.com/dictionary/hot%20key (accessed May 25, 2022).
24. Goldstein B. International consensus conference on pediatric sepsis. international pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. (2005) 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6
25. Nadkarni LD, Roskind CG, Auerbach MA, Calhoun AW, Adler MD, Kessler DO. The development and validation of a concise instrument for formative assessment of team leader performance during simulated pediatric resuscitations. Simul Healthc. (2018) 13:77–82. doi: 10.1097/SIH.0000000000000267
26. Cheng A, Eppich W, Epps C, Kolbe M, Meguerdichian M, Grant V. Embracing informed learner self-assessment during debriefing: the art of plus-delta. Adv Simul. (2021) 6:22. doi: 10.1186/s41077-021-00173-1
27. Cheng A, Kolbe M, Grant V, Eller S, Hales R, Symon B, et al. A practical guide to virtual debriefings: communities of inquiry perspective. Adv Simul. (2020) 5:1–9. doi: 10.1186/s41077-020-00141-1
28. Stone K, Reid J, Caglar D, Christensen A, Strelitz B, Zhou L, et al. Increasing pediatric resident simulated resuscitation performance: a standardized simulation-based curriculum. Resuscitation. (2014) 85:1099–105. doi: 10.1016/j.resuscitation.2014.05.005
29. Cheng A, Lang TR, Starr SR, Pusic M, Cook DA. Technology-enhanced simulation and pediatric education: a meta-analysis. Pediatrics. (2014) 133:e1313–23. doi: 10.1542/peds.2013-2139
30. Duff J, Kardong-Edgren S, Chang TP, Elkin RL, Ramachandra G, Stapleton S, et al. Closing the gap: a call for a common blueprint for remote distance telesimulation. BMJ Simul Technol Enhanc Learn. (2021) 7:185–7. doi: 10.1136/bmjstel-2021-000875
31. Balmaks R, Auzina L, Gross IT. Remote rapid cycle deliberate practice simulation training during the COVID-19 pandemic. BMJ Simul Technol Enhanc Learn. (2020) 7:176–7. doi: 10.1136/bmjstel-2020-000671
32. Jewer J, Parsons MH, Dunne C, Smith A, Dubrowski A. Evaluation of a mobile telesimulation unit to train rural and remote practitioners on high-acuity low-occurrence procedures: pilot randomized controlled trial. J Med Internet Res. (2019) 21:e14587. doi: 10.2196/14587
33. Ikeyama T, Shimizu N, Ohta K. Low-cost and ready-to-go remote-facilitated simulation-based learning. Simul Healthc. (2012) 7:35–9. doi: 10.1097/SIH.0b013e31822eacae
34. Okrainec A, Vassiliou M, Kapoor A, Pitzul K, Henao O, Kaneva P, et al. Feasibility of remote administration of the fundamentals of laparoscopic surgery (FLS) skills test. Surg Endosc. (2013) 27:4033–7.
35. Kirkpatrick DL, Kirkpatrick JD. Evaluating Training Programs: The Four Levels. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers (2006).
36. Lane RD, Funai T, Reeder R, Larsen GY. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics. (2016) 138:e20154153. doi: 10.1542/peds.2015-4153
37. Oliveira CF, Nogueira de Sá FR, Oliveira DS, Gottschald AF, Moura JD, Shibata AR, et al. Time-and fluid-sensitive resuscitation for hemodynamic support of children in septic shock: barriers to the implementation of the American college of critical care medicine/pediatric advanced life support guidelines in a pediatric intensive care unit in a developing world. Pediatr Emerg Care. (2008) 24:810–5. doi: 10.1097/PEC.0b013e31818e9f3a
Keywords: telesimulation, simulation-based education, COVID-19 educational innovations, hotkeys, septic shock
Citation: James EJG, Vyasam S, Venkatachalam S, Sanseau E, Cassidy K, Ramachandra G, Rebekah G, Adhikari DD, Deutsch E, Nishisaki A and Nadkarni VM (2022) Low-Cost “Telesimulation” Training Improves Real Patient Pediatric Shock Outcomes in India. Front. Pediatr. 10:904846. doi: 10.3389/fped.2022.904846
Received: 26 March 2022; Accepted: 30 May 2022;
Published: 26 July 2022.
Edited by:
Avihu Z. Gazit, Washington University in St. Louis, United StatesReviewed by:
Kenneth E. Remy, Case Western Reserve University, United StatesCopyright © 2022 James, Vyasam, Venkatachalam, Sanseau, Cassidy, Ramachandra, Rebekah, Adhikari, Deutsch, Nishisaki and Nadkarni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ebor Jacob G. James, ZWJvcmphY29iQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.