- 1General and Thoracic Pediatric Surgery Unit, Bambino Gesù Children's Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 2Pathology Unit, Department of Laboratories, Bambino Gesù Children's Hospital, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
Cystic dysplasia of the rete testis (CDRT) is a rare cause of testicular masses in children. The pathogenesis of this malformation remains unclear. It is often associated with other genitourinary anomalies, commonly presenting as agenesis or dysplasia of the ipsilateral kidney. A case involving a 9-year-old boy with a testicular lesion and ipsilateral renal agenesis, who was diagnosed with CDRT after histological examination, is reported. In addition, a systematic review of the literature was performed to better understand this pathology to design the most appropriate treatment and follow-up strategy for patients with CDRT.
Introduction
Cystic dysplasia of the rete testis (CDRT) is a rare cause of testicular masses in children. It was first described by Leissring and Oppenheimer in 1973 as a rare benign testicular lesion (1). CDRT is characterized by irregular cystic spaces lined by cuboidal epithelium in the mediastinum or rete testes (2). It is often associated with genitourinary tract anomalies, primarily with renal agenesis (3). This malformation is likely the result of a disorder(s) in the connection between the mesonephric duct and germinal epithelium and represents a diagnostically challenging condition in the pediatric population (1). The purpose of this study was to investigate CDRT in a 9-year-old boy with a right testicular lesion and ipsilateral renal agenesis. Furthermore, we performed a systematic review of the literature to better understand this pathology to design the most appropriate treatment and follow-up strategy for patients with CDRT.
Case Report
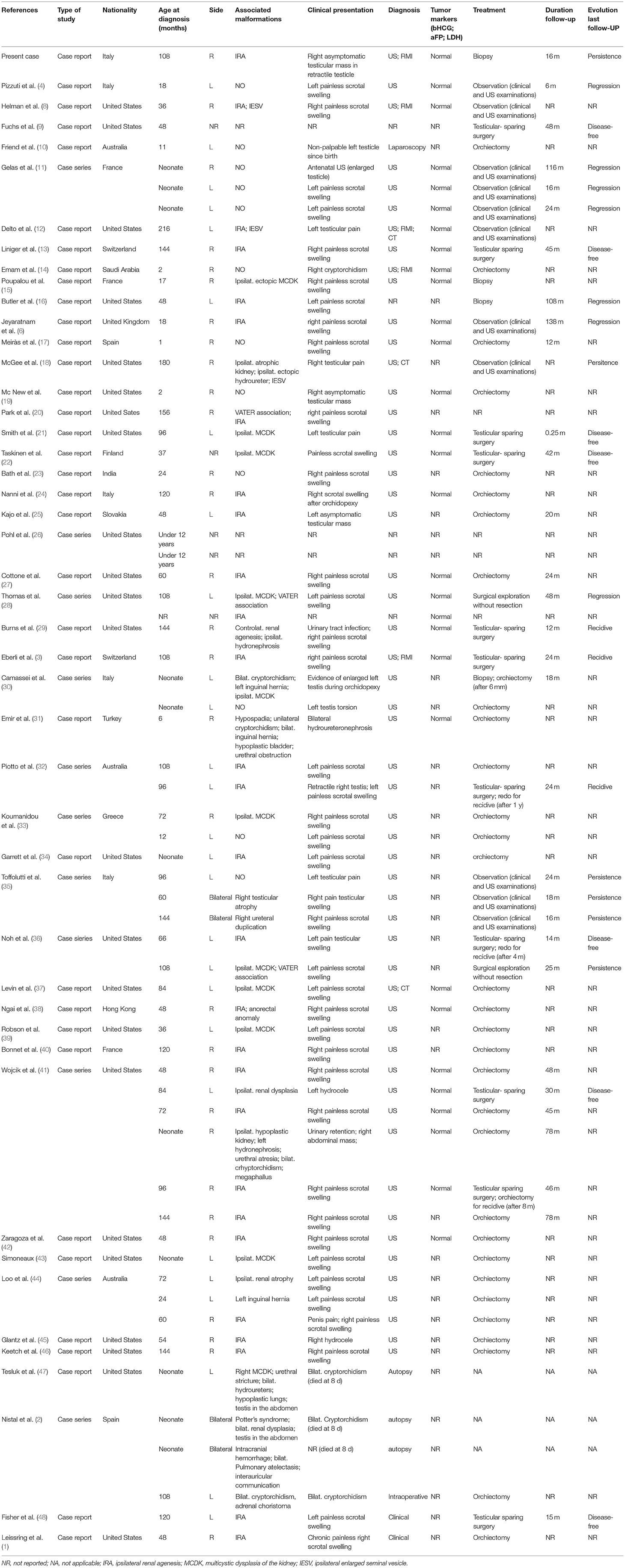
A 9-year-old boy with a right retractile testicle underwent testicular ultrasonography, which revealed a testicular lesion and, accordingly, was referred to the authors' hospital. On physical examination, the testicles were in the scrotum, with normal volume and consistency. The right testicle exhibited a palpable upper pole lesion. Testicular ultrasound examination was repeated, revealing a circumscribed area, measuring 20 × 10 × 9 mm, containing several minuscule cysts of varying sizes in the right mediastinal testis (Figure 1). The lesion did not appear vascularised on color Doppler ultrasound and was surrounded by normal testicular parenchyma; in addition, the left testicle was normal. Screening for associated urinary anomalies was performed using abdominal ultrasound and magnetic resonance imaging (MRI). The right kidney was not visualized on abdominal ultrasound, which suggested right renal agenesis, whereas the left kidney and bladder appeared to be normal. Moreover, MRI confirmed right renal agenesis and revealed enlargement of the right epididymis with an extended area of altered signal (16.5 × 9.5 × 12 mm), hypointensity on T1, and hyperintensity on diffusion-weight imaging sequences, with no contrast uptake and without calcific or fat images. Markers for testicular tumors, including alpha-fetoprotein, human chorionic gonadotropin, and lactate dehydrogenase, were within normal limits. Considering the ultrasound characteristics of the lesion associated with renal agenesis, CDRT was suspected, and a conservative treatment strategy was chosen. The patient was followed-up with periodic clinical and ultrasonographic examinations. Testicular ultrasound performed on the lesion 3 and 5 months later revealed no variations in morphology, size, or structural characteristics. Surgical exploration was performed using the inguinal approach to exclude possible malignancy. Intraoperatively, the testicular parenchyma was mostly substituted with spongiform tissue; accordingly, biopsy of the right testicle and epididymis was performed. Histological examination revealed irregular cystic spaces located in the mediastinum of the testis, displacing the testicular parenchyma. The cysts were lined with flattened cuboidal epithelium and separated by fibrous septa. No germ cell tumors were identified (Figure 2). Histological examination, along with renal agenesis, confirmed the diagnosis of CDRT. Four months after surgery, testicular ultrasound revealed no change in the right testicular lesion. One year later, testicular ultrasound was repeated, and the lesion exhibited the same morphological characteristics, with no internal blood flow, although its dimensions increased to 33 × 12 × 27 mm.
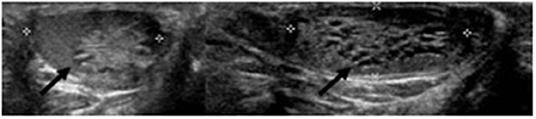
Figure 1. Pre-operative ultrasound of present case. Cysts in the right mediastinum testis (black arrow).
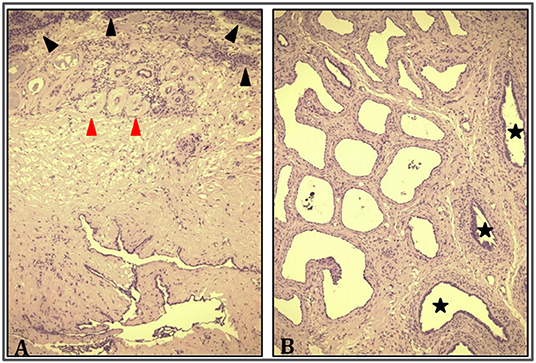
Figure 2. Cystic dysplasia of rete testis. (A) Rete testis consisting of irregularly ectasic spaces lined by cuboidal epithelium and surrounded by fibrous stroma (bottom); testicular parenchyma (top) is characterized by prebuberal (black arrowhead) and atrophic seminiferous tubules (red arrowhead) (HE 10×). (B) Dilatation and irregularity of rete testis was global and reached the epididymis (black stars) (HE 20×).
At the last follow-up-−28 months after surgery—the patient was in good general condition. Testicular ultrasound revealed a lesion with similar dimensions (35 mm × 16 mm × 16 mm), with unchanged parenchymal characteristics. Tumor marker levels were also determined and remained normal. Finally, it was decided to continue ultrasound follow-up every year due to the benign nature of the lesion.
Discussion
CDRT is a rare, benign cause of testicular masses in the pediatric population. The differential diagnosis of CDRT includes other intrascrotal pathologies, including hydrocele, hernia, other benign and malignant testicular masses, and testicular torsion. All cystic or multicystic testicular lesions must be considered, including simple intratesticular cysts, epidermoid cysts, tunica albuginea cysts, testicular teratomas or lymphomas, juvenile granulosa cell tumors, gonadal stromal tumors, and cystic lymphangiomas. Ultrasonography can be used for the initial diagnosis and differentiation of these diseases (4).
CDRT has a histological and sonographic appearance similar to tubular ectasia of the rete testis, a benign polycystic testicular disorder caused by obstruction in the epididymis or efferent ductulus in the adult population. This condition is often bilateral and is usually not associated with urinary abnormalities (5). In addition, CDRT is usually unilateral, with no predominance on either side and a mean age at presentation of approximately of 6 years, as reported by Jeyaratnam et al. (6).
CDRT usually manifests as painless scrotal swelling. It is frequently associated with genitourinary anomalies, ipsilateral renal agenesia, and multicystic dysplasia of the kidney (3). Nevertheless, the exact pathogenesis of CDRT remains unclear. Leissring and Oppenheimer suggested that the lack of connection between the mesonephric duct and germinal epithelium at the level of the rete testis leads to progressive degeneration of the mediastinum testis into small cysts. This hypothesis could also explain urinary tract abnormalities that are frequently associated with CDRT. The mesonephric duct originates from the ureteral bud, which eventually forms the kidney (1). In addition, Nistal et al. proposed another hypothesis involving the over-secretion of fluid in immature seminiferous tubules with no lumen. Spontaneous regression of the cysts could be explained by progressive canalization of the tubules during childhood (2).
Owing to the rarity and importance of CDRT, the aim of the present study was to describe a case of CDRT in a 9-year-old child and to perform a systematic review of the literature on which to base the treatment and follow-up of this pathology. Accordingly, a systematic literature review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (i.e., “PRISMA”) guidelines. Eligible studies included those that investigated CDRT and were published as full-text articles by indexed journals in the Cochrane, MEDLINE via PubMed, Embase, or Scopus databases. Keywords used in the literature search included “cystic dysplasia rete testis” and their MeSH terms in any possible combination. Only articles published in English with available abstracts were included, with no limits on publication date. The reference lists of relevant studies were screened to identify other potentially eligible studies. The search was repeated up to November 30, 2021.
Editorial comments, letters to the editor, studies involving animals, adults, unpublished reports, studies on deceased fetuses, abstracts from scientific meetings, and book chapters were excluded from the review.
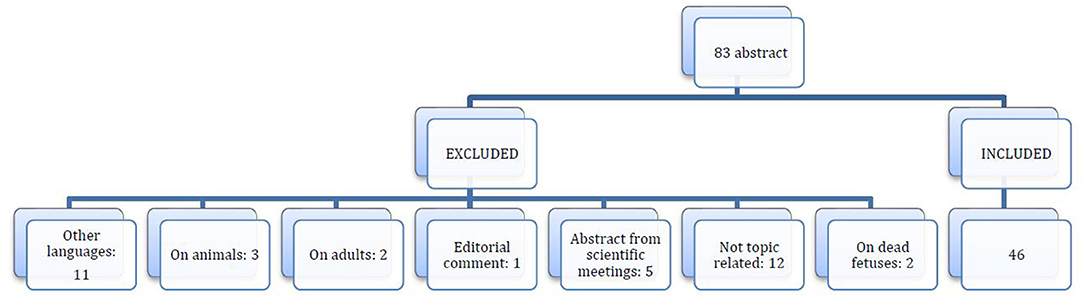
The risk of bias for observational studies was appraised using the methodological index for non-randomized studies (MINORS) (7). Risk of bias was assessed by two reviewers (GC and SF), who extracted data from the included studies. Any discordance was resolved by consensus with the third author (VP). For each study included in the analysis, the following data were extracted: demographic features; number of patients; clinical features; treatment performed; and follow-up. After eliminating duplicates, the initial literature search retrieved 83 potential studies, as shown in the PRISMA flow diagram (Figure 3). After applying the inclusion and exclusion criteria, 46 articles, including 65 patients (66 including our case report), were selected (Table 1).
Results of this study confirmed the many characteristics of this disease and highlighted further interesting aspects.
Data are reported as mean or rate, and were analyzed using GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA; http://www.graphpad.com) for Windows (Microsoft Corporation, Redmond, WA, USA).
The age at presentation ranged from birth to 18 years, with a mean of 5.2 years (62.5 months). Only two cases involving adults, 23 and 63 years of age, respectively, have been reported and were excluded from this study.
CDRT was usually unilateral [n = 62 (93.9%)], while bilateral lesions were reported in only four (6%) patients. In addition, CDRT exhibited no predominance on either side [right, n = 30 (45.5%); and left, n = 27 (41%)]. The most frequent clinical presentation was painless scrotal swelling [n = 40 (60.6%)], followed by penile pain (n = 1), asymptomatic testicular mass [n = 3 (4.5%)], testicular pain [n = 6 (9%)], and testicular torsion [n = 1 (1.5%)]. In one (15%) case, the lesion was suspected after antenatal ultrasound, while in four (6%), the lesion was discovered after diagnostic examinations for an undescended testicle. Two patients with non-palpable testes at birth died of comorbidities, and the diagnosis was made after autopsy. Another patient with testes in the scrotum was born in a tenuous clinical condition and received an autopsy diagnosis (2). Two (5.7%) patients presented with hydrocele, one (1.5%) with bilateral hydroureteronephrosis, and one (1.5%) with urinary retention, with a right abdominal mass for urethral atresia. Clinical presentation was not described in four (6%) patients.
CDRT was frequently associated with urogenital system anomalies [n = 50 (75.8%)], with the most common being ipsilateral renal agenesis [n = 28 (50%)], whereas contralateral renal agenesis was found in only one (1.5%) case. Other anomalies were also reported, including multicystic dysplasia of the kidney [n = 11 (16.6%)], one of which was contralateral, hypoplastic/atrophic kidney [n = 3 (4.5%)], enlarged seminal vesicle [n = 3 (4.5%)], renal dysplasia [n = 2 (3%)], duplication of ureters [n = 2 (3%)], pyeloureteral stenosis [n = 2 (3%)], hydroureter [n = 2 (3%)], urethral stricture [n = 2 (3%)], hypospadia [n = 1 (1.5%)], and megaphallus [n = 1 (1.5%)]. Other malformations associated with CDRT included Vater association [n = 3 (4.5%)], Potter's syndrome [n = 1 (1.5%)], and anorectal malformation [n =1 (1.5%)].
Ultrasound is the gold standard for the diagnosis and follow-up of CDRT. MRI can be used as a complementary examination (14). In our review, testicular ultrasound was used in 54 (81.8%) patients. The description of ultrasound imaging for CDRT was similar in all reported cases. The affected testicle was usually enlarged, with a mass of small cysts (2–8 mm in size) in the rete testis. The surrounding testicular tissue and epididymis were normal but compressed. As an exception, Robson et al. reported a case of CDRT in which ultrasonography revealed a solid lesion (39). When cysts are extremely small, they can appear as echogenic foci, mimicking testicular microlithiasis (3).
Markers of testicular tumors (i.e., alpha-fetoprotein, beta-human chorionic gonadotropin, and lactate dehydrogenase) were normal when tested [n = 30 (45.5%)] in all reported cases of CDRT. In 45 (68%) patients, histological examinations were available and were similar in all cases reported.
Histologically, CDRT is typically characterized by a multicystic lesion primarily located in the mediastinum testis. The cystic spaces were separated by connective tissue lined by cuboidal cells. Cysts usually differ in shape and size (ranging from several millimeters) (3). Moreover, cystic spaces express keratin and vimentin in a cytoplasmic pattern, as well as epithelial membrane antigens, such as that of the ductular epithelium of the mediastinum testis (45).
There are no clear diagnostic criteria for CDRT. Levin et al. suggested that CDRT can be suspected if the lesion is well-circumscribed with normal surrounding parenchyma and is composed of multiple small cysts revealed on ultrasound, if tumor markers are normal, and if there are associated mesonephric anomalies (37). If these criteria are fulfilled, as in the present case, open biopsy of the lesion is not immediately necessary to confirm the diagnosis. No standard treatment has been defined.
Previously, orchiectomy was the treatment of choice (1). Recently, because of better understanding of the benign nature of this pathology, a conservative approach was proposed, such as testicular-sparing surgery or observation (11). Poupalou et al. proposed testicular-sparing surgery for large lesions at diagnosis or enlarging lesions under observation (15). More specifically, we found that in all reported cases of CDRT, 32 (48.5%) were treated with orchiectomy as a definitive treatment. In 11 (16.7%) cases, testicular-sparing surgery with excision of the lesion was performed with preservation of the normal testicular parenchyma. Biopsy was performed in three (4.5%) patients. Only two (3%) patients underwent surgical exploration without biopsy or excision of the lesion. In 11 (16.7%) patients, an observational approach (without biopsy) was adopted, and the patients were monitored with periodic clinical and ultrasonographic follow-up. Follow-up data after orchiectomy were available for only nine cases. No recurrence was reported after a mean follow-up of 41 months. In contrast, of the 11 patients treated using the testicular-sparing approach, five (45.5%) experienced recurrence of the cyst after a median of 12 months, confirming the importance of radical treatment in conservative surgery, maintaining a safety margin between the removed mass and the healthy parenchyma.
Different treatments for recurrence have been used. The testicular-sparing approach was chosen in two patients, one of which experienced another recurrence after the second surgery. Orchiectomy was performed in two patients without evidence of recurrence at follow-up, while in one case, reoperation was not performed. Of the 16 patients who did not undergo orchiectomy or testicular-sparing surgery, regression of the lesion was reported in seven (43.8%). These patients were followed up with cyclical ultrasound until complete resolution of CDRT after a median of 59.6 months from diagnosis. Remarkably, four (57.1%) of these patients did not have an associated malformation. Helman et al. recently suggested a diagnostic and management algorithm reserving surgical intervention only for cases in which the diagnosis was unclear. Yearly scrotal ultrasound was proposed for patients who met the criteria for CDRT (8). When non-surgical management is chosen, close follow-up during the first few months after diagnosis is mandatory (11). However, surgical biopsy and histological confirmation are indispensable for definitive diagnosis and to rule out malignant cystic testicular lesions, especially when there is no ultrasound regression of the lesion.
In conclusion, CDRT is a rare diagnosis of testicular masses in the pediatric population. It is usually unilateral with no predominance on either side, and the mean age at presentation is ~5–6 years. CDRT usually manifests as painless scrotal swelling. It is frequently associated with genitourinary anomalies, particularly ipsilateral renal agenesis; however, its pathogenesis remains unclear. Ultrasound is the gold standard for the diagnosis and follow-up of CDRT, although there are no clear diagnostic criteria for CDRT. However, it can be suspected if the lesion is well-circumscribed with normal surrounding parenchyma and is composed of multiple small cysts on ultrasound, if tumor markers are normal, or if there are associated mesonephric anomalies. When non-surgical management is chosen, close follow-up during the first few months after diagnosis is mandatory. Surgical biopsy and histological confirmation are indispensable for definitive diagnosis and for ruling out malignant cystic testicular lesions, especially when there is no ultrasound evidence of regression of the lesion.
The principal limitation of this systematic review was the presence of only case report and case series with short to intermediate follow up in the international literature. Moreover, not all data were reported in the analyzed studies.
Data Availability Statement
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Author Contributions
GC, SF, VP, FD-C, and AI have made substantial contributions to the development of the manuscript, read and approved the version submitted, and share responsibility for the content.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leissring JC, Oppenheimer RO. Cystic dysplasia of the testis: a unique congenital anomaly studied by microdissection. J Urol. (1973) 110:362–3. doi: 10.1016/S0022-5347(17)60218-0
2. Nistal M, Regadera J, Paniagua R. Cystic dysplasia of the testis. Light and electron microscopic study of three cases. Arch Pathol Lab Med. (1984) 108:579–83.
3. Eberli D, Gretener H, Dommann-Scherrer C, Pestalozzi D, Fehr JL. Cystic dysplasia of the testis: a very rare paediatric tumor of the testis. Urol Int. (2002) 69:1–6. doi: 10.1159/000064351
4. Pizzuti G, Di Renzo D, Persico A, Lelli Chiesa P. Spontaneous regression of cystic dysplasia of the rete testis in an 18-month-old boy: the key role of ultrasonography. J Ultrasound. (2021) 24:81–84. doi: 10.1007/s40477-019-00391-4
5. Mahlknecht A, Mahlknecht P, Fallaha M, Wieser A. Tubular ectasia of the rete testis (TERT). Differential diagnosis of cystic testicular disorders. Arch Ital Urol Androl. (2015) 87:5–7. doi: 10.4081/aiua.2015.1.5
6. Jeyaratnam R, Bakalinova D. Cystic dysplasia of the rete testis: a case of spontaneous regression and review of published reports. Urology. (2010) 75:687–90. doi: 10.1016/j.urology.2009.05.067
7. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chioppini J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
8. Helman T, Epelman M, Ellsworth P. Cystic dysplasia of the rete testis: does pathophysiology guide management? Urology. (2020) 141:150–3. doi: 10.1016/j.urology.2020.03.026
9. Fuchs ME, Atkinson TH, DaJusta DG. Cystic dysplasia of the testis in an intraabdominal undescended testicle. Urol Case Rep. (2017) 13:143–4. doi: 10.1016/j.eucr.2017.04.013
10. Friend J, Barker A, Khosa J, Samnakay N. Benign scrotal masses in children - some new lessons learned. J Pediatr Surg. (2016) 51:1737–42. doi: 10.1016/j.jpedsurg.2016.07.016
11. Gelas T, Margain Deslandes L, Mestrallet G, Pracros JP, Mouriquand P. Spontaneous regression of suspected cystic dysplasia of the rete testis in three neonates. J Pediatr Urol. (2016) 12:387.e1–4. doi: 10.1016/j.jpurol.2016.05.032
12. Delto JC, Mittal AG, Castellan M. Testicular mass in late adolescence: not always malignant. Urology. (2016) 94:7–9. doi: 10.1016/j.urology.2016.04.039
13. Liniger B, Fleischmann A, Zachariou Z. Benign cystic lesions in the testis of children. J Pediatr Urol. (2012) 8:226–33. doi: 10.1016/j.jpurol.2011.06.008
14. Emam AT, Awad FM, Elsayed EA, Algashi M. Cystic dysplasia of the rete testis: ultrasound and magnetic resonance imaging findings. Saudi J Kidney Dis Transpl. (2012) 23:559–61.
15. Poupalou A, Spyridis G, Vakaki M, Giamarelou P, Petousis G, Nikolaidis P. A case of cystic dysplasia of the rete testis in a 17-months-old boy. Case Rep Med. (2011) 2011:389857. doi: 10.1155/2011/389857
16. Matthew Butler, Sabah Servaes, Richard Bellah. Cystic dysplasia of the testis: spontaneous regression. Pediatr Radiol. (2011) 41:1346–8. doi: 10.1007/s00247-011-2014-6
17. Meirás FC, Fraile AG, Ruiz GD, Tomás IE, Robinot DC, Bramtot AA. Cystic dysplasia of the rete testis: case report. J Pediatr Urol. (2009) 5:513–5. doi: 10.1016/j.jpurol.2009.04.003
18. McGee SM, Hutcheson JC, Vandersteen DR, Reinberg Y, Wolpert JJ. Cystic dysplasia of testis associated with ectopic ureter causing chronic orchalgia. Urology. (2009) 73:1423.e7–8. doi: 10.1016/j.urology.2008.04.025
19. Mac New HG, Terry NE, Fowler CL. Cystic dysplasia of the rete testis. J Pediatr Surg. (2008) 43:768–70. doi: 10.1016/j.jpedsurg.2007.12.054
20. Park E, Morrison S. Cystic dysplasia of the rete testis in an adolescent with VATER association. Pediatr Radiol. (2008) 38:123. doi: 10.1007/s00247-007-0646-3
21. Smith PJ, DeSouza R, Roth DR. Cystic dysplasia of the rete testis. Urology. (2008) 72:230.e7–10. doi: 10.1016/j.urology.2007.11.132
22. Seppo Taskinen Riitta Fagerholm Johanna Aronniemi Testicular tumors in children and adolescents. J Pediatr Urol. (2008) 4:134–7. doi: 10.1016/j.jpurol.2007.10.002
23. Bhat ML, Rasool Z, Kadri SM, Wani N, Hassan G, Mumtaz D, et al. Cystic dysplasia of testis: a case report. J Clin Pathol. (2006) 59:1002–3. doi: 10.1136/jcp.2005.032888
24. Nanni L, Buonuomo V, Gessi M, Lauriola L, Pintus C. Cystic dysplasia of the rete testis associated to cryptorchidism: a case report. Arch Ital Urol Androl. (2005) 77:199–201.
25. Kajo K, Matoska J, Javorka K, Macháleková K, Tomaskin R, Kliment K. Cystic dysplasia of the rete testis. Case report. APMIS. (2005) 113:720–3. doi: 10.1111/j.1600-0463.2005.apm_342.x
26. Pohl HG, Shukla AR, Metcalf PD, Cilento BG, Retik AB, Bagli DJ, et al. Prepubertal testis tumors: actual prevalence rate of histological types. J Urol. (2004) 172(6 Pt 1):2370–2. doi: 10.1097/01.ju.0000144402.13556.74
27. Cottone JL Jr, Redman JF. Cystic dysplasia of the testis with terminal ureterectasis and renal absence: evidence of involution of a dysplastic kidney? South Med J. (2003) 96:56–7. doi: 10.1097/01.SMJ.0000047842.84124.61
28. Thomas AD, Wu HY, Canning DA, Snyder HM 3rd. Spontaneous regression of cystic dysplasia of the testis. J Urol. (2003) 169:645. doi: 10.1016/S0022-5347(05)63982-1
29. Burns JA, Cooper CS, Austin JC. Cystic dysplasia of the testis associated with ipsilateral renal agenesis and contralateral crossed ectopia. Urology. (2002) 60:344. doi: 10.1016/S0090-4295(02)01729-6
30. Camassei FD, Francalanci P, Ferro F, Capozza N, Boldrini R. Cystic dysplasia of the rete testis: report of two cases and review of the literature. Pediatr Dev Pathol. (2002) 5:206–10. doi: 10.1007/s10024001-0112-4
31. Emir L, Karabulut A, Agras K, Germiyanoglu C, Ozer E, Erol D. A rare cause of cystic testicular mass in an infant–cystic dysplasia of the testis. Scand J Urol Nephrol. (2001) 35:153–5. doi: 10.1080/003655901750170641
32. Piotto L, LeQuesne GW, Gent R, Bourne AJ, Freeman J, Ford WD. Congenital cystic dysplasia of the rete testis. Pediatr Radiol. (2001) 31:724–6. doi: 10.1007/s002470100541
33. Koumanidou C, Theofanopoulou M, Nikas J, Vakaki M, Pitsoulakis G, Kakavakis K. Cystic dysplasia of the testis: a rare cause of painless hemiscrotal enlargement in childhood. Eur Radiol. (2000) 10:1653–4. doi: 10.1007/s003300000453
34. Garrett JE, Cartwright PC, Snow BW, Coffin CM. Cystic testicular lesions in the pediatric population. J Urol. (2000) 163:928–36. doi: 10.1016/S0022-5347(05)67855-X
35. Toffolutti T, Gamba PG, Cecchetto G, Talenti E, Tchaprassian Z. Testicular cystic dysplasia: evaluation of 3 new cases treated without surgery. J Urol. (1999) 162:2146–8. doi: 10.1016/S0022-5347(05)68146-3
36. Noh PH, Cooper CS, Snyder HM III. Conservative management of cystic dysplasia of the testis. J Urol. (1999) 162:2145. doi: 10.1016/S0022-5347(05)68145-1
37. Levin TL, Kogan SJ. Cystic dysplasia of the testis: sonographic diagnosis and differentiation from testicular microlithiasis. Pediatr Radiol. (1998) 28:955–7. doi: 10.1007/s002470050508
38. Ngai RL, Yeung BK, Tsui WM, Cheng FY. Cystic dysplasia of the testis associated with ipsilateral renal agenesis and high anorectal anomalies. J Pediatr Surg. (1998) 33:787–8. doi: 10.1016/S0022-3468(98)90222-6
39. Robson M, Thomson MA, Minette LJ. Cystic dysplasia of the testis associated with multicystic dysplasia of the kidney. Pediatr Urol. (1998) 51:477–9. doi: 10.1016/S0090-4295(97)00688-2
40. Bonnet JP, Aigrain Y, Ferkadji L. Cystic dysplasia of the testis with ipsilateral renal agenesis. A case report and review of the literature. Eur J Pediatr Surg. (1997) 7:57–9. doi: 10.1055/s-2008-1071054
41. Wojcik LJ, Hansen K, Diamond DA, Koyle M, Koff SA, Coplen DE, et al. Cystic dysplasia of the rete testis: a benign congenital lesion associated with ipsilateral urological anomalies. J Urol. (1997) 158:600–4. doi: 10.1016/s0022-5347(01)64566-x
42. Zaragoza MR, Buckler LB, Parikh MJ. Cystic dysplasia of the testis: an unusual cause of a pediatric scrotal mass. Urology. (1996) 47:244–7. doi: 10.1016/S0090-4295(99)80425-7
43. Simoneaux SF, Atkinson GO, Ball TI. Cystic dysplasia of the testis associated with multicystic dysplastic kidney. Pediatr Radiol. (1995) 25:379–80. doi: 10.1007/BF02021711
44. Loo CK, Yung T. Cystic dysplasia of the testis: a report of three cases and review of the literature. Pediatr Pathol Lab Med. (1995) 15:885–93. doi: 10.3109/15513819509027025
45. Glantz L, Hansen K, Caldamone A, Medeiros LJ. Cystic dysplasia of the testis. Hum Pathol. (1993) 24:1142–5. doi: 10.1016/0046-8177(93)90197-O
46. Keetch DW, McAlister WH, Manley CB, Dehner LP. Cystic dysplasia of the testis. Sonographic features with pathologic correlation. Pediatr Radiol. (1991) 21:501–3. doi: 10.1007/BF02011723
47. Tesluk H, Blankenberg TA. Cystic dysplasia of testis. Urology. (1987) 29:47–9. doi: 10.1016/0090-4295(87)90597-8
Keywords: CDRT, cystic dysplasia, rete testis, testicle, children, testicular mass
Citation: Contini G, Frediani S, Pardi V, Diomedi-Camassei F and Inserra A (2022) Cystic Dysplasia of the Rete Testis: Case Report and Systematic Review of the Literature. Front. Pediatr. 10:898038. doi: 10.3389/fped.2022.898038
Received: 16 March 2022; Accepted: 25 April 2022;
Published: 18 May 2022.
Edited by:
Antonino Morabito, University of Florence, ItalyReviewed by:
Alberto Mantovani, Meyer Children's Hospital, ItalyRossella Angotti, University of Siena, Italy
Copyright © 2022 Contini, Frediani, Pardi, Diomedi-Camassei and Inserra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simone Frediani, c2ltb25lLmZyZWRpYW5pQG9wYmcubmV0
 Giorgia Contini
Giorgia Contini Simone Frediani
Simone Frediani Valerio Pardi
Valerio Pardi Francesca Diomedi-Camassei
Francesca Diomedi-Camassei Alessandro Inserra
Alessandro Inserra