
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 09 June 2022
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.896086
A correction has been applied to this article in:
Corrigendum: Specifically increased rate of infections in children post measles in a high resource setting
 Daniel Bühl1
Daniel Bühl1 Olga Staudacher1,2
Olga Staudacher1,2 Sabine Santibanez3
Sabine Santibanez3 Rainer Rossi4†
Rainer Rossi4† Hermann Girschick5†
Hermann Girschick5† Volker Stephan6†
Volker Stephan6† Beatrix Schmidt7†
Beatrix Schmidt7† Patrick Hundsdoerfer8†
Patrick Hundsdoerfer8† Arpad von Moers9†
Arpad von Moers9† Michael Lange10†
Michael Lange10† Michael Barker11†
Michael Barker11† Marcus A. Mall1,12,13
Marcus A. Mall1,12,13 Ulrich Heininger14
Ulrich Heininger14 Dorothea Matysiak-Klose15
Dorothea Matysiak-Klose15 Annette Mankertz3
Annette Mankertz3 Horst von Bernuth1,2,12,16*
Horst von Bernuth1,2,12,16*Objectives: Post-measles increased susceptibility to subsequent infections seems particularly relevant in low-resource settings. We tested the hypothesis that measles causes a specifically increased rate of infections in children, also in a high-resource setting.
Methods: We conducted a retrospective cohort study on a large measles outbreak in Berlin, Germany. All children with measles who presented to hospitals in Berlin were included as cases, children with non-infectious and children with non-measles infectious diseases as controls. Repeat visits within 3 years after the outbreak were recorded.
Results: We included 250 cases, 502 non-infectious, and 498 infectious disease controls. The relative risk for cases for the diagnosis of an infectious disease upon a repeat visit was 1.6 (95% CI 1.4–2.0, p < 0.001) vs. non-infectious and 1.3 (95% CI 1.1–1.6, p = 0.002) vs. infectious disease controls. 33 cases (27%), 35 non-infectious (12%) and 57 (18%) infectious disease controls presented more than three times due to an infectious disease (p = 0.01, and p = 0.02, respectively). This results in a relative risk of more than three repeat visits due to an infection for measles cases of 1.8 (95% CI 1.3–2.4, p = 0.01), and 1.4 (95% CI 1.0–1.9, p = 0.04), respectively.
Conclusion: Our study demonstrates for the first time in a high-resource setting, that increased post-measles susceptibility to subsequent infections in children is measles-specific—even compared to controls with previous non-measles infections.
Measles causes significant mortality and morbidity in children (1, 2). In 2019 the WHO estimated 9,828,400 measles cases with 207,500 associated deaths worldwide, mostly affecting children under the age of 5 years (3). In high-resource regions, vaccine hesitancy remains the major risk for outbreaks and thus is a declared threat to global health (4–8). Neither Europe, nor the United States have met the WHO goal of more than 95% of the population being vaccinated with two doses against measles according to the nationally recommended schedule (3, 9, 10). Measles was assumed to cause short-term immunosuppression as early as 1765 by Francis Homes and 1908 by Clemens von Pirquet (11). The first observed a higher susceptibility to diphtheria, the latter the loss of cutaneous reactivity to tuberculin post measles (11, 12). However, this loss of delayed hypersensitivity as measured by skin reaction to tuberculin has also been reported in humans infected with influenza, varicella and polio viruses (13, 14). In 2012 immunosuppression post measles was coined “measles-induced immune amnesia” (15).
There are three major lines of evidence for measles-induced immune amnesia: First, studies at population level demonstrated an unexpectedly large reduction of all-cause mortality and morbidity in children after introduction of measles vaccination (16–18). This reduction exceeded the expected avoidance of directly measles-related sequelae, and is attributed to a general reduction of infections due to assumed unspecific effects of the vaccination (16–18). Second, 7 weeks post measles, significant skewing of the preexisting diversity of antibody repertoires and a shift to immature B cell-receptor repertoires was observed in a cohort of Dutch children with low vaccination coverage (19–21). A similar skewing of the B cell-receptor repertoire along with a strong skewing of the memory T cell pool were observed in macaques upon experimental infections with measles, attributed to the infection and depletion of CD150-positive immune cells (15, 19, 20, 22, 23). These studies identified two phases of immunologic effects: A short-term immunosuppression in the first weeks after measles-infection and a longer lasting susceptibility to infections of up to 5 years (18, 24–27). Third, one ecological and three epidemiological studies observed an increased susceptibility to infections after measles (24–27).
Yet, studies that compare clinical effects post measles on the population level with the one after other infections remain scarce. Taking advantage of a cohort of children in the largest geographically and temporally confined measles outbreak in Germany in the past 15 years, we tested for the first time the hypothesis that measles is associated with a specifically increased rate of infections also in a high-resource setting (28–33). To test for a specific effect of measles we compared the rate of infections in children post measles not only with the one in children without measles but also with the rate of infections in children post non-measles infections. Since sequela post measles are mostly observed in children, we conducted a retrospective cohort study to estimate the relative risk for subsequent infectious diseases in children. The observational period was set to 3 years as measles-induced immune amnesia waned after 26 months, 30 months and 1 year, respectively, in previous studies in high resource settings (24, 26, 27).
To our knowledge, this is the first investigation that compares the number of infections post measles not only with the number of infections in children who had not contracted measles, but also in children who had contracted other, non-measles infections. Taking advantage of a single geographically and temporally confined measles outbreak allowed us to follow on the specific impact of measles on morbidity among children in a genetically and socially highly diverse population in a high resource setting.
The study was approved by the ethics committee of the Charité—Universitätsmedizin Berlin (EA2_028_20). The retrospective cohort study was conducted in all nine children’s hospitals in Berlin. Children (age 18 years) who fulfilled the WHO case definition for measles and presented to any emergency room during the measles epidemic between 20th October 2014 and 30th August 2015 were included as cases (28, 29, 31). “Laboratory confirmed” measles was defined as a laboratory confirmed infection. “Clinically compatible” measles was defined by the presence of the following measles-defining symptoms: fever, rash and cough, coryza or conjunctivitis. “Suspected” measles was defined by the presence of two out of three measles-defining symptoms. Patients with chronic diseases (e.g., asthma, diabetes, cancer, etc.) were excluded, as well as children with permanent residency outside Berlin.
The outbreak in the Berlin area between the 20th October 2014 and the 30th August 2015 comprised 1,344 patients with measles, of whom 720 were children (29, 31). Measles strain D8-Rostov on Don-2987 was imported from Serbia to Germany, and spread among the Berlin population (29, 31). In 2014/2015, the population of Berlin comprised 3,562,166 inhabitants with diverse and heterogeneous ancestry [e.g., 573,342 (16%) non-German citizens and 444,257 (12.5%) German citizens with a migrant background from over 190 countries], 538,326 (15%) of whom where under the age of 18 (28, 34).
For each patient with measles four controls were identified: two who presented with an acute non-infectious disease and two further controls with an acute infectious, non-measles disease (Supplementary Table 1). Controls were matched for sex, age (± 10%), date of first visit to the hospital (±1 month), and duration of in-patient care if applicable (± 20% in full days). The age of controls was chosen in a relative interval of ± 10% of the age of cases, because a relative interval reflects differences in growth and development among different age groups more realistically than a fixed time span. Study design is illustrated in Figure 1. Children with chronic diseases or measles before or during the follow-up period were excluded. If less than four controls could be identified by these criteria, the search was first broadened to any sex (in 114 controls matching 64 cases), second to a broader range of the first visit ± 2 months (in 113 controls matching 70 cases) and last to any length of stay in the hospital (in 25 controls matching 15 cases) (details are shown in Supplementary Table 2).
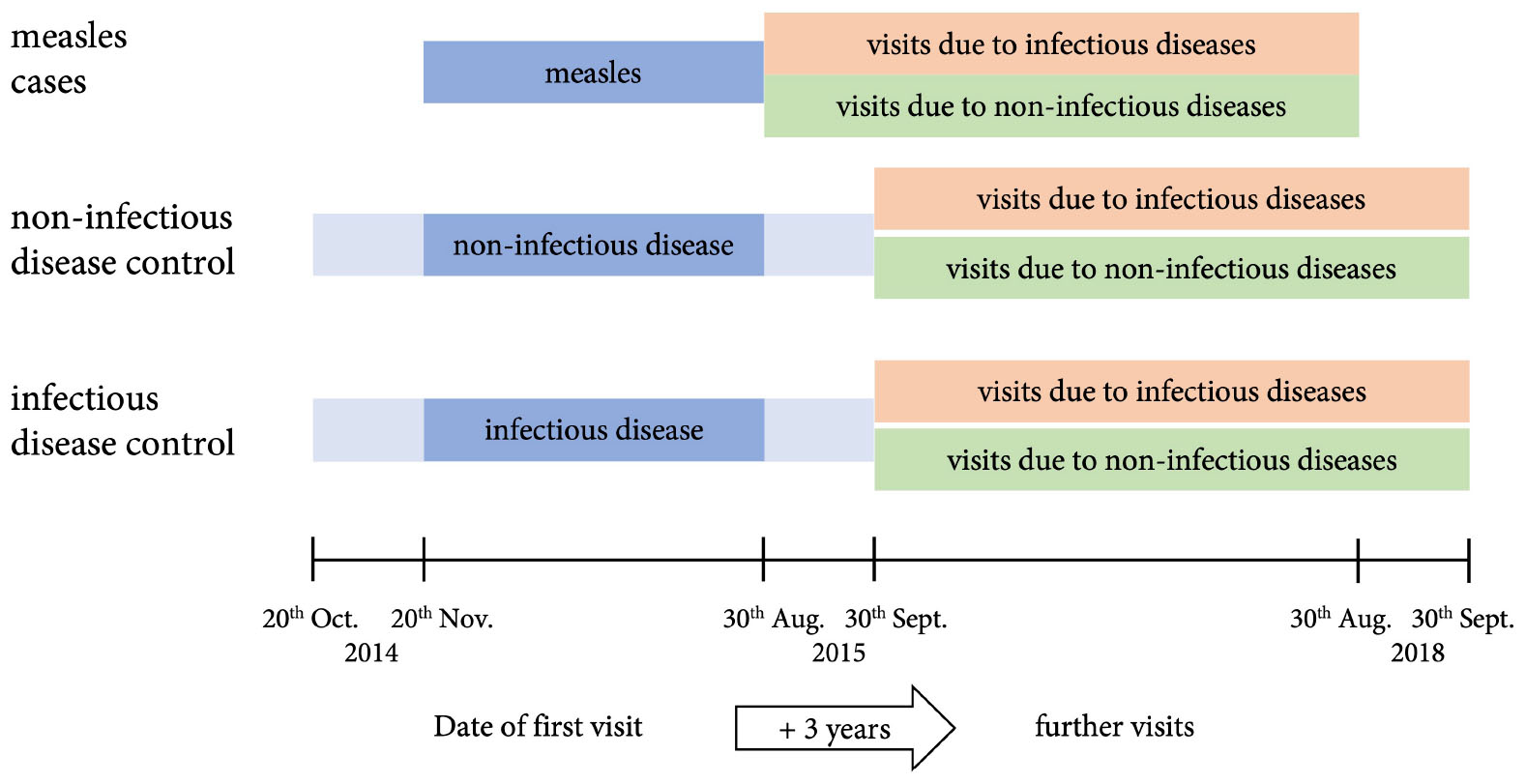
Figure 1. Study design. Children and adolescents (age ≤ 18 years) who fulfilled the WHO case definition for measles and presented to the emergency room during the measles epidemic between 20th October 2014 and 30th August 2015 were included as cases. Controls were matched for sex, age (± 10), date of first visit to the hospital (±1 month, illustrated), and duration of in-patient care if applicable (± 20% in full days). All patients were followed up for 3 years.
The following data were retrieved from the hospitals’ patient management systems: Age (years and months) at first visit, sex, postal code of residence, asylum status (if documented), hospital, date of admission, date of discharge, primary ICD-10-diagnosis, stay on intensive care unit (ICU) (yes/no), measles immunization status, WHO case definition for measles and all repeat visits to the emergency room during the following 3 years (regardless whether patients remained outpatients or were admitted). The information outlined above was equally recorded for repeat visits. The ICD-10-diagnoses of repeat visits were grouped as infectious or non-infectious (Supplementary Table 1). Repeat visits directly related to the previous one were disregarded: Related visits due to non-infectious diagnoses were identified by the same ICD-10-code. Related visits due to infectious diagnoses were clustered into body regions and assigned an approximate duration of infection in which a visit with an ICD-10-code from the same cluster was most likely related (Supplementary Table 1). Calculations on repeat visits due to infectious and non-infectious diseases were calculated among patients and controls with repeat visits.
IBM SPSS Statistics 27 and R (Version 4.0.4.) were used to perform statistical tests. Proportions were compared by (two sided) two proportion z-tests. One-sided p values were calculated using Fishers Exact or the Mann–Whitney U test. Risk Ratios (RRs) are reported with a confidence interval (CI) of 95%. The comparability of study and outbreak populations was calculated by Kolmogorov-Smirnov test for age distribution and Chi-Square test for residential district.
We identified 262 children with measles who presented to an emergency room of the nine children hospitals in Berlin during the 2014/2015 outbreak. This comprises 36.4% of the total of 720 children reported with measles during this outbreak (31). 12 had to be excluded because of a chronic disease (n = 9), permanent residence outside of Berlin (n = 2), or death related to measles (n = 1). Baseline characteristics of cases are shown in Table 1. 182 cases (73%) were categorized as “laboratory confirmed,” 65 (26%) as “clinically compatible” and 3 (1%) as “suspected” according to the WHO classification. The distribution of cases per district of Berlin is shown in Supplementary Figure 1. Comparison of age distribution, proportion of asylum seekers and residential districts yielded no significant differences between study cases and the group of all children affected by measles in Berlin (p = 0.05, p = 0.24, p = 0.92, respectively—Supplementary Figures 2, 3). For each case four controls were identified, resulting in a total of 502 children with a non-infectious diagnosis and 498 with an infectious diagnosis at the time of inclusion, as two patients had to be reassigned from the infectious to the non-infectious disease control group later in the process. The most frequent diagnoses among non-infectious disease controls on first visit were concussions and fractures, while infectious disease controls mainly presented with acute upper respiratory infections, gastroenteritis, bronchitis, and otitis media caused by unspecified pathogens. Detailed characteristics of controls are summarized in Table 1.
Totally 124 (50%) cases, 285 (57%) non-infectious disease controls and 311 (62%) infectious disease controls presented to an emergency room at least once again during the observation period of 3 years and accounted for 311, 680 and 842 repeat visits, respectively. The number of repeat visits due to infections per patient is shown in Supplementary Figure 4. A detailed description of patients with and the respective number of repeat visits is depicted in Tables 2, 3.
Most common infectious diagnoses throughout all groups were acute upper respiratory infections, gastroenteritis, unspecified viral infection, bronchitis, otitis media, pharyngitis and laryngitis, while most common non-infectious diagnoses were concussion, superficial head injury, subluxation of radial head, nausea and vomiting. While diagnoses were broadly distributed, not showing an increase on infections in specific regions, the overall quantity and proportions showed significant differences.
The proportion of patients who presented to an emergency room during the follow up interval was comparable in cases and non-infectious disease controls (124 (50%) cases vs. 285 (57%) controls, p = 0.08). When stratified by diagnosis given upon a repeat visit, significantly more cases with repeat visits returned because of an infectious disease [90 (73%) cases vs. 166 (58%) controls, p = 0.006] and were more frequently admitted (19 (15%) cases vs. 24 (8%) controls, p = 0.03). We observed the same distribution of diagnoses at repeat visits when analyzing the total number of visits instead of patients (see Table 3).
Also, when comparing cases to infectious disease controls, differences were present. The proportion of patients with any repeat visit to the hospital was significantly higher in controls [124 (50%) cases vs. 311 (62%) controls, p = 0.001]. However, there was no significant difference when these patients were stratified by diagnoses at repeat visits. Though, when analyzing the number of visits per diagnosis category, the proportion of infectious diagnoses was significantly higher in cases [212 (64%) visits in cases vs. 464 (56%) in controls, p = 0.01] (see Tables 2, 3).
Children post measles who were seen in the emergency room had a risk ratio of 1.6 (95% CI 1.4–2.0, p < 0.001) for being diagnosed with an infection compared to non-infectious disease controls and a risk ratio of 1.3 (95% CI 1.1–1.6, p = 0.002) compared to infectious disease controls. When comparing the two control groups, infectious disease controls had an increased risk of 1.2 (95% CI 1.1–1.3, p < 0.001) for being diagnosed with an infection compared to non-infectious disease controls. Additionally, patients who presented more than three times due to an infectious disease during follow-up accounted for a significantly higher proportion in cases [33 (27%) cases, 35 (12%) non-infectious, p < 0.001, and 57 (18%) infectious disease controls, p = 0.04], with an elevated risk ratio for cases of 1.8 (95% CI 1.3–2.4, p = 0.01) compared to non-infectious controls and 1.4 (95% CI 1.0–1.9, p = 0.04) compared to infectious controls (see Figure 2).
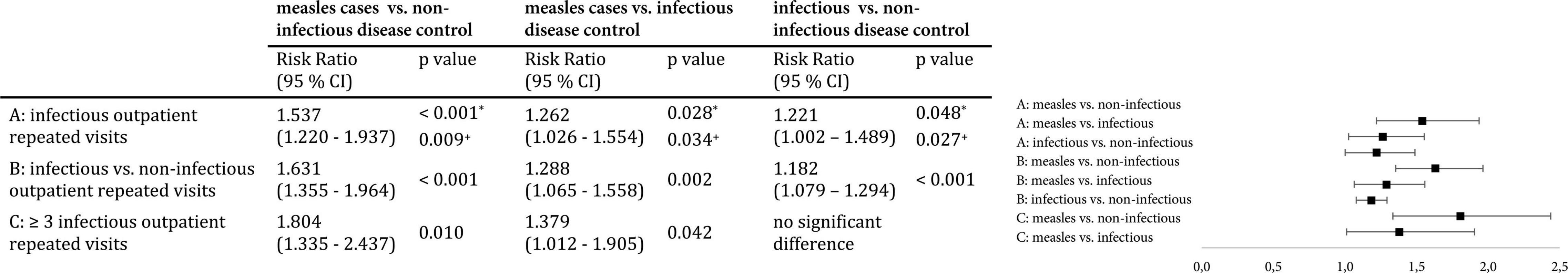
Figure 2. Risk ratio for repeat visits to the emergency room due to infections. (A) Relative risk of cases for an outpatient repeat visit with an infectious diagnosis, calculated using the Man-Whitney-U test (regarding only patients with repeat visits). (B) Relative risk of cases for an outpatient repeat visit with an infectious diagnosis compared to a non-infectious diagnosis, calculated using the Chi-Square Test. (C) Relative risk of cases for three or more infectious outpatient repeat visits, calculated using the Chi-Square Test.
The Berlin measles outbreak in 2014/2015 constitutes the largest outbreak in Germany during the past 15 years. It offered the unique opportunity to test the hypothesis that there is a specifically increased rate of infections post measles, compared to effects post other infections, also in high-resource settings. To our knowledge, our results indicate for the first time an elevated relative risk of repeat visits to the emergency room due to infections for children who contracted measles (cases) compared to not only non-infectious (relative risk of 1.6) but also infectious disease controls (relative risk of 1.3). To our knowledge, these data are also the first to demonstrate an increased risk for infections after a geographically as well as temporally confined measles outbreak in a high-resource setting among a genetically and socially highly diverse population.
In the United Kingdom 2,228 children post measles had been investigated through population based data between 1990 and 2014 (26). During a maximum of 5 years follow-up there was an increased relative risk for infections in children after measles compared to children free of measles (26). This relative risk decreased but remained significant, from 1.44 in the first month to 1.15, between 2.5 and 5 years of follow-up (26). Even within our by far smaller sample size of 250 cases we found a comparably increased relative risk for infectious diseases after measles. In 2020, a Swiss study reported on an increased rate of rehospitalization due to infectious diseases for children in the first year who had been hospitalized for measles between 2000 and 2015 (27). The authors found a higher relative risk of 5.2 for rehospitalization due to infections in the first year post measles compared to non-infectious controls (27). However, these results were based on 113 cases only and two non-infectious disease controls per case, with a total of twelve and six children who were readmitted to the hospital, respectively (27). In contrast, within our sample of 250 cases, we saw an increased susceptibility to infections post measles over the whole period of 3 years. Also, we included all repeat visits to the emergency room in our analysis, regardless of the patient’s admission to hospital. This allowed a more realistic statistical estimation of the relative risk for further infections. Additionally, we noticed an increased risk ratio for repeat visits due to infections after measles not only compared to non-infectious, but also to infectious disease controls, which was also higher than the risk ratio of infectious compared to non-infectious disease controls, indicating a measles-specific effect on further infections. To our knowledge, no previous study included children with other, non-measles infections as controls. While at least 61% of cases were not vaccinated against measles (documentation in the hospitals’ patient management systems was not complete with respect to the vaccination status), vaccination history was hardly ever recorded in controls. All infectious diseases among cases and controls were not vaccine-preventable, with the exception of one visit in each group. So differences between cases and controls cannot be attributed to general differences in the vaccination history. A further strength of our measles cohort is its heterogeneous composition (28, 31, 34). Compared to the detailed studies on T and B cell immunity post measles, based on an ecological sample of socially and geographically highly clustered Dutch children of a community of orthodox protestants, the diverse composition of our sample makes a shallow gene pool as a possible confounder for an increased susceptibility to infections post measles highly unlikely. By including all patients who presented with measles to one of the nine children’s hospitals of Berlin, we were able to comprise 35% of all known children infected with measles during the outbreak (31). We found no significant differences in age, documented asylum seekers and residence district between our sample and the city’s population that contracted measles at that time.
Germany mainly provides primary pediatric care in private practices and usually only during off hours by emergency rooms in hospitals. Keeping this in mind, our study design has several limitations. First, the sample size was limited to 250 children with measles. Yet, despite this rather small size [at least compared to the United Kingdom-study (26)], differences in repeat visits for infectious diseases and the relative risk for subsequent infections compared to controls were significant. Second, we were not able to collect data on how many repeat visits occurred to pediatricians in private practice. Third, we were not able to identify patients that sought emergency care for any illness following a measles infection diagnosed outside a hospital. Forth, we must acknowledge that more patients among measles cases were asylum seekers than among controls. It is plausible that these children relocated to places outside Berlin during the 3 years of follow-up after the measles outbreak, adding to the percentage of cases lost to follow-up. Additionally, we found a significantly higher proportion of patients with at least one repeat visit due to non-infectious diagnoses in both control groups, compared to cases. Since the incidence of non-infectious symptoms should be the same in cases and controls, it seems at least possible (if not likely) that we lost a bigger proportion of patients to follow-up in the measles group. Last, we were not able to show a shift toward more severe subsequent infections after measles compared to controls. Although few severe, life-threatening and even deadly infections post measles had been observed in Berlin during or shortly after the outbreak on the individual level, there was no significant increase of severe infections post measles on the population level (28). Additionally, although the control groups show similar patient characteristics and no significant difference in visits due to non-infectious diseases, we recorded significantly more visits due to an infection in the infectious disease compared to the non-infectious disease control group. We assume that the included infectious disease controls might display further undocumented risk factors making these patients more susceptible to infections (or at least to repeat visits due to infections). Even so, our study demonstrates for the first time that measles cases are at significantly increased risk for subsequent infections, also if compared to controls post other infectious diseases.
Our findings underline the hypothesis that measles is associated with a specifically increased rate of infections in children, even in a high-resource setting like Germany. While measles-induced immune amnesia is considered to cause measles-related mortality particularly in low-resource settings, our study suggests increased post-measles morbidity in children in any country, regardless of medical resources. Our results reinforce the necessity to overcome vaccine hesitancy. Vaccination of > 95% of the population against measles with two doses will not only expedite the rather abstract WHO long-term and global goal to eliminate measles, but immediately lower local measles related all-cause morbidity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
DB and HB designed the study, had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis, and wrote the manuscript. DB collected the data. DB, OS, and HB analyzed the data. HG, RR, VS, BS, PH, AMo, ML, and MB provided access to the hospital patient management systems. SS and AMa provided access to laboratory data. UH, DM-K, HG, RR, SS, AMa, MB, and MM critically revised the manuscript. UH initially proposed to investigate the Berlin measles outbreak of 2014/2015. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
DB was supported by a scholarship of the Konrad-Adenauer Foundation and a doctoral scholarship of the Sonnenfeld Foundation.
HB is an associated member of the standing committee on vaccination against measles at the Robert Koch Institute. DM-K is a member of the standing committee on vaccination against measles at the Robert Koch Institute. AMa and SS are consulting members of the standing committee on vaccination at the Robert Koch Institute. UH is a member of the standing committee on vaccination at the Robert Koch Institute.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DB presented preliminary results on the following meetings: Congress of the Austrian Society of Pediatrics and Adolescent Medicine 2020 and Congress of the German Society of Pediatrics and Adolescent Medicine 2021.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.896086/full#supplementary-material
1. Hübschen JM, Gouandjika-Vasilache I, Dina J. Measles. Lancet. (2022) 399:678–90. doi: 10.1016/s0140-6736(21)02004-3
2. Portnoy A, Jit M, Ferrari M, Hanson M, Brenzel L, Verguet S. Estimates of case-fatality ratios of measles in low-income and middle-income countries: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e472–81. doi: 10.1016/S2214-109X(18)30537-0
3. Patel MK, Goodson JL, Alexander JP Jr., Kretsinger K, Sodha SV, Steulet C, et al. Progress toward regional measles elimination - Worldwide, 2000-2019. MMWR Morb Mortal Wkly Rep. (2020) 69:1700–5. doi: 10.15585/mmwr.mm6945a6
4. Larson HJ, Jarrett C, Eckersberger E, Smith DMD, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. (2014) 32:2150–9. doi: 10.1016/j.vaccine.2014.01.081
5. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. (2015) 33:4161–4. doi: 10.1016/j.vaccine.2015.04.036
6. The Lancet Child Adolescent Health. Vaccine hesitancy: a generation at risk. Lancet Child Adolescent Health. (2019) 3:281. doi: 10.1016/S2352-4642(19)30092-6
7. World Health Organization [WHO].Immunization Agenda 2030 (1 April 2020) [10.02.2021]. Geneva: World Health Organization (2021).
8. World Health Organization [WHO].World Health Statistics 2020: Monitoring Health for the Sdgs, Sustainable Development Goals. Geneva: World Health Organization (2020).
9. World Health Organization [WHO].WHO Vaccine-Preventable Diseases: Monitoring System. 2020 Global Summary [updated 12.10.202010.02.2021]. Geneva: World Health Organization (2020).
10. Hill HA, Yankey D, Elam-Evans LD, Singleton JA, Pingali SC, Santibanez TA. Vaccination coverage by age 24 months among children born in 2016 and 2017 - national immunization survey-child, United States, 2017-2019. MMWR Morb Mortal Wkly Rep. (2020) 69:1505–11. doi: 10.15585/mmwr.mm6942a1
11. Pirquet CV. Das Verhalten Der Kutanen Tuberkulinreaktion Während Der Masern. Dtsch Med Wochenschr. (1908) 34:1297–300.
12. Home F. An Inquiry into the Nature, Cause, and Cure of the Croup. Edinburgh: A. Kincaid and J. Bell (1765). p. 15, 35, 41
13. Notkins AL, Mergenhagen SE, Howard RJ. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. (1970) 24:525–38. doi: 10.1146/annurev.mi.24.100170.002521
14. Lischner HW. Viral suppression of delayed hypersensitivity. J Pediatr. (1972) 80:174–7. doi: 10.1016/s0022-3476(72)80489-x
15. de Vries RD, McQuaid S, van Amerongen G, Yüksel S, Verburgh RJ, Osterhaus AD, et al. Measles immune suppression: lessons from the macaque model. PLoS Pathog. (2012) 8:e1002885. doi: 10.1371/journal.ppat.1002885
16. Higgins JPT, Soares-Weiser K, López-López JA, Kakourou A, Chaplin K, Christensen H, et al. Association of Bcg, Dtp, and measles containing vaccines with childhood mortality: systematic review. BMJ. (2016) 355:i5170. doi: 10.1136/bmj.i5170
18. Mina MJ. Measles, immune suppression and vaccination: direct and indirect nonspecific vaccine benefits. J Infect. (2017) 74:S10–7. doi: 10.1016/S0163-4453(17)30185-8
19. Laksono BM, de Vries RD, Verburgh RJ, Visser EG, de Jong A, Fraaij PLA, et al. Studies into the mechanism of measles-associated immune suppression during a measles outbreak in the Netherlands. Nat Commun. (2018) 9:4944. doi: 10.1038/s41467-018-07515-0
20. Mina MJ, Kula T, Leng Y, Li M, de Vries RD, Knip M, et al. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. (2019) 366:599–606. doi: 10.1126/science.aay6485
21. Petrova VN, Sawatsky B, Han AX, Laksono BM, Walz L, Parker E, et al. Incomplete genetic reconstitution of B cell pools contributes to prolonged immunosuppression after measles. Sci Immunol. (2019) 4:eaay6125. doi: 10.1126/sciimmunol.aay6125
22. de Swart RL, Ludlow M, de Witte L, Yanagi Y, van Amerongen G, McQuaid S, et al. Predominant infection of Cd150+ lymphocytes and dendritic cells during measles virus infection of Macaques. PLoS Pathog. (2007) 3:e178. doi: 10.1371/journal.ppat.0030178
23. Nelson AN, Putnam N, Hauer D, Baxter VK, Adams RJ, Griffin DE. Evolution of T Cell responses during measles virus infection and RNA clearance. Sci Rep. (2017) 7:11474. doi: 10.1038/s41598-017-10965-z
24. Mina MJ, Metcalf CJ, de Swart RL, Osterhaus AD, Grenfell BT. Long-Term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science. (2015) 348:694–9. doi: 10.1126/science.aaa3662
25. Le NTH, Ho NT, Grenfell B, Baker S, Geskus RB. Biphasic pattern in the effect of severe measles infection; the difference between additive and multiplicative scale. BMC Infecti Dis. (2021) 21:1249. doi: 10.1186/s12879-021-06930-x
26. Gadroen K, Dodd CN, Masclee GMC, de Ridder MAJ, Weibel D, Mina MJ, et al. Impact and longevity of measles-associated immune suppression: a matched Cohort Study using data from the thin general practice database in the Uk. BMJ Open. (2018) 8:e021465. doi: 10.1136/bmjopen-2017-021465
27. Behrens L, Cherry JD, Heininger U. The susceptibility to other infectious diseases following measles during a three year observation period in Switzerland. Pediatr Infect Dis J. (2020) 39:478–82. doi: 10.1097/inf.0000000000002599
28. Robert Koch-Institut.Berliner Masernausbruch 2014/2015. Berlin: Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung (2015).
29. Kühne A, Gilsdorf A. [Infectious Disease Outbreaks in Centralized Homes for Asylum Seekers in Germany from 2004-2014]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2016) 59:570–7. doi: 10.1007/s00103-016-2332-9
30. Santibanez S, Hübschen JM, Ben Mamou MC, Muscat M, Brown KE, Myers R, et al. Molecular surveillance of measles and rubella in the WHO European Region: new challenges in the elimination phase. Clin Microbiol Infect. (2017) 23:516–23. doi: 10.1016/j.cmi.2017.06.030
31. Werber D, Hoffmann A, Santibanez S, Mankertz A, Sagebiel D. Large measles outbreak introduced by asylum seekers and spread among the insufficiently vaccinated resident population, Berlin, October 2014 to August 2015. Eurosurveillance. (2017) 22:30599. doi: 10.2807/1560-7917.ES.2017.22.34.30599
32. Bitzegeio J, Majowicz S, Matysiak-Klose D, Sagebiel D, Werber D. Estimating age-specific vaccine effectiveness using data from a large measles outbreak in Berlin, Germany, 2014/15: evidence for waning immunity. Euro Surveill. (2019) 24:1800529. doi: 10.2807/1560-7917.Es.2019.24.17.1800529
33. Arendt F, Scherr S. News-stimulated public-attention dynamics and vaccination coverage during a measles outbreak: an observational study. Soc Sci Med. (2020) 265:113495. doi: 10.1016/j.socscimed.2020.113495
34. Berlin-Brandenburg AFS.Statistischer Bericht / A / I / 5. Einwohnerinnen Und Einwohner Im Land Berlin. Berlin: (2015). Available online at: https://www.statistischebibliothek.de/mir/receive/BBHeft_mods_00018984
Keywords: measles, outbreak, immune amnesia, Europe, high resource setting
Citation: Bühl D, Staudacher O, Santibanez S, Rossi R, Girschick H, Stephan V, Schmidt B, Hundsdoerfer P, von Moers A, Lange M, Barker M, Mall MA, Heininger U, Matysiak-Klose D, Mankertz A and von Bernuth H (2022) Specifically Increased Rate of Infections in Children Post Measles in a High Resource Setting. Front. Pediatr. 10:896086. doi: 10.3389/fped.2022.896086
Received: 14 March 2022; Accepted: 11 May 2022;
Published: 09 June 2022.
Edited by:
Dimitri Van der Linden, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Daan Van Brusselen, GZA, BelgiumCopyright © 2022 Bühl, Staudacher, Santibanez, Rossi, Girschick, Stephan, Schmidt, Hundsdoerfer, von Moers, Lange, Barker, Mall, Heininger, Matysiak-Klose, Mankertz and von Bernuth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Horst von Bernuth, aG9yc3Qudm9uLWJlcm51dGhAY2hhcml0ZS5kZQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.