
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr. , 19 August 2022
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.893815
This article is part of the Research Topic Biomarkers to Predict, Prevent and Find the Appropriate Treatments of Disorders in Childhood View all 14 articles
Cytokines are an important modulator of the immune system and have been found to be altered significantly in many neurological and psychiatric disorders, like obsessive compulsive disorder (OCD) and movement disorders. Also, in pediatric autoimmune neuropsychiatric disorders associated with group A streptococcal infections (PANDAS), which are characterized by abrupt debut of symptoms of OCD and /or movement disorder symptoms, alterations in the immune system have been suggested. The aim of this paper was to review the current literature on the cytokine profile of pediatric patients with symptoms of OCD and/or movement disorder symptoms. A search of PubMed and Medline was performed with specific keywords to review studies measuring cytokines in pediatric patients with symptoms of OCD and/or movement disorders. Nineteen studies were found, twelve of which included a healthy control group, while four studies had control groups of children with other disorders, primarily neurological or psychiatric. One study compared cytokines measurements to reference intervals, and two studies had a longitudinal design. Many cytokines were found to have significant changes in patients with symptoms of OCD and/or movement disorders compared to both healthy controls and other control groups. Furthermore, differences were found when comparing cytokines in periods of exacerbation with periods of remission of symptoms in study participants. The cytokines that most studies with healthy control groups found to be significantly altered were TNF-α, IL-1β and IL-17. Although the exact role of these cytokines in OCD and movement disorder symptoms remains unclear, the available literature suggests a proinflammatory cytokine profile. This offers interesting perspectives on the pathogenesis of OCD and/or movement disorder symptoms in children, and further research into the implications of cytokines in neuropsychiatric disorders is warranted.
Autoimmunity is the failure of the immune system to recognize the organism as itself. The classic component of autoimmune disorder is the inflammation (1), which is a normal physiological defense against infection and tissue damage. However, in many autoimmune disorders an abnormal inflammatory response is associated with tissue and organ damage (2). Autoimmunity can be induced through many different mechanisms. One common etiology is post-infectious, as is seen in Guillain Barré Syndrome, rheumatic fever, and glomerulonephritis. Although many pathogens can cause autoimmunity, group A streptococci (GAS) is especially potent (3). Many autoimmune diseases, for example systemic lupus erythematosus, have comorbid psychiatric symptoms, suggesting a connection between disorders of the immune system and psychiatric disorders (1). Major depressive disorder has been studied extensively. In patients suffering from depression cardinal features of inflammation, such as elevated cytokines in peripheral blood and cerebrospinal fluid (CSF) as well as other acute inflammatory mediators, have been seen (4). In other psychiatric disorders such as obsessive-compulsive disorder (OCD), chronic tic disorder (CTD) and Tourette's Syndrome (TS) it has also been suggested that a subgroup of patients might have immune-related and/or post-infectious autoimmune etiology (5).
Historically, several studies have described patients with OCD and/or movement disorder after infections. In 1978, Kondo and Kabasawa reported a sudden and abrupt debut of a tic disorder after fever in a 11 year- old boy who had elevated antistreptolysin antibodies and responded well to treatment with corticosteroids (6). In the 1980s and 1990s, patients with OCD symptoms developing simultaneously with Sydenham's Chorea (SC) related to GAS infections were described (5). In 1990, children with movement disorders were found to have elevated antistreptococcal titers, and a link between an antecedent GAS infection and movement disorders was suggested (7). In 1995, Allen et al. (8) reported four cases of abrupt, severe onset or a worsening of OCD and/or movement disorder in form of tics. All patients had had recent infections, GAS or viral, and the essential symptoms were determined to be pediatric, infection-triggered, autoimmune neuropsychiatric disorders (PITANDS) (8). Pediatric autoimmune neuropsychiatric disorders associated with streptococcal (group A) infections (PANDAS) were first described in 1998 by Swedo et al. (9) and were described as presence of OCD and/or a tic disorder temporally associated with a GAS infection.
The role of cytokines in neuroinflammation and as pathophysiological mechanism in psychiatric disorders is of interest. Cytokines are small glycoproteins which can be produced by many different cells in all organs. They play an important role in brain development and promotion of normal brain function (10) and can, amongst many other things, create or hinder inflammation and recruit cellular components of the immune system (11). However, they can turn detrimental for the brain if strongly activated by infection or injury, as high levels of pro-inflammatory cytokines can negatively impact memory, neural plasticity and neurogenesis (10).
However, not much is known about the cytokine profile in children with neuropsychiatric symptoms. An improved understanding of the cytokine profile of these patients could offer insight into the pathogenesis of these disorders. In this article we review the available literature, to determine the cytokine profile of children with neuropsychiatric symptoms as seen in OCD, TS, SC, CTD, PANS and PANDAS.
In the present review, a literature search in the Pubmed, PMC and MEDLINE databases was performed. The initial search with the following terms; {[PANDAS (Body-Key Terms)] OR [PANS (Body-Key Terms)] OR [OCD (Body-Key Terms)] OR [Sydenham's chorea (Body-Key Terms)] OR [Tourette's disorder (Body-Key Terms)]} AND {[cytokine (Body-Key Terms)] OR [immune(Body-Key Terms)]} was conducted through the PubMed Central (PMC) database and yielded 342 results. A supplementary search with the terms “(cytokine) AND [(OCD) OR (PANDAS) OR (PANS) OR (Tourette's disorder) OR (tics) OR (Sydenham's chorea)] AND [(pediatric) or (children)]” was carried out on Pubmed.gov database, which includes PMC and MEDLINE, to see if any supplementary materials not found in the original search could be added. This yielded 90 results.
A total of 432 article titles and abstracts were assessed for relevance for the review. Exclusion criteria were studies written in other languages than English, letters to the editor, conference presentations, editorials, comments, or opinions. Seventy articles were included for close reading of the full text. Furthermore, 52 articles of potential interest were added through the references of the aforementioned articles.
In total, 122 article abstracts were systematically read to clarify if the articles documented any kind of cytokine measurement in subjects with either PANDAS, PANS, OCD, TS or SC, and 33 articles were identified. From these 33 articles, 19 had pediatric populations and 14 had only adult populations and were therefore excluded.
Of the 19 articles describing cytokines in pediatric patients with obsessive/compulsive symptoms and/or movement disorder symptoms, 12 had included a healthy control group, 5 had control groups with other disorders or no control group and 2 had a longitudinal study design.
The studies examining cytokines in pediatric patients compared to healthy controls are summarized in Table 1. The studies primarily used ELISA but also other immune assays to examine the different cytokine levels in primarily serum or plasma. The studies subjects differed in included number, ages, diagnosis and in extent of use of psychotropic medication. The cytokines the studies have chosen to examine also differed between studies, however many studies chose to examine TNF-a, IL-6, IL-1b, IL-2, IL-17A and IL-12, and many of the studies found significant alterations of these cytokines when compared to healthy controls. The cytokines with significant results from Table 1 are summarized in Table 2.
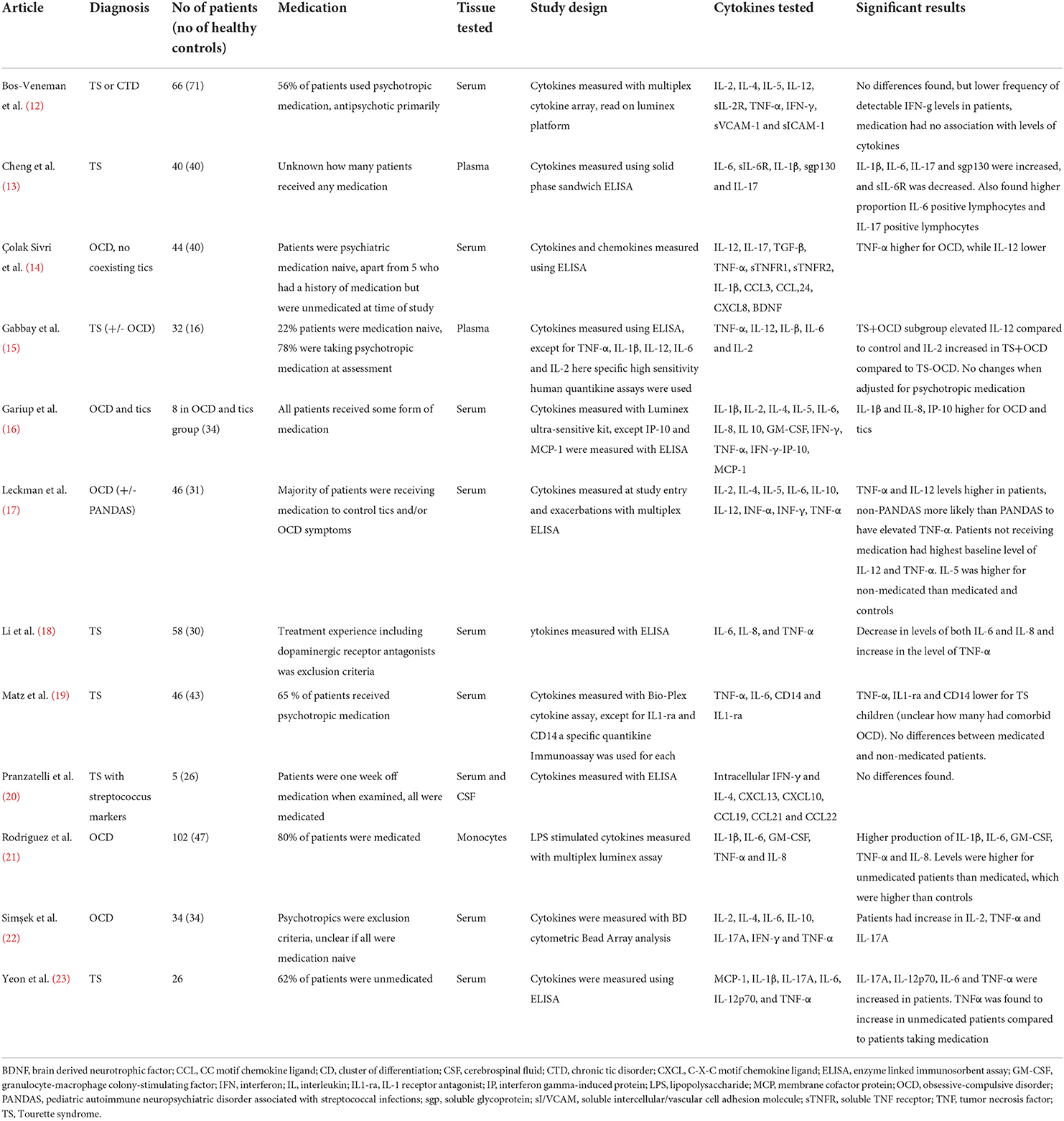
Table 1. Studies examining cytokines in pediatric patients with obsessive-compulsive and/or tic symptoms compared to healthy controls.
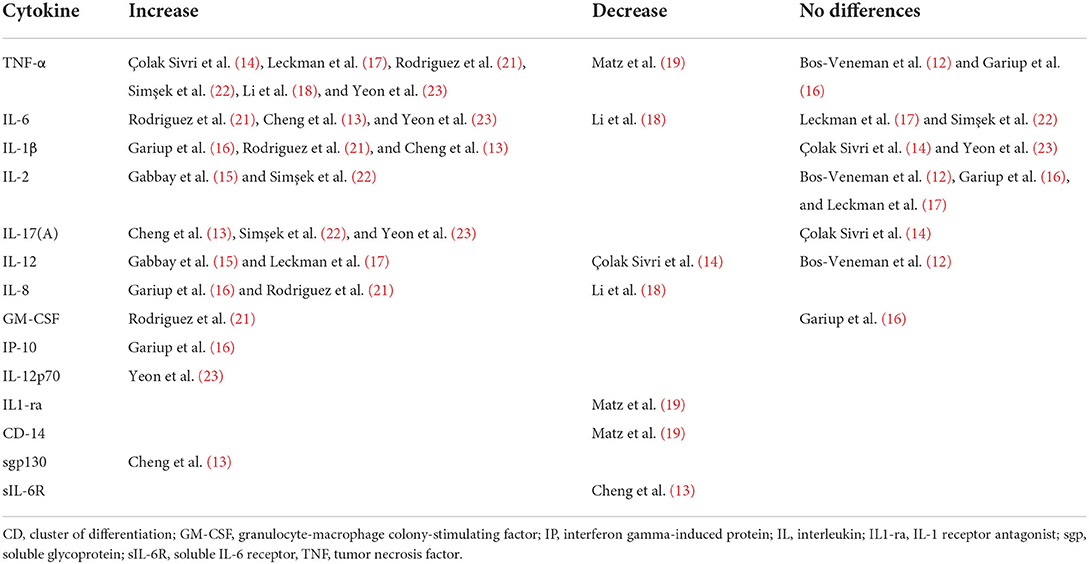
Table 2. Overview of cytokine changes in studies in pediatric patients with tics and/or obsessive-compulsive symptoms compared to healthy controls.
Five studies have included controls with other disorders, other neurological diseases, patients undergoing tonsillectomy (24–28). In general, these studies have included less participants (N = 12–24) and they tested a broad range of cytokines through primarily ELISA. The medication status was not always described. The significant results included a higher level of IL-2 in patients with OCD compared to controls with ADHD or schizophrenia (24). Elevated IL-6 and IL-17A in D2R specific T-cells from subset of patients with SC, TS or PANS were seen compared to controls with neurological disease (25). Compared with children with non-inflammatory neurological diseases like epilepsy, IL-4 was found to be increased in patients with acute and persistent SC and Il-10 and IL-12 were only elevated in patients with acute SC (26). Another study analyzed cytokines in tonsils from PANDAS children compared to children undergoing tonsillectomy for either obstructive sleep apnea or chronic tonsilitis (29). A significant increase of TNF-a and eoxtaxin-3 was found in patients with PANDAS, while IL-8, IP-10, IL-17A IL-10 and IL-12 were significantly decreased (27). Another study compared serum cytokines from PANS children to standard reference values and found them to be within the normal reference (28).
Two studies had a longitudinal design (30, 31). The first study investigated children with TS and CTD with or without OCD and compared periods of exacerbation of symptoms to periods of remission. TNF-α was higher in exacerbation compared to remission. They found no differences in serum cytokine levels between tic-OCD patients and tic+OCD patients (30). The second longitudinal study compared PANDAS debut to periods of exacerbations and no significant differences in cytokines were found (31).
The aim of this study was to review the cytokine profile of pediatric patients with neuropsychiatric symptoms as seen in OCD, TS, SC, PANDAS, PANS or CTD. We found that cytokines for these patient groups appear to be affected in a proinflammatory direction. Of special interest were TNF-α, IL-17 and IL-1β, as most studies measuring these cytokines found a significant increase compared to healthy controls. The studies that had included healthy controls found significant increases in especially the cytokines TNF-α, IL-17 and IL-1β, but many other cytokines were also reported as being significantly increased or decreased in patients compared to healthy controls, as shown in Table 2. The studies with other control groups had more heterogenous results, most likely due to smaller sample sizes and more heterogeneity in the control groups. Only two cytokines were reported significantly altered in more than one study with a non-healthy control group (Il-17 and IL-10), however these were reported as significantly increased in one study and significantly decreased in the other. The studies with longitudinal design were also challenged by their sample size. One study found significantly increased TNF-α in periods of exacerbations, while the other did not.
TNF-α is a proinflammatory cytokine, as it can initiate a strong inflammatory response in nucleated cells, but it can also act as an immunosuppressive mediator by limiting the inflammatory responses. Furthermore, it has a role in inhibiting the development of autoimmune diseases (32). TNF-α has been found to be of importance in many neurological and psychiatric disorders. A recent study found that maternal OCD was related to a significantly higher level of cord blood TNF-α which also was positively correlated with maternal anxiety level (33). Some polymorphisms of the TNF-α gene have found to be associated to OCD susceptibility (29, 34) and others to TS (35). In this review, increased levels of TNF-α were seen across different diagnoses. Significantly increased levels were seen in two studies with patients with TS (18, 23) and in three studies with patients with OCD (14, 21, 22). Furthermore, one longitudinal study found that TNF-α levels were increased in periods of exacerbations in children with TS/CTD (30). Although these findings not have been replicated by other studies (12, 19, 31), they do suggest that TNF-α might be involved in children with obsessive-compulsive and/or tic-related symptoms. Based on the findings in this review, we suggest that a dysregulation or increase of TNF-α, perhaps on a genetic basis, could be associated with obsessive-compulsive and tic symptoms.
IL-1β is also a proinflammatory cytokine, and has been of interest in various central nervous system (CNS) diseases, like multiple sclerosis (36). In children with febrile seizures, elevated levels of IL-1β (as well as TNF-α) in CSF were seen (37). In a meta-analysis, an association was found between the risk of febrile seizures and epilepsy and polymorphism in the IL-1β (511) gene (38). IL-1β has been found to be associated with Post-Traumatic Stress Disorder and bipolar disorder (39, 40). However, the literature on IL-1β and its association to obsessive-compulsive and/or tic symptoms is scarce. One systematic review and meta-analysis found significant reduction in IL-1β compared to healthy controls; importantly this was almost exclusively based on data from adults (41). In a Chinese population with OCD no association was found in IL-1β−511 polymorphism compared to healthy controls (42).
IL-17 (often also called IL-17A) is a proinflammatory cytokine and is mainly expressed by CD4+ TH17 cells (32). In CNS, IL-17 is a mediator between immune cells and tissue, and it was found that an artificial overexpression of IL-17 activates glial cells and enhances neuroinflammation (43). IL-17 has been reported to synergize with other proinflammatory cytokines, such as TNF-α, and potentiate their effects (44, 45). IL-17 has also been reported to be associated to different neurological and psychiatric disorders. In Parkinson's Disease patients, increased levels of IL-17 were correlated with higher levels of anxiety and depression (46). Depression has also been associated with IL-17. One study found IL-17 to be significantly increased in peripheral blood in depressive patients compared to healthy controls (47), although a correlation between severity of depression and IL-17 levels was not found (48). In children with autism, IL-17 levels were elevated compared to healthy controls, and were significantly correlated with the severity of autism (49). Although dysregulation of IL-17 has been found to be triggering several autoimmune diseases in murine models (50), literature on its role in obsessive-compulsive or tic symptoms remains sparse (41). We suggest therefore that studies investigating cytokines in children with obsessive-compulsive and/or tic symptoms in the future should include this cytokine in order to elucidate its role.
An important consideration regarding the methods used in the included articles is that most of the studies measured peripheral cytokines, in serum, or plasma, or tonsil tissue. It can be argued that peripheral cytokines are unreliable surrogate markers of the cytokines in the CNS, as peripheral cytokines can be influenced by many other variables such as age, body mass index, medication, smoking, stress and circadian fluctuation (51). On the other hand, it is important to recognize that the blood-brain barrier (BBB), initially giving reason for the immune privilege hypothesis, can be impaired in various ways, for example by inflammatory cytokines which appear to play a crucial role in allowing antibodies to cross the BBB by impacting (52) stability of the BBB (53). TNF-α induces formation of gaps in BBB by internalizing tight junction protein via upregulation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF κB) and its transcription myosin light chain kinase (54, 55). IL-17 also disrupts the BBB tight junctions and promotes the transmigration of CD4+ lymphocytes through TH17 cells' ability (activated by IL-17) to permeabilize the BBB (56). Peripheral inflammation has also been reported to affect the BBB permeability in other psychiatric disorders (schizophrenia, bipolar disorder and major depressive disorder) (57).
The included studies have used various immune assays for measuring cytokines, performed on various biological materials (serum, plasma, CSF and tonsil tissue) from differing patient groups (TS, OCD, SC, CTD, PANS and PANDAS with different comorbid combinations). Only some of the studies reported on medication status, and not all of them included medication status as confounder. These considerations, and the relatively small number of patients and controls make meta-analysis and subgroup analysis challenging.
In summary, there appears to be an increase in proinflammatory cytokines most clearly for TNF-α, but probably also for IL-17 and IL-1β, in children with obsessive-compulsive and movement disorder symptoms compared to healthy controls. These cytokines can through their effect on the BBB give rise to neuroinflammation. This can potentially offer important insights into the pathogenesis of obsessive-compulsive and tic symptoms, as it implies that at least a subgroup of the affected patients could have an autoimmune pathogenesis. This could offer new treatment options for the afflicted children. However, more knowledge on the role of the immune system, including that of pro-inflammatory cytokines, is needed in the future. The knowledge from the existing studies is still limited and challenged by the heterogeneity of used methods and their relatively small sample size, and thus larger studies are needed to thoroughly examine the cytokine profile of children with obsessive-compulsive and movement disorder symptoms.
RF conducted the review and authored the manuscript with supervision and revisions from CS, ND, and LS. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor UL-T declared a shared parent affiliation with the author ND at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Davison K. Autoimmunity in psychiatry. Br J Psychiatry. (2012) 200:353–5. doi: 10.1192/bjp.bp.111.104471
2. Duan L, Rao X, Sigdel KR. Regulation of inflammation in autoimmune disease. J Immunol Res. (2019) 28:2019. doi: 10.1155/2019/7403796
3. Wucherpfennig KW. Mechanisms for the induction of autoimmunity by infectious agents. J Clin Invest. (2001) 108:1097–104. doi: 10.1172/JCI200114235
4. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
5. Murphy TK, Kurlan R, Leckman J. The immunobiology of tourette's disorder, pediatric autoimmune neuropsychiatric disorders associated with streptococcus, and related disorders: a way forward. J Child Adolesc Psychopharmacol. (2010) 20:317–31. doi: 10.1089/cap.2010.0043
6. Macerollo A, Martino D. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an evolving concept. Tremor Hyperkinetic Mov. (2013) 25:3. doi: 10.5334/tohm.167
7. Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics. (1993) 92:39–43. doi: 10.1542/peds.92.1.39
8. Allen AJ, Leonard HL, Swedo SE. Case study: a new infection-triggered, autoimmune subtype of pediatric OCD and tourette's syndrome. J Am Acad Child Adolesc Psychiatry. (1995) 34:307–11. doi: 10.1097/00004583-199503000-00015
9. Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. (1998) 155:264–71. doi: 10.1176/ajp.155.2.264
10. Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. (2011) 25:181–213. doi: 10.1016/j.bbi.2010.10.015
11. Marazziti D, Mucci F, Lombardi A, Falaschi V, Dell'Osso L. The cytokine profile of OCD: pathophysiological insights. Int J Interferon Cytokine Mediat Res. (2015) 7:35–42. doi: 10.2147/IJICMR.S76710
12. Bos-Veneman NGP, Bijzet J, Limburg PC, Minderaa RB, Kallenberg CG, Hoekstra PJ. Cytokines and soluble adhesion molecules in children and adolescents with a tic disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2010) 34:1390–5. doi: 10.1016/j.pnpbp.2010.06.028
13. Cheng Y-h, Zheng Y, He F, Yang J-h, Li W-b, Wang M-l, et al. Detection of autoantibodies and increased concentrations of interleukins in plasma from patients with Tourette's syndrome. J Mol Neurosci. (2012) 48:219–24. doi: 10.1007/s12031-012-9811-8
14. Çolak Sivri R, Bilgiç A, Kilinç I. Cytokine, chemokine and BDNF levels in medication-free pediatric patients with obsessive–compulsive disorder. Eur Child Adolesc Psychiatry. (2018) 27:977–84. doi: 10.1007/s00787-017-1099-3
15. Gabbay V, Coffey BJ, Guttman LE, Gottlieb L, Katz Y, Babb JS, et al. A cytokine study in children and adolescents with Tourette's disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2009) 33:967–71. doi: 10.1016/j.pnpbp.2009.05.001
16. Gariup M, Gonzalez A, Lázaro L, Torres F, Serra-Pagès C, Morer A. IL-8 and the innate immunity as biomarkers in acute child and adolescent psychopathology. Psychoneuroendocrinology. (2015) 62:233–42. doi: 10.1016/j.psyneuen.2015.08.017
17. Leckman JF, Katsovich L, Kawikova I, Lin H, Zhang H, Krönig H, et al. Increased serum levels of interleukin-12 and tumor necrosis factor-alpha in Tourette's syndrome. Biol Psychiatry. (2005) 57:667–73. doi: 10.1016/j.biopsych.2004.12.004
18. Li E, Ruan Y, Chen Q, Cui X, Lv L, Zheng P, et al. Streptococcal infection and immune response in children with Tourette's syndrome. Childs Nerv Syst. (2015) 31:1157–63. doi: 10.1007/s00381-015-2692-8
19. Matz J, Krause DL, Dehning S, Riedel M, Gruber R, Schwarz MJ, et al. Altered monocyte activation markers in Tourette's syndrome: a case–control study. BMC Psychiatry. (2012) 12:29. doi: 10.1186/1471-244X-12-29
20. Pranzatelli MR, Tate ED, Allison TJ. Case-control, exploratory study of cerebrospinal fluid chemokines/cytokines and lymphocyte subsets in childhood Tourette syndrome with positive streptococcal markers. Cytokine. (2017) 96:49–53. doi: 10.1016/j.cyto.2017.03.003
21. Rodríguez N, Morer A, González-Navarro EA, Serra-Pages C, Boloc D, Torres T, et al. Inflammatory dysregulation of monocytes in pediatric patients with obsessive-compulsive disorder. J Neuroinflammation. (2017) 28:14. doi: 10.1186/s12974-017-1042-z
22. Simşek S, Yüksel T, Çim A, Kaya S. Serum cytokine profiles of children with obsessive-compulsive disorder shows the evidence of autoimmunity. Int J Neuropsychopharmacol. (2016) 19:pyw027. doi: 10.1093/ijnp/pyw027
23. Yeon S-M, Lee JH, Kang D, Bae H, Lee KY, Jin S, et al. A cytokine study of pediatric Tourette's disorder without obsessive compulsive disorder. Psychiatry Res. (2017) 247:90–6. doi: 10.1016/j.psychres.2016.11.005
24. Mittleman BB, Castellanos FX, Jacobsen LK, Rapoport JL, Swedo SE, Shearer GM. Cerebrospinal fluid cytokines in pediatric neuropsychiatric disease. J Immunol. (1950) 159:2994–9. Available online at: https://www.jimmunol.org/content/159/6/2994
25. Pilli D, Zou A, Dawes R, Lopez JA, Tea F, Liyanage G, et al. Pro-inflammatory dopamine-2 receptor-specific T cells in paediatric movement and psychiatric disorders. Clin Transl Immunol. (2020) 9:e1229. doi: 10.1002/cti2.1229
26. Church AJ, Dale RC, Cardoso F, Candler PM, Chapman MD, Allen ML, et al. CSF and serum immune parameters in Sydenham's chorea: evidence of an autoimmune syndrome? J Neuroimmunol. (2003) 136:149–53. doi: 10.1016/S0165-5728(03)00012-2
27. Walls A, Cubangbang M, Wang H, Raiji M, Knight J, Steehler M, et al. Pediatric autoimmune neuropsychiatric disorder associated with streptococcus immunology: a pilot study. Otolaryngol Neck Surg. (2015) 153:130–6. doi: 10.1177/0194599815577784
28. Gromark C, Harris RA, Wickström R, Horne A, Silverberg-Mörse M, Serlachius E, et al. Establishing a pediatric acute-onset neuropsychiatric syndrome clinic: baseline clinical features of the pediatric acute-onset neuropsychiatric syndrome cohort at karolinska institutet. J Child Adolesc Psychopharmacol. (2019) 29:625–33. doi: 10.1089/cap.2018.0127
29. Jiang C, Ma X, Qi S, Han G, Li Y, Liu Y, et al. Association between TNF-α-238G/A gene polymorphism and OCD susceptibility. Medicine. (2018) 97:e9769. doi: 10.1097/MD.0000000000009769
30. Parker-Athill EC, Ehrhart J, Tan J, Murphy TK. Cytokine correlations in youth with tic disorders. J Child Adolesc Psychopharmacol. (2015) 25:86–92. doi: 10.1089/cap.2014.0103
31. Singer HS, Gause C, Morris C, Lopez P, Tourette Syndrome Study Group. Serial immune markers do not correlate with clinical exacerbations in pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Pediatrics. (2008) 121:1198–205. doi: 10.1542/peds.2007-2658
32. Akdis M, Aab A, Altunbulakli C, Azkur K, Costa RA, Crameri R, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: Receptors, functions, and roles in diseases. J Allergy Clin Immunol. (2016) 138:984–1010. doi: 10.1016/j.jaci.2016.06.033
33. Uguz F, Onder Sonmez E, Sahingoz M, Gokmen Z, Basaran M, Gezginc K, et al. Neuroinflammation in the fetus exposed to maternal obsessive–compulsive disorder during pregnancy: a comparative study on cord blood tumor necrosis factor-alpha levels. Compr Psychiatry. (2014) 55:861–5. doi: 10.1016/j.comppsych.2013.12.018
34. Cappi C, Muniz RK, Sampaio AS, Cordeiro Q, Brentani H, Palácios SA, et al. Association study between functional polymorphisms in the TNF-alpha gene and obsessive-compulsive disorder. Arq Neuropsiquiatr. (2012) 70:87–90. doi: 10.1590/S0004-282X2012000200003
35. Keszler G, Kruk E, Kenezloi E, Tarnok Z, Sasvari-Szekely M, Nemoda Z. Association of the tumor necrosis factor−308 A/G promoter polymorphism with Tourette syndrome. Int J Immunogenet. (2014) 41:493–8. doi: 10.1111/iji.12147
36. Mendiola AS, Cardona AE. The IL-1β phenomena in neuroinflammatory diseases. J Neural Transm. (2018) 125:781–95. doi: 10.1007/s00702-017-1732-9
37. Kwon A, Kwak BO, Kim K, Ha J, Kim SJ, Bae SH, et al. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. (2018) 59:5–10. doi: 10.1016/j.seizure.2018.04.023
38. Saghazadeh A, Gharedaghi M, Meysamie A, Bauer S, Rezaei N. Proinflammatory and anti-inflammatory cytokines in febrile seizures and epilepsy: systematic review and meta-analysis. Rev Neurosci. (2014) 25:281–305. doi: 10.1515/revneuro-2013-0045
39. Söderlund J, Olsson S, Samuelsson M, Walther-Jallow L, Johansson C, Erhardt S, et al. Elevation of cerebrospinal fluid interleukin-1β in bipolar disorder. J Psychiatry Neurosci. (2011) 36:114–8. doi: 10.1503/jpn.100080
40. Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. (2015) 2:1002–12. doi: 10.1016/S2215-0366(15)00309-0
41. Gray SM, Bloch MH. Systematic review of proinflammatory cytokines in obsessive-compulsive disorder. Curr Psychiatry Rep. (2012) 14:220–8. doi: 10.1007/s11920-012-0272-0
42. Bo Y, Liu S, Yin Y, Wang Z, Cui J, Zong J, et al. Association study between IL-1β-511 C/T polymorphism and obsessive-compulsive disorder (OCD) in Chinese han population. Int J Psychiatry Med. (2013) 46:145–52. doi: 10.2190/PM.46.2.b
43. Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. (2015) 129:625–37. doi: 10.1007/s00401-015-1402-7
44. Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-α is mediated by CCAAT/Enhancer-binding protein family members*. J Biol Chem. (2004) 279:2559–67. doi: 10.1074/jbc.M308809200
45. Maertzdorf J, Osterhaus ADME, Verjans GMGM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol. (2002) 169:5897–903. doi: 10.4049/jimmunol.169.10.5897
46. Green HF, Khosousi S, Svenningsson P. Plasma IL-6 and IL-17A Correlate with severity of motor and non-motor symptoms in Parkinson's disease. J Park Dis. (2019) 9:705–9. doi: 10.3233/JPD-191699
47. Davami MH, Baharlou R, Ahmadi Vasmehjani A, Ghanizadeh A, Keshtkar M, Dezhkam I, et al. Elevated IL-17 and TGF-β serum levels: a positive correlation between T-helper 17 cell-related pro-inflammatory responses with major depressive disorder. Basic Clin Neurosci. (2016) 7:137–42. doi: 10.15412/J.BCN.03070207
48. Beurel E, Lowell JA. Th17 cells in depression. Brain Behav Immun. (2018) 69:28–34. doi: 10.1016/j.bbi.2017.08.001
49. AL-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. (2012) 9:158. doi: 10.1186/1742-2094-9-158
50. Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. (2008) 29:628–36. doi: 10.1016/j.immuni.2008.07.018
51. Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O. Neuroinflammation and psychiatric illness. J Neuroinflammation. (2013) 10:43. doi: 10.1186/1742-2094-10-43
52. Diamond B, Honig G, Mader S, Brimberg L, Volpe BT. Brain-reactive antibodies and disease. Annu Rev Immunol. (2013) 31:345–85. doi: 10.1146/annurev-immunol-020711-075041
53. Platt MP, Agalliu D, Cutforth T. Hello from the other side: how autoantibodies circumvent the blood–brain barrier in autoimmune encephalitis. Front Immunol. (2017) 21:8. doi: 10.3389/fimmu.2017.00442
54. Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, et al. Caveolin-1–dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. (2010) 189:111–26. doi: 10.1083/jcb.200902153
55. Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta. (2011) 1812:252–64. doi: 10.1016/j.bbadis.2010.06.017
56. Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. (2007) 13:1173–5. doi: 10.1038/nm1651
Keywords: cytokines, autoimmune, pro-inflammatory, obsessive-compulsive, movement disorders
Citation: Fabricius RA, Sørensen CB, Skov L and Debes NM (2022) Cytokine profile of pediatric patients with obsessive-compulsive and/or movement disorder symptoms: A review. Front. Pediatr. 10:893815. doi: 10.3389/fped.2022.893815
Received: 10 March 2022; Accepted: 28 July 2022;
Published: 19 August 2022.
Edited by:
Ulrik Lausten-Thomsen, Copenhagen University Hospital Rigshospitalet, DenmarkCopyright © 2022 Fabricius, Sørensen, Skov and Debes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rebecca Alison Fabricius, cmViZWNjYS5hbGlzb24uZmFicmljaXVzQHJlZ2lvbmguZGs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.