
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 06 July 2022
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.888498
 Anna Kern1†
Anna Kern1† Pia H. Kuhlmann1†
Pia H. Kuhlmann1† Stefan Matl1
Stefan Matl1 Markus Ege1,2
Markus Ege1,2 Nicole Maison1,2,3
Nicole Maison1,2,3 Jana Eckert1
Jana Eckert1 Ulrich von Both1,4
Ulrich von Both1,4 Uta Behrends5
Uta Behrends5 Melanie Anger5
Melanie Anger5 Michael C. Frühwald6
Michael C. Frühwald6 Michael Gerstlauer6
Michael Gerstlauer6 Joachim Woelfle7
Joachim Woelfle7 Antje Neubert7
Antje Neubert7 Michael Melter8
Michael Melter8 Johannes Liese9
Johannes Liese9 David Goettler9
David Goettler9 Andreas Sing10
Andreas Sing10 Bernhard Liebl10
Bernhard Liebl10 Johannes Hübner1‡
Johannes Hübner1‡ Christoph Klein1*,‡
Christoph Klein1*,‡ the COVID Kids Bavaria Consortium1
the COVID Kids Bavaria Consortium1Introduction: Here we report our results of a multi-center, open cohort study (“COVID-Kids-Bavaria”) investigating the distribution of acute SARS-CoV-2 infections among children and staff in 99 daycare facilities and 48 elementary schools in Bavaria, Germany.
Materials and Methods: Overall, 2,568 children (1,337 school children, 1,231 preschool children) and 1,288 adults (466 teachers, 822 daycare staff) consented to participate in the study and were randomly tested in three consecutive phases (September/October 2020, November/December 2020, March 2021). In total, 7,062 throat swabs were analyzed for SARS-CoV-2 by commercial RT-PCR kits.
Results: In phase I, only one daycare worker tested positive. In phase II, SARS-CoV-2 was detected in three daycare workers, two preschool children, and seven school children. In phase III, no sample tested positive. This corresponds to a positive test rate of 0.05% in phase I, 0.4% in phase II and 0% in phase III. Correlation of a positive PCR test result with the local-7-day incidence values showed a strong association of a 7-day-incidence of more than 100/100,000 as compared to <100/100,000 (OR = 10.3 [1.5–438], p < 0.005). After phase III, antibody testing was offered to 713 study participants in elementary schools. A seroprevalence rate of 7.7% (students) and 4.5% (teachers) was determined.
Discussion: During the initial waves of the SARS-CoV-2 pandemic, the risk of a positive SARS-CoV-2 result correlated positively with the local 7-day incidence. Hence, the occurrence of SARS-CoV-2 infections were reflected in schools and daycare facilities. An increased risk of SARS-CoV-2 transmission in the setting of daycare and elementary schooling was unlikely.
When the current coronavirus disease 19 (COVID-19) pandemic started spreading around the world, this scenario immediately invoked concerns with respect to earlier pandemic situations, such as the 1918 influenza pandemic. Back then, predominantly the younger age groups had a high morbidity and mortality burden, in part due to transmission dynamics. Closing schools was an effective measure to control the spread of the disease (1). Also, during seasonal influenza waves primarily young children played a significant role in disease spreading (2). Initially, this raised concerns that children may be a major contributing factor for spreading severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As a mitigation measure many countries—including Germany—closed their schools. This disrupted education for billions of learners worldwide (3) but also led to impaired physical and mental health as well as increased socioeconomic and gender inequalities (4).
Even though infection rates in children were eventually found to be as high as in adults (5), COVID-19 generally causes only mild disease in children, including infants (5, 6). Many children remain completely asymptomatic (7). Thus, the impact of asymptomatic individuals on operating schools and daycare facilities remained unclear.
Hence, the COVID Kids Bavaria study was initiated to investigate the occurrence and transmission rates of SARS-CoV-2 infections among children and staff in daycare facilities and elementary schools in Bavaria, Germany. Here, we report the occurrence of incident and prevalent cases in relation to the overall Bavarian incidence rates in three consecutive test phases between September 2020 and March 2021. We also determined the seroprevalence rate after completion of the PCR-based analysis.
The COVID Kids Bavaria study is a large, multi-center, open cohort study investigating the distribution of SARS-CoV-2 among children and staff in daycare facilities and elementary schools in Bavaria, Germany. Six study centers were involved representing all Bavarian university children's hospitals (Ludwig-Maximilians-Universität München, Technische Universität München, Augsburg, Erlangen, Regensburg, Würzburg).
Local ethics committees approved the study protocol and all related documents. COVID Kids Bavaria is registered with the German Clinical Trials Register (http://www.drks.de/DRKS00022380).
Bavaria is the largest of 16 federal states in Germany with a population of 13.2 million (8). In 2,408 elementary schools, approximately 442,000 children are being educated. The vast majority of these schools (2,257) are public schools (9). For representativeness, one public elementary school was selected from each of the 46 electoral districts, which divide the federal state of Bavaria in similar shares with respect to population size (Figure 1). Each elementary school was matched by two daycare facilities in the surrounding area (one nursery and one kindergarten or combined facilities). Facilities were characterized as urban or rural according to data from the Federal Statistical Bureau of Germany (10).
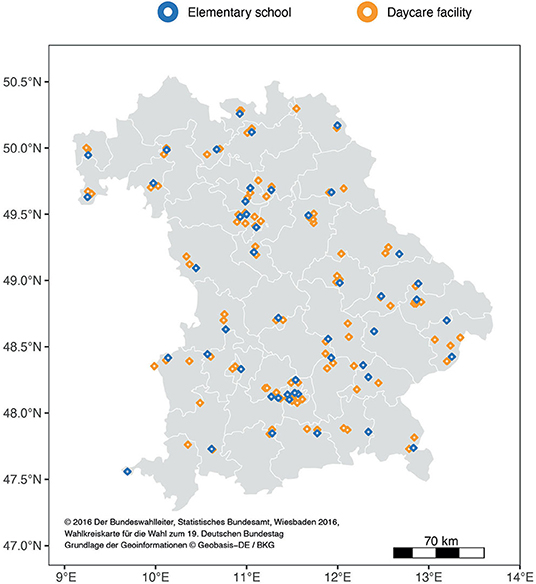
Figure 1. Participating facilities in Bavaria: The white lines delineate the 46 electoral districts (Bundestag constituencies) with approximately equal population shares.
Participants were eligible for enrollment if they met the following inclusion criteria: child aged 1 to 10 years or teachers/daycare staff attending the participating facilities at the day of assessment and written informed consent provided by participants or their legal representatives. In addition, individuals had to be in a state of health permitting to visit the facilities as outlined by the latest corona guidelines from the department of health at the time of assessment (11).
Eligible participants were approached from June 2020 and recruitment was continued throughout all phases. Study visits occurred in each facility once in every phase. Phase I took place from 2020-09-23 till 2020-11-05, phase II from 2020-11-23 till 2020-12-16. Phase III was initially scheduled for 2021-01-25–2021-02-12 but was shifted to 2021-03-01–2021-03-26 due to lock down measures. Phase I was dominated by the SARS-CoV-2 wild type. Additionally, from December 2020 onwards variants of concern were detected in the German population [Alpha (B.1.1.7); Beta (B.1.351); Gamma (P.1)] until completion of throat swab sampling. To exclude contamination effects by infections acquired during holidays or lock down periods, each study phase started with a delay of at least 2 weeks from reopening of the facilities.
A pilot phase was conducted in July 2020 before the summer holiday break in two elementary schools to validate measurement instruments.
The majority of children enrolled in the pilot phase were visiting the last grade of elementary school. By design, the enrolled children of the last grade of the elementary school could not be followed up after the summer break. Therefore, the results of the pilot phase are reported separately.
For assessing non-response, an additional anonymous questionnaire was sent to parents of school children of 15 selected elementary schools via an online survey platform. Here, we asked for their reason to participate or not to participate in our study as well as compliance with SARS-CoV-2 protection measures and general demographics.
To correlate SARS-CoV-2 cases detected by this study with the occurrence of infections across Bavaria, numbers of the local 7-day incidence were drawn from the publicly available LGL database (12). The correlation was based on the 7-day incidence of the administrative district of each individual sampling site on the day of testing.
Each visit entailed testing of a random sample for SARS-CoV-2 by oral throat swabs and subsequent pseudonymized RT-PCR analysis. Sample size for PCR-testing was calculated estimating a point prevalence of SARS-CoV-2 of 3% and a detection of every sixth case by testing only on a single day per class or group. This results in an expected proportion of positive tests of 0.5%. To determine the point prevalence on an alpha level of 5% with a power of 80%, 3000 samples per study phase were necessary.
For PCR testing, random subsamples were selected with 18 school children/preschool children and 4 teachers/daycare staff per facility during phase I. This figure was increased to 40 school children/20 preschool children and 20 teachers/10 daycare staff during the subsequent phases. In addition, the full set of pilot samples were subjected to PCR testing.
PCR analyses for SARS-CoV-2 was conducted by laboratories of the Bavarian Health and Food Safety Authority (LGL) using the RT-PCR method. The tests detected at least 2 gene regions of the viral RNA. (E gene & S gene, RealStar SARS-CoV-2 RT-PCR Kit, Altona Diagnostics; E gene & Orf 1a gene, ampliCube Coronavirus SARS-CoV-2, Mikrogen Diagnostik; E gene & N2 gene, Xpert Xpress SARS-CoV-2,Cepheid; N gene & RdRp gene, Abbott RealTime SARS-CoV-2-Amplificaton Reagent Kit, Abbott Molecular). Positive test results were immediately reported to participants and local health authorities. Performance of Next Generation Sequencing was feasible for five samples.
Following a standardized questionnaire, secondary attack rates and infection circumstances were assessed retrospectively via telephone interviews with participants tested positive in the PCR.
To assess seroprevalence in our cohort, antibody-testing against SARS-CoV-2 was offered to staff and children in 15 selected elementary schools in June/July 2021.
Antibody testing was conducted by the LMU Klinikum, Department of Infectious Disease and Tropical Medicine, using a pan IgG-antibody test (13). The N-Antigen was detected analyzing capillary blood on standardized filter paper (Anti-SARS-CoV-2 N, Roche). At the same time participants completed a questionnaire regarding the history of a SARS-CoV-2 infection confirmed by laboratory testing.
Descriptive statistics included summaries of participation rates at the facility and individual level. Key characteristics of participants (age, sex) were summarized as mean (range) or count (percentage). Missing values are listed in the respective tables; they were not imputed.
Characteristics of responders and non-responders were compared by Fisher's exact test. Odds ratios (OR) with 95%-confidence intervals (CI) were also calculated by Fisher's exact test; Wilcoxon rank sum test was used to compare continuous variables.
The positive test rate was calculated as a percentage of positive tests among all tests performed. Seroprevalence was calculated as a percentage of positive tests among all tests performed and stratified by age groups (children/adults). The 95% confidence intervals for binomial values were calculated by exact binomial tests.
All statistical analyses were performed with R (version 4.0.0) (14) including the packages tidyr, dplyr for data processing and ggplot2 and ggmap for data visualization.
Of all 149 enrolled facilities, 147 facilities were visited by study teams (Figure 1). The selected facilities represented urban (23 elementary schools, 54 daycare facilities) and rural areas (25 elementary schools, 45 daycare facilities). Two facilities were not visited as they were locked down during sampling periods.
Extrapolating from the size and number of facilities, we expected 10,723 school children, 682 teachers, 8586 preschool children, 1,299 daycare staff to be eligible. Of these, 2568 (66.6%) children [1337 (34.7%) school children, 1,231 (31.9%) preschool children] and 1,288 (33.4%) adults [466 (12.1%) teachers, 822 (21.3%) daycare staff] consented to participate in the study and to provide throat swabs for SARS-CoV-2 testing (Figure 2).
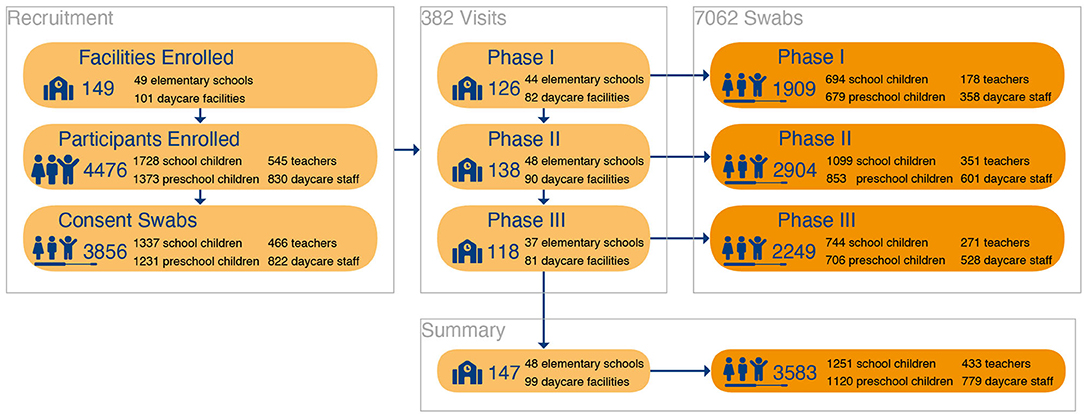
Figure 2. Participant Flow: Inclusion and participation of study individuals for throat swab- PCR-testing.
Preschool children covered an age range from 0 to 7 years and school children from 6 to 12 years (Table 1). Sex distribution was equal in children, whereas teachers and daycare staff were predominantly female (89 and 95%, respectively). The non-response assessment questionnaire was answered almost equally by the parents of participating and non-participating individuals (46.9 and 53.1% respectively). Participating and non-participating children were similar in demographics and their parents had a similar educational level and felt equally affected by the COVID-19 pandemic (Supplementary Table 1). Non-participation in the study was associated with considering general hygiene measures (e.g., wearing masks, social distancing, and washing hands) to be less important, lower interest in vaccinating their children against SARS-CoV-2 and being less anxious about contracting SARS-CoV-2. The most common reasons for parents not to participate were (i) the wish to spare their children a throat swab and (ii) rejection of their children to participate.
PCR-testing for SARS-CoV-2 was performed in 7062 throat swab samples, 4,775 (67.6%) samples from children [2,537(35.9%) school children, 2,238 (31.7%) preschool children] and 2287 (32,4%) samples from adults [800 (11.3%) teachers, 1,487 (21.1%) daycare staff]. Of these, 13 samples tested positive for SARS-CoV-2 with one daycare worker in phase I (1 positive sample out of 1.909, 95% CI: 0.0–5.6) and three daycare workers, two preschool children, and seven school children in phase II (12 positive samples out of 2.904, 95% CI: 6.2–20.9) (Figure 3). The throat swab samples obtained in phase III yielded no positive SARS-CoV-2 PCR results (0 positive samples out of 2.249, 95% CI: 0.0–3.7). These figures correspond to a positive test rate of 0.05% in phase I, 0.4% in phase II and 0% in phase III.
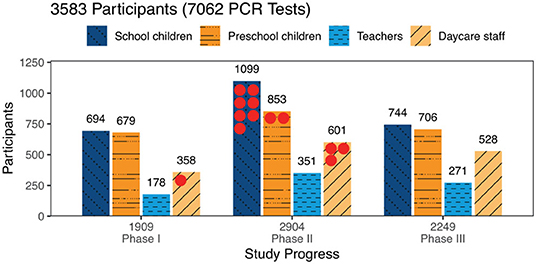
Figure 3. Participants tested by phase and study group: Numbers of participants tested are given by study group and study phase. Altogether 7,062 tests were performed with no available result in 9 tests. The 13 positive PCR tests are marked by red dots.
Four daycare workers and six children were newly detected by our study. Three other children with positive PCR tests turned out to have been diagnosed with SARS-CoV-2 by other measures in the past. They were allowed to reenter their school after completing a 14-days isolation period and still tested positive after readmission (Table 2).
Two potential clusters with two positively tested children each within one school, but different classes, were identified. In the retrospective case analysis, five families stated that their children were presumably infected by other family members. With a cycle threshold (ct) of <30, only one child was presumably highly infectious at the time of testing. This child had already been quarantined as a contact person on the subsequent day before our test result arrived (Table 2).
The results of the pilot study were analyzed separately (see Methods). In July 2020, two elementary schools were visited, and 60 swabs were taken in 32 school children and 28 teachers. At the time, schools reduced the number of students per class following public health policy measures. Therefore, classes were divided into two groups. In one group of fourth-grade students, two children tested positive, whereas the other group could not be tested as they had been quarantined shortly before.
The countrywide incidence of COVID-19 varied considerably over the study phases (Figure 4). The local 7-day incidence used for calculation refers to the 7-day incidence of the administrative district of each individual sampling site on the day of testing.
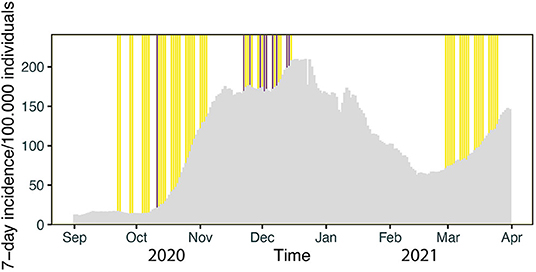
Figure 4. Timeline of testing: Gray bars denote the daily 7-day incidence/100,000 in Bavaria. The vertical yellow lines reflect the days of PCR testing in Phase I, II and III, respectively. Days with positive PCR test results for SARS-CoV-2 are marked by purple lines.
During phase I the mean local 7-day-incidence was 55/100,000 (range: 4–255/100.000), during phase II 184/100,000 (range: 84-580/100.000) and during phase III 77/100,000 (range: 12-238/100.000), yielding a mean local 7-day-incidence of 115/100,000 over all tests performed. Positive PCR tests were obtained on days with a local 7-day incidence of 212/100,000 on average, whereas negative PCR tests corresponded to an average 7-day incidence of 115/100,000 (Figure 5). During data collection a local 7-day incidence of more than 100/100,000 triggered stricter governmental containment measures including school closures or schooling in smaller groups. Hence, the threshold of 100/100,000 was of societal interest. Our correlation shows that a positive PCR test result was strongly associated with a local 7-day incidence of more than 100/100,000 as compared to <100/100,000 (OR = 10.3 [1.5–438], p < 0.005).
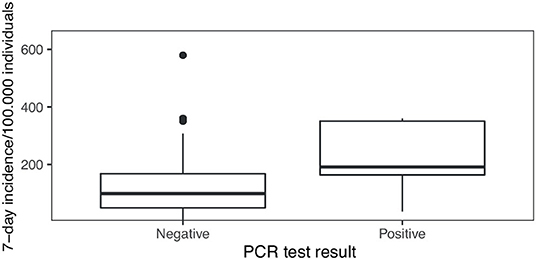
Figure 5. PCR test results in the context of local 7-day incidence numbers: The local 7-day incidence per 100,000 individuals differs between days when negative and positive PCR test results were obtained in our study (p < 0.0005).
Upon completion of the three phases of PCR-testing, IgG antibody-testing for SARS-CoV-2 was performed in 713 individuals attending elementary schools [511 (71.7%) children, 202 (28.3%) teachers]. Of these, 39 school children and 9 teachers tested positive for SARS-CoV-2-antibodies, whereas negative results were obtained in 470 school children and 193 teachers. This corresponds to a seroprevalence of 7.6% (95% confidence interval: 5.5–10.3%) in school children and 4.5% (95% confidence interval: 2.1–8.3%) in teachers. In 2 samples from school children, serum antibody assays failed for technical reasons and therefore measurements were not available.
Of all individuals tested for SARS-CoV-2-antibodies, 438 provided information on whether a potential previous infection confirmed by laboratory testing has occurred or not (Table 3). The recall of a prior infection with SARS-CoV-2 confirmed by a positive PCR test result yielded a false negative rate of 56% in children and 50% in adults (OR = 1.24 [0.14–11.05]).
The COVID Kids Bavaria study assessed the occurrence of SARS-CoV-2 in healthy individuals attending elementary schools and day care facilities in three different phases covering the second and third wave of the COVID-19 pandemic in Bavaria. Of 7,062 PCR-tests in 3,856 participants, 13 yielded a positive test result (4 daycare workers, 2 preschool children, 7 school children), of whom three children were known cases testing still positive after a required isolation period of 14 days. A positive PCR test result was strongly associated with a local 7-day incidence of more than 100/100,000 as compared to <100/100,000 (OR = 10.3 [1.5–438], p < 0.005). Half of the individuals with detectable IgG antibodies against SARS-CoV-2 were unaware of a previous infection.
Essentially, we did not intend to quantify the overall prevalence of disease within the specified target population, as this is already done by other scientific studies (15, 16) and the health authorities (17). Rather we aimed to assess the spread of the disease in healthy individuals attending day care facilities and elementary schools on a regular basis. A strength of our study is the broad coverage of urban and rural areas and the equal representation of all Bavarian districts. This has been facilitated by a collaborative effort of all University Children's Hospitals of Bavaria, the support of the health authorities, and the involved facilities. An anonymous non-responder questionnaire showed no significant differences with respect to demographics and experience of personal limitations due to restrictions of everyday life. However, participants and non-participants differed in their perception of their personal risk and of the necessity of hygiene measures. These moderate differences were expected and indicate a minor but no major selection bias.
The initial sample size calculation was based on the assumption that 0.5% of PCR samples would be positive. This figure was derived from an estimated point prevalence of 3% and an average incubation period of 6 days (18). The 3% estimate was estimated based on the 7-day-incidence in Bavaria during study protocol preparation. Additionally, similar prevalence values were reported by a Spanish seroepidemiological study (19). Bavarian seroepidemiological prevalences had not been published at that time. When testing an individual only on 1 day, as in our study by design, 5 out of 6 individuals might escape. Retrospectively the assumed figure of positive samples was an overestimation, and the projected sample size was not reached in phase I due to a low recall rate and in phase III due to lockdown measures. However, intensified recruitment in phase II led to an inclusion of 2900 individuals and a detection of 0.4% positive samples thereby almost meeting the prior assumptions suggesting that the incidence in children and staff was not higher than in the general population.
The low number of detected cases could suggest that established hygiene measures worked reliably and prevented daycare facilities and elementary schools from major outbreaks. These findings build upon the results of a previous study conducted in the Munich metropolitan area (20). Moreover, the risk of asymptomatic SARS-CoV-2 infections in children visiting daycare or elementary schools was rather low, as hardly any new cases were detected. Only nine children were tested positive, three of these were known cases detected upon recovery and after 14 days of isolation. These three children had a low virus load as indicated by Ct values > 30 and thus were unlikely to spread SARS-CoV-2.
Our detection rate was closely related to the local incidence numbers in the respective administrative districts of Bavaria, which is illustrated by the particularly strong association of detection of cases in our population with the concurrent local incidence values above 100/100,000. Together with the exact timing of the detection of positive samples (Figure 4), this demonstrates that the COVID Kids Bavaria study mirrored well the spread of SARS-CoV-2 across Bavaria. Similar findings were reported by a study from Public Health England, which describes a strong association of SARS-CoV-2 outbreaks among staff and students in educational settings during June/July 2020 in England with the regional COVID-19 incidence (21). Studies from other countries have also highlighted that community transmission of SARS-CoV-2 is a risk factor for transmission in daycare facilities and schools (5, 22). Collectively, these results are in line with the concept that the spread of SARS-CoV-2 in the population were not primarily “driven” by children attending elementary schools and daycare facilities in Germany prior to delta and omicron waves (20, 23–25). Furthermore, the findings of a Catalonian study and a recent report of the European Center for Disease Prevention and Control (ECDC) report fewer SARS-CoV-2 outbreaks in preschools and primary schools compared to secondary schools (5, 26).
The study was not designed to track chains of infection, to assess local outbreaks, or to determine the secondary attack rate. Our random sampling strategy thus cannot offer a complete picture of the epidemic activity in entire school classes and daycare groups. Nevertheless, retrospective interviews with parents suggested that transmission of the virus to children occurred predominantly at home and not within daycare facilities or elementary schools. This is in line with findings of a case control study from the U.S. in children and adolescents: A positive SARS-CoV-2 test was not associated with in-person school or childcare attendance during the preceding 2 weeks (27). In our survey, only a single individual was found to have a high viral load (i.e., a Ct value below 30) and thus a marked potential to spread the virus. Of note, even before the return of our PCR test result, this child had been quarantined because a close SARS-CoV-2 positive contact person had been identified by the health authorities. In other words, only one person day of the about 7,000 person days covered by our tests bore a marked risk of spreading the disease.
Our study population was characterized by the absence of symptoms suggestive of a SARS-CoV-2 infection as we deliberately tested children and adults who were allowed to attend their respective facilities only in good health. This is in sharp contrast to the predominant testing strategy of the health authorities, which focused mainly on symptomatic individuals or those with a high likelihood of relevant exposure. Therefore, asymptomatic individuals might have been missed by the authorities, thereby underestimating the true incidence in the entire population systematically. Conversely, our approach missed symptomatic individuals as these were not present at their facilities during the assessment.
Upon completion of the PCR-test phases, we added a serological study module to estimate the numbers of recorded and unrecorded cases in the population of school children and their teachers. The detected seroprevalence of 7.7% among school children in summer 2021 corresponds to the figure of 8.4% [95% confidence interval: 6.4-10.9%] determined in 15,771 children in a seroepidemiological study in Bavaria, Germany (28) and 7.8% [95% credible interval 6.2–9.5%] determined in 2500 Swiss children (29) in fall 2020. The proportion of undetected cases in our cohort, i.e., individuals unaware of a previous infection, was about 50%. The share of undetected cases was much lower as compared to an earlier seroepidemiological study in Bavaria: In fall 2020, infections among children up to 18 years old were 6-fold higher than the actual reported incidence. In the beginning of 2021, the numbers were still three to four times higher (28, 30). This gradual decline may reflect an increase in the coverage of tests, particularly in asymptomatic individuals. This notion is supported by our antibody sub-study with an even lower share of unrecorded cases.
At the time of writing this manuscript, Health Authorities in Bavaria have intensified the screening efforts by rolling out RT-PCR pool testing and Antigen testing 2–3 times per week, thereby missing hardly any incident case in children, including asymptomatic individuals. In week 48 (December) 2021, the corresponding 7-day-incidence values were 1,172 in school children (aged 6–11 years) and 579 in middle-aged adults (35–59 years) (12). Consequently, our data from the first waves of the COVID pandemic cannot easily be compared to the current situation. Moreover, at the time of our field phases few adults and virtually no children were immunized, and infection dynamics have been changing substantially with newly emerging SARS-CoV-2 strains characterized by increased transmissibility. Regarding the increased transmissibility, a 6-8-fold higher incidence of SARS-CoV-2 infections within the age group of 0–4 years associated with Omicron compared to Delta was shown by a retrospective cohort study (09/2021-01/2022) conducted in the United States of America (31).
Nevertheless, our study is in line with the idea that hygiene measures and testing strategies during the early waves of Covid-19 contained the pandemic in elementary schools and daycare settings, particularly when the Bavarian incidence was high (i.e., phase II during the second wave). With systematic and comprehensive testing strategies (32, 33), maintaining hygiene measures and increasing vaccination rates we may be in a better situation to face the currently prevailing Delta and Omicron variants without falling back to less sophisticated measures such as extensive and complete closing of schools and daycare facilities. Recent German studies have clearly pointed out the magnitude of side effects of school closures including a lack of opportunities to learn and socialize leading to an increased risk of psychiatric problems and chronic disease (4, 34) as well as a rise in educational inequality (35). Hence, an informed cost-benefit analysis prior to reinstall such mitigation measure needs to be performed.
While closing schools may have been a rational strategy during the influenza pandemic about 100 years ago, today, we have access to highly improved diagnostic and preventive measures. Our data, along with other studies, provide an argument for reserving complete shutdown of schools and daycare facilities as an ultima ratio measure.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of the Medical Faculty of the LMU Munich. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Hannah Kindermann, Tilmann Schober, Patricia Schmied, Alexander Neuner, Laura Dech, Nikolaus Rieber, Jonas Geisperger, Philip Oehler, Anna Mittermeier, Katrin Moritz, Christopher Schulze, Irmgard Toni, Ludwig Seebauer, Christoph Härtel, Andrea Streng, Patricia Niekler, Ute Eberle, Nikolaus Ackermann, Andreas Wieser, Raquel Rubio Acero. The Covid Kids Bavaria Consortium consists of scientists and partners, who contributed important work to the study as they recruited participants and performed specimen collection (PS, AN, LD, JG, PO, AM, KM, CS, IT, CH, AS, PN, LS), developed and performed the serological testing (AW, RR) and RT-qPCR testing (UE, NA), managed the administrative frameworks and databases (HK), and partnered as conceptual advisors (TS, NR).
CK, JH, AK, ME, NM, UvB, and PK contributed to conception and design of the study. SM organized the database. The statistical analysis was planned by SM, ME, AK, and PK. SM performed the statistical analysis and created the corresponding figures. AK and PK wrote the first draft of the manuscript. ME wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The study was funded by the Bavarian Ministry of Science and the Arts (Bayerisches Staatsministerium für Wissenschaft und Kunst). The study was also supported by the Care-for-Rare Foundation and its philanthropic partners.
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank all study participants for accepting our invitation and being part of this assessment. We acknowledge great financial support by the Bavarian Ministry of Science and the Arts. We are indebted to our enthusiastic clinical and laboratory teams; without their help the study would not have been possible. This manuscript is currently published as a preprint on medrxiv.org (36).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.888498/full#supplementary-material
1. Short KR, Kedzierska K, van de Sandt CE. Back to the future: lessons learned from the 1918 influenza pandemic. Front Cell Infect Microbiol. (2018) 8:343. doi: 10.3389/fcimb.2018.00343
2. Tsang TK, Fang VJ, Chan KH, Ip DK, Leung GM, Peiris JS, et al. Individual Correlates of Infectivity of Influenza A Virus Infections in Households. PLoS ONE. (2016) 11:e0154418. doi: 10.1371/journal.pone.0154418
3. UNESCO. School Closures Caused by Coronavirus (COVID-19). Unesco (2020). Available online at: https://en.unesco.org/covid19/educationresponse (accessed December 14, 2021).
4. Ravens-Sieberer U, Kaman A, Erhart M, Devine J, Schlack R, Otto C. Impact of the COVID-19 pandemic on quality of life and mental health in children and adolescents in Germany. Eur Child Adolesc Psychiatry. (2021) 31:879–89. doi: 10.1007/s00787-021-01726-5
5. ECDC. COVID-19 in Children and the Role of School Settings in Transmission - Second Update. Eurpean Centre for Disease Prevention and Control, Stockholm (page last updated July 8th, 2021) (2021). Available online at: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission (accessed December 14, 2021).
6. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Calò Carducci FI, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolescent Health. (2020) 4:653–61. doi: 10.1016/S2352-4642(20)30177-2
7. Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 Among Children in China. Pediatrics. (2020) 145:e20200702. doi: 10.1542/peds.2020-0702
8. Statistisches Bundesamt (Destatis). 14. koordinierte Bevölkerungsvorausberechnung nach Bundesländern - Moderate Entwicklung der Geburtenhäufigkeit, der Lebenserwartung und des Wanderungssaldos (G2-L2-W2): Statistisches Bundesamt (Destatis). (2019). Available online at: https://service.destatis.de/laenderpyramiden/ (accessed December 14, 2021).
9. Bayerisches Staatsministerium für Unterricht und Kultus. I 1. Überblick. Bayerns Schulen in Zahlen 2018/2019. 2019; Heft 67 (Schriften des Bayerischen Staatsministeriums für Unterricht und Kultus, Reihe A, Bildungsstatistik,):6, Bayerisches Staatsminsterium für Unterricht und Kultus, München (2021).
10. Thünen-Institut für Ländliche Räume, Cartographer Landatlas (www.landatlas.de). Braunschweig (2021). Available online at: https://karten.landatlas.de/app/landatlas/ (accessed December 14, 2021).
11. Bayerisches- Staatsministerium für Unterricht und Kultus. Coronavirus aktuell- Aktualisierter Rahmen-Hygieneplan für bayerische Schulen. (2021). Available online at: https://www.km.bayern.de/allgemein/meldung/7061/aktualisierter-rahmen-hygieneplan-fuer-bayerische-schulen.html (accessed December 14, 2021).
12. Bayerisches, Landesamt für Gesundheit und Lebensmittelsicherheit 2020. Corona-Fälle in Bayern - Archiv der Fallzahlen 2021 [updated 14th of December, 2021]. Available online at: https://www.lgl.bayern.de/gesundheit/infektionsschutz/infektionskrankheiten_a_z/coronavirus/karte_coronavirus/archiv.htm (accessed December 14, 2021).
13. Beyerl J, Rubio-Acero R, Castelletti N, Paunovic I, Kroidl I, Khan ZN, et al. A dried blood spot protocol for high throughput analysis of SARS-CoV-2 serology based on the Roche Elecsys anti-N assay. EBioMedicine. (2021) 70:103502. doi: 10.1016/j.ebiom.2021.103502
14. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2020).
15. Tonshoff B, Muller B, Elling R, Renk H, Meissner P, Hengel H, et al. Prevalence of SARS-CoV-2 infection in children and their parents in Southwest Germany. JAMA Pediatr. (2021) 175:586–93. doi: 10.1001/jamapediatrics.2021.0001
16. Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. (2020) 382:2302–15. doi: 10.1056/NEJMoa2006100
17. Robert Koch-Institut; Abteilung für Epidemiologie und Gesundheitsmonitoring (2021). COVID-19: Fallzahlen in Deutschland und weltweit -Fallzahlen in Deutschland – Tuesday, December 14, 2021, 00:00 AM (updated online 06:30 AM) 2021. Available online at: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Fallzahlen.html (accessed December 14, 2021).
18. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. (2020) 396:535–44. doi: 10.1016/S0140-6736(20)31483-5
19. Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. (2020) 172:577–82. doi: 10.7326/M20-0504
20. Hoch M, Vogel S, Kolberg L, Dick E, Fingerle V, Eberle U, et al. Weekly SARS-CoV-2 Sentinel Surveillance in Primary Schools, Kindergartens, and Nurseries, Germany, June–November 2020. Emerg Infect Dis. (2021) 27:2192–6. doi: 10.3201/eid2708.204859
21. Ismail SA, Saliba V, Lopez Bernal J, Ramsay ME, Ladhani SN. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis. (2021) 21:344–53. doi: 10.1016/S1473-3099(20)30882-3
22. Macartney K, Quinn HE, Pillsbury AJ, Koirala A, Deng L, Winkler N, et al. Transmission of SARS-CoV-2 in Australian educational settings: a prospective cohort study. Lancet Child Adolescent Health. (2020) 4:807–16. doi: 10.1016/S2352-4642(20)30251-0
23. Haag L, Blankenburg J, Unrath M, Grabietz J, Kahre E, Galow L, et al. Prevalence and transmission of severe acute respiratory syndrome coronavirus type 2 in childcare facilities: a longitudinal study. J Pediatr. (2021) 237:136–42. doi: 10.1016/j.jpeds.2021.07.054
24. Buchholz U, Lehfeld AS, Otte im Kampe E, Lindahl M, Lewandowsky M, Hauer B, et al. Epidemiologie von COVID-19 im Schulsetting. Epid Bull. (2021) 13:23–36.
25. Kern A, Diebenbusch J, Berner R, Krägeloh-Mann I, De Bock F, Renz-Polster H, et al. Welche Rolle spielen Kinder in Schulen und Kindertagesstätten bei der Übertragung von SARS-CoV-2? – Eine evidenzbasierte Perspektive. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. (2021) 64:1492–9. doi: 10.1007/s00103-021-03454-2
26. Alonso S, Alvarez-Lacalle E, Català M, López D, Jordan I, García-García JJ, et al. Age-dependency of the Propagation Rate of Coronavirus Disease 2019 Inside School Bubble Groups in Catalonia, Spain. Pediatr Infect Dis J. (2021) 40:955–61. doi: 10.1097/INF.0000000000003279
27. Hobbs CV, Martin LM, Kim SS, Kirmse BM, Haynie L, McGraw S, et al. Factors Associated with Positive SARS-CoV-2 Test Results in Outpatient Health Facilities and Emergency Departments Among Children and Adolescents Aged <18 Years—Mississippi, September–November 2020. Morb Mortal Wkly Rep. (2020) 69:1925. doi: 10.15585/mmwr.mm6950e3
28. Hippich M, Sifft P, Zapardiel-Gonzalo J, Bohmer MM, Lampasona V, Bonifacio E, et al. A public health antibody screening indicates a marked increase of SARS-CoV-2 exposure rate in children during the second wave. Med (N Y). (2021) 2:571–2. doi: 10.1016/j.medj.2021.03.019
29. Ulyte A, Radtke T, Abela IA, Haile SR, Berger C, Huber M, et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. (2021) 372:n616. doi: 10.1136/bmj.n616
30. Hippich M, Holthaus L, Assfalg R, Zapardiel-Gonzalo J, Kapfelsperger H, Heigermoser M, et al. A Public Health Antibody Screening Indicates a 6-Fold Higher SARS-CoV-2 Exposure Rate than Reported Cases in Children. Med (N Y). (2021) 2:149–63 e4. doi: 10.1016/j.medj.2020.10.003
31. Wang L, Berger NA, Kaelber DC, Davis PB, Volkow ND, Xu R. Incidence Rates and Clinical Outcomes of SARS-CoV-2 Infection With the Omicron and Delta Variants in Children Younger Than 5 Years in the US. JAMA Pediatr. (2022) 1:e220945. doi: 10.1001/jamapediatrics.2022.0945. [Epub ahead of print].
32. Joachim A, Dewald F, Suarez I, Zemlin M, Lang I, Stutz R, et al. Pooled RT-qPCR testing for SARS-CoV-2 surveillance in schools - a cluster randomised trial. EClinicalMedicine. (2021) 39:101082. doi: 10.1016/j.eclinm.2021.101082
33. Forster J, Streng A, Rudolph P, Rücker V, Wallstabe J, Timme S, et al. Feasibility of SARS-CoV-2 Surveillance Testing Among Children and Childcare Workers at German Day Care Centers: A Nonrandomized Controlled Trial. JAMA Network Open. (2022) 5:e2142057-e. doi: 10.1001/jamanetworkopen.2021.42057
34. Langmeyer A, Guglhör-Rudan A, Naab T, Urlen M, Winklhofer U. Kind sein in Zeiten von Corona. Ergebnisbericht zur Situation von Kindern während des Lockdowns im Frühjahr 2020. Bericht des Deutschen Jugendinstituts, (2020) p. 101. Available online at: https://www.dji.de/fileadmin/user_upload/bibs2020/Ergebnisbericht_Kindsein_Corona_2020.pdf (accessed December 14, 2021).
35. Grewenig E, Lergetporer P, Werner K, Woessmann L, Zierow L. COVID-19 and educational inequality: How school closures affect low- and high-achieving students. Eur Econ Rev. (2021) 140:103920. doi: 10.1016/j.euroecorev.2021.103920
Keywords: SARS-CoV-2, surveillance, children, elementary school, preschool, PCR, seroprevalence
Citation: Kern A, Kuhlmann PH, Matl S, Ege M, Maison N, Eckert J, von Both U, Behrends U, Anger M, Frühwald MC, Gerstlauer M, Woelfle J, Neubert A, Melter M, Liese J, Goettler D, Sing A, Liebl B, Hübner J, Klein C and the COVID Kids Bavaria Consortium (2022) Surveillance of Acute SARS-CoV-2 Infections in Elementary Schools and Daycare Facilities in Bavaria, Germany (09/2020–03/2021). Front. Pediatr. 10:888498. doi: 10.3389/fped.2022.888498
Received: 02 March 2022; Accepted: 03 June 2022;
Published: 06 July 2022.
Edited by:
Dimitri Van der Linden, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Joanna Merckx, McGill University, CanadaCopyright © 2022 Kern, Kuhlmann, Matl, Ege, Maison, Eckert, von Both, Behrends, Anger, Frühwald, Gerstlauer, Woelfle, Neubert, Melter, Liese, Goettler, Sing, Liebl, Hübner, Klein and the COVID Kids Bavaria Consortium. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christoph Klein, Q2hyaXN0b3BoLmtsZWluQG1lZC51bmktbXVlbmNoZW4uZGU=
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.