- 1The Children's Hospital of the Zhejiang University School of Medicine, National Clinical Research Center for Child Health, Hangzhou, China
- 2Department of Pediatrics, Shaoxing Maternal and Child Health Care Hospital, Hangzhou, China
- 3Endocrinology, Genetics, and Metabolism, Beijing Diabetes Center for Children and Adolescents, Medical Genetics Department, Beijing Children's Hospital, Beijing, China
- 4Department of Pediatric Endocrinology and Inherited Metabolic Diseases, Children's Hospital of Fudan University, Shanghai, China
- 5Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 6Department of Pediatric, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 7Department of Endocrinology, Children's Hospital of Chongqing Medical University, Chongqing, China
- 8Department of Endocrinology, Shanghai Children's Hospital of Shanghai Jiao Tong University, Shanghai, China
- 9Laboratory for Translational Genetics, Department of Human Genetics, KU Leuven, Leuven, Belgium
Objective: To investigate the clinical incidence and characteristics of type 1 diabetes mellitus (T1DM) of children and adolescents at the time of initial diagnosis in China.
Methods: Data on all pediatric patients with newly diagnosed T1DM were retrospectively collected from 34 medical centers in 25 major cities in China from January 2015 to January 2020. Patients were classified into three age groups: <5 years, 5 to <10 years, and ≥10 years of age. The same patient population was also categorized into diabetic ketoacidosis (DKA) and non-DKA groups based on clinical criteria.
Results: The mean annual clinical incidence of T1DM was 3.16/100,000 from the years 2015 to 2019. A total of 6,544 patients with newly diagnosed T1DM aged 0–16 years (median 7.84 ± 3.8) were studied [ages <5 years (29.3%), 5 to <10 years (38.7%), and ≥10 years (32%)], 52.4% of them were women. In total, 90.5% of the cases were occurred in individuals without a family history. Patients had lower C-peptide (CP) and body mass index (BMI) z scores when compared with healthy children, 41.8% of them had measurable T1DM-related antibodies and 52.7% had DKA. Among all three age groups, the <5 years group had the lowest BMI z score, CP, and glycated hemoglobin (HbA1c) on average, while it had the highest incidence rate of DKA (56.9%). Compared to the non-DKA group, the DKA group was significantly younger, with a lower BMI z score and CP, higher antibody positive rate, HbA1c, and the rate of insulin pump therapy.
Conclusion: The clinical incidence of T1DM in children and adolescents in China was 3.16/100,000. Patients with DKA at the first diagnosis of T1DM have a worse β-cell function. Public health measures for the prevention and treatment of T1DM should focus on preschoolers (aged <5 years) in particular, considering the severity and the highest frequency of DKA in this age group. More efforts should be dedicated to early screening and diagnosis of the T1DM.
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized by insulin deficiency and resultant hyperglycemia, accounting for about 90% of diabetes in children and adolescents. It is a major pediatric endocrine disease that endangers children's health. According to the data released by International Diabetes Federation (IDF) in 2019, 1.1 million children and adolescents (<20 years of age) have T1DM around the world, and the number of patients continues to increase (1, 2). The global average increase in incidence has been 3–4% per year over the past decades, with an especially steeper rise in low-incidence countries (3). The incidence of T1DM remains unstable in China, a longitudinal population-based registry study showed that it significantly increased from 2.72/100,000 in 2007 to 3.60/100,000 in 2017 (4). One nationwide registry study based on multiple centers showed that the incidence of T1DM in children aged between 0 and 14 years was 1.9/100,000 (5). Regional surveys in Beijing, Shanghai, and Zhejiang have found that the compound annual growth rate (CAGR) in T1DM incidence in China is much higher than the world average (6–8). Additionally, the CAGR in T1DM incidence in the children under 5 years old is approximately 5–34%, suggesting that the onset of the disease has an increasing trend at younger ages (9). The earlier age at onset of T1DM in childhood, the greater risk of dying from related acute and chronic complications. The average life expectancy of children with T1DM is reduced by about 12 years even in western developed countries (10, 11). However, there is a terrific lack of comprehensive information related to the clinical incidence and characteristics of T1DM in Chinese children and adolescents, particularly about the declining pancreatic β-cell function, T1DM-related antibodies, status of diabetic ketoacidosis (DKA), diagnosis, and treatment of the disease, etc. The aim of this study was to investigate these clinical features and test the hypothesis that children and adolescents who present with DKA at the diagnosis of T1DM have a higher antibody positive rate, worse pancreatic β-cell function, and need more efficient insulin therapy.
Methods
Participants
In a national observational cohort study from the National Diabetes Register, we recruited hospitalized patients <16 years of age with newly diagnosed T1DM from 34 medical centers in 25 major cities of China between January 2015 and January 2020. These cities include regions of different economic development levels throughout the country according to geographical location, climate, culture, and population (northeast, north, northwest, southwest, central, east, and south), involving 16 provinces, 2 autonomous regions, and 4 municipalities and covering 79.4% of the whole population of China. For the purpose of achieving a real representative profile of T1DM, all of the participating medical centers are the most influential ones in the local medical institutions attended by excellent pediatric endocrinologists. The inclusion criteria for T1DM were according to the standards of medical care in diabetes from American Diabetes Association (ADA) (12–14). DKA was defined by the International Society of Pediatric and Adolescent Diabetes (ISPAD) as follows: blood glucose above 200 mg/dl, metabolic acidosis (venous pH under 7.30 or serum bicarbonate under 15 mEq/L), and ketosis (ketonemia or ketonuria). According to venous pH, DKA can be classified into mild (under 7.30), moderate (under 7.20), or severe (under 7.10) (15, 16). Diabetic ketosis (DK) was defined as ketonemia and/or ketonuria. The present study excluded patients with an uncertain diagnosis of T1DM and those with diabetes secondary to some other conditions, such as hemochromatosis, cystic fibrosis, or chemotherapy. Participants were classified into three age groups: <5 years, 5 to <10 years, and ≥10 years. The same patient population was also categorized as DKA and non-DKA groups based on clinical criteria. Additionally, the patients with DKA were further grouped by the severity levels (mild/moderate/severe) for the study.
This study was approved by the University of Zhejiang's Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Written informed consent and assent, when appropriate, were obtained from all the participants or parents or legal guardians at the beginning of the study.
Anthropometric and Laboratory Measures
The body weight and height of the participants were measured following a standardized procedure by trained investigators. The body mass index (BMI) was calculated as weight (kg)/height (m2) and was converted to BMI z score as [BMI–mean (BMI)]/SD (BMI), the mean (BMI) and SD (BMI) were obtained from the height and weight standardized growth charts of Chinese children and adolescents (17).
All subjects had blood drawn for plasma glucose, C-peptide (CP), glycated hemoglobin (HbA1c), pH, ketone bodies, serum bicarbonate along with T1DM-related antibodies, such as glutamic acid decarboxylase antibody (GADA), insulinoma antigen-2 antibody (IA−2A), insulin autoantibodies (IAA), Zinc Transporter 8 antibody (ZnT8A), and islet cell antibody (ICA). The urine sample was also collected for a test of glycosuria and ketonuria. Meanwhile, a questionnaire survey about the birth history and family history of diabetes was obtained. Insulin therapeutic regimens (continuous subcutaneous insulin infusion [CSII] with an insulin pump or multiple daily subcutaneous injections of insulin) were recorded and also both hospitalization and post-hospitalization durations.
Statistical Analysis
Means (± SD), medians (interquartile range), or geometric means (95% CI) for continuous variables were calculated based on their distributions and proportions and were estimated for categorical variables. Continuous variables were compared using the independent sample t-test, Mann-Whitney U test, Univariate ANOVA, or Kruskal-Wallis test, depending on the distribution of variables and the numbers of groups compared. Categorical variables were compared using the chi-squared (χ2) test. The correlation analysis and partial correlation analysis were adopted for analyzing the relationship between variables. In the examination of <5 years, 5 to <10 years, and ≥10 years groups, we used analysis of covariance (ANCOVA) to compare the geometric means of adjusting for BMI z score as covariate, the ordinal logistic regression was used when the data distribution was skewed. In the study of DKA and non-DKA groups, the binary logistic regression was used, and age and BMI z score were used as independent variables. All comparisons were exploratory. A value of p < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS 20.0 statistical software.
Clinical Incidence of T1DM
All newly diagnosed diabetes cases were outpatient or emergency department visits before hospitalization. Because the population of children and adolescents in all of the areas where the study centers located is not available, we cannot calculate the incidence of T1DM in the general population. However, the clinical incidence rate of T1DM, which reflects the incidence of disease in all children and adolescents attending clinical clinics, can be obtained. The clinical incidence rate of T1DM was calculated as the sum of newly diagnosed cases of both children and adolescents each year divided by the annual number of outpatient and emergency visits in 34 medical centers in China.
Results
Clinical Incidence of T1DM
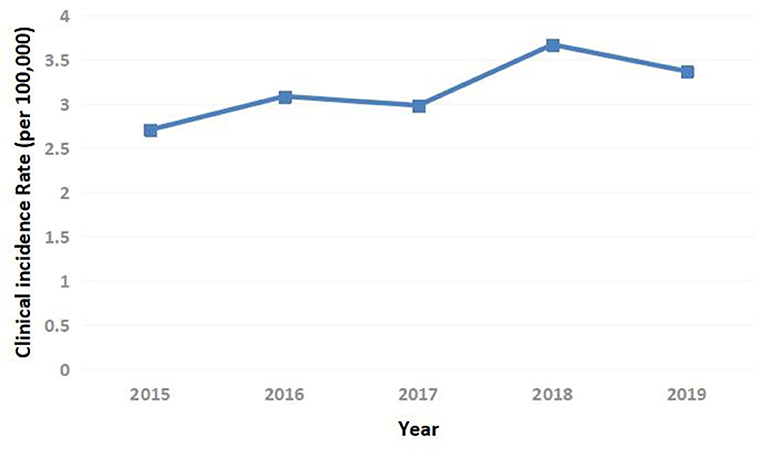
The annual clinical incidence of T1DM had gradually increased from 2.71/100,000 in 2015, 3.08/100,000 in 2016, 2.98/100,000 in 2017, 3.67/100,000 in 2018 to 3.37/100,000 in 2019. Besides, within the country variation was seen with rates 6–8 times higher in the northeast than the southwest (Figures 1, 2). The mean annual clinical incidence of T1DM was 3.16/100,000, and the CAGR was 5.6% during the period 2015–2019.
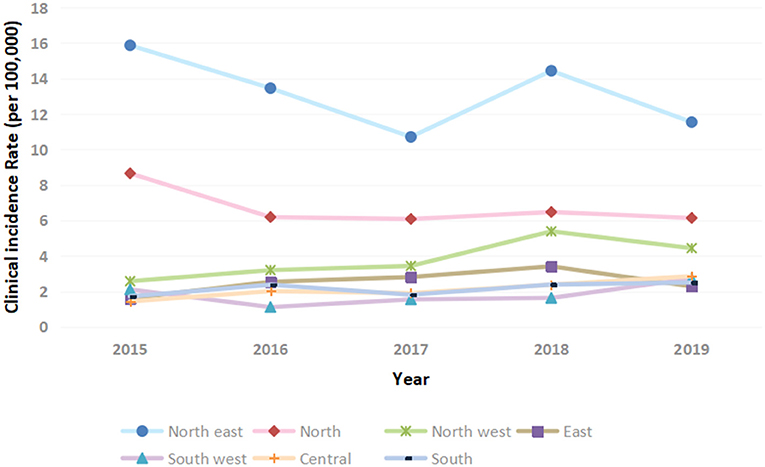
Figure 2. Clinical incidence of T1DM in children and adolescents in China stratified by geographical region.
Participants Characteristics
In total, 7,700 patients aged 0–16 years were approached based on the inclusion criteria of T1DM. Of these, 7,206 patients were recruited. Among the participants, 34 were excluded because of the presence of diabetes secondary to some other conditions, such as hemochromatosis, cystic fibrosis, or chemotherapy. Additional 628 patients were excluded because of missing CP and/or T1DM-related antibody data. In the end, 6,544 patients with newly diagnosed T1DM were eligible for the study, with a median age of 7.84 ± 3.8 years, the proportion of each age group: <5 years (29.3%), 5 to <10 years (38.7%), and ≥10 years (32%), and 52.4% of women. The clinical characteristics of the participants were summarized as follows: BMI z score, −0.22 ± 1.58 (kg/m2); HbA1c, 11.8 ± 2.72 (%); pH of DKA, 7.15 ± 0.16; and CP, 0.25 ± 0.4 (ng/ml). Among the participants, the incidence rates of DK and DKA were 75.6 and 52.7%, respectively, at the first diagnosis. Overall 41.8% of the patients had measurable T1DM-related antibodies, such as GADA (27.7%), IA−2A (15%), IAA (13.2%), ZnT8A (10.4%), and ICA (9.6%); 19.8% of the patients showed two or more serum antibodies positive. In total, 90.5% of T1DM cases occurred in individuals without a family history of diabetes mellitus, only 2.1, 6.7, and 0.7% of the cases had a family history in first-, second-, or third-degree relatives, respectively. All of the newly diagnosed patients with T1DM received the treatment of insulin during their period of hospitalization. In total, 27.4% of them were treated with insulin pump (CSII) therapy, and the others (72.6%) were treated with multiple daily subcutaneous injections of insulin. However, a very low percentage (5.5%) of patients adopted insulin pump therapy post-hospitalization.
Relationships Between the Clinical Parameters of Participants
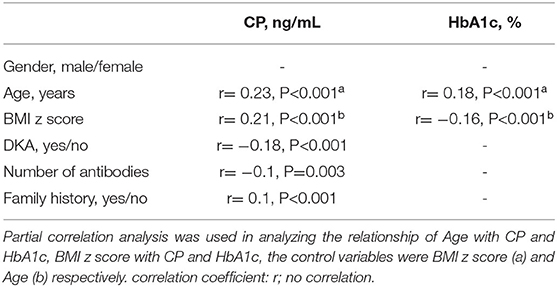
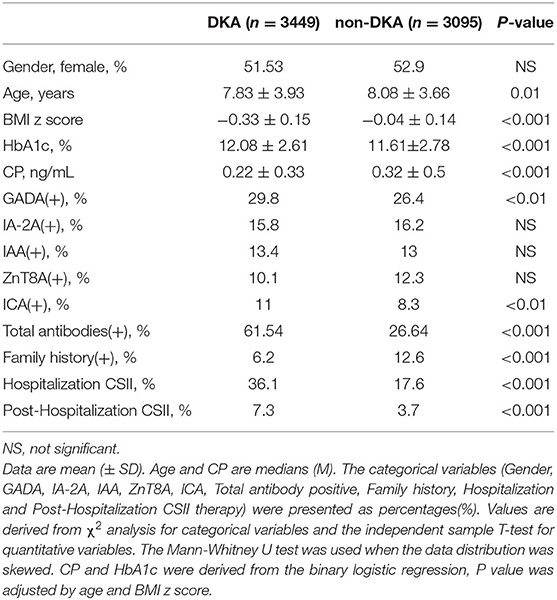
There were noticeable positive correlations between CP and age, CP and BMI z score. CP had a weak correlation with the number of antibodies and family history, respectively. HbA1c was positively correlated with age, but it was inversely correlated with BMI z score. DKA status (yes/no) was positively correlated with the number of existing antibodies (r = 0.17, p = 0.000), while it was negatively correlated with CP. There was no noticeable correlation between HbA1c and CP (Table 1).
Comparative Analysis Among the age Groups
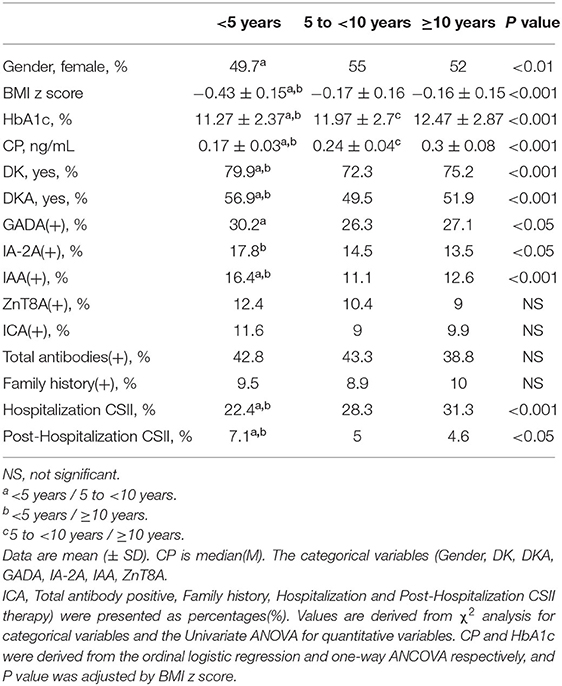
Compared with the 5 to <10 years and ≥10 years groups, the <5 years group had significantly lower BMI z score, adjusted CP, and adjusted HbA1c (Figures 3A,B), and meanwhile, markedly higher the incidence of DK and DKA. Patients in the group of <5 years had a notably lower rate of CSII therapy during the period of hospitalization, while remarkably higher during the post-hospitalization sequential treatment when compared with the 5 to <10 years and ≥10 years groups. Although there was no significant difference in total antibody positive rates among the three age groups, the group of <5 years still had higher positive rates of GADA, IA−2A, and IAA than the others. There was no notably difference in family history among the three age groups (Table 2).
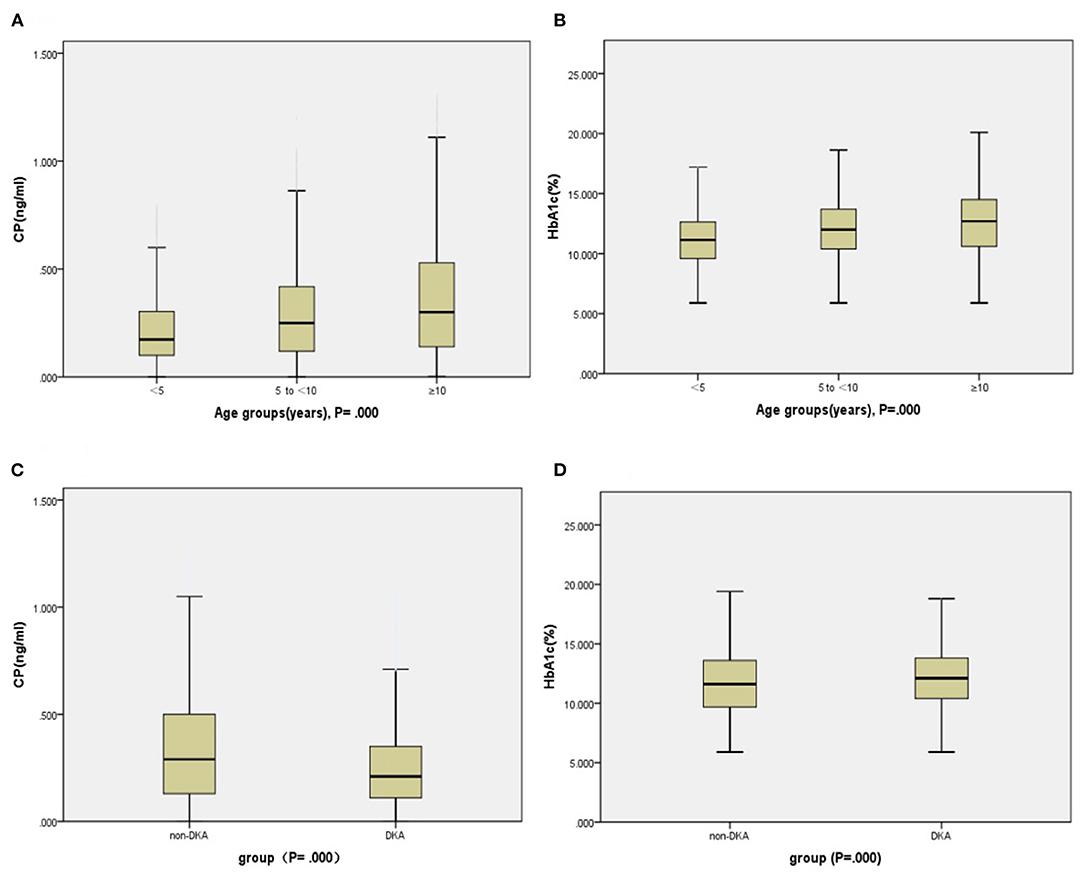
Figure 3. Indices of insulin secretion (CP) and HbA1c in the participants. Panels (A,B): Age groups. Panels (C,D): DKA and non-DKA groups.
Comparative Analysis Between the DKA and Non-DKA Groups
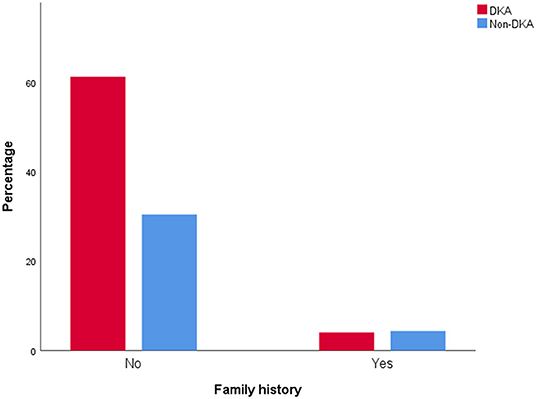
Compared with the non-DKA group, the DKA group had significantly lower age, BMI z score, CP (Figure 3C), and rate of family history positive (Figure 4), but in contrast, dramatically higher GADA, ICA, total antibodies positive rate, HbA1c (Figure 3D), and rate of CSII therapy. There was no notable difference in gender between the groups (Table 3).
Besides, there was no significant difference in gender, age, BMI z score, CP, HbA1c, total antibodies positive rate, family history, and the rate of CSII therapy among the DKA subgroups (mild, moderate, and severe groups).
Discussion
Type 1 diabetes mellitus is a chronic autoimmune disease characterized by the loss of insulin-producing pancreatic β-cells. The pathogenesis of the disease is multifactorial and involves a genetic susceptibility that predisposes to abnormal immune responses in the presence of environmental triggers that insults to the pancreatic islets (18, 19). There is no single clinical feature, which can perfectly distinguish type 1 from non-type 1 diabetes at diagnosis. The classification depends on consideration of risk factors for type 1 vs. other subtypes and the integration of clinical features (e.g., age of diagnosis, BMI, and CP) with biomarkers (e.g., pancreatic autoantibodies) (20). In this cohort study, we found that the clinical incidence of T1DM in children and adolescents in China was 3.16/100,000, and it has been gradually increasing annually. That can indirectly reflect the incidence of T1DM in the general population. The peak period of disease onset was in school-aged children (aged 5 to <10 years, 38.7%), which is similar to the report of the SEARCH (SEARCH for Diabetes in Youth) study in the United States (21). Women had a slightly higher morbidity of T1DM, consistent with the previous findings (4), which may be one of the features of childhood T1DM in China. Although obesity is associated with the increasing presentation of T1DM for a potentially excess β-cell stress (22, 23), the patients were rarely obese at diagnosis in our study. We found that the patients were generally underweight with a lower BMI z score, furthermore, the BMI z score was negatively correlated with the severity of the disease. Despite T1DM is a heritable polygenic disease with identical twin concordance of 30–70%, sibling risk of 6–7%, and a risk of 1–9% for children who have a parent with diabetes (24, 25), the overwhelming majority of the cases occurred in individuals without a family history of diabetes mellitus (90.5%) in our study. It indicated that environmental risk factors may play a crucial role in the pathogenesis of T1DM in addition to genetic factors.
The pathogenesis of T1DM results from a complex interaction between the pancreatic β-cell and innate and adaptive immune systems, inflammation, and selective autoimmune destruction of the pancreatic β-cells resulting in insulin deficiency (26–28). The presence of autoantibodies is associated with the destruction of pancreatic β-cell. Previous studies have reported that over 90% of people with newly diagnosed T1DM have measurable antibodies, they are one of the most reliable biomarkers for autoimmune diabetes in both children and adults (29). In our study, overall 41.8% of the patients with newly diagnosed T1DM had measurable-related antibodies, and 19.8% of the total patients showed two or more serum antibodies positive. The rate of antibody positive is similar to Indian (30), but significantly lower than Caucasian, Japanese, and Korean (31, 32), the discrepancy may be due to the different levels of quality control in the test. Under the background of a low antibody positive rate, in order to avoid misdiagnosing other forms of diabetes among patients with negative autoantibodies, the classification should depend on consideration of risk factors for type 1 and the clinical features (e.g., age of diagnosis, BMI, and CP) and should get the genetic testing if necessary (e.g., it is difficult to distinguish from monogenic diabetes). We also found that patients aged <5 years had a higher positive rate of GADA, IA-2A, and IAA than the older age groups. Previous investigations have suggested that IAA is often the first expressed, especially in younger children, GADA positivity represents a propensity for general autoimmunity, while IA2-A positivity may be a more specific marker of β-cell destruction (19). It could be concluded that patients aged <5 years may be engaged in a very early autoimmunity leading to β-cell destruction and finally the onset of T1DM. People with T1DM have reduced β-cell function at diagnosis when compared with healthy ones (33, 34). Usually, low CP as a marker of severe endogenous insulin deficiency is useful to guide both classification and treatment in cases of diabetes (35). In this cohort, the β-cell function of participants has been severely impaired (reflected by a lower CP). Furthermore, we also find that younger age, lower BMI z score, and the presence of more antibodies are the destructive factors to the β-cell function. In addition, a worsening β-cell function means an increasing incidence of DKA.
Diabetic ketoacidosis is a serious complication of T1DM, which is characterized by the triad of hyperglycemia, acidosis, and ketosis caused by insulin deficiency. It is the main cause of morbidity and mortality in children and adolescents with T1DM due to the short-term risks and long-term consequences (36–39). Previous studies have shown that the frequency of DKA at diagnosis has ranged from 12.8% (Sweden) to 80% (United Arab Emirates), and it is inversely associated with gross domestic product, latitude, and background incidence of T1DM (40, 41). In our study, the incidence rate of DKA at the onset of T1DM was 52.7%, much higher than developed countries and similar to India (5, 42). It can be explained by different levels of disease awareness and healthcare provision, in countries with a low incidence of T1DM, the awareness of DKA tends to be low while the rate of DKA is high, conversely, in countries with higher incidence and prevalence rates of T1DM, people are more effective in the detection of symptoms of new-onset T1DM prior to DKA, and then many children with new-onset T1DM receive insulin therapy in time to avoid DKA. That also can be indirectly explained by the newly diagnosed patients with a family history of T1DM who usually had a low incidence of DKA. That suggests the ways to reduce the rate of DKA even further following appropriate campaigns, such as increased medical awareness and healthcare provision (43). We also find that factors associated with increased risk of DKA are younger age, lower BMI z score, lower CP, higher positive rate of antibodies, and the number of existing antibodies, while the protective factor is having a family history of T1DM at the time of diagnosis. Our finding is supported by a number of preceding observational studies (44, 45). Despite there was no significant correlation between HbA1c and DKA, the DKA group had a significantly higher HbA1c (11.8 ± 2.72, %) when compared with the non-DKA group. We have found that HbA1c is lower in younger patients in the study of age groups, but it is conversely higher in the younger patients with DKA, implying that young children with DKA at diagnosis already have more aggressive dysglycemia and β-cell destruction, which is associated with a low rate of partial remission (37). Unfortunately, we found that the youngest patients (aged <5 years) had the highest incidence rate of DKA at the onset of T1DM (56.9%).
The Diabetes Control and Complications Trial (DCCT) showed that intensive glycemic control improved the outcomes in patients with T1DM (46, 47), and insulin therapy is the cornerstone. In this study, all of the newly diagnosed patients with T1DM received the treatment of insulin during the period of hospitalization, only 27.4% of them adopted insulin pump therapy (CSII), and the rate had plummeted to 5.5% during the post-hospitalization sequential treatment. The percentage of CSII therapy is much lower than in developed countries, such as the United States (50%), Germany, and Australia (74%) (48), that may be caused by multifactors that include economic factors, availability of resources, and patient's propensity to the treatment, etc. It has been previously reported that insulin pump therapy is more efficient in glycemic control and minimizes short- and long-term complications (49, 50). We also found that patients with DKA had a higher rate of CSII treatment both during hospitalization and post-hospitalization when compared with patients with non-DKA, indicating that patients with DKA need more efficient insulin therapy. DKA at diagnosis of T1DM in children predicts poor long-term glycemic control, increasing the risk for long-term complications. Targeted DKA prevention programs could result in substantial healthcare cost reduction and reduced patient morbidity and mortality (51). Public health measures for the prevention and treatment of T1DM should be directed toward the preschoolers (aged <5 years), considering the severity and highest frequency of DKA in this age group. Levels of disease awareness and healthcare provision should be improved timely. Previous studies have shown that immune-mediated β-cell destruction, marked by the presence of diabetes autoantibodies, occurs before and continues after the clinical diagnosis of T1DM, most patients will have complete destruction of β-cells within a few years after diagnosis without a targeted intervention to sustain β-cell function (28, 52). Multiple studies have reported that even the modest preservation of β-cell function could achieve better glycemic control and long-term benefits (53–55). Hence, more efforts should be dedicated to achieve early screening and diagnosis of the disease and preserve β-cell function as early as possible.
The primary limitation of this study is that as a multicenter study, the laboratory tests, such as HbA1c, autoantibody, and CP, were not measured in a central laboratory and this could introduce bias, although all the tests were performed using a standardized assay protocol. Furthermore, differences in race/ethnicity factors among the participants may explain some of the outcome differences, though the number of participants of racial and ethnic minorities is very small, we cannot make a comparative study between different ethnic groups due to the lack of relevant data. Further research studies are urgently required.
The T1DM China Study Group for Children and Adolescents
Guo-Hua Li, Ke Huang, Guan-Ping Dong, Jian-Wei Zhang, Chun-Xiu Gong, Fei-Hong Luo, Xiao-Ping Luo, Chun-Lin Wang, Min Zhu, Pin Li, Zhi-Ya Dong, Hua-Mei Ma, Li Liu, Hong-Wei Du, Lan-Wei Cui, Yu Yang, Zhi-Hua Wang, Gui-Mei Li, Xin-Ran Cheng, Mireguli.Maimaiti, Zhe Su, Hai-Yan Wei, Rong-Xiu Zheng, Ying Xin, Tang Li, Rui-Min Chen, Lin-Qi Chen, Fan Yang, Shao-Ke Chen, Yu-Qing Chen, Ying-Xue, Yu-Liu, Ling Wang, Jun-Fen Fu*
Guo-Hua Li, Ke Huang, Guan-Ping Dong, Jun-Fen Fu*, Department of Endocrinology, The Children's Hospital of the Zhejiang University School of Medicine, National clinical research center for child health, Hangzhou, China. Jian-Wei Zhang, Department of Pediatrics, Shaoxing Women and Children's Hospital, Hangzhou, China. Chun-Xiu Gong, Department of Endocrinology, Genetics, and Metabolism, Beijing Diabetes Center for Children and Adolescents, Medical Genetics Department, Beijing Children's Hospital, Beijing, China. Fei-Hong Luo, Department of Pediatric Endocrinology and Inherited Metabolic Diseases, Children's Hospital of Fudan University, Shanghai, China. Xiao-Ping Luo, Department of Pediatrics, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. Chun-Lin Wang, Department of Pediatric, The First Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China. Min Zhu, Department of Endocrinology, Children's Hospital of Chongqing Medical University, Chongqing, China. Pin Li, Department of Endocrinology, Shanghai Children's Hospital of Shanghai Jiao Tong University, Shanghai, China. Zhi-Ya Dong, Department of Pediatrics, Ruijin Hospital, Shanghai Jiao Tong University, Shanghai, China. Hua-Mei Ma, Department of Pediatrics, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China. Li Liu, Department of Genetics and Endocrinology, Guangzhou Women and Children's Medical Center, Guangzhou, China. Hong-Wei Du, Department of Pediatric Endocrinology, The First Bethune Hospital of Jilin University, Changchun, China. Lan-Wei Cui, Department of Pediatric, The First Affiliated Hospital of Harbin Medical University, Harbin, China. Yu Yang, Department of Endocrinology, Children's Hospital of Nanchang University & Jiangxi Provincial Children's Hospital, Nanchang, China. Zhi-Hua Wang, Department of Endocrinology, Xi'an Children's Hospital, Xi'an, China. Gui-Mei Li, Department of Pediatric, Shandong Provincial Hospital, Jinan, China. Xin-Ran Cheng, Department of Endocrinology, Chengdu Women's and Children's Central Hospital, Chengdu, China. Mireguli. Maimaiti Department of Pediatric, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China. Zhe Su, Department of Endocrinology, Shenzhen Children's Hospital, Shenzhen, China. Hai-Yan Wei, Department of Pediatric Endocrinology and Inherited Metabolic Diseases, Henan Children's Hospital, Zhengzhou, China. Rong-Xiu Zheng, Department of Pediatric, Tianjin Medical University General Hospital, Tianjin, China. Ying Xin, Department of Endocrinology, Shengjing hospital of China Medical University, Shenyang, China. Tang Li, Department of Pediatrics, Qingdao women and children's hospital, Qingdao University, Qingdao, China. Rui-Min Chen, Department of Endocrinology, Children's Hospital of Fuzhou, Fujian Province, Fuzhou, China. Lin-Qi Chen, Department of Pediatric Endocrinology, Children's Hospital Affiliated to Soochow University, Suzhou, China. Fan Yang, Department of Pediatrics, West China 2nd University Hospital, Sichuan University, China. Shao-Ke Chen, Department of Pediatrics, The Second Affiliated Hospital of Guangxi Medical University, Nanning, China. Yu-Qing Chen, Department of Endocrinology and Rheumatology, Anhui Provincial Children's Hospital, Hefei, China. Ying Xue, Department of Pediatric Endocrinology, Xuzhou Children's Hospital, Xuzhou, China. Yu Liu, Department of Pediatric Endocrinology and Inherited Metabolic Diseases, Guiyang Maternal and Child Health Hospital, Guiyang, China. Ling Wang, Laboratory for Translational Genetics, Department of Human Genetics, KU Leuven, Leuven, Belgium. These authors contributed equally to the study. The T1DM China Study Group for Children and Adolescents is officially authorized by the Bureau of Medical Administration, Inspection and Supervision of National Health and Family Planning Commission of the People's Republic of China, funded by China International Medical Foundation, and supervised by the Chinese Medical Association.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Zhejiang's Institutional Review Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
G-HL and J-FF conceptualized and designed the clinical study. KH, G-PD, J-WZ strengthened the coordination and development of the project among various research centers. G-HL accomplished the data collection and analysis with the assistance of LW, and wrote the manuscript. All authors were responsible for the project carried out in their own centers. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the project of Prevalence and Risk of Obesity and Diabetes in Youth (PRODY) and was funded by the National Key Research and Development Program of China (no. 2016YFC1305301), the National Natural Science Foundation of China (no. 81570759), the Fundamental Research Funds for the Central Universities (2020XZZX002-22), the Research Fund of Zhejiang Major Medical and Health Science and Technology & National Ministry of Health (WKJ-ZJ-1804), Zhejiang Provincial Key Science and Technology Project (LGF21H070004), Zhejiang Provincial Natural Science Foundation of China [grant number LQ20H070003], and Zhejiang Province Natural Sciences Foundation Zhejiang Society for Mathematical Medicine (LSZ19H070001). The effect of intestinal flora and its metabolites on the changes in islet function in children with type 1 diabetes mellitus during the honeymoon (no. LGF21H070004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to thank all centers participating in the study. The authors are also deeply grateful for the commitment of the study participants and their parents. Special thanks to Prof. Yan Ni for her professional suggestion on data analysis.
Abbreviations
T1DM, type 1 diabetes mellitus; CP, C-peptide; CAGR, compound annual growth rate; DKA, diabetic ketoacidosis; DK, Diabetic ketosis; BMI, body mass index; HbA1c, glycated hemoglobin; GADA, glutamic acid decarboxylase antibody; IA-2A, insulinoma antigen-2 antibody; IAA, insulin autoantibodies; ZnT8A, Zinc Transporter 8 antibody; ICA, islet cell antibody; CSII, continuous subcutaneous insulin infusion.
References
1. Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, Ogle GD. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. (2019) 157:107842. doi: 10.1016/j.diabres.2019.107842
2. Green A, Hede SM, Patterson CC, Wild SH, Imperatore G, Roglic G, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. (2021) 64:2741–50. doi: 10.1007/s00125-021-05571-8
3. Tuomilehto J, Ogle GD, Lund-Blix NA, Stene LC. Update on worldwide trends in occurrence of childhood type 1 diabetes in 2020. Pediatr Endocrinol Rev. (2020) 17:198–209. doi: 10.17458/per.vol17.2020.tol.epidemiologychildtype1diabetes
4. Liu C, Yuan YC, Guo MN, Xin Z, Chen GJ, Bentley AR, et al. Incidence of type 1 diabetes may be underestimated in the Chinese population: evidence from 21.7 million people between 2007 and 2017. Diabetes Care. (2021) 44:2503–9. doi: 10.2337/dc21-0342
5. Weng J, Zhou Z, Guo L, Zhu D, Ji L, Luo X, et al. T1D China Study Group. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. (2018) 360: j5295. doi: 10.1136/bmj.j5295
6. Gong C, Meng X, Jiang Y, Wang X, Cui H, Chen X. Trends in childhood type 1 diabetes mellitus incidence in Beijing from 1995 to 2010: a retrospective multicenter study based on hospitalization data. Diabetes Technol Ther. (2015) 17:159–65. doi: 10.1089/dia.2014.0205
7. Zhao Z, Sun C, Wang C, Li P, Wang W, Ye J, et al. Rapidly rising incidence of childhood type 1 diabetes in Chinese population: epidemiology in Shanghai during 1997-2011. Acta Diabetol. (2014) 51:947–53. doi: 10.1007/s00592-014-0590-2
8. Wu HB, Zhong JM, Hu RY, Wang H, Gong WW, Pan J, et al. Rapidly rising incidence of type 1 diabetes in children and adolescents aged 0-19 years in Zhejiang, China, 2007 to 2013. Diabet Med. (2016) 33:1339–46. doi: 10.1111/dme.13010
9. Subspecialty Subspecialty Group of Endocrinologic Hereditary and Metabolic Diseases the the Society of Pediatrics Chinese Medical Association; Editorial Board Chinese Chinese Journal of Pediatrics. [Expert consensus on the standardized diagnosis and management of type 1 diabetes mellitus in Chinese children]. Zhonghua Er Ke Za Zhi. (2020) 58:447–54. doi: 10.3760/cma.j.cn112140-20200221-00124
10. Morgan E, Black CR, Abid N, Cardwell CR, McCance DR, Patterson CC. Mortality in type 1 diabetes diagnosed in childhood in Northern Ireland during 1989-2012: a population-based cohort study. Pediatr Diabetes. (2018) 19:166–70. doi: 10.1111/pedi.12539
11. Rawshani A, Sattar N, Franzén S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. (2018) 392:477–86. doi: 10.1016/S0140-6736(18)31506-X
12. American Diabetes Association. Standards of medical care in diabetes−2014. Diabetes Care. (2014) 37 (Suppl. 1):S14–80. doi: 10.2337/dc14-S014
13. American Diabetes Association. Classification and diagnosis of diabetes. Sec. 2. In standards of medical care in diabetes-2017. Diabetes Care. (2017) 40(Suppl. 1):S11–24 doi: 10.2337/dc17-S005
14. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care. (2019) 42(Suppl. 1):S13–28. doi: 10.2337/dc19-S002
15. Wolfsdorf JI, Allgrove J, Craig ME, Edge J, Glaser N, Jain V, et al. ISPAD Clinical Practice Consensus Guidelines 2014. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatric Diabetes. (2014) 15:154–79. doi: 10.1111/pedi.12165
16. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. Clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. (2018) 19 Suppl 27:155–77. doi: 10.1111/pedi.12701
17. Li H, Ji CY, Zong XN, et al. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years[J]. Zhonghua er ke za zhi. (2009) 47:487–49223.
18. Anaya JM. The diagnosis and clinical significance of polyautoimmunity. Autoimmun Rev. (2014) 13:423–6. doi: 10.1016/j.autrev.2014.01.049
19. Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev. (2016) 15:644–8. doi: 10.1016/j.autrev.2016.02.017
20. Shields BM, Peters JL, Cooper C, Lowe J, Knight BA, Powell RJ, et al. Can clinical features be used to differentiate type 1 from type 2 diabetes? A systematic review of the literature. BMJ Open. (2015) 5:e009088. doi: 10.1136/bmjopen-2015-009088
21. Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Search for diabetes in youth study. incidence trends of type 1 and type 2 diabetes among youths, 2002-2012. N Engl J Med. (2017) 376:1419–29. doi: 10.1056/NEJMoa1610187
22. Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. (2016) 387: 2340–48. doi: 10.1016/S0140-6736(16)30507-4
23. Ferrara CT, Geyer SM, Liu YF, et al. Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care. (2017) 40:698–7011. doi: 10.2337/dc16-2331
24. Triolo TM, Fouts A, Pyle L, Yu L, Gottlieb PA, Steck AK. Type 1 diabetes trialnet study group. identical and nonidentical twins: risk and factors involved in development of islet autoimmunity and type 1 diabetes. Diabetes Care. (2019) 42:192–9. doi: 10.2337/dc18-0288
25. Pociot F, Lernmark Å. Genetic risk factors for type 1 diabetes. Lancet. (2016) 387:2331–39. doi: 10.1016/S0140-6736(16)30582-7
26. Hull CM, Peakman M, Tree TIM. Regulatory T cell dysfunction in type 1 diabetes:what's broken and how can we fix it? Diabetologia. (2017) 60:1839–50. doi: 10.1007/s00125-017-4377-1
27. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. (2010) 464:1293–300. doi: 10.1038/nature08933
28. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. (2018) 391:2449–62. doi: 10.1016/S0140-6736(18)31320-5
29. Mayer-Davis EJ, Kahkoska AR, Jefferies C, Dabelea D, Balde N, Gong CX, et al. Clinical practice consensus guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. (2018) 19 Suppl 27:7–19. doi: 10.1111/pedi.12773
30. Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. (2016) 12:357–70. Epub 2016 Apr 15. PMID: 27080137. doi: 10.1038/nrendo.2016.53
31. Kawasaki E. Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol. (2014) 23:99–105. doi: 10.1297/cpe.23.99
32. Kim KY, Kim MS, Lee YJ, Lee YA, Lee SY, Shin CH, Kim JH. Glutamic acid decarboxylase and tyrosine phosphatase-related islet antigen-2 positivity among children and adolescents with diabetes in korea. Diabetes Metab J. (2022). doi: 10.4093/dmj.2021.0332. [Epub ahead of print].
33. Evans-Molina C, Sims EK, DiMeglio LA, Ismail HM, Steck AK, Palmer JP, et al. Type 1 Diabetes TrialNet Study Group. β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight. (2018) 3:e120877. doi: 10.1172/jci.insight.120877
34. Dabelea D, Mayer-Davis EJ, Andrews JS, Dolan LM, Pihoker C, Hamman RF, et al. Clinical evolution of beta cell function in youth with diabetes: the SEARCH for diabetes in youth study. Diabetologia. (2012) 55:3359–68. doi: 10.1007/s00125-012-2719-6
35. Jones AG, Hattersley AT. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet Med. (2013) 30:803–17. doi: 10.1111/dme.12159
36. Hursh BE, Ronsley R, Islam N, Mammen C, Panagiotopoulos C. Acute kidney injury in children with type 1 diabetes hospitalized for diabetic ketoacidosis. JAMA Pediatr. (2017) 171:e170020. doi: 10.1001/jamapediatrics.2017.0020
37. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long-term glycemic control. Diabetes Care. (2017) 40:1249–55. doi: 10.2337/dc17-0558
38. Cameron FJ, Scratch SE, Nadebaum C, Northam EA, Koves I, Jennings J, et al. DKA Brain Injury Study Group. Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care. (2014) 37:1554–62. doi: 10.2337/dc13-1904
39. Jawaid A, Sohaila A, Mohammad N, Rabbani U. Frequency, clinical characteristics, biochemical findings and outcomes of DKA at the onset of type-1 DM in young children and adolescents living in a developing country - an experience from a pediatric emergency department. J Pediatr Endocrinol Metab. (2019) 32:115–9. doi: 10.1515/jpem-2018-0324
40. Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. (2012) 55:2878–94. doi: 10.1007/s00125-012-2690-2
41. Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with Human Development Index (HDI): an updated systematic review, meta-analysis, and meta-regression. Horm Metab Res. (2018) 50:209–22. doi: 10.1055/s-0044-102090
42. Kanwal SK, Bando A, Kumar V. Clinical profile of diabetic ketoacidosis in Indian children. Indian J Pediatr. (2012) 79:901–4. doi: 10.1007/s12098-011-0634-3
43. Martin Holder, Stefan Ehehalt. Significant reduction of ketoacidosis at diabetes onset in children and adolescents with type 1 diabetes-the stuttgart diabetes awareness campaign, Germany. Pediatr Diabetes. (2020) 21:1227–1231. doi: 10.1111/pedi.13064
44. Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. (2011) 343:d4092. doi: 10.1136/bmj.d4092
45. de Vries L, Oren L, Lazar L, Lebenthal Y, Shalitin S, Phillip M. Factors associated with diabetic ketoacidosis at onset of Type 1 diabetes in children and adolescents. Diabet Med. (2013) 30:1360–6. doi: 10.1111/dme.12252
46. Danne T, Phillip M, Buckingham BA, Jarosz-Chobot P, Saboo B, Urakami T, et al. Clinical practice consensus guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. (2018) 19 Suppl 27:115–35. doi: 10.1111/pedi.12718
47. American Diabetes Association. 13. Children and adolescents: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43(Suppl. 1):S163–82. doi: 10.2337/dc20-S013
48. Maahs DM, Hermann JM, DuBose SN, Miller KM, Heidtmann B, DiMeglio LA, et al. DPV Initiative; T1D Exchange Clinic Network. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D exchange and German/Austrian DPV registries. Diabetologia. (2014) 57: 1578–85. 10.1007/s00125-014-3272-2. doi: 10.1007/s00125-014-3272-2
49. Karges B, Schwandt A, Heidtmann B, Kordonouri O, Binder E, Schierloh U, et al. Association of insulin pump therapy vs insulin injection therapy with severe hypo, Ketoacidosis, and Glycemic control among children, adolescents, and young adults with T1DM. JAMA. (2017) 318:1358–66. doi: 10.1001/jama.2017.13994
50. Maria Ida Maiorino, Ofelia Casciano, Elisabetta Della Volpe, Giuseppe Bellastella, Dario Giugliano, Katherine Esposito. Reducing glucose variability with continuous subcutaneous insulin infusion increases endothelial progenitor cells in type 1 diabetes: an observational study. Endocrine. (2016) 52: 244–52. doi: 10.1007/s12020-015-0686-7
51. Maahs DM, Hermann JM, Holman N, Foster NC, Kapellen TM, Allgrove J, et al. National paediatric diabetes audit and the royal college of paediatrics and child health, the DPV initiative, and the T1D exchange clinic network. rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the US, Austria, and Germany. Diabetes Care. (2015) 38:1876–82. doi: 10.2337/dc15-0780
52. Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. (2017) 3:17016. PMID: 28358037. doi: 10.1038/nrdp.2017.16
53. Pinckney A, Rigby MR, Keyes-Elstein L, Soppe CL, Nepom GT, Ehlers MR. Correlation among hypoglycemia, glycemic variability, and C-peptide preservation after alefacept therapy in patients with type 1 diabetes mellitus: analysis of data from the immune tolerance network T1DAL trial. Clin Ther. (2016) 38:1327–39. doi: 10.1016/j.clinthera.2016.04.032
54. Sørensen JS, Johannesen J, Pociot F, et al. Residual β-cell function 3–6 years after onset of type 1 diabetes reduces risk of severe hypoglycemia in children and adolescents. Diabetes Care. (2013) 36:3454–59. doi: 10.2337/dc13-0418
Keywords: type 1 diabetes, incidence, diabetic ketoacidosis, β-cell function, children and adolescences
Citation: Li G-H, Huang K, Dong G-P, Zhang J-W, Gong C-X, Luo F-H, Luo X-P, Wang C-L, Zhu M, Li P, Wang L, Fu J-F and The T1DM China Study Group for Children Adolescents (2022) Clinical Incidence and Characteristics of Newly Diagnosed Type 1 Diabetes in Chinese Children and Adolescents: A Nationwide Registry Study of 34 Medical Centers. Front. Pediatr. 10:888370. doi: 10.3389/fped.2022.888370
Received: 02 March 2022; Accepted: 19 April 2022;
Published: 15 June 2022.
Edited by:
Stefano Passanisi, University of Messina, ItalyReviewed by:
Giulio Maltoni, Sant'Orsola-Malpighi Polyclinic, ItalyFrancesco Maria Rosanio, Federico II University Hospital, Italy
Copyright © 2022 Li, Huang, Dong, Zhang, Gong, Luo, Luo, Wang, Zhu, Li, Wang, Fu and The T1DM China Study Group for Children Adolescents. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun-Fen Fu, ZmpmNjhAemp1LmVkdS5jbg==; ZmpmNjhAcXEuY29t
 Guo-Hua Li
Guo-Hua Li Ke Huang
Ke Huang Guan-Ping Dong
Guan-Ping Dong Jian-Wei Zhang2
Jian-Wei Zhang2 Chun-Xiu Gong
Chun-Xiu Gong Fei-Hong Luo
Fei-Hong Luo Xiao-Ping Luo
Xiao-Ping Luo Chun-Lin Wang
Chun-Lin Wang Jun-Fen Fu
Jun-Fen Fu