
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr., 19 May 2022
Sec. General Pediatrics and Pediatric Emergency Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.886551
This article is part of the Research TopicHot Topics in PediatricsView all 50 articles
 Laura Polloni1,2
Laura Polloni1,2 Ileana Baldi3
Ileana Baldi3 Margherita Amadi4
Margherita Amadi4 Valentina Tonazzo4
Valentina Tonazzo4 Roberta Bonaguro1
Roberta Bonaguro1 Francesca Lazzarotto1
Francesca Lazzarotto1 Alice Toniolo1
Alice Toniolo1 Dario Gregori3
Dario Gregori3 Antonella Muraro1*
Antonella Muraro1*Background: Anaphylaxis is a life-threatening event, but it is frequently undertreated in pediatric patients with food allergies. Previous studies showed that auto-injectable adrenaline (AAI) is underused by patients and parents. This is especially troubling since fatal anaphylaxis has been associated with delayed adrenaline administration.
Objectives: This study aimed to investigate parental practice and knowledge in anaphylaxis management, and perceived barriers and facilitators in using AAI.
Results: A retrospective survey was completed by 75 parents (41 mothers, 34 fathers) of children with food allergy and AAI prescription attending the Food Allergy Referral Center of Veneto, Italy. Results showed poor parental preparedness and reluctance to use AAI despite a high/moderate self-rated knowledge (median total score of 23–min. 3, max. 30). Most parents (77%) declared they were carrying AAI but only 20% used it in case of a severe reaction. Most reported Fear/Fear of making mistakes (46 parents) and Concern about possible side effects as barriers (35), while Poor knowledge of the correct AAI use (1) and Lack of knowledge/ incorrect assessment of symptoms (2) were reported less frequently. Theoretical-practical courses for parents on AAI use (65), Psycho-education/Psychological support (3) for better dealing with the emotional aspects of anaphylaxis and Written instructions (1) have been suggested as main facilitators.
Conclusion: Understanding parents' experience and perspective on managing anaphylaxis is crucial to implement effective educational programs. A multidisciplinary approach should be considered.
Anaphylaxis is a life-threatening reaction characterized by acute onset of symptoms involving different organ systems and requiring immediate intervention (4–6). Although the fatality rate due to anaphylaxis remains low (7), the frequency of hospitalization from food-induced anaphylaxis has been increasing in recent years (8). The symptoms of anaphylaxis are highly variable. Skin, mucosal and gastrointestinal symptoms occur most frequently (>90% of cases) followed by symptoms involving the respiratory and cardiovascular systems (>50%) (5, 6). Food is the most common elicitor of anaphylactic reactions among children and adolescents (9, 10). Intramuscular adrenaline is the treatment of choice for food-related anaphylactic reactions and adrenaline autoinjectors (AAI) are recommended for the first-line management of anaphylaxis in the community, since they are relatively safe, have a low risk of error and are fast to administer (5, 6). Although AAI are routinely prescribed for patients at risk of serious reactions, previous studies have shown an underuse by patients and parents (11–20). A retrospective study on children with a history of anaphylaxis (15) found that recurrent generalized allergic reactions occurred with a frequency of 0.98 episodes per patient per year and were more common in those with food compared with insect venom anaphylaxis. The AAI was only used in 29% of recurrent anaphylactic reactions. Parental knowledge was deficient in recognition of the symptoms of anaphylaxis and use of the AAI device. Those children in whom the AAI was used were less likely to require hospital admission (15).
Another study on parental use of AAI for children with food-induced anaphylaxis reported that only 8% of parents had administered it. Neither a history of anaphylaxis nor knowledge correlated with an increased level of comfort with administration (16). Similarly, a further research showed that 69% of parents were unable to use the AAI, did not have it available, or did not know when it should be administered (12).
This pattern is worrying given that most cases of reported deaths from anaphylaxis have been associated with delayed administration of epinephrine (13, 21). It is essential to understand the parents' perspective in order to facilitate the use of AAI. The present study is part of a larger project on quality of life and management of FA and anaphylaxis in the family. This preliminary part investigated parental previous experience of managing food-induced anaphylaxis, perceived knowledge, and perceived barriers and facilities about the use of AAI focusing mainly on descriptive and qualitative analysis of parents' viewpoint.
A total of 75 Italian parents of children with FA took part in the study. Inclusion criteria comprised that (4) children were confirmed suffering from immunoglobulin E (IgE)-mediated food allergy by an allergist considering their clinical history, the evidence of sensitization and a positive food challenge or positive skin prick test and/or serum-specific IgE results; (5) children were prescribed with an AAI.
All participants developed food allergy in early childhood and did not suffer from serious concomitant non-allergic disease. Food involved in patients' food allergy were milk, egg, wheat, fish and nuts.
Parents have been trained by an allergist to use AAI at least once a year through practical demonstration with an AAI training device and verbal instructions. They also received a written emergency plan for the school.
A survey about parental management and perceived knowledge of anaphylaxis was carried out at the Food Allergy Referral Center, Veneto Region in Padua (North Eastern Italy). Parents were invited to participate (as part of a larger project on quality of life and management of FA and anaphylaxis in the family) while accompanying the children to clinical visits. They were given an information sheet outlining the study and encouraged to ask the researcher questions. If interested in participating, they signed a written consent form. Participants were recruited sequentially over a period of 12 months. The project was approved by the Ethic Committee of Psychological Research of Padua University (registration number 7A30FE81F8F234480D5A94C49C270FF3). It was performed in respect of the European regulation regarding potential sensitive data and according to World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects.
The survey was made up of four sections:
1. Socio-demographic and clinical data: Clinical data reported by parents were cross-checked with medical record data.
2. Questions on previous anaphylactic experience: Parents were asked if they had ever managed an anaphylactic reaction (4–6) of their child, and if so, they were asked how they acted, choosing from a list of possible behaviors (showed in Figure 1). This list was devised following a focus group with some parents on how they behaved when managing a severe allergic reaction and based on current European guidelines on anaphylaxis management (5).
3. Parental self-rated knowledge on anaphylaxis management: Parents were asked to answer the following questions on a Likert scale from 1 (completely insufficient) to 10 (excellent)
• How they defined their degree of knowledge of food-induced anaphylaxis (e.g., knowledge of symptoms)
• How did they define their degree of knowledge of the correct use of AAI (e.g., how the AAI is used in practice)
• How did they define their degree of knowledge of the management of anaphylaxis (e.g., what to do in case of anaphylaxis).
4. Questions on barriers and facilities about AAI parental use: Mothers and fathers were asked why, in their opinion, parents may be reluctant to administer AAI (barriers) and what could help them (facilities). Both questions were open-ended.
Parents reported having managed at least one of their child's anaphylaxis were asked to complete all the four sections of the survey. Parents who never managed their child's anaphylaxis completed sections 1, 3, and 4.
Descriptive analysis is presented stratified by parent's gender and overall, with percentages (number of cases) or median (first, third quartile), as appropriate. Fisher and Chi square test were used for univariable analysis. A linear regression model was used to investigate the effect of socio-demographic characteristics and previous anaphylactic experience on perceived knowledge total score. The model building strategy was established on the basis of a backward selection procedure and the Akaike Information Criterion (AIC). The concept underlying the AIC is to examine the complexity of the model together with its goodness-of-fit to the sample data and to produce a measure balancing the two. The usual procedure is to compute the AIC for each model and select that corresponding to the lowest value.
In addition, a qualitative analysis was carried out on parents' open responses about the barriers and facilitators of using AAI. The analysis of the texts was carried out by two independent judges (MA and VT) who identified and attributed categories that could describe parental responses in a salient way. The identified categories were supervised by LP and AM. In the phase of attribution of the categories, in case of lack of agreement between the judges, the category was discussed until 100% agreement was reached.
Parents and children's characteristics are shown in Table 1. Thirty-four fathers and 41 mothers of children with FA and AAI prescription (age 3–14) participated. Most children had multiple FA (69.3%) and comorbidity with asthma and/or eczema (77.3%). Sixty-four parents (85.3%) reported having managed at least one of their child's anaphylaxis and fully completed the second section of the survey (Questions on previous anaphylactic experience), while the remaining parents completed only section 1, 3, and 4. As showed in Figure 1, 77% of parents were carrying AAI and 20% would have used AAI often or always. Most parents, 81%, administered antihistamines, 52% asked the other parent for help, 48% went to the hospital or called the doctor, 35% administered bronchodilator and 36% cortisone, while 17% called the emergency service. No differences appear when the responses are stratified by gender (Supplementary Table 1).
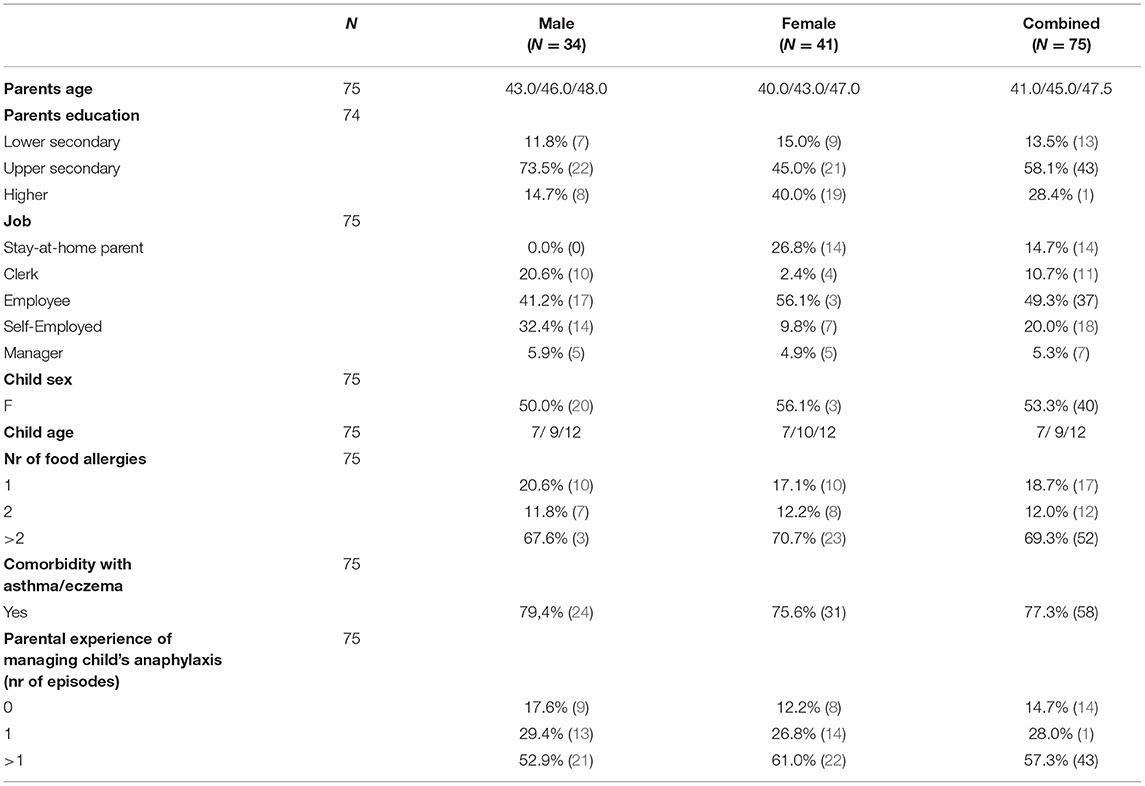
Table 1. Participants' characteristics stratified by parent's gender and overall, with percentages (number of cases) or median (first, third quartile), as appropriate.
With regard to Parental self-rated knowledge on anaphylaxis management, parents reported a median score of 7 on a 1–10 Likert scale on knowledge of anaphylaxis symptoms, a median score of 8 on knowledge of AAI Use, a median score of 7 on knowledge of anaphylaxis management and a median total score of 23 (min. 3, max. 30). The Fisher test found no significant differences between mothers and fathers' scores (Supplementary Table 2).
Stepwise regression results (Table 2) indicate that one age increase in patient age and previous episodes of managing anaphylaxis (as compared to none) significantly increase the mean Total score of 0.53 and 3.15, respectively.
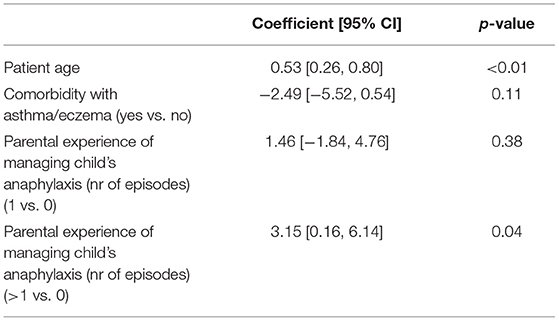
Table 2. Linear regression on perceived knowledge total score with backward selection of variables based on AIC.
As showed in Table 3 qualitative analysis on parents' open responses about the barriers and facilitators of using AAI led to the identification of four categories for each. The barriers identified by the parents are in order of frequency: (4) Fear/Fear of making mistakes; (5) Concern about possible side effects resulting from the administration of adrenaline; (6) Poor knowledge of the correct procedure for using the AAI device; (7) Lack of knowledge/ incorrect assessment of symptoms that require AAI. The facilitators identified in order of frequency are: (4) Theoretical-practical courses on AAI use for parents; (5) Psycho-education/Psychological support; (6) Written Instructions; (7) Parent support groups. More than one answer was possible.
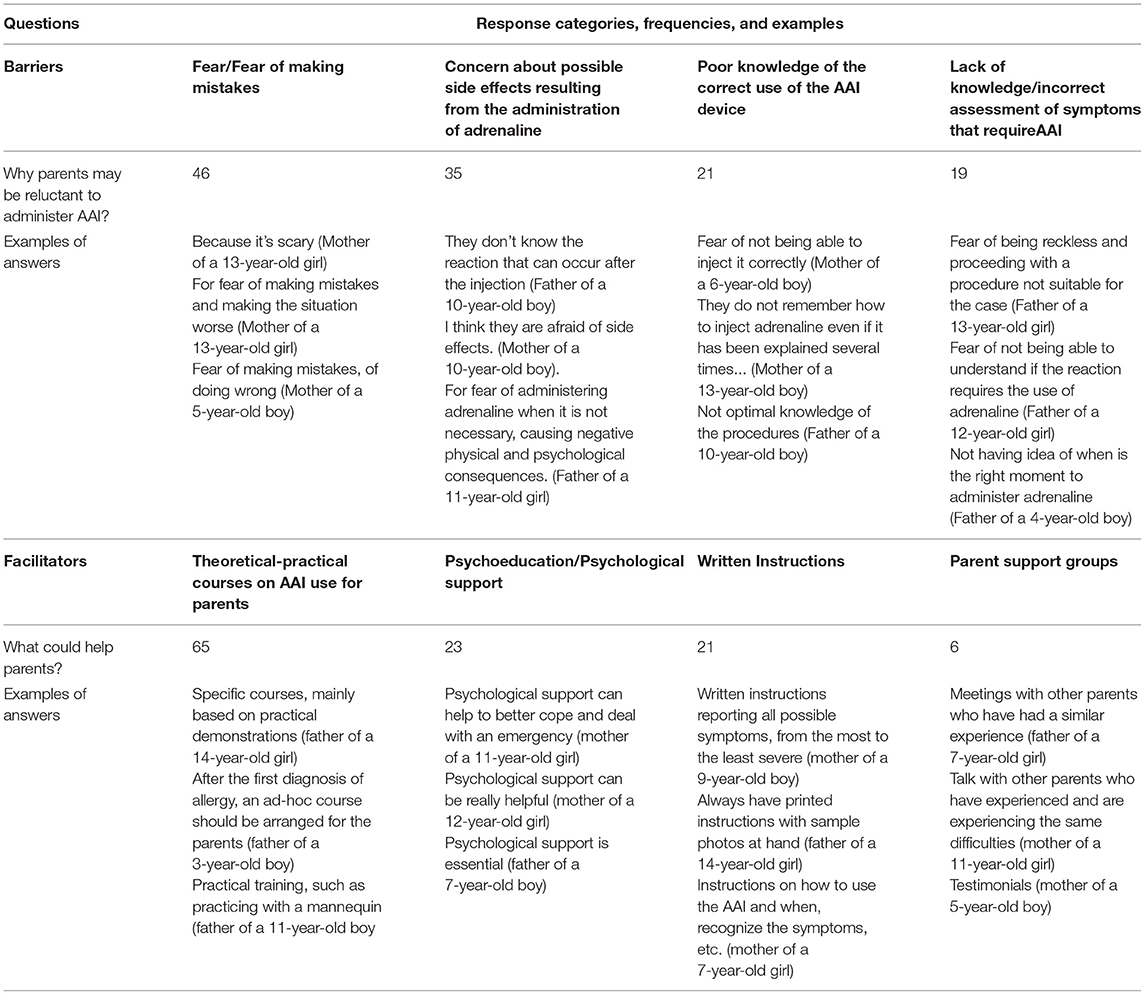
Table 3. Qualitative analysis on parents' open responses about the barriers and facilitators of using AAI: categories, frequencies, and examples.
The results of this survey showed poor parental preparedness for managing food-induced anaphylaxis and reluctance to use AAI despite a routine training (at least once a year) and a high/moderate self-rated knowledge on anaphylaxis management. The majority of parents (81%) administered antihistamines, and 36% cortisone, although they are both not recommended for the acute management of anaphylaxis (2). If used prior to adrenaline administration, antihistamines/glucocorticoids administration could lead to a delay in first-line treatment of anaphylaxis. Adrenaline is the first-line treatment of anaphylaxis because it has a faster onset of action and more appropriate and robust pharmacologic action (2, 6, 10). Most parents (77%) declared they were carrying AAI at the time of their children severe allergic reactions but only 20% would have used it often or always. Results are in line with previous researches (11–19) and support the importance of investigating the reasons why parents do not act properly in case of anaphylaxis. A recent study involving 164 caregivers (17) reported that the majority did not give their allergic children AAI at the time of their most severe allergic reactions, despite declaring confidence in their ability to treat allergic reactions. Reasons caregivers indicated for not administering the AAI included the following: reactions did not seem severe enough; use of other medication; and fear of using AAI. A qualitative study (ten parents involved) exploring parental experiences with AAI use for their child's anaphylaxis found that parents uniformly described feelings of isolation, fear/anxiety, hesitation and guilt during reactions, that constitute emotional barriers. Accordingly, parents in the present study mostly reported Fear/Fear of making mistakes and Concern about possible side effects as barriers, while Poor knowledge of the correct AAI use and Lack of knowledge/ incorrect assessment of symptoms were reported less frequently. A number of previous studies on parental preparedness for managing anaphylaxis focused on knowledge (e.g., recognition of the symptoms of anaphylaxis and use of the AAI device) (1, 12, 15, 25) highlighting specific knowledge deficits that could impact negatively. In addition to deficiencies in knowledge, given the critical nature of anaphylaxis, the psychological component (e.g., fear or anxiety) has been found preponderant to the underuse of AAI (3, 16). A study involving 165 parents reported that parental empowerment directly correlated with increased comfort with AAI use, but knowledge did not (16). Other researches (3, 11, 17) found that the most common reasons among parents for not using adrenaline despite carrying was concern about adverse effects, fear about the injection, and not wanting to activate emergency services or go to the emergency department.
Fear might be a psychological factor that paralyzes instead of enabling a parent to act accordingly in the event of anaphylaxis in their child (3, 16). Prior studies (22, 24, 26–28) have confirmed the psychological effect that food allergy has on families functioning and wellbeing. These burdens might also impair a parent's response to an acute life-threatening event. It has been demonstrated that among parents who experienced their child anaphylaxis, 14.6% developed an acute stress disorder and this was comparable to the percentage of PTSD in adults who experienced anaphylaxis themselves (23). Further researches should investigate psychological factors (e.g., fear/anxiety, self-efficacy) contributing to parental preparedness for managing food-induced anaphylaxis in order to remove or mitigate barriers.
To our knowledge this is the first study exploring parental perspective not only about barriers but also facilitators. Parents reported Theoretical-practical courses on AAI use for parents as main help and Written available instructions. This underscores the need for continued focus on educating patients and caregivers to recognize signs and symptoms of anaphylaxis, understanding the role of different allergy medications and effectively using AAI (17). Many participants suggested Psycho-education/Psychological support for better dealing with the emotional aspects of anaphylaxis management (e.g., fear/anxiety, stress) and a limited number reported Parent support groups to share experiences. This highlights the importance to develop effective training strategies to prepare caregivers to act during stressful situations. It is worth to note that the Food Allergy Center of Veneto Region has a dedicated psychological service for supporting patients and families in the psycho-socio-emotional management of food allergy and anaphylaxis.
This preliminary study is very descriptive in its nature and some limitations need to be acknowledged. The sample is quite homogeneous in its characteristics and all patients were attending a third level Food Allergy Referral Center, which limits generalizability of the results. In addition, the retrospective design is subject to recall bias, as participants were asked to describe their child's anaphylaxis, which could have occurred months to years before the survey was completed. Despite these limitations, this study offers a unique overview on parents experience and perspective on managing food-induced anaphylaxis considering not only barriers but also facilitators. The psychological component seems to be crucial and need to be addressed. This is essential to develop novel interventions to improve food allergy management in pediatric patients. We previously confirmed that multidisciplinary training for anaphylaxis management in the school setting, that included theoretical, practical and psychological aspects, showed efficacy in improving management by school personnel (29). This should be implemented also for parents in order to improve safety and wellbeing of children with severe food allergy.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethic Committee of Psychological Research of Padua University. The patients/participants provided their written informed consent to participate in this study.
LP contributed to study design, data collection and interpretation, and writing of the draft of the manuscript. IB contributed to statistical design and analysis, data interpretation, and writing of the manuscript. MA and VT contributed to study design, data collection, data interpretation, and critically reviewed the manuscript. RB, FL, and AT contributed to study design, data interpretation, and critically reviewed the manuscript. DG contributed to statistical design and analysis, data interpretation, and critically reviewed the manuscript. AM contributed to study design, data interpretation, project administration, supervision, and critically reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.886551/full#supplementary-material
1. Pouessel G, Deschildre A, Castelain C, Sardet A, Sagot-Bevenot S, De Sauve-Boeuf A, et al. Parental knowledge and use of epinephrine auto-injector for children with food allergy. Pediatr Allergy Immunol. (2006) 17:221–6. doi: 10.1111/j.1399-3038.2006.00391.x
2. Dodd A, Hughes A, Sargant N, Whyte AF, Soar J, Turner PJ. Evidence update for the treatment of anaphylaxis. Resuscitation. (2021) 163:86–96. doi: 10.1016/j.resuscitation.2021.04.010
3. Chad L, Ben-Shoshan M, Asai Y, Cherkaoui S, Alizadehfar R, St-Pierre Y, et al. A majority of parents of children with peanut allergy fear using the epinephrine auto-injector. Allergy. (2013) 68:1605–9. doi: 10.1111/all.12262
4. Simons FER, Ardusso LR, Bilò MB, Cardona V, Ebisawa M, El-Gamal YM, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. (2014) 7:9. doi: 10.1186/1939-4551-7-9
5. Muraro A, Roberts G, Worm M, Bilò MB, Brockow K, Fernández Rivas M, et al. Anaphylaxis: guidelines from the European academy of allergy and clinical immunology. Allergy. (2014) 69:1026–45. doi: 10.1111/all.12437
6. Muraro A, Worm M, Alviani C, Cardona V, DunnGalvin A, Garvey LH, et al. EAACI guideline: anaphylaxis (2021 update). Allergy. (2021) 77:357–77. doi: 10.1111/all.15032
7. Turner PJ, Gowland MH, Sharma V, Ierodiakonou D, Harper N, Garcez T, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. (2015) 135:956–63.e1. doi: 10.1016/j.jaci.2014.10.021
8. Greenhawt M, Gupta RS, Meadows JA, Pistiner M, Spergel JM, Camargo CA, et al. Guiding principles for the recognition, diagnosis, and management of infants with anaphylaxis: an expert panel consensus. J Allergy Clin Immunol Pract. (2019) 7:1148–56.e5. doi: 10.1016/j.jaip.2018.10.052
9. Grabenhenrich LB, Dölle S, Moneret-Vautrin A, Köhli A, Lange L, Spindler T, et al. Anaphylaxis in children and adolescents: the European anaphylaxis registry. J Allergy Clin Immunol. (2016) 137:1128–37.e1. doi: 10.1016/j.jaci.2015.11.015
10. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy Eur J Allergy Clin Immunol. (2014) 69:1008–25. doi: 10.1111/all.12429
11. Ratanaprug C, Srisuwatchari W, Jirapongsananuruk O, Visitsunthorn N, Pacharn P. Carrying rates of epinephrine devices in children with food-induced anaphylaxis. Asia Pac Allergy. (2019) 9:e12. doi: 10.5415/apallergy.2019.9.e12
12. Arkwright PD, Farragher AJ. Factors determining the ability of parents to effectively administer intramuscular adrenaline to food allergic children. Pediatr Allergy Immunol. (2006) 17:227–9. doi: 10.1111/j.1399-3038.2006.00392.x
13. Prince BT, Mikhail I, Stukus DR. Underuse of epinephrine for the treatment of anaphylaxis: missed opportunities. J Asthma Allergy. (2018) 11:143–51. doi: 10.2147/JAA.S159400
14. Parke L, Senders AS, Bindslev-Jensen C, Lassen AT, Oropeza AR, Halken S, et al. Adherence to adrenaline autoinjector prescriptions in patients with anaphylaxis. Clin Transl Allergy. (2019) 9:59. doi: 10.1186/s13601-019-0297-0
15. Gold MS, Sainsbury R. First aid anaphylaxis management in children who were prescribed an epinephrine autoinjector device (EpiPen). J Allergy Clin Immunol. (2000) 106:171–6. doi: 10.1067/mai.2000.106041
16. Kim JS, Sinacore JM, Pongracic JA. Parental use of EpiPen for children with food allergies. J Allergy Clin Immunol. (2005) 116:164–8. doi: 10.1016/j.jaci.2005.03.039
17. Glassberg B, Nowak-Wegrzyn A, Wang J. Factors contributing to underuse of epinephrine autoinjectors in pediatric patients with food allergy. Ann Allergy Asthma Immunol. (2021) 126:175–9. doi: 10.1016/j.anai.2020.09.012
18. Stockhammer D, Katelaris CH, Simpson MD, Vanniasinkam T. Parent perceptions in managing children with food allergy: an Australian perspective. World Allergy Organ J. (2020) 13:100468. doi: 10.1016/j.waojou.2020.100468
19. Murata MA, Yamamoto LG. Patient/parent administered epinephrine in acute anaphylaxis. Am J Emerg Med. (2021) 46:499–502. doi: 10.1016/j.ajem.2020.10.060
20. Noimark L, Wales J, Du Toit G, Pastacaldi C, Haddad D, Gardner J, et al. The use of adrenaline autoinjectors by children and teenagers. Clin Exp Allergy. (2012) 42:284–92. doi: 10.1111/j.1365-2222.2011.03912.x
21. Pumphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. (2000) 30:1144–50. doi: 10.1046/j.1365-2222.2000.00864.x
22. Polloni L, Cavallin F, Lolli E, Schiavo R, Bua M, Volpe B, et al. Psychological wellbeing of parents with infants admitted to the neonatal intensive care unit during SARS-CoV-2 pandemic. Child. (2021) 8:755. doi: 10.3390/children8090755
23. Laura P, Sabrina B, Lucia R, Roberta B, Francesca L, Alice T, et al. Post-anaphylaxis acute stress symptoms: a preliminary study on children with food-induced anaphylaxis and their parents. J Allergy Clin Immunol Pract. (2020) 8:3613–5. doi: 10.1016/j.jaip.2020.06.036
24. Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. (2010) 65:933–45. doi: 10.1111/j.1398-9995.2010.02342.x
25. Wang J, Young MC, Nowak-Wegrzyn A. International survey of knowledge of food-induced anaphylaxis. Pediatr Allergy Immunol. (2014) 25:644–50. doi: 10.1111/pai.12284
26. Polloni L, Schiff S, Ferruzza E, Lazzarotto F, Bonaguro R, Toniolo A, et al. Food allergy and attitudes to close interpersonal relationships: an exploratory study on attachment. Pediatr Allergy Immunol. (2017) 28:458–63. doi: 10.1111/pai.12732
27. Polloni L, Ferruzza E, Ronconi L, D'Ovidio G, Bonaguro R, Lazzarotto F, et al. Maternal anxiety and previous anaphylaxis are associated with alexithymia in young patients with food allergy. Pediatr Allergy Immunol. (2022) 33:e13680. doi: 10.1111/pai.13680
28. Feng C, Kim J-H. Beyond Avoidance: the psychosocial impact of food allergies. Clin Rev Allergy Immunol. (2018) 57:74–82. doi: 10.1007/s12016-018-8708-x
Keywords: food allergy, children, anaphylaxis, parents, adrenaline, autoinjector, management, education
Citation: Polloni L, Baldi I, Amadi M, Tonazzo V, Bonaguro R, Lazzarotto F, Toniolo A, Gregori D and Muraro A (2022) Management of Children With Food-Induced Anaphylaxis: A Cross-Sectional Survey of Parental Knowledge, Attitude, and Practices. Front. Pediatr. 10:886551. doi: 10.3389/fped.2022.886551
Received: 28 February 2022; Accepted: 26 April 2022;
Published: 19 May 2022.
Edited by:
Jérémie F. Cohen, Necker-Enfants Malades Hospital, FranceReviewed by:
Umit Murat Sahiner, Hacettepe University, TurkeyCopyright © 2022 Polloni, Baldi, Amadi, Tonazzo, Bonaguro, Lazzarotto, Toniolo, Gregori and Muraro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonella Muraro, bXVyYXJvQGNlbnRyb2FsbGVyZ2llYWxpbWVudGFyaS5ldQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.