
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 29 July 2022
Sec. Child and Adolescent Psychiatry
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.886324
Children's spatial cognition abilities are a vital part of their learning and cognitive development, and important for their problem-solving capabilities, the development of mathematical skills and progress in Science, Technology, Engineering and Maths (STEM) topics. As many children have difficulties with STEM topic areas, and as these topics have suffered a decline in uptake in students, it is worthwhile to find out how learning and performance can be enhanced at an early age. The current study is the first to investigate if dog-assisted and relaxation interventions can improve spatial abilities in school children. It makes a novel contribution to empirical research by measuring longitudinally if an Animal-Assisted Intervention (AAI) or relaxation intervention can boost children's development of spatial abilities. Randomized controlled trials were employed over time including dog intervention, relaxation intervention and no treatment control groups. Interventions were carried out over 4 weeks, twice a week for 20 min. Children were tested in mainstream schools (N = 105) and in special educational needs (SEN) schools (N = 64) before and after interventions, after 6 weeks, 6 months and 1 year. To assess intervention type and to provide advice for subsequent best practice recommendations, dog-assisted interventions were run as individual or small group interventions. Overall, children's spatial abilities improved over the year with highest increases in the first 4 months. In Study 1, typically developing children showed higher scores and more continuous learning overall compared to children with special educational needs. Children in the dog intervention group showed higher spatial ability scores immediately after interventions and after a further 6 weeks (short-term). Children in the relaxation group also showed improved scores short-term after relaxation intervention. In contrast, the no treatment control group did not improve significantly. No long-term effects were observed. Interestingly, no gender differences could be observed in mainstream school children's spatial skills. In study 2, children in SEN schools saw immediate improvements in spatial abilities after relaxation intervention sessions. No changes were seen after dog interventions or in the no treatment control group. Participants' pet ownership status did not have an effect in either cohort. These are the first findings showing that AAI and relaxation interventions benefit children's spatial abilities in varied educational settings. This research represents an original contribution to Developmental Psychology and to the field of Human-Animal Interaction (HAI) and is an important step towards further in-depth investigation of how AAI and relaxation interventions can help children achieve their learning potential, both in mainstream schools and in schools for SEN.
Children's visuospatial abilities are important in early development, and processing information about space is involved in infant's object location and locomotion (1). Spatial abilities develop gradually with age, and spatial reasoning encompasses the processing of space, shape, distance, direction, and angles, in addition to understanding these with reference to the self and the wider environment (1, 2). Children's egocentric representation (explaining the reference of objects relative to the self) gradually matures to include an allocentric representation (describing locations using external frames of reference such as objects relative to each other) (3–5). Accordingly, limits in performance on visuospatial tasks may therefore be due to the immaturity of neural networks involved in such functions (2).
Spatial cognition is intricately linked with problem-solving capabilities and high-level processing in the cognitive system (6). For example, spatial ability is associated with the development of mathematical skills in children (6–9) and plays a critical role in achievement in STEM topics (science, technology, engineering and mathematics) (10–12). Additionally, as spatial reasoning is part of humans' integrated neuro-cognitive system, wider functioning such as children's inhibitory control and attentional functioning are also likely to affect processing capabilities. For example, Beattie, Schutte, and Cortesa (13) found that children with better inhibitory and attentional ability had greater spatial working memory. These related abilities are integral to the learning process overall and affect academic performance.
It is noteworthy that spatial abilities may be influenced differently by the differing cognitive abilities in typically developing children and in those with special educational needs (SEN). For instance, children with Down Syndrome typically have a cognitive profile with impaired verbal processing abilities, but less impaired visuospatial processing abilities (14–18). Certain visuospatial abilities can also differ between those with Autism Spectrum Disorder (ASD) and Attention Deficit Hyperactivity Disorder (ADHD), and typically developing children (19, 20), for example in spatial perspective-taking (21) and other spatial tasks. Studies have reported that those with ASD can show superior ability in visuospatial processing tasks with particular strength in tasks focussed on detail and local feature processing, and poorer ability to attend to the spatial configuration as a whole, employing more global processing as detailed in Central Coherence Theory (22). However, these findings are not replicated in all circumstances-mixed evidence exists and more complex solutions, taking other cognitive processing function into account, are offered (23–26).
As with typically developing children, children with the same diagnosis vary in terms of their cognitive profile (23).
Additionally, the picture is more complex when taking gender differences in SEN populations into account as the over-representation of males makes the generalization of findings problematic (27).
Gender differences in spatial processing have been found in typical populations (28–30) with males often outperforming females (29, 31–34). Indeed Reilly, Neumann, and Andrews (35) suggest that of all cognitive functions, spatial processing shows the largest difference between genders. With its integral role for the development of quantitative reasoning skills important for mathematics and science subjects, this difference could contribute to gender differences in STEM subjects and underrepresentation of women in STEM fields. Theories have been offered to explain such differences on the basis of biological/hormonal factors (35–39), gender orientation and gender stereotypes [(35, 38), for wider discussion see (40)], socialization and play experience (35) and evolutionary pressures (41, 42). However, others argue that differences in spatial ability are often not present or are small (30, 40, 43, 44). Furthermore, evidence suggests that spatial skills are malleable, and can be improved through training (45–47), and that environmental factors and experience also play a large part in these observed differences (35, 48, 49).
As spatial processing and problem-solving are crucial to educational outcomes, for example in mathematics and in STEM subjects, it is important that such abilities are adequately supported in the school environment. This is especially pertinent as in recent years pupils' interest in STEM subjects, and uptake of STEM subjects by students, has dropped alarmingly (50).
One intervention which may enhance spatial abilities in children is an animal-assisted intervention (AAI). AAIs are becoming increasingly popular in educational settings as pets in the classroom may be beneficial to children's learning success (51), classroom behavior (52) and their emotional and cognitive development [see (53–57) for recent reviews and overview] as well as contribute to lower stress levels (58, 59). While animal-assisted interventions show some beneficial effects on human health and emotional well-being, learning and memory (55–57), it has been demonstrated that research in the field is growing, but also that the knowledge base still needs to be strengthened with many areas still under-investigated (60). In the past, research in this area has often suffered from small sample sizes, lack of control groups and overall insufficient scientific rigor (56, 61, 62). However, in recent years steady progress has been made in more thorough investigation of the effects of AAI on human health, well-being and cognition with improvements also found in executive function [e.g. (60, 63–65)].
Next to AAI, relaxation, meditation and yoga interventions have become increasingly popular in schools. They can help to improve mental and physical well-being, regulate stress, enhance performance on selective attention, concentration and mental flexibility tasks and psychomotor speed (66–74). Broderick and Metz (75) found that girls demonstrated increased feelings of calmness, relaxation and self-acceptance after mindfulness interventions. However, overall, the field is suffering from similar methodological problems as the earlier field of AAI.
Currently, only very few studies have been carried out on the effects of AAI on children's specific cognitive abilities. Previous studies have highlighted the beneficial effects of a dog's presence during a task on young children's cognitive functions such as memory (76), object recognition performance (77) and object categorisation tasks (78, 79). In addition, studies such as those of Hergovich, Monshi, Semmler and Zieglmayer (80) and Kotrschal and Ortbauer (52) reported increased classroom cohesion and improved behavior of children with a dog present which is an important factor in ensuring that conditions are optimal for learning. There are currently no studies investigating effects of dog-assisted interventions on children's cognitive development, and more specifically, there are no studies focusing on spatial abilities.
Explanations as to why dogs can have beneficial effects on humans are proposed by adapted and dynamic biopsychosocial models which integrate biological, physiological, psychological and social support (81–87), while others provide historical and social explanations [e.g., (88, 89)]. Physiological indices for arousal and affiliative behaviors have been identified as biological mechanisms underlying the human-animal bond (e.g., lower stress levels as indicated by lower cortisol, higher oxytocin levels, lowered blood pressure, reduced skin conductance, lower heart rate; (59, 90–93). Improved concentration, attention and motivation have been observed with the dog's presence creating a positive social atmosphere [for overview (90)]. Thus, an overarching, integrative approach combining neurobiological processes, attachment, biophilia and caregiving to pets may be best-suited to explain the resulting human-animal relationships, their development and their physiological and endocrine basis (83). Gee, Gryphon and McCardle (94) proposed a theoretical framework organizing the results of research and predicting unexplored pathways of indirect effects on learning through social-emotional development. This framework includes direct effects of classroom activities involving animals (mostly dogs) on children's motivation, engagement, self-regulation, and social interaction, as well as indirect effects on socio-emotional development and learning. This framework, though broadly useful, was not intended to serve as the basis for specific predictions within individual areas of cognitive development.
Despite spatial cognition being a crucial part of cognitive development and highly important to mathematics and STEM subjects, studies have so far not been carried out on the effects of animal-assisted interventions (AAI) or relaxation interventions on children's spatial cognition. The current study closes this knowledge gap and makes a novel and unique contribution to the field of animal-assisted and relaxation interventions within Developmental Psychology. We tested if dog-assisted interventions lead to enhanced spatial ability in children compared to relaxation interventions and compared to a no treatment control group.
Effects of AAI and relaxation interventions on children's spatial ability were investigated employing randomized controlled trials longitudinally, thus guaranteeing high scientific rigor. We tested typically developing children and children with special educational needs (SEN) to maximize knowledge gain in the field. Additionally, to provide practical advice for best practice in schools, intervention type was also assessed as to which works best [as the evidence is ambiguous (94)], and interventions were carried out as individual or small group interventions. This adds to the knowledge base as, depending on results, it may be possible to reduce direct contact time for therapy dogs per setting adding to animal welfare (95), and it may help to introduce the most cost-efficient intervention provision in educational settings (94).
In line with the above research, we predicted spatial ability improvements in the dog-assisted interventions compared to the control group when comparing pre- and post intervention periods. We expected intermediate effects for relaxation interventions and no or only maturational change in spatial abilities in the control condition. Concerning longer lasting effects, our longitudinal design allowed for exploration of such effects.
The current study was part of a larger, longitudinal, randomized controlled trial systematically examining the effects of dog- and relaxation-interventions on school children's academic performance, social and emotional well-being and measuring physiological changes (Lincoln Education Assistance with Dogs; https://lead.blogs.lincoln.ac.uk/) (95). The longitudinal studies described here investigated specifically the effects of AAI and relaxation interventions on spatial cognition in typically developing children (Study 1) and children with SEN (Study 2).
This research was approved by the University of Lincoln Research Ethics Committee (SOPREC) and is in line with British Psychological Society Ethics guidelines. In addition, the WALTHAM Animal Welfare and Ethical Review Board also approved the research.
Children were recruited through mainstream and special educational needs schools in Lincolnshire and Gloucestershire, UK. In Study 1, 105 children took part in Lincolnshire, UK (N = 54 males, 51 females, mean age = 8.91 yrs, SD = 0.39, min = 8.21, max = 10.07; 4 mainstream schools). In Study 2, 64 children (N = 54 males, 10 females, mean age = 9.27 yrs, SD = 0.79, min = 8.0, max = 11.5) from 7 SEN schools in Lincolnshire and Gloucestershire, UK, participated. Diagnoses for the latter included 15 children with ASD, 16 with ADHD, 12 with ASD and ADHD, 12 with learning disorder not otherwise specified (LD NOS), and 9 with unknown diagnoses as parents did not provide this information. Please see (Table 1) below for numbers of children taking part at each assessment point per condition and school type, and (Table 2) for retention rates and reasons for attrition.
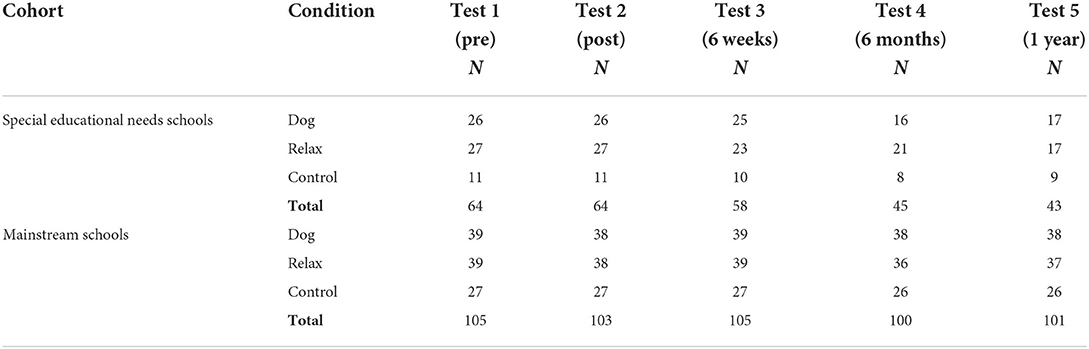
Table 1. Number of children taking part in the test task at each assessment point per condition and school type.
All children attended school full-time. Researchers and dog handlers were in possession of enhanced police cheques, and researchers were highly experienced in research with school children.
Twenty-two different dogs and their handlers (N = 21) took part in the interventions on a volunteer basis. All handlers had insurance: N = 19 through their registration with Pets as Therapy, N =1 obtained separate insurance, and N = 1 was insured via their registration with Therapy Dogs Nationwide. All dog-handlers were required to attend safety training on understanding dog stress signaling behaviors before the study started. Dog breeds included: 1 Greek Hare-Hound, 2 Cavalier King Charles Spaniel and Miniature Poodle crossbreeds, 1 Labrador and miniature Poodle crossbreed, 2 German Short-Haired Pointers, 2 Miniature Schnauzers, 3 Labradors and 1 Labrador crossbreed, 2 Tibetan Mastiffs, 1 Border Terrier, 1 Scottish Terrier, 1 Lurcher, 1 Clumber Spaniel, 1 Yorkshire Terrier, 1 Pekingese, 1 Smooth Collie, 1 Cocker Spaniel and 1 Golden Retriever. All dogs were healthy and had been assessed by independent canine behavioral experts to ensure their suitability to work with children.
The British Ability Scales (BAS-3) (96) were used to measure children's spatial ability (SA). The BAS-3 is a standardized cognitive scale normed for use from 5:00 to 17:11 (years: months) and designed to measure mental abilities significant for learning and educational performance (see https://www.gl-assessment.co.uk/assessments/products/bas3/ for more details). Two assessments within the BAS-3 were administered: Recognition of Designs and Pattern Construction to provide a Spatial Ability cluster score (SA). Children's performance in the Recognition of Designs task reflects their visual-spatial processing, short term visual memory, perception of spatial orientation and visualization abilities. Performance in the Pattern Construction task reflects the following: a child's visual-motor skills; spatial visualization (including matching abilities, perception of relative orientation, the ability to reproduce designs with objects, and to perceive and analyze visual information); non-verbal reasoning abilities (including skills in decomposing and reconstructing a design; the use of systematic strategies, for example, sequential assembly, hypothesis testing and trial and error); and the ability to follow verbal instructions and use verbal mediation strategies. After extensive assessment tool search and piloting, we chose the BAS-3 tool kit for the following reasons: it contained a range of assessments of the specific areas our research aimed to investigate, it was feasible to carry these out in realistic time slots with suitable duration for children of the chosen age group, it was usable and normed for both cohorts, and it was normed for British-English speaking children.
Parents gave consent for all children to take part in the study and provided details of any allergies and phobias to dogs. Children's assent was acquired prior to all test and intervention sessions, and parents and children were informed of their (and their children's) right to withdraw from the study at any time without having to give a reason. Dog handlers consented to taking part with their dogs and were free to withdraw at any time. Dogs were monitored continuously throughout the study for potential signs of wanting to withdraw. They also were free to retreat at any time.
All children took part in detailed safety training with dog body language training (95, 97) and further safety information before the study began, in order to set clear expectations for children's behavior around the dogs. This reduced the potential risk of incidents, and was designed to foster respect and uphold high standards of animal welfare. Children were familiarized with the dogs prior to study begin to eliminate potential novelty effects (95).
Children's performance was assessed before interventions began (baseline), immediately after interventions, and was repeated after 6 weeks, 6 months and after 1 year to assess if interventions provided immediate, short-term, mid-term or long-term improvements to children's spatial ability. See Figure 1 below for overview of procedure details.
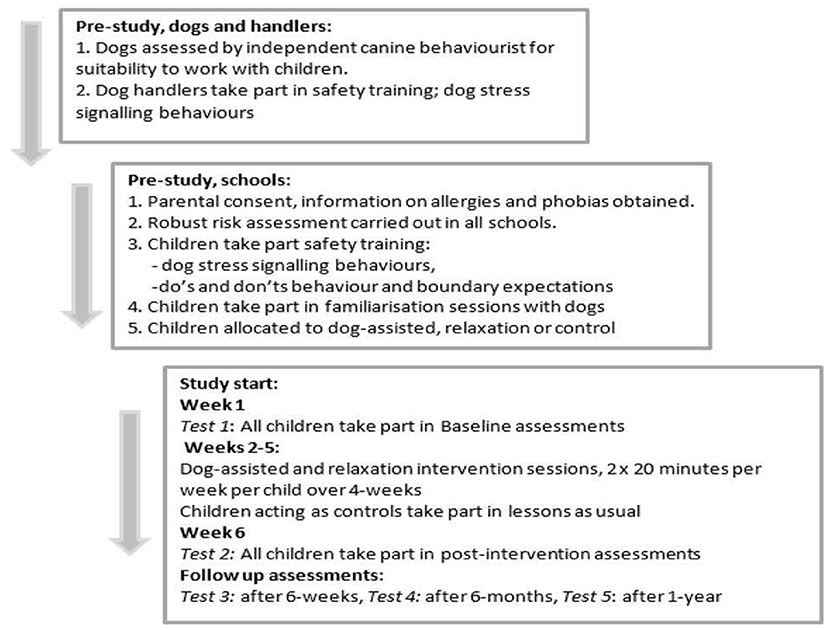
Figure 1. Welfare, safety, familiarization and consent and assent procedures carried out before and at study start for dogs and handlers, children, parents and schools taking part in the longitudinal randomized controlled trial.
Stratified randomization was used to place children in the different intervention groups. This method ensured that we did not confound dog ownership, socio-economic status or children's academic ability with intervention condition. Testing was carried out in schools in waves with 1/3 of participants in the dog group, 1/3 in the relaxation control group and 1/3 in the no treatment control group to avoid potential effects of seasonal affective disorder (SAD). For example, if the dog intervention would have taken place in summer, and the control groups in autumn or winter, we would have confounded the study and not been able to say if effects are due to SAD or our intervention conditions. Hence, to avoid confounding the study, all testing with all groups took place over the whole year as described above.
Children were randomly assigned to take part either in individual or in small group interventions.
Interventions took place in a separate room in schools during the normal school day. During the interventions, the researcher and the dog handler were present as were the dog and the children. Having completed all safety training, children were taken to the room, with the dog handler and dog waiting outside the room to greet the children (the dog had been familiarized to the room and with the children beforehand, see above). Children were asked to sit down and remain seated unless the activities taking place required them to do otherwise. Intervention sessions were 20 min and structured with approximately 5 min for initial dos and don'ts (e.g., “don't hug/kiss/crowd the dog,” etc.) and greeting the dog and handler. Then approximately 10 min were spent on learning facts about the dogs via the handler, talking about and interacting with the dog as deemed suitable by handler and researcher who were constantly observing the dog's signaling and body language in order to safeguard the dog's welfare. As all sessions were child-led, they varied somewhat in content. The last 5 min were spent saying goodbye to dog and handler and petting the dog as appropriate (again decided by dog handler and researcher).
Relaxation sessions took part in a separate room and involved child age-appropriate meditation consisting of “Jellyfish” and “Butterfly” recordings from Enchanted Meditations for Kids (98) presented alternately across the sessions. Children were asked to lie down on a yoga mat and close their eyes; children who did not feel comfortable doing this, or who were unable due to mobility issues (mainly in SEN schools), were allowed to sit and relax with their eyes open or closed as they preferred. Again, the duration was 20 min, with timings approximately 5 min of active relaxation (body scanning with children moving toes, legs, fingers, etc.), followed by 10 min of meditation, and 5 min of active relaxation to match the profile of the dog sessions as closely as possible.
Children assigned to the no treatment control group condition took part in their usual class lessons.
A robust risk assessment was carried out for all settings taking part in the study (95). This incorporated strict protocols for animal welfare which were followed at all times. Care plans were completed for all dogs. Dogs were not required to work more than 2 h per day and had short breaks every 20 min as children moved between classrooms. Typical working times for most sessions were 1 h and 20 min in total. Dogs always had access to their own bedding for “time out” and water was freely available. Interventions would be stopped if dogs showed any signs of discomfort or being tired, and handlers were free to take their dog outside for a break as they felt appropriate. However, this did not occur.
Before study start, a priori power calculations were undertaken to determine sample size for the main repeated measures ANOVA with 3 conditions (dog intervention, relaxation intervention, and control group) and 5 measurement repetitions (GPower 3.1.9) (99); to obtain statistical power at the recommended 0.80 level for our analyzes (alpha at 0.05, for effect size up to 0.25), we required 27 children per cohort. For analysis including dog ownership, 42 participants were needed and for complex analysis with gender and dog ownership included (only possible in mainstream cohort due to expected typical male gender majority SEN samples), 60 participants were ideal. Due to the 5 repeated measures with the full study duration of 1 year, we overrecruited as feasible to avoid loss of power due to potential attrition.
Repeated measures ANOVAS were carried out overall, and for Study 1 (Mainstream school children) on Condition (dog intervention, relaxation intervention, no treatment control) and Time (before and after intervention, 6 weeks, 6 months, 1 year), also including Gender and Dog ownership for children. Analysis was then split into group and individual testing conditions. As we predicted a complex interaction pattern of improvements in spatial ability in children in the dog intervention group over the relaxation group, with no improvements expected in the no treatment control group, planned comparisons with Bonferroni corrections were calculated to investigate these specific predicted effects.
A similar pattern was followed for children in SEN schools (Study 2). However, due to the sample consisting mainly of boys, and due to missing information on dog ownership, we did not include Gender and Dog Ownership as factors in this analysis (see footnote, p. 12).
It is important to note that for all intervention conditions specific predictions, calculated with planned comparisons, were of core interest as it was predicted specifically that children in the dog intervention would show clear improvement after the intervention compared to the no treatment control group. Some improvement was expected in the relaxation group between pre and post intervention test times, and no significant improvement in the control group. Hence, planned comparisons were crucial to our analysis.
Significance testing follows the usual p-value criterion of smaller than 0.05 for significant results, and for planned comparisons smaller significance levels were used employing Bonferroni corrections. Statistical analysis was carried out using Statistica 12 as well as IBM SPSS, version 26. No data was excluded or replaced.
Assumptions of normality were not violated for scores of spatial ability for either SEN schools: skewness of 0.678 (SE.297) and kurtosis of 1.482 (SE.586): Shapiro-Wilk (W = 0.966, p =0.075) or mainstream schools: skewness of 0.220 (SE 0.236) and kurtosis −0.031 (SE 0.467); Shapiro-Wilk (W = 0.992, p =0.797). Data from both cohorts were therefore analyzed using parametric analysis. Data were first assessed per cohort, then comparing cohorts. Within each analysis, results were first calculated for all children followed by analyzes for individual and group interventions.
A one-way analysis of variance revealed that scores for spatial ability were significantly lower for children who attended SEN schools (M = 82.67) compared to those in mainstream schools (M = 95.30) [F(1, 168) = 28.168, p < 0.001, =0.144]. A further one-way analysis to assess the effect of differing diagnosis on spatial ability within the SEN cohort showed no significant differences [F(4, 59) = 1.094, p =0.368, =0.069] [ASD (M = 83.1); ADHD (M = 85.0); ASD-ADHD (M = 87.5); other (M = 77.0); unknown (M = 78.6)].
A repeated measures ANOVA of Time (pre-intervention baseline, post-intervention, 6 weeks, 6 months, 1 year) x Condition (dog, relaxation, control) x Gender (male, female) x Dog ownership (dog, no dog) was conducted. Children showed a significant main effect of Time [F(4, 336) = 32.358, p < 0.001, =0.278] with time accounting for a large amount of variance within the model. Spatial ability scores improved significantly over time overall, and most strikingly between the baseline and post-intervention [t(102) = −5.070, p < 0.001, d = 0.49] and post-intervention and the 6-week assessments [t(102) = −5.744, p < 0.001, d = 0.56]. No significant main effect for Condition [F(2, 84) = 0.787, p = 0.459, = 0.018] occurred. No interaction for Time with Condition was revealed either [F(8, 336) =0.728, p =0.667, =0.017].
To investigate our main question and the predicted effects of the dog and the relaxation intervention on spatial ability, planned comparisons with Bonferroni correction (p < 0.004) revealed that children in the dog interventions showed highly significant and immediate improvements in spatial ability from pre-intervention baseline to post-intervention (t(37) = −3.499, p = 0.001, d = 0.56) and from post-intervention to 6-week testing [t(37) = −4.507, p < 0.001, d =0.73], [M = 92.68 (baseline), 99.30 (post), 105.18 (6 weeks)].
Children who had taken part in the relaxation interventions showed no immediate, but significant short-term improvements in spatial ability scores from post-intervention to 6-week test times only [t(37) = −3.861, p < 0.001, d =0.62], [M = 98.68 (post), 105.33 (6 weeks)]. The control group showed no significant improvements. No further significant main effects of Gender and Dog ownership, nor any interactions, were found (p < 0.05).
A repeated measures ANOVA of Time (pre-intervention baseline, post-intervention, 6 weeks, 6 months, 1 year) x Condition (dog, relaxation, control) x Gender (male, female) x Dog ownership (dog, no-dog) for individual intervention sessions showed a significant main effect of Time [F(4, 204) = 21.436, p < 0.001, = 0.296], with children making significant improvements in spatial ability scores overall, and in particular from pre-intervention baseline to post-intervention [t(67) = −4.575, p < 0.001, d =0.55] and post-intervention to 6-week tests [t(67) = −3.784, p < 0.001, d =0.45]. There was no main effect for Condition [F(2, 51) = 0.486, p = 0.618, = 0.019]. The interaction for Time with Condition [F(8, 204) = 2.091, p = 0.038, = 0.076] reached significance and, in line with our predictions, further planned comparisons revealed that children in the individual dog interventions showed significantly improved scores {[t(20) = −3.725, p = 0.002, d = 0.77]; (Bonferroni-corrected with p = 0.004) from pre-intervention baseline to post-intervention tests [M = 91.35 (pre), 101.30 (post)]}. Neither relaxation intervention [t(20) = −1.428, p = 0.169, d = 0.31], nor the no treatment control group [t(26) = −5.70, p = 0.010, d = 0.53] showed significant changes in scores immediately after intervention. No significant improvements in scores were visible from post intervention to week 6 for any of the groups. As above, no further significant main effects or interactions of Gender and Dog ownership were found.
To assess the effects of AAI in group interventions, the same repeated measures ANOVA of Time (pre-intervention baseline, post-intervention, 6 weeks, 6 months, 1 year) x Condition (dog, relaxation, control) x Gender (male, female) x Dog ownership (dog, no-dog) was conducted. The significant overall effect of Time [F(4, 184) = 20.726, p < 0.001, =0.311] was analyzed further and showed that children taking part in group interventions made significant improvements in spatial ability from baseline to post-intervention [t(61) = −3571, p = 0.001, d = 0.45] and post-intervention to 6-week test times [t(61) = −4.407, p < 0.001, d = 0.55]. No Condition main effect[F(2, 46) = 1.238, p = 0.300, = 0.051] or interaction for Time with Condition reached significance [F(8, 184) = 1.313, p = 0.239, = 0.054].
As above, we predicted specific improvements per condition, and planned comparisons revealed significant improvements in spatial ability for children in the group dog interventions. These occurred only from post-intervention to the 6-week test time [t(17) = −3.713, p = 0.002, d = 0.87] [M = 97.06 (post), 104.58 (6 weeks)], i.e., not immediately following intervention (pre-intervention to post-intervention: t (17) = −1.113, p =0.281, d =0.26), but somewhat delayed compared to the individual interventions.
No significant relaxation effects were seen pre to post (t (16) = −2.124, p =0.050, d =0.51) or post to 6-weeks (t (16) = −2.880, p =0.011, d =0.69). The no treatment contros group also failed to show significant improvements (pre-post (t (26) = −2.794, p =0.010, d =0.53); post to 6-weeks (t (26) = −1.535, p =0.137, d =0.29). There were no further significant main effects or interactions of Gender and Dog ownership.
A repeated measures ANOVA of Time (pre-intervention baseline, post-intervention, 6 weeks, 6 months, 1 year) x Condition (dog, relaxation, control) was conducted1. A significant main effect of Time demonstrated SEN school children's significant improvements in spatial ability scores [F(4, 128) = 4.926, p = 0.001, = 0.133) with time accounting for a large amount of variance within the model. Significant improvements occurred from baseline to post-intervention test times (t(632) = −4.577, p < 0.001, d = 0.57). No differences were found between other consecutive test times across the length of the study. As expected, also for this cohort, there was no significant main effect for Condition [F(2, 32) = 0.149, p = 0.862, =0.009] and no interaction with Time [F(8, 128) = 0.312, p = 0.960, = 0.019].
As with the typical cohort, planned comparisons were calculated to find out if interventions had an effect on spatial ability (Bonferroni significance level: p < 0.004). Only children in the relaxation condition [t(26) = −3.521, p = 0.002, d = 0.67) made significant improvements from pre-intervention baseline to post-intervention assessments [M= 81.19 (baseline), 87.67 (post)]. However, children in the dog [t(25) = −2.654, p = 0.014, d = 0.52] and control conditions [t(10) = −1.314, p = 0.218, d = 0.39] did not improve their scores significantly.
For individual interventions a repeated measures ANOVA of Time (pre-intervention baseline, post-intervention, 6 weeks, 6 months, 1 year) x Condition (dog, relaxation, control) only showed a significant main effect of Time [F(4, 60) = 3.891, p = 0.007, = 0.206], with children with special educational needs significantly improving from baseline to post-intervention test times only [t(25) = −3.840, p = 0.001, d = 0.75]. No main effect for Condition [F(2, 15) = 0.935, p = 0.414, = 0.111] or interaction for Time with Condition reached significance [F(8, 60) = 0.605, p = 0.770, = 0.075].
To investigate the predicted intervention effects, planned comparisons showed that scores for children did not increase significantly between the pre-and post-intervention {dog [t(6) = −1.837, p = 0.116, d = 0.69], relaxation [t(7) = −4.080, p = 0.005, d = 1.44], control [t(10) = −1.314, p = 0.218, d = 0.39] with the relaxation group just missing significance (Bonferroni-adjusted significance level: p = 0.004)}.
No significant increases in scores were found either from post-intervention to 6-week test times {dog [t(6) = −1.276, p = 0.249, d = 0.48], relaxation [t(4) = 0.607, p = 0.577, d = 0.27], control [t(9) = −1.664, p = 0.130, d = 0.52]. No other comparisons reached significance}.
To assess results for children who took part in group interventions, the repeated measures ANOVA of Time (pre-intervention baseline, post-intervention, 6 weeks, 6 months, 1 year) x Condition (dog, relaxation, control) revealed a significant main effect of Time [F(4, 84) = 3.231, p = 0.016, = 0.133). Children taking part in group interventions made significant improvements in spatial ability from baseline to post-intervention test times [t(48) = −3.092, p = 0.003, d = 0.44]. No significant Condition main effect [F(2, 21) = 0.493, p = 0.617, = 0.045] or interaction for Time with Condition occurred [F(8, 84) = 0.325, p = 0.954, = 0.030]. Planned comparisons investigating the predicted intervention effects showed that children did not improve significantly from pre to post-intervention {dog [t(18) = −1.998, p = 0.061, d = 0.45], relaxation [t(18) = −1.882, p = 0.076, d = 0.43], control [t(10) = −1.314, p = 0.218, d = 0.39]} and post-intervention to 6-week test times {dog [t(17) = 0.170, p = 0.897, d = 0.04), relaxation [t(17) = −1.239, p = 0.232, d = 0.29], control [t(9) = −1.664, p = 0.130, d = 0.52]}. No other consecutive tests were significant. Figures 2–4 below illustrate in overview the main results.
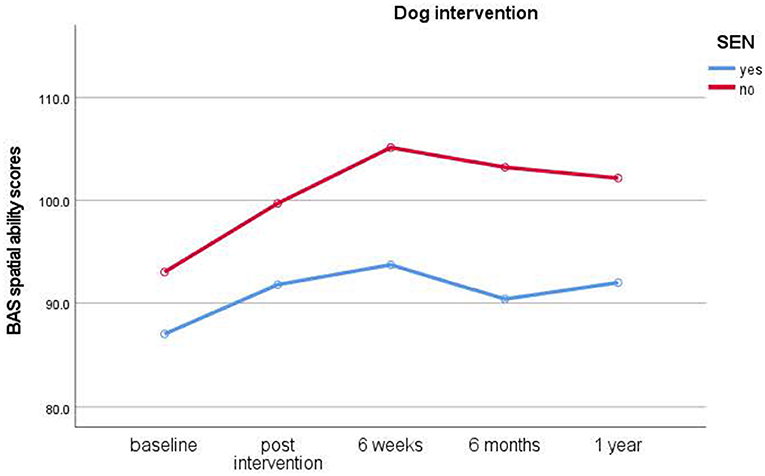
Figure 2. Results of longitudinal assessments in the dog intervention: means for British Ability Scale spatial ability scores (y-axis) over time (x-axis) in the dog intervention group for children with and without special educational needs (SEN). Higher scores imply higher ability.
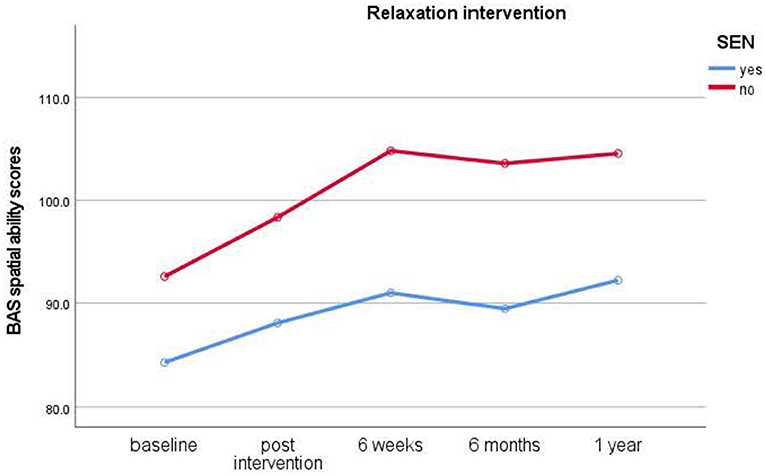
Figure 3. Results of longitudinal assessments in the relaxation intervention: means for British Ability Scale spatial ability scores (y-axis) over time (x-axis) in the relaxation intervention group for children with and without special educational needs (SEN). Higher scores imply higher ability.
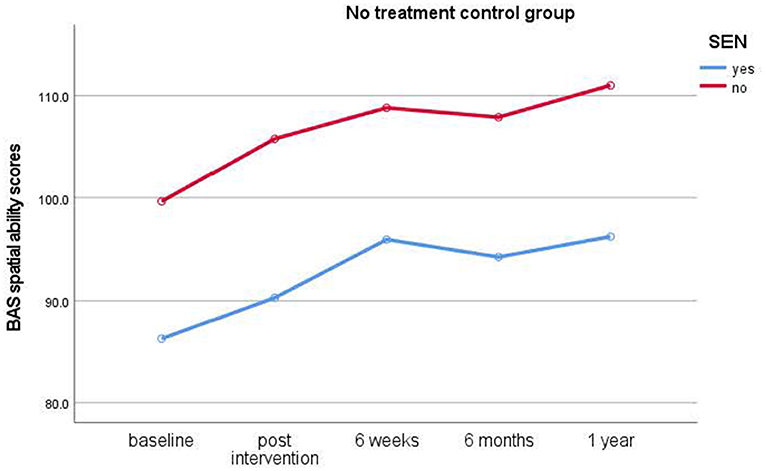
Figure 4. Results of longitudinal assessments in the no treatment control group: means for British Ability Scale spatial ability scores (y-axis) over time (x-axis) in the no treatment control group for children with and without special educational needs (SEN). Higher scores imply higher ability.
Children's spatial abilities are a crucial part of their learning and cognitive development, and important for children's problem-solving capabilities, the development of mathematical skills and progress in STEM topic areas. As many children, with and without SEN, struggle with maths and STEM topics, and as these topics have suffered a significant loss of interest by school children and decline in uptake in students (50), it is worthwhile to study how learning and performance can be enhanced at an early age.
This study is the first to investigate if dog-assisted and relaxation interventions can improve spatial abilities in school children. The study employed high scientific rigor by using randomized controlled trials and a longitudinal design. We also broadened the scope of the research by including both children attending mainstream and special educational needs schools (SEN). As it has been hitherto unknown if individual or group interventions work better, the study also assessed the effects of individual and group interventions to make recommendations for best and most efficient practice.
The results outline how typically developing children and children with SEN developed over the year during which time all children's spatial ability scores increased significantly from baseline over the 1-year study duration and thus showing the expected general learning and maturation effects. Immediate and short-term improvements were also revealed after 4-week interventions.
Study 1 results indicate that typically developing children benefitted from the dog intervention. Improvements in spatial ability scores occurred immediately after the intervention and lasted up to 6 weeks, with effect sizes ranging from medium to large. Interestingly, individual dog interventions showed more immediate effects, while group interventions had somewhat delayed effects with children showing better scores after intervention end to 6 weeks. Likewise, children in mainstream schools who took part in the relaxation intervention also benefitted from these overall, albeit relaxation interventions showing no immediate, but significant short-term improvements in spatial ability scores from post-intervention to 6-week test times. In contrast, it is noteworthy that no significant improvements in spatial ability scores were seen in the no treatment control group.
Overall, the results show that dog and relaxation interventions enhance mainstream school children's spatial abilities, and it noteworthy to point out that the dog intervention shows significant results throughout, with individual sessions having a more immediate effect and group sessions a delayed effect. It could be argued that individual sessions involved more intensive interaction between children and dogs and therefore stronger calming effects in line with Beetz and colleagues (90). This may have had a beneficial effect on children's processing of spatial tasks shortly after the interventions. Children in the group sessions had less intensive contact time with the dogs, but they had instead other group members to share the experience with which could contribute to a delayed effect. Future research will need to establish if the less intense animal experience combined with peer contact and potential later discussions may have led to a delayed beneficial effect.
Study 2 revealed, in contrast to the typically developing cohort, that children with special educational needs (SEN) showed a significant increase in spatial ability in the relaxation condition only. They showed significant improvements from baseline to post-intervention assessments with medium effect sizes. While children in the dog condition also showed improved scores, these differences did not reach significance. Likewise, children in the no treatment control condition also did not show a significant improvement in scores. In the SEN cohort, no clear advantage for either individual or group interventions became evident from the data. Thus, this cohort benefitted from relaxation interventions instead of dog-assisted interventions.
The integrative dynamic biopsychosocial model (82, 83) is best suited to explain the result patterns for both cohorts, based on the stress-reducing and calming effects of both interventions, including the creation of a positive atmosphere, beneficial to learning, in dog-assisted interventions and relaxation interventions (82, 83, 85, 86, 89, 90, 92). Concerning specifically spatial ability tasks it should be highlighted that these involve working memory, which incorporates integrated systems of the central executive, phonological loop and visual-spatial sketchpad (100). These flexible integrated cognitive systems can be affected by individual factors and wider influences such as learning, emotion and stress (101). Previous AAI research has shown positive effects on memory during cognitive tasks (76–79, 102). Positive emotions can also have a beneficial effect on spatial working memory (103–106), and affective states can influence working memory (101). Relaxation and stress reduction as shown in other research on cortisol level buffering in AAI is likely involved during both dog and relaxation interventions (58, 59), and may have benefitted the spatial ability tasks. Likewise, improvements in executive functioning, which have been linked to the presence of a dog in college students (63) and school children (64), may be driving improvements in spatial ability scores. As one is able to inhibit irrelevant thoughts, relax and focus on the task at hand, general cognitive abilities, such as spatial ability, also improve.
Regarding the developmental pattern over the year, it is noteworthy that children's scores did not rise as steeply (or significantly) after the 6-week follow up point. This may be as children's cognitive scores may fluctuate as the school term progresses, as learning and development do not always represent a linear process (105, 106).
Potentially, the repeated use of the BAS tool kit could present a limitation of the current study in case the closer test intervals at the beginning of the study (baseline / after the 4-week intervention / 6 weeks later) may have resulted in practice effects enhancing the test results up to the 6-week time point, and which may dissipate after a longer break of 6-months. However, it is unclear how likely this scenario is given the complexity of the tasks and given the differences in results in the experimental groups and the no treatment control group. Further studies may also include a different, or a combination of, cognitive instruments. In the current study we were limited to choose one cognitive assessment tool due to other measures taking place within the overall larger-scale project as mentioned above.
The lack of further significant improvements suggests that dog interventions may not show longevity past the post-intervention test time or the later assessment 6 weeks after post intervention testing (in week 12).
Concerning cohort differences, children's scores of spatial reasoning were significantly higher for those attending mainstream schools than for those with special educational needs (2, 19, 20). This is in line with previous research showing differing performance in children with neurotypical and non-neurotypical developmental profiles. Within the SEN cohort, processing of spatial ability was not significantly different based on the diagnoses of the children. This is a noteworthy finding, given that different diagnoses have diverse aetiologies and so differ in terms of their neural systems, memory, attention and executive function which are integral to efficient visuospatial processing. The current results are therefore consistent with those studies that did not find superior ability in visuospatial processing tasks in participants with ASD (24–26). As spatial reasoning is important in many other areas of learning such as the STEM topics and is malleable (46) it would be interesting and worthwhile to assess whether AAI paired with specific skills training can foster long term benefits for children's spatial ability.
As pet ownership may have additional beneficial effects on the health and well being of children (107), and as it is unknown how this may interact with the effects of AAI and relaxation interventions, dog ownership was included in this longitudinal study. However, no effects of dog ownership were found, nor were there any interactions. This finding suggests that for populations (typical and SEN) of 8–10-year-old children, dog ownership is not necessary for the accrual of benefits from interacting with a therapy dog in interventions. It may be useful in future to investigate attachment to pets and attitude to pet dogs to find out if this potentially influences interaction outcomes.
With interventions taking place twice a week over 4 weeks, and the cognitive assessments carried out without the presence of a dog in the room, the current study adds to previous research showing beneficial effects on cognitive tasks with a dog present during testing [see (76–79)]. As there are no comparable studies into the effects of AAI on children's spatial cognition over time (56), the current research pioneers longitudinal investigation of AAI and relaxation interventions.
Interestingly, despite previous research and theories reporting gender differences in spatial skills, this study found no significant differences between girls and boys on the standardized tests. These results are in line with research showing small or no differences (30, 42–44, 108) and potentially highlight the influence of teaching [e.g., (35, 47)].
While individual intervention sessions require more working hours with dogs and handlers, group sessions could mean cost efficiencies for schools and reduced working time for therapy dogs. However, the results of the current study indicate that the individual dog interventions may be more effective. To our knowledge, there are no systematic studies on how dosage of interventions may relate to intervention type (individual or group) – future research should be carried out to enable effective interventions.
Concerning potential feasibility, organizational, ethical and safety challenges in school settings, the following should be highlighted: For this longitudinal, randomized controlled trial in schools with two child cohorts to be feasible, it required early and meticulous planning. Next to the usual complex planning involved in longitudinal studies, further protocols concerning ethics and safety had to be established and implemented, including, for example, school, parent and child consent/assent. We operated with a timetable that was agreed in advance with schools and dog handlers and we managed to maintain schools' and children's continued interest and cooperation. Concerning human and animal safety and welfare, we have successfully employed the Lincoln Education Assistance with Dogs (LEAD) risk assessment tool (95) for this study – the tool not just ensured a thorough risk assessment, and provided a structure with clear areas of responsibility, but also enabled consistent, safe and welfare-guided practice for all involved.
We would therefore recommend the following steps as vital for successful AAI and AAI research in schools:
(1) Timing and Commitment: Following appropriate ethics approval, ensure significant advance recruitment of schools with clear information as to what the requirements are concerning time and space (e.g., separate room for specific duration). It is useful to be clear about the amount of commitment needed from schools and teachers so all involved can agree to researchers spending a substantial amount of time in schools with the children.
(2) Clarity of Information: Transparency concerning the study to inform teachers, parents and children of all that is involved is essential to obtain consent/assent as well as to maintain ongoing interest.
(3) Safety and welfare: Human and animal safety and welfare need to be ensured at all times. The LEAD tool (95) for AAIs as well as safety training for all involved as described above [e.g., on dog body language (97)] is efficient and helps to raise awareness of potential risk and ensure the safety and welfare of all involved.
In conclusion, this longitudinal RCT study is the first to demonstrate how children's spatial abilities can benefit from AAI with dogs and from relaxation interventions. In Study 1, typically developing children showed improvements in spatial abilities especially over the first 12 weeks, but also beyond, and those in the dog group showed significant improvement immediately after the intervention and also short-term (a further 6 weeks after intervention end). They also showed significantly enhanced performance short-term after relaxation interventions. In contrast, no significant improvements in spatial abilities were found in the no treatment control group.
In Study 2, the cohort of children with SEN showed lower scores overall, showed most learning only in the first 6 weeks, and benefitted only from relaxation interventions. Intervention effects did not extend to the second testing point after the end of the intervention.
As immediate and short-term effects, but not long-term effects were evident, and as spatial abilities are important for wider academic skills such as maths and STEM topics in both cohorts, it is recommended that further research assesses how AAI and relaxation interventions may be incorporated into training applications to enhance such skills. Furthermore, we need to understand better why a dog intervention may improve these skills in typical children, but not in SEN children. It is possible that with SEN children a longer or more intensive period of intervention (higher dosage) may be required to accrue benefits, if any, of an AAI.
This study provides information about the time course of effects of one type of AAI on spatial ability, but many variables need to be examined in the future such as dosage of intervention (number of days of AAI per week, number of weeks of AAI), details of the intervention (do the children need to touch the dog), and delivery of the intervention (free form vs planned pedagogy). The underlying mechanisms of action and the potential for interaction among these mechanisms need to be investigated in further depth in future so that we may make effective recommendations for the use of AAI in typical and SEN children in future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by University of Lincoln Psychology Research Ethics Committee (SOPREC) and also WALTHAM Animal Welfare and Ethical Review Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
KM conceived the study and obtained the research funding. VB contributed to conception of the project. KM, NG, and ER advised on data collection and analysis. VB and MD collected the data. ER oversaw the data base. VB, MD, KM, and ER collated and/or analyzed the data. All authors contributed to the final research design of the current study. All authors contributed to advice on analysis and to writing the manuscript, and read and approved the final manuscript.
This research was funded with a research grant from the WALTHAM Petcare Science Institute (formerly Waltham Centre for Pet Nutrition), Mars Petcare, and a grant from the Waltham Foundation.
We would like to thank all the children and schools taking part in our research and welcoming us to their environments. Thank you to all heads of schools, teachers, teaching assistants, and other school staff for enabling this research to take place. Thank you also to all dog handlers and their dogs for taking part, and to Pets as Therapy for supporting our research. We would furthermore like to thank our independent dog behavior specialists Dr. Hannah Wright, Alison Winters, and Dr. Muriel Brasseur for assessing the dogs for us.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^The majority of SEN children were boys, hence, we could not include Gender in our analysis. Information on Dog Ownership for this cohort was incomplete due to parental non-responses. Due to expected higher attrition in this group, we calculated the power a priori: with assumed power of 0.8, 3 groups and 5 measurement repetitions, a sample size of 27 is required (significance level at 0.05, effect size 0.25).
1. Vasilyeva M, Lourenco SF. Development of spatial cognition. Wiley Interdiscip Rev Cogn Sci. (2012) 3:349–62. doi: 10.1002/wcs.1171
2. Atkinson J, Nardini M. Visuospatial and visuomotor development. In: Reed J, Rogers JW, editors. Child Neuropsychology. Oxford: Blackwell (2007).
3. Nardini M, Burgess N, Breckenridge K, Atkinson J. Differential developmental trajectories for egocentric, environmental and intrinsic frames of reference in spatial memory. Cognition. (2006) 101:153–72. doi: 10.1016/j.cognition.2005.09.005
4. Newcombe N, Huttenlocher J. Children's early ability to solve perspective taking problems. Dev Psychol. (1992) 28:635–43. doi: 10.1037/0012-1649.28.4.635
5. Bremner JG, Bryant P. Place vs. response as the basis of spatial errors made by young infants. J Exp Child Psychol. (1977) 23:162–71. doi: 10.1016/0022-0965(77)90082-0
6. Falomir Z, Olteteanu AM. Special issue on problem-solving, creativity and spatial reasoning. Cogn Syst Res. (2019) 58:31–4. doi: 10.1016/j.cogsys.2019.05.001
7. Newcombe NS, Levine SC, Mix KS. Thinking about quantity: the intertwined development of spatial and numerical cognition. Wiley Interdiscip Rev Cogn Sci. (2015) 6:491–505. doi: 10.1002/wcs.1369
8. Newcombe N. Picture this: increasing math and science learning by improving spatial thinking. American Educ. (2010) 34:29–35.
9. Zhang X, Koponen T, Räsänen P, Aunola K, Lerkkanen MK, Nurmi JE. Linguistic and spatial skills predict early arithmetic development via counting sequence knowledge. Child Dev. (2014) 85:1091–107. doi: 10.1111/cdev.12173
10. Hodgliss A, Gilligan KA, Tollmie AK, Thomas MCC, Farran EK. Spatial cognition and science achievements: the contribution of intrinsic and extrinsic spatial skills from 7 to 1years. Br J Educ Psychol. (2018) 88:675–97. doi: 10.1111/bjep.12211
11. Wai J, Lubinski D, Benbow CP. Spatial ability for STEM domains: aligning over 50 years of cumulative psychological knowledge solidifies its importance. J Edu Psychol. (2009) 101:817–35. doi: 10.1037/a0016127
12. Lubinski D. Spatial ability and STEM: a sleeping giant for talent identification and development. Pers Individ Diff. (2010) 49:344–51. doi: 10.1016/j.paid.2010.03.022
13. Beattie HL, Schutte AR, Cortesa CS. The relationship between spatial working memory precision and attention and inhibitory control in young children. Cogn Dev. (2018) 47:32–45. doi: 10.1016/j.cogdev.2018.02.002
14. Abbeduto L, Warren SF, Conners FA. Language development in down syndrome: from the pre-linguistic period to the acquisition of literacy. Ment Retard Dev Disabil Res Rev. (2007) 13:247–61. doi: 10.1002/mrdd.20158
15. Silverman W. Down syndrome: cognitive phenotype. Ment Retard Dev Disabil Res Rev. (2007) 13:228–36. doi: 10.1002/mrdd.20156
16. Chapman RS. Language development in children and adolescents with down syndrome. Ment Retard Dev Disabil Res Rev. (1997) 3:307–12. doi: 10.1002/(SICI)1098-2779(1997)3:4<307::AID-MRDD5>3.0.CO;2-K
17. Wang PP, Bellugi U. Evidence from 2 genetic syndromes for a dissociation between verbal and visual-spatial short-term-memory. J Clin Exp Neuropsychology. (1994) 16:317–22. doi: 10.1080/01688639408402641
18. Lott IT, Dierssen M. Cognitive deficits and associated neurological complications in individuals with downs syndrome. Lancet Neurol. (2010) 9:623–33. doi: 10.1016/S1474-4422(10)70112-5
19. Alpanda S. The investigation of the relationship between ADHD and visual-spatial functions. Soc Behav Sci. (2015) 174:2219–25. doi: 10.1016/j.sbspro.2015.01.878
20. Mitchell P, Ropar D. Visuo-spatial abilities in autism: a review. Infant Child Dev. (2004)13:185–98. doi: 10.1002/icd.348
21. Cardillo R, Erbì C, Mammarella IC. Spatial perspective-taking in children with autism spectrum disorders: the predictive role of visuospatial and motor abilities. Front Hum Neurosci. (2020) 14:208. doi: 10.3389/fnhum.2020.00208
22. Frith U, Happe F. Autism: beyond “theory of mind”. Cognition. (1994) 50:115–32. doi: 10.1016/0010-0277(94)90024-8
23. Sahyoun CP, Soulieres I, Belliveau JW, Mottron L, Mody M. Cognitive differences in pictoral reasoning between high-functioning autism and aspergers syndrome. J Autism Dev Disord. (2010) 39:1014–23. doi: 10.1007/s10803-009-0712-9
24. Chabani E, Hommel B. Visuospatial processing in children with Autism: no evidence for (training resistant) abnormalities. J Autism Dev Disord. (2014) 44:2230–43. doi: 10.1007/s10803-014-2107-9
25. Mottron L, Burack JA, Iarocci G, Belleville S, Enns JT. Locally oriented perception with intact global processing among adolescents with high-functioning autism: evidence from multiple paradigms. J Child Psychol Psychiatry. (2003) 44:904–13. doi: 10.1111/1469-7610.00174
26. Edgin JO, Pennington BF. Spatial cognition in autism spectrum disorders: superior, impaired, or just intact? J Autism Dev Disord. (2005) 35:729–45. doi: 10.1007/s10803-005-0020-y
27. Vogel SA. Gender differences in intelligence, language, visual-motor abilities, and academic achievement in students with learning disabilities: a review of the literature. J Learn Disabil. (1990) 23:44–52. doi: 10.1177/002221949002300111
28. Halpern DF. Gender differences in cognitive abilities. 3rd ed. New York, NY: Psycology Press (2000).
29. Levine SC, Huttenlocher J, Taylor A, Langrock A. Early gender differences in spatial skill. Dev Psychol. (1999) 35:940–9. doi: 10.1037/0012-1649.35.4.940
30. Voyer D, Voyer S, Bryden MP. Magnitude of gender differences in spatial abilities: a meta-analysis and consideration of critical variables. Psychol Bull. (1995) 117:250–70. doi: 10.1037/0033-2909.117.2.250
31. Collaer ML, Hill E. Large gender difference in adolescents on a timed line judgment task: Attentional contributors and task relationship to mathematics. Percept. (2006) 35:561–72. doi: 10.1068/p5003
32. Cherney ID, Collaer ML. Gender differences in line judgment: relation to mathematics preparation and strategy use. Percept Motor Skills. (2005) 100: 615–27. doi: 10.2466/pms.100.3.615-627
33. Collaer ML, Lane C, Maxwell M. Human Males, But not Females, Are Sensitive to Visuospatial Task Geometry. Amherst, MA: Society for Behavioral Neuroendocrinology (2002).
34. Collaer ML, Nelson JD. Large visuospatial gender difference in line judgment: possible role of attentional factors. Brain Cogn. (2002) 49:1–12. doi: 10.1006/brcg.2001.1321
35. Reilly D, Neumann DL, Andrews G. Gender differences in spatial ability: implications for STEM education and approaches to reducing the gender gap for parents and educators. In: Khine M, editors. Visual-Spatial Ability in STEM Education. Cham: Springer (2016).
36. Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. (1994) 57:5–14. doi: 10.1016/0031-9384(94)00197-D
37. Moody MS. Changes in test scores on the mental rotations test during the menstrual cycle. Percept Motor Skills. (1997) 84:955–61. doi: 10.2466/pms.1997.84.3.955
38. Neave N, Menaged M, Weightman DR. Sex differences in cognition: the role of testosterone and sexual orientation. Brain Cogn. (1999) 41:245–62. doi: 10.1006/brcg.1999.1125
39. Hausmann M, Slabbekoorn D, van Goozen SHM, Cohen-Kettenis PT. Gender hormones affect spatial abilities during the menstrual cycle. Behav Neurosci. (2001) 114:1245–50. doi: 10.1037/0735-7044.114.6.1245
40. Jones CM, Braithwaite VA, Healy SD. The evolution of sex differences in spatial ability. Behav Neurosci. (2003) 117:403–11. doi: 10.1037/0735-7044.117.3.403
41. Silverman II, Choi J, Mackewn A, Fisher M, Moro J, Olshansky E. Evolved mechanisms underlying wayfinding. Further studies on the hunter-gather theory of spatial Gender differences. Evol Hum Behav. (2000) 21:201–13. doi: 10.1016/S1090-5138(00)00036-2
42. Collaer ML, Reimers S, Manning JT. Visuospatial performance on an internet line judgement task and potential markers: sex, sexual orientation, and 2D:4D. Arch Sex Behav. (2007) 36:177–92. doi: 10.1007/s10508-006-9152-1
43. Caldera YM, McCulp AD, O'Brien M, Truglio RT, Alvarez M, Huston AC. Children's play preferences, construction play with blocks, and visual-spatial skills: are they related? Internat J Behav Dev. (1999) 23:855–72 doi: 10.1080/016502599383577
44. Linn MC, Petersen AC. Emergence and characterization of gender differences in spatial ability: a meta-analysis. Child Dev. (1985) 56:1479–98. doi: 10.2307/1130467
45. Blüchel M, Lehmann J, Kellner J, Jansen P. The improvement in mental rotation performance in primary school-aged children after a two-week motor-training. Edu Psychol. (2013) 33:75–86. doi: 10.1080/01443410.2012.707612
46. Uttal DH, Meadow NG, Tipton E, Hand LL, Alden AR, Warren C, et al. The malleability of spatial skills: a meta-analysis of training studies. Psychol Bull. (2013) 139:352–402. doi: 10.1037/a0028446
47. Tzuriel D, Egozi G. Gender differences in spatial ability of young children: the effects of training and processing strategies. Child Dev. (2010) 81:1417–30. doi: 10.1111/j.1467-8624.2010.01482.x
48. Baenninger M, Newcombe NS. The role of experience in spatial test performance: a meta-analysis. Gender Roles. (1989) 20:327–44. doi: 10.1007/BF00287729
49. Baenninger M, Newcombe NS. Environmental input to the development of gender-related differences in spatial and mathematical ability. Learn Individ Differ. (1995) 7:363–79. doi: 10.1016/1041-6080(95)90007-1
50. IET report. Studying STEM – What Are the Barriers? (2008). Available online at: https://mei.org.uk/files/pdf/Studying_Stem.pdf (accessed February 23, 2022).
51. Hummel E, Randler C. Living animals in the classroom: a meta-analysis on learning outcome and a treatment–control study focusing on knowledge and motivation. J Sci Educ Technol. (2011) 21:95–105. doi: 10.1007/s10956-011-9285-4
52. Kotrschal K, Ortbauer B. Behavioural effects of the presence of a dog in the classroom. Anthrozoös. (2003) 16:147–59. doi: 10.2752/089279303786992170
53. Gee NR, Fine A, Schuck S. Animals in educational settings: research and practice. In: Fine AH, editor. Handbook of Animal-Assisted Therapy 4th ed. London: Academic Press (2015) 195–210. doi: 10.1016/B978-0-12-801292-5.00014-6
56. Brelsford VL, Meints K, Gee NR, Pfeffer K. Animal-assisted interventions in the classroom- a systematic review. Internat J Environ Res Public Health. (2017) 14:E669. doi: 10.3390/ijerph14070669
57. Gee NR, Fine A, McCardle P. How Animals Help Students Learn: Research and Practice for Educators and Mental Health Professionals. New York, NY: Routledge (2017).
58. Beetz A, Julius H, Turner D, Kotrschal K. Effects of social support by a dog on stress modulation in male children with insecure attachment. Front Psychol. (2012) 3:352. doi: 10.3389/fpsyg.2012.00352
59. Meints K, Brelsford VL, Dimolareva M, Maréchal L, Pennington K, Rowan E, et al. Can dogs reduce stress levels in school children? Effects of dog-assisted interventions on salivary cortisol in children with and without special educational needs using randomised controlled trials under review. PlosONE. (2022) 17:e0269333. doi: 10.1371/journal.pone.0269333
60. Griffin JA, Hurley K, McCune S. Human-animal interaction research: progress and possibilities. Front Psychol. (2019) 10:2803. doi: 10.3389/fpsyg.2019.02803
61. Kazdin AE. Methodological standards and strategies for establishing the evidence base of animal-assisted therapies. In: Fine A,. editor. Handbook on Animal-Assisted Therapy, 3rd ed. San Diego, CA: Elsevier (2010). p. 519–46.
62. Herzog HA. The impact of pets on human health and psychological well-being: fact, fiction, or hypothesis. Curr Direct Psychol Sci. (2011) 24:236–9. doi: 10.1177/0963721411415220
63. Pendry P, Carr AM, Gee NR, Vandagriff JL. Incorporating human-animal interaction into academic stress management programs: effects on typical and at-risk college students' executive function. AERA Open. (2021) 7:1–18. doi: 10.1177/23328584211011612
64. Schuck SEB, Emmerson NA, Abdullah MM, Fine AH, Stehli A, Lakes KD. A randomized controlled trial of traditional psychosocial and canine-assisted intervention for children with ADHD. Hum Anim Interact Bull. (2018) 6:64–80.
65. Schuck SEB, Johnson HL, Abdullah MM, Stehli A, Fine AH, Lakes KD. The role of animal assisted intervention on improving self-esteem in children with attention deficit/hyperactivity disorder. Front Pediatr. (2018) 6:300. doi: 10.3389/fped.2018.00300
66. Waters L, Barsky A, Ridd A, Allen K. Contemplative education: a systematic, evidence-based review of the effect of medication intervention in schools. Edu Psychol Rev. (2014) 27:103–34. doi: 10.1007/s10648-014-9258-2
67. Ferreira-Vorkapic C, Feitoza JM, Marchioro M, Simoes J, Kozasa E, Telles S. A systematic review of randomised control trials of yoga-based interventions. Evid Based Complement Alternat Med. (2015) 2015:345835. doi: 10.1155/2015/345835
68. Telles S, Singh N, Bhardwaj AK, Kumar A, Balkrishna A. Effect of yoga or physical exercise on physical, cognitive and emotional measures in children: a randomised controlled trial. Child Adolesc Psychiatry Ment Health. (2013) 7:37. doi: 10.1186/1753-2000-7-37
69. Hagen I, Nayar US. Yoga for children and young people's mental health and well-being: research review and reflections on the mental health potentials of yoga. Front Psychiatry. (2014) 5:1–6. doi: 10.3389/fpsyt.2014.00035
70. Mendelson T, Greenberg MT, Dariotis JK, Gould LF, Rhoades BI, Leaf PJ. Feasibility and preliminary outcomes of a school-based mindfulness intervention for urban youth. J Abnorm Psychol. (2010) 38:985–984. doi: 10.1007/s10802-010-9418-x
71. Tang Y, Ma Y, Wang J, Fan Y, Feng S, Lu Q, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. (2007) 104:17152–6. doi: 10.1073/pnas.0707678104
72. Sarokte AS, Rao MV. Effects of Medhya Rasayana and Yogic practices in improvement of short-term memory among school-going children. Int Quart J Res Ayurveda. (2013) 34:383–9. doi: 10.4103/0974-8520.127720
73. Pradhan B, Nagendra HR. Effect of yoga relaxation techniques on performance of digit-letter substitution task by teenagers. Int J Yoga. (2009) 2:30–4. doi: 10.4103/0973-6131.43293
74. Sarang SP, Telles S. Immediate effect of two yoga-based relaxation techniques on performance in a letter cancellation task. Percept Mot Skills. (2007) 105:379–85. doi: 10.2466/pms.105.2.379-385
75. Broderick PC, Metz S. Learning to BREATHE: a pilot trial of a mindfulness curriculum for adolescents. Adv Sch Ment Health Promot. (2009) 2:35–55. doi: 10.1080/1754730X.2009.9715696
76. Gee NR, Church MT, Altobelli CL. Pre-schoolers make fewer errors on an object categorisation task in the presence of a dog. Anthrozoös. (2010) 23:223–30. doi: 10.2752/175303710X12750451258896
77. Gee NR, Belcher JM, Grabski JL, DeJesus M, Riley W. The presence of a therapy dog results in improved object recognition performance in preschool children. Anthrozoös. (2012) 25:289–300. doi: 10.2752/175303712X13403555186172
78. Gee NR, Gould JK, Swanson CC, Wagner AK. Pre-schoolers categorise animate objects better in the presence of a dog. Anthrozoös. (2012) 25:187–98. doi: 10.2752/175303712X13316289505387
79. Gee NR, Crist EN, Carr DN. Preschool children require fewer instructional prompts to perform a memory task in the presence of a dog. Anthrozoös. (2010) 23:173–84. doi: 10.2752/175303710X12682332910051
80. Hergovich A, Monshi B, Semmler G, Zieglmayer V. The effects of the presence of a dog in the classroom. Anthrozoös. (2002) 15:37–50. doi: 10.2752/089279302786992775
81. Engel G. The need for a new medical model: a challenge for biomedical science. Science. (1977) 196:26–129. doi: 10.1126/science.847460
82. Lehman BJ, David DM, Gruber JA. Rethinking the biopsychosocial model of health: understanding health as a dynamic system. Soc Person Psychol Compass. (2017) 11:e12328. doi: 10.1111/spc3.12328
83. Gee NR, Rodriguez KE, Fine AH, Trammell JP. Dogs Supporting human health and well-being: a biopsychosocial approach. Front Vet Sci. (2021) 8:630465. doi: 10.3389/fvets.2021.630465
84. Friedmann E, Gee NR. Companion animals as moderators of stress responses: Implications for academic performance, testing and achievement. In NR Gee, A Fine, P McCardle, editors. How Animals Help Students Learn: Research and Practice for Educators and Mental Health Professionals. New York, NY; London: Routledge; Taylor Francis Group (2017).
85. Julius H, Beetz A, Kotrschal K, Turner D, Uvn?s-Moberg K. Attachment to Pets: an integrative view of human-animal relationships with implications for therapeutic practice. Cambridge, MA: Hogrefe Publishing (2013).
86. O'Haire ME, McKenzie SJ, Beck AM, Slaughter V. Animals may act as social buffers: Skin conductance arousal in children with autism spectrum disorder in a social context. Dev Psychobiol. (2015) 57:584–95. doi: 10.1002/dev.21310
87. Gee NR, Mueller MK. (2019). A systematic review of research on pet ownership and animal interactions among older adults Anthrozoös. (2019) 32:183–207. doi: 10.1080/08927936.2019.1569903
88. Serpell JA. Animal-assisted interventions in historical perspective. In: Fine AH, editor. Animal-Assisted Therapy: Foundations and Guidelines for Animal-Assisted Interventions. London: Elsevier (2015).
89. McNicholas J, Collis GM. Dogs as catalysts for social interactions: robustness of the effect. Br J Psychol. (2000) 91:61–70. doi: 10.1348/000712600161673
90. Beetz AM. Theories and possible processes in action in animal-assisted interventions. Appl Dev Sci. (2017) 21:139–49. doi: 10.1080/10888691.2016.1262263
91. Handlin L, Nilsson A, Ejdeback M, Hydbring-Sandberg E, Uvn?s-Moberg K. Associations between the psychological characteristics of the human-dog relationship and oxytocin and cortisol levels. Anthrozoös. (2012) 25:215–28. doi: 10.2752/175303712X13316289505468
92. Petersson M, Uvn?s-Moberg K, Nilsson A, Gustafson L, Hydbring-Sandberg E, Handlin L. Oxytocin and cortisol levels in dog owners and their dogs are associated with behavioural patterns: an exploratory study. Front in Psy. (2017) 8:1796. doi: 10.3389/fpsyg.2017.01796
93. Friedmann E, Thomas SA, Son H, Chapa D, McCune S. Pet's presence and owner's blood pressures during the daily lives of pet owners with pre- to mild hypertension. Anthrozoös. (2013) 26:535–50. doi: 10.2752/175303713X13795775536138
94. Gee NR, Griffin JA, McCardle P. Human-animal interaction research in school settings: current knowledge and future directions. AERA Open. (2017) 3:1–9. doi: 10.1177/2332858417724346
95. Brelsford VL, Dimolareva M, Gee NR, Meints K. Best practice standards in animal-assisted interventions: how the LEAD risk assessment tool can help. Animals. (2020) 10:974. doi: 10.3390/ani10060974
97. Meints K, Brelsford VL, De Keuster T. Teaching children and parents to understand dog signaling. Front Vet Sci. (2018) 5:257. doi: 10.3389/fvets.2018.00257
99. Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/BF03193146
100. Baddeley A. Hitch, G. Working Memory. Psychol Learn Motiv. (1974) 8:47–89. doi: 10.1016/S0079-7421(08)60452-1
101. Blasiman RN, Was CA. Why is working memory performance unstable? A Review of 21 Factors. Eur J Psychol. (2018) 14:188–231. doi: 10.5964/ejop.v14i1.1472
102. Hediger K, Turner D. Can dogs increase children's attention and concentration performance? A randomised controlled trial. Hum Anim Interact Bull. (2014) 2:21–39.
103. Storbeck J, Maswood R. Happiness increases verbal and spatial working memory capacity where sadness does not: emotion, working memory and executive control. Cogn Emot. (2016) 30:925–38. doi: 10.1080/02699931.2015.1034091
104. Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proc Natl Acad Sci U S A. (2002) 99:4115–20. doi: 10.1073/pnas.062381899
105. Ayoub CC, Fischer KW. Developmental pathways and intersections among domains of development. In: McCartney K, Phillips KD, editors. Blackwell Handbook of Early Childhood Development. Oxford: Blackwell (2006). p. 62–81.
106. Reilly D. Linear or nonlinear? A metacognitive analysis of educational assumptions and reform efforts. Intern J Edu Manag. (2000) 14:7–15. doi: 10.1108/09513540010310350
107. Purewal R, Christley R, Kordas K, Joinson C, Meints K, Gee NR, et al. Companion animals and child/adolescent development: a systematic review of the evidence. Int J Environ Res Public Health. (2017) 14:234. doi: 10.3390/ijerph14030234
Keywords: animal-assisted intervention (AAI), dog-assisted, randomized controlled trial, longitudinal, child, special educational needs (SEN), cognition, spatial ability
Citation: Brelsford VL, Dimolareva M, Rowan E, Gee NR and Meints K (2022) Can dog-assisted and relaxation interventions boost spatial ability in children with and without special educational needs? A longitudinal, randomized controlled trial. Front. Pediatr. 10:886324. doi: 10.3389/fped.2022.886324
Received: 28 February 2022; Accepted: 08 July 2022;
Published: 29 July 2022.
Edited by:
Kimberley D. Lakes, University of California, Riverside, United StatesReviewed by:
Isabelle Esther Bauer, University of Texas Health Science Centre at Houston, United StatesCopyright © 2022 Brelsford, Dimolareva, Rowan, Gee and Meints. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerstin Meints, a21laW50c0BsaW5jb2xuLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.