
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr. , 28 April 2022
Sec. Pediatric Hematology and Hematological Malignancies
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.885876
This article is part of the Research Topic Risk Factors and New Treatment Options in Pediatric Venous Thromboembolism View all 7 articles
In children with gastrointestinal disorders such as inflammatory bowel disease (IBD) and intestinal failure (IF), the risk of venous thromboembolism (VTE) is increased. VTE may lead to pulmonary embolism, sepsis and central line infection, stroke and post-thrombotic syndrome. The purpose of this review is to summarize current knowledge and recent advances around VTE management in pediatric gastroenterology with a focus on IBD and IF. The VTE incidence in children with IBD is reported to be around 4–30 per 10,000 patient-years, with higher incidences for hospitalized children. While in general, IF is less common than IBD, the VTE incidence in children with IF is around 750 per 10,000 patient-years. The most common risk factors for development of VTE involve deviations leading to Virchow's triad (endothelial damage, stasis, and hypercoagulability) and include active inflammation, particularly with colonic involvement, presence of a central venous catheter, underlying thrombophilia, reduced mobility, surgery, and hospitalization. Classes of anticoagulants used for treatment of VTE are low molecular weight heparins and vitamin K antagonists. However, the use of direct oral anticoagulants for treatment or prevention of VTE has not been studied in this pediatric population yet. Pediatric gastroenterologists apply different VTE prevention and treatment strategies due to lack of literature and lack of consensus. We discuss the role of primary and secondary prophylactic use of anticoagulants, and provide tools and recommendations for screening, prevention and management for the specific pediatric populations.
Venous thromboembolism (VTE) involves deep venous thrombosis, pulmonary embolism, cerebral sinovenous thrombosis (CSVT), central venous catheter (CVC) related VTE, and abdominal vein thrombosis, such as portal, or mesenteric vein thrombosis (1). According to Virchow's triad (dating from 1856), VTE results from endothelial damage, stasis, and hypercoagulability (2). In general, VTE may lead to post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension, associated with substantial morbidity and high healthcare expenses (3).
Although VTE is rare in childhood, pediatricians, and more specific pediatric gastroenterologists, may encounter children with VTE, either with gastrointestinal symptoms or consequences of VTE, or with VTE as complication of gastrointestinal diseases. Abdominal vein thrombosis, which can be secondary to intra-abdominal infections, pancreatitis, surgery, and sickle cell disease, is often asymptomatic, but may eventually manifest with symptoms of acute abdomen, or with gastrointestinal complications reflecting portal hypertension, including liver dysfunction, splenomegaly, or upper gastrointestinal bleeding (4). On the other hand, gastrointestinal diseases that may be complicated by development of VTE are cystic fibrosis, celiac disease, inflammatory bowel disease (IBD) and intestinal failure (IF) (5, 6). Within this spectrum, children with IBD and IF are most at risk of developing VTE.
The estimated prevalence of IBD is 58.9–66.3 per 100,000 children in Western Europe (7). The worldwide incidence is increasing, especially in younger children (7–9). Recently, emerging data has demonstrated that VTE is associated with IBD in children, but the precise mechanism is not yet fully understood. VTE in children with IBD is associated with longer hospital stay, increased need for intensive care unit admission, higher costs, and increased in-hospital mortality (10).
In children with IF, the gut insufficiently absorbs nutrients and fluids needed for growth and maintaining homeostasis (11). Therefore, these children depend on long-term parenteral nutrition (PN) given through a CVC. Prevalence of long-term PN varies between 9.6 and 14.1 per million children (12, 13). Children with IF may develop VTE, in IF mostly referred to as central venous thrombosis (CVT), because it is related to the presence of a CVC (14). Recurrent VTE is a major barrier for delivering PN and can result in loss of vascular access. Loss of vascular access is one of the criteria for consideration of small bowel transplantation (15).
Given the risk and consequences of VTE in children with IBD and IF, pediatric gastroenterologists need practical tools for diagnostics, prevention and treatment of VTE in these populations, while considering disease-specific risk factors and underlying mechanisms. Therefore, this review aims to describe incidence, mechanisms and risk factors of VTE, and to outline screening, prevention and treatment strategies for VTE in pediatric gastroenterology with a focus on IBD and IF. We offer insight into controversies, current research gaps and new developments in the field.
In a recent meta-analysis, VTE incidence in the general pediatric population was found to be 0.27 per 10,000 person-years (PY) (16).
In recent years, several studies have evaluated the VTE risk in children with IBD. A Canadian population-based study reported a 5-year incidence of 31.2 per 10,000 PY for VTE, compared to 0.78 per 10,000 PY in children without IBD (17). The incidence was highest in the 1st year after diagnosis (81.2 per 10,000 PY). Another Danish population-based study found an incidence rate of 8.9 per 10,000 PY for VTE in children with IBD, with a hazard ratio of 6.6 compared to age-matched controls. The prospective international Safety Registry, covering 24,802 children with IBD, reported an incidence of 3.09 per 10,000 PY, 14-fold higher than the incidence rate in the general pediatric population (16). Altogether, these studies clearly show an increased VTE risk in children with IBD, though with varying incidence rates, which is likely the result of a different methodology and background population of the studies. Interestingly, CSVT has been described as a relatively common VTE type in children with IBD. According to two studies, including a systematic review, CSVTs made up to almost 50% of VTE cases, with a reported incidence rate of 1.86 per 10,000 PY in the Safety Registry (16, 18). Another single-center retrospective study found that in 4/154 (2.6%) newly-diagnosed children with IBD a CSVT occurred within 5 years. Although this was not supported by data from other pediatric studies (17, 19), physicians should be vigilant about CSVT occurrence in children with IBD, especially considering the significant morbidity and mortality.
Although pediatric IF is much less prevalent than pediatric IBD (PIBD), VTE itself is much more common in children with IF. Reported incidences of VTE vary among studies, depending on the population studied, the follow-up duration period and the time since diagnosis of IF. In a recent meta-analysis (from 2019) including 1,277 children with IF, the authors reported thrombosis to be present in 25.7%, resulting in an event rate of 7.5% per year and an incidence of 750 per 10,000 PY (14). When looking at individual studies published after publication of the meta-analysis, ranging prevalences have been reported. Asouzo et al. reported one or more thrombi in 30 patients from a cohort of 65 patients with IF (46%) with a median age of 6.83 years (20), while Schmidt et al. reported thrombosis in 95 out of 388 patients with IF (24%) during a median follow-up time of 398 days (21). Secondary (i.e., recurrent) VTE occurred in 44% of the patients of the latter study. LaRusso et al. retrospectively included 37 patients with IF, of which 8 developed venous thrombosis (22%) (22).
In PIBD, the increased VTE risk results from a combination of increased prevalence of general VTE-related risk factors and prothrombotic conditions in this patient population, such as immobilization or dehydration, and the systemic inflammation leading to the development of Virchow's triad (Figure 1). Prothrombotic abnormalities observed in IBD include initiation and activation of the coagulation system (e.g., increased coagulants such as fibrinogen, factor V, VIII, IX, Von Willebrand factor or thrombin-antithrombin complexes), reduced activity of natural anticoagulant mechanisms (e.g., decreased antithrombin III, decreased protein C and S, and decreased tissue factor pathway inhibitor), abnormality of fibrinolysis, platelet abnormalities (thrombocytosis, increased activation and aggregation), and reactivity and dysfunction of the endothelium (23–26). Prothrombogenic cytokines that are implicated to be the driver of thrombus formation in patients with IBD are IL-1b, TNF-a, and IL6 (23).
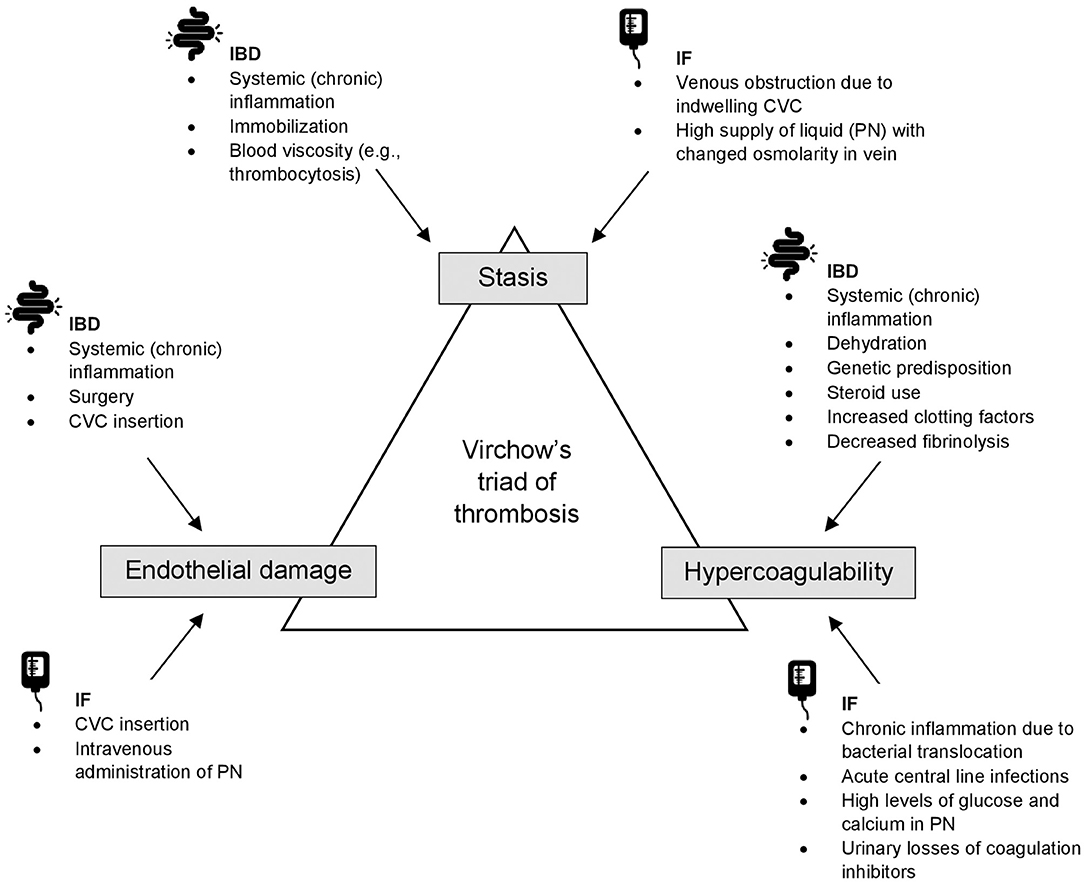
Figure 1. An overview of Virchow's triad of thrombosis in IBD and IF. CVC, central venous catheter; IBD, inflammatory bowel disease; IF, intestinal failure; PN, parenteral nutrition.
Several risk factors may contribute to the higher VTE risk in children with IBD (Table 1). Generally, VTE is more common in adolescents than in younger children (27). So far, there is no evidence confirming this is also the case in PIBD.
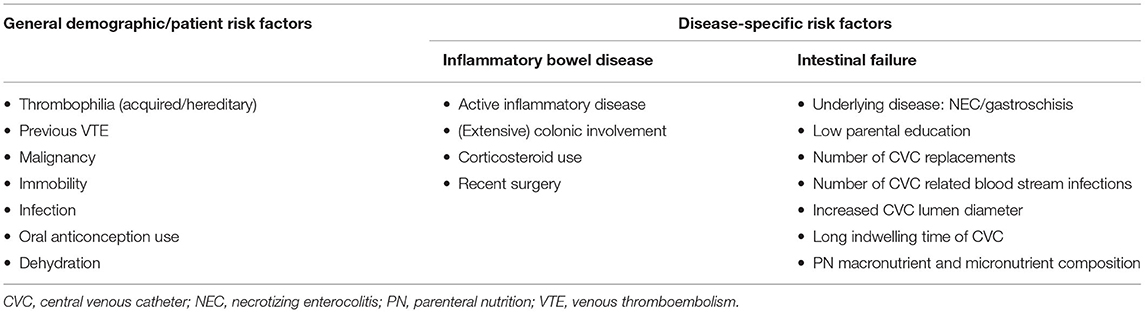
Table 1. Risk factors for developing venous thromboembolic events in children with inflammatory bowel disease and/or intestinal failure.
By far one of the most precipitating factors is hospitalization. One study reported an incidence of 117.9 per 10,000 PY in hospitalized children with IBD (28). The Safety Registry study reported a relative risk of 559 for hospitalization (16). However, VTE development is not limited to hospitalized patients. According to a recently published study, VTE was the indication for admission in 11/23 (48%) cases (29), and in the Safety Registry study, 9/21 (43%) cases occurred in non-hospitalized patients (16). Therefore, awareness of VTE occurrence and prevention should not be limited to admitted patients.
Active inflammation is another important factor contributing to increased VTE risk in patients with IBD. This was already proven by several large adult studies (30, 31) and has recently been demonstrated in a pediatric study, in which all but one patient had clinical or biochemical evidence of active disease (16).
Another independent risk factor is surgery. McKie et al. demonstrated that children with IBD undergoing a major surgical procedure had a 1.98 greater odds of developing a VTE, compared to children with IBD without major surgery (32). Bence et al. performed a retrospective review of children who underwent colorectal resection for IBD and found that 4.3% of patients developed postoperative VTE after a median of 14 days postoperatively (33). The increased risk of VTE after surgery might even extend after hospital-discharge, as has been demonstrated in adults with ulcerative colitis (UC) after urgent surgery (34).
Pediatric UC patients are likely to be more prone to thromboembolic events than Crohn's disease (CD) patients. A meta-analysis in adults showed similar risks in patients with UC and CD (RR 2.57 vs. 2.12) (35), while several studies in children found a lower incidence rate in CD patients (17, 19). The risk difference between adult and pediatric UC patients could be explained by the fact that children more often present with pancolitis, which is more extensive disease and has been associated with VTE in adults with IBD (36, 37). In the Safety Registry, all six CD patients had colonic involvement and all 14 UC patients had pancolitis (16). These findings suggest that extensive colonic involvement confers a higher risk of VTE, though the association with endoscopic disease severity has not been evaluated yet.
There is little evidence regarding the relationship between IBD therapy and VTE risk in children with IBD. In the previously mentioned Safety Registry, 9/20 patients received corticosteroids, and 4/20 received anti-TNF prior to the VTE event (16). Another nested case-control study in hospitalized patients with IBD reported that steroid use was an independent risk factor of VTE (OR 12.7) (29). To the best of our knowledge, no other studies assessed the association between VTE risk in children with IBD and therapy use. Generally, corticosteroids are considered thrombogenic. Steroids contribute to the hypercoagulable state by increasing levels of factor VIII/von Willebrand factor complex and by inducing a hypofibrinolytic state by increasing plasminogen activator inhibitor 1 (38). In adults, corticosteroid use is associated with a higher risk of VTE (OR 2.2) (39). However, this could be secondary to active disease rather than corticosteroid use alone. There is conflicting data regarding the risk of VTE in adults with IBD receiving biological therapy, such as TNF-alfa inhibitors. Sarlos et al. suggested a 5-fold decreased VTE risk of TNF-alfa inhibitors compared to steroids (39). Another nationwide observational study showed no difference in VTE risk between patients with IBD treated with TNF-alfa inhibitors compared with non-biologic immunomodulating agents (e.g., thiopurines, methotrexate), though in a subgroup of patients <45 years, TNF-alfa inhibitors were associated with VTE risk reduction in CD patients (40).
Several unspecific risk factors that are common in patients with IBD may increase the risk of VTE. These include dehydration due to increased gastrointestinal losses and impaired nutritional status, presence of CVCs, total PN and reduced mobilization due to active disease or fatigue.
In pediatric IF, the increased VTE risk likely results from vessel wall injury due to CVC insertion and intravenous administration of PN. Also, a hypercoagulable state may be present due to chronic inflammation, acute central line infections, and high levels of glucose and calcium in PN (41). Proteinuria, which is common in children with IF on long-term PN (42), is accompanied by urinary losses of coagulation inhibitors such as antithrombin and protein S and C, also leading to a hypercoagulable state (43). In Figure 1, mechanisms in IF leading to the development of Virchow's triad are presented.
VTE risk factors in IF (Table 1) are mostly related to the CVC. VTE may be due to catheter insertion and PN, multiple surgical procedures to replace catheters and repeated episodes of CVC related blood stream infections (44–46). LaRusso et al. found no significant difference in thrombosis rates between different types of catheters used: in tunneled CVC use, incidence was 0.15/1,000 catheter days; and in peripherally inserted central catheter (PICC) use, incidence was 0.55/1,000 catheter days (22). In another study, not only CVC related risk factors were found, but VTE was also found to be associated with parental education (20). Possibly, in parents with lower education, there is a lack of understanding of line care and delay in recognition of line complications and infection. Another risk factor is underlying disease, in which necrotizing enterocolitis and gastroschisis were predictors of VTE (20). This may be because these diseases involve inflammation of the gut. Inflammation increases fibrinogen concentration, induces tissue factor expression on the cell surface of leucocytes, and increases platelet production through inflammatory mediators such as interleukin-6; all involved in the coagulation cascade (47).
Table 2 includes special considerations for identification of risk factors, VTE prevention, VTE recognition, and VTE management in children with IBD and IF.
Treatment of VTE in children with IBD and IF traditionally consists of heparin (unfractionated heparin or low-molecular-weight heparin (LMWH)), followed by LMWH or vitamin K antagonists (VKAs) (48). Direct oral anticoagulants (DOACs), such as apixaban, rivaroxaban and edoxaban (direct factor Xa inhibitors), and dabigatran (direct thrombin inhibitor), offer advantages over these traditional agents, such as oral route of administration, in general no need for laboratory monitoring of anticoagulant activity, and no dose adjustment. The first completed pediatric studies show that DOACs could be a safe and effective alternative for treatment of VTE in children (49). However, it remains unknown if these results can be extrapolated to specific patient groups at risk of worse outcome (e.g., bleeding), such as children with IBD or IF (50). Altered pharmacokinetics or drug interactions in these patients might require dose adjustments and better monitoring by measuring trough or peak plasma concentrations. Absorption of DOACs occurs in different locations, with apixaban being mainly absorbed in the distal small intestine and proximal colon, and edoxaban, rivaroxaban and dabigatran absorbed in the proximal gastrointestinal tract (e.g., stomach and proximal small intestine) (51). Therefore, these latter agents might be an option in stable children with (active) IBD. DOACs seem less useful in children with IF due to absorption problems in the proximal gastrointestinal tract. Despite absorption problems, VKAs are also administered orally in some children with IF, who tolerate a little oral nutrition. Monitoring of INR values ensures that therapeutic VKA levels can be achieved. If children with IF or IBD receive a DOAC, laboratory monitoring is obligatory to guarantee therapeutic anticoagulation levels, depending on the site of absorption and location of the gastro-intestinal disease.
The role of DOACs as thromboprophylaxis in children is yet to be established. When providing thromboprophylaxis with DOACs for children with IBD or IF, the same considerations apply as for treatment of VTE.
Genetic risk seems to be associated with development of VTE in patients with IBD (52). The contribution of hereditary or acquired thrombophilia to VTE development in patients with IBD is likely to be limited (16, 32, 53), and routine screening should thus be reserved for unusual cases. To decide which patients might benefit from thromboprophylaxis, personalized risk-stratification based on general and disease-specific VTE risk factors risk factors is needed. However, until recently, there has been a lot of reluctance in prescribing thromboprophylaxis. In a survey among 162 pediatric gastroenterologists, 92% agreed children with IBD are at increased risk for VTE, but only 1/3 of them ever provided prophylaxis for their patients. Eighty-one percent reported this was because of a paucity of data (54). Because of limited evidence, current ESPGHAN guidelines only recommend thromboprophylaxis with low molecular weight heparin in the setting of adolescents with an acute severe colitis (ASC) with additional risk factors (55). Another issue of prescribing thromboprophylaxis is safety concerns, especially the presumed bleeding risk in patients with active disease (54, 56, 57). However, in a retrospective study in children hospitalized for ASC that received enoxaparin (LMWH) as thromboprophylaxis, no differences in hemoglobin levels or need for blood transfusions were observed. (58) Further support for the safety of thromboprophylaxis comes from adult studies with IBD and from a meta-analysis on thromboprophylaxis in children in general (59).
Fortunately, prevention of VTE in PIBD, based on personalized risk-stratification, has recently received more attention. According to a RAND appropriateness panel, prophylactic anticoagulation was deemed appropriate in all children with IBD admitted with ASC during the COVID-19 pandemic (60). A limitation was that the clinical scenarios did not take into consideration specific patient factors and thus VTE risk factors. Therefore, and based on cumulative evidence of VTE risk, another RAND appropriateness panel was convened this year, comprising pediatric gastroenterologists and experts in pediatric surgery and hematology, that rated the appropriateness of thromboprophylaxis in other settings, while taking into consideration VTE risk factors such as age and sex (61). These initiatives highlight the expanding interest of PIBD experts and current advances in the field of VTE prevention. Despite the increased awareness and vigilance of VTE risk in children with IBD, thromboprophylaxis has not yet been routinely used in this high-risk population. This should be done based on personalized risk-stratification, weighing both VTE risk factors and bleeding risk.
In 2018, Neelis et al. explored organization and clinical practice of IF teams in an international survey (62). According to the ESPGHAN/ESPEN guidelines on PN in infants, children and adolescents (from 2005, applicable at that time), VKAs or LMWH may be given prophylactically to patients on long-term PN at risk of thromboembolism or with previous thromboembolism (63). According to the survey, different VTE prevention and treatment strategies were applied across Europe: 46% of 59 IF teams prescribed prophylactic anticoagulation. Types of anticoagulation used were LMWH (standard in 14%, sometimes in 19% of the teams), heparin lock (standard in 12%, sometimes in 17% of the teams), and VKAs (standard in 2%, sometimes in 14% of the teams). The main reason for not prescribing prophylactic anticoagulation was lack of evidence. Prophylactic anticoagulation was used significantly more frequently in the teams with >10 patients compared with teams with ≤ 10 patients on home PN (59 vs. 28%, respectively) (62).
Already in 2003, the use of prophylactic warfarin was examined. No new VTE and no bleeding complications were found in this study including eight patients (64). In a study comparing LMWH and VKA prophylaxis with no prophylaxis in 32 patients, less occlusions, less infections, and no bleeding complications were found in the group receiving prophylaxis (65). Recently, Nagelkerke et al. assessed VTE and bleeding incidence in a cohort of 55 children with IF on home PN and prophylactic anticoagulation (LMWH or VKA) (66). Cumulative thrombosis-free survival was 96% after 2 years, and 78% after 5 years. Eight cases of VTE occurred on LMWHs (0.14/1,000 anticoagulation days) and 2 on VKAs (0.17/1,000 anticoagulation days). Cumulative bleeding-free survival was 81% after 2 years, and 73% after 5 years. The incidences of clinically relevant and major bleeding were 0.1 and 0.03 per 1,000 catheter days, respectively. These are relatively low rates of VTE and slightly elevated rates of bleeding, compared with studies in which no prophylaxis was used (65, 67).
It remains unclear what the best anticoagulant regimen is for preventing VTE. In the current ESPGHAN/ESPEN guidelines on venous access in pediatric PN (from 2018), the authors state that there is insufficient evidence to recommend prophylactic anticoagulants (68). We need studies comparing prophylaxis with no prophylaxis by means of randomized controlled trials or by comparing thrombosis and bleeding outcomes between centers with different regimens. In the ESPGHAN/ESPEN guidelines, it is recommended to use the antiseptic agent taurolidine during long-term CVC use to prevent line infections (68). This recommendation is mostly based on evidence in adult studies, but also in children the effectiveness of taurolidine has been shown (69, 70). Since recurrent CVC related blood stream infections are associated with higher risk of CVT development, taurolidine might also prevent CVT indirectly.
There are no guidelines for children with IF when it comes to secondary prophylaxis to prevent recurrent thrombosis. However, in the new NASPGHAN recommendations (from 2021), the authors state that children with incomplete resolution of thrombus should be maintained on prophylactic anticoagulation with LMWH (71). Schmidt et al. retrospectively compared two different regimens for secondary anticoagulation prophylaxis with LMWH in two pediatric IF centers (21). In the short-term regimen, children with CVT received therapeutic dosing until thrombus resolution or up to 3 months without prophylactic dosing thereafter, and in the long-term regimen, therapeutic dosing was given up to 3 months and prophylactic dosing continued thereafter until line removal. The authors recommend long-term secondary anticoagulation prophylaxis in children with CVT that require PN for prolonged periods of time, since secondary thrombosis occurred in eight of 13 (62%) patients in the short-term group and in nine of 26 (35%) in the long-term protocol group (P = 0.019) after a median time of 144.5 and 689 days, respectively (P = 0.01).
Routine screening for CVT in children on long-term PN is subject of debate. Nagelkerke et al. recently recommended routine, annual radiologic screening for CVT development in these children, since 40% of the CVT they found with ultrasound screening were asymptomatic (66). However, in a study concerning critically ill children with asymptomatic CVT, no radiographic extensions of thrombi during follow-up were seen and acute or long-term complications were rare despite absence of treatment (72). However, in these critically ill children, the CVC was removed because PN was no longer needed, which is not possible for children with IF. Routine radiographic screening could be considered in patients without thromboprophylaxis.
In this review, we have shown that both children with IBD and IF are at increased risk of VTE compared to the general pediatric population. Risk factors are both catheter-related and disease-specific, but also more generic underlying mechanisms such as thrombophilia, hospitalization and inflammation play a key role in the development of VTE. Once VTE is diagnosed, close collaboration with pediatric hematologists is needed for adequate treatment and subsequent follow-up of VTE. However, as prevention is key, thromboprophylaxis for high-risk patients might be of even more importance. Due to lack of evidence, there is no clear guidance on prevention of VTE in both IBD and IF. Future studies are therefore required to evaluate the safety and efficacy of thromboprophylaxis, including DOACs.
RK and LV reviewed the literature and wrote and edited the manuscript. BK and LR supervised the study and revised the manuscript. All authors agreed with the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank C. Heleen van Ommen for reviewing the manuscript for important intellectual content.
1. Chan AK, Deveber G, Monagle P, Brooker LA, Massicotte PM. Venous thrombosis in children. J Thromb Haemost. (2003) 1:1443–55. doi: 10.1046/j.1538-7836.2003.00308.x
2. Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol. (2008) 143:180–90. doi: 10.1111/j.1365-2141.2008.07323.x
3. Winter MP, Schernthaner GH, Lang IM. Chronic complications of venous thromboembolism. J Thromb Haemost. (2017) 15:1531–40. doi: 10.1111/jth.13741
4. Kumar R, Kerlin BA. Thrombosis of the abdominal veins in childhood. Front Pediatr. (2017) 5:188. doi: 10.3389/fped.2017.00188
5. Barker M, Thoenes D, Dohmen H, Friedrichs F, Pfannenstiel C, Heimann G. Prevalence of thrombophilia and catheter-related thrombosis in cystic fibrosis. Pediatr Pulmonol. (2005) 39:156–61. doi: 10.1002/ppul.20158
6. Deutschmann A, Schlagenhauf A, Leschnik B, Hoffmann KM, Hauer A, Muntean W. Onset of thrombin generation occurs more rapidly in pediatric patients with celiac disease. J Pediatr Gastroenterol Nutr. (2015) 61:230–3. doi: 10.1097/MPG.0000000000000786
7. Kuenzig ME, Fung SG, Marderfeld L, Mak JW, Kaplan GG, Ng SC, et al. Twenty-first century trends in the global epidemiology of pediatric-onset inflammatory bowel disease: systematic review. Gastroenterology. (2022) 162:1147–59.e4. doi: 10.1053/j.gastro.2021.12.282
8. Benchimol EI, Bernstein CN, Bitton A, Carroll MW, Singh H, Otley AR, et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am J Gastroenterol. (2017) 112:1120–34. doi: 10.1038/ajg.2017.97
9. Benchimol EI, Fortinsky KJ, Gozdyra P, Van den Heuvel M, Van Limbergen J, Griffiths AM. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. (2011) 17:423–39. doi: 10.1002/ibd.21349
10. Chien KA, Cooley V, Prishtina F, Grinspan ZM, Gerber LM, Kucine N. Health and financial burdens associated with venous thrombosis in hospitalized children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2021) 72:748–51. doi: 10.1097/MPG.0000000000003052
11. Goulet O, Ruemmele F. Causes and management of intestinal failure in children. Gastroenterology. (2006) 130(2Suppl.1):S16–28. doi: 10.1053/j.gastro.2005.12.002
12. Diamanti A, Capriati T, Gandullia P, Di Leo G, Lezo A, Lacitignola L, et al. Pediatric chronic intestinal failure in italy: report from the 2016 survey on behalf of Italian Society for Gastroenterology, Hepatology and Nutrition (Sigenp). Nutrients. (2017) 9:111217. doi: 10.3390/nu9111217
13. Neelis EG, van Oers HA, Escher JC, Damen GM, Rings EH, Tabbers MM. Treatment of children with intestinal failure: intestinal rehabilitation, home parenteral nutrition or small intestine transplantation? [Behandeling Van Kinderen Met Darmfalen: Darmrevalidatie, Parenterale Voeding Thuis of Dunnedarmtransplantatie?] Ned Tijdschr Geneeskd. (2014) 158:A7771.
14. Pierret ACS, Wilkinson JT, Zilbauer M, Mann JP. Clinical outcomes in pediatric intestinal failure: a meta-analysis and meta-regression. Am J Clin Nutr. (2019) 110:430–6. doi: 10.1093/ajcn/nqz110
15. Middleton SJ, Jamieson NV. The current status of small bowel transplantation in the UK and Internationally. Gut. (2005) 54:1650–7. doi: 10.1136/gut.2004.062612
16. Aardoom MA, Klomberg RCW, Kemos P, Ruemmele FM, van Ommen H, de Ridder L, et al. The incidence and characteristics of venous thromboembolisms in paediatric-onset inflammatory bowel disease; a prospective international cohort study based on the pibd-setquality safety registry. J Crohns Colitis. (2021) 2021:jjab171. doi: 10.2139/ssrn.3844854
17. Kuenzig ME, Bitton A, Carroll MW, Kaplan GG, Otley AR, Singh H, et al. Inflammatory bowel disease increases the risk of venous thromboembolism in children: a population-based matched cohort study. J Crohns Colitis. (2021) 2021:jjab113. doi: 10.1093/ecco-jcc/jjab113
18. Lazzerini M, Bramuzzo M, Maschio M, Martelossi S, Ventura A. Thromboembolism in pediatric inflammatory bowel disease: systematic review. Inflamm Bowel Dis. (2011) 17:2174–83. doi: 10.1002/ibd.21563
19. Kappelman MD, Horvath-Puho E, Sandler RS, Rubin DT, Ullman TA, Pedersen L, et al. Thromboembolic risk among danish children and adults with inflammatory bowel diseases: a population-based nationwide study. Gut. (2011) 60:937–43. doi: 10.1136/gut.2010.228585
20. Asouzu MA, Shroyer M, Graham JS, Wilkinson L, Galloway DP, Martin CA. Development of venous thrombi in a pediatric population of intestinal failure. J Pediatr Surg. (2019) 54:2145–8. doi: 10.1016/j.jpedsurg.2018.12.022
21. Schmidt ML, Wendel D, Horslen SP, Lane ER, Brandao LR, Gottschalk E, et al. Secondary anticoagulation prophylaxis for catheter-related thrombosis in pediatric intestinal failure: comparison of short- vs. long-term treatment protocols. J Parenter Enteral Nutr. (2020) 45:1432–40. doi: 10.1002/jpen.2055
22. LaRusso K, Schaack G, Fung T, McGregor K, Long J, Dumas MP, et al. Should you pick the Picc? prolonged use of peripherally inserted central venous catheters in children with intestinal failure. J Pediatr Surg. (2019) 54:999–1004. doi: 10.1016/j.jpedsurg.2019.01.052
23. Senchenkova E, Seifert H, Granger DN. Hypercoagulability and platelet abnormalities in inflammatory bowel disease. Semin Thromb Hemost. (2015) 41:582–9. doi: 10.1055/s-0035-1556590
24. Papa A, Danese S, Grillo A, Gasbarrini G, Gasbarrini A. Review article: inherited thrombophilia in inflammatory bowel disease. Am J Gastroenterol. (2003) 98:1247–51. doi: 10.1111/j.1572-0241.2003.07491.x
25. Danese S, Papa A, Saibeni S, Repici A, Malesci A, Vecchi M. Inflammation and coagulation in inflammatory bowel disease: the clot thickens. Am J Gastroenterol. (2007) 102:174–86. doi: 10.1111/j.1572-0241.2006.00943.x
26. Irving PM, Pasi KJ, Rampton DS. Thrombosis and inflammatory bowel disease. Clin Gastroenterol Hepatol. (2005) 3:617–28. doi: 10.1016/S1542-3565(05)00154-0
27. Chalmers EA. Epidemiology of venous thromboembolism in neonates and children. Thromb Res. (2006) 118:3–12. doi: 10.1016/j.thromres.2005.01.010
28. Nylund CM, Goudie A, Garza JM, Crouch G, Denson LA. Venous thrombotic events in hospitalized children and adolescents with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2013) 56:485–91. doi: 10.1097/MPG.0b013e3182801e43
29. Mitchel EB, Rosenbaum S, Gaeta C, Huang J, Raffini LJ, Baldassano RN, et al. Venous thromboembolism in pediatric inflammatory bowel disease: a case-control study. J Pediatr Gastroenterol Nutr. (2021) 72:742–7. doi: 10.1097/MPG.0000000000003078
30. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. (2010) 375:657–63. doi: 10.1016/S0140-6736(09)61963-2
31. Bollen L, Vande Casteele N, Ballet V, van Assche G, Ferrante M, Vermeire S, et al. Thromboembolism as an important complication of inflammatory bowel disease. Eur J Gastroenterol Hepatol. (2016) 28:1–7. doi: 10.1097/MEG.0000000000000495
32. McKie K, McLoughlin RJ, Hirsh MP, Cleary MA, Aidlen JT. Risk factors for venous thromboembolism in children and young adults with inflammatory bowel disease. J Surg Res. (2019) 243:173–9. doi: 10.1016/j.jss.2019.04.087
33. Bence CM, Traynor MD Jr, Polites SF, Ha D, Muenks P, St Peter SD, et al. The incidence of venous thromboembolism in children following colorectal resection for inflammatory bowel disease: a multi-center study. J Pediatr Surg. (2020) 55:2387–92. doi: 10.1016/j.jpedsurg.2020.02.020
34. McCurdy JD, Ellen Kuenzig M, Spruin S, Fung OW, Mallik R, Williams L, et al. Surgery and the subtype of inflammatory bowel disease impact the risk of venous thromboembolism after hospital discharge. Dig Dis Sci. (2021). doi: 10.1007/s10620-021-07064-5 [Epub ahead of print].
35. Yuhara H, Steinmaus C, Corley D, Koike J, Igarashi M, Suzuki T, et al. Meta-analysis: the risk of venous thromboembolism in patients with inflammatory bowel disease. Aliment Pharmacol Ther. (2013) 37:953–62. doi: 10.1111/apt.12294
36. Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. (2008) 103:2272–80. doi: 10.1111/j.1572-0241.2008.02052.x
37. Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. (2004) 99:97–101. doi: 10.1046/j.1572-0241.2003.04026.x
38. Johannesdottir SA, Horváth-Puhó E, Dekkers OM, Cannegieter SC, Jørgensen JO, Ehrenstein V, et al. Use of glucocorticoids and risk of venous thromboembolism: a nationwide population-based case-control study. J Am Med Assoc Intern Med. (2013) 173:743–52. doi: 10.1001/jamainternmed.2013.122
39. Sarlos P, Szemes K, Hegyi P, Garami A, Szabo I, Illes A, et al. Steroid but not biological therapy elevates the risk of venous thromboembolic events in inflammatory bowel disease: a meta-analysis. J Crohns Colitis. (2018) 12:489–98. doi: 10.1093/ecco-jcc/jjx162
40. Desai RJ, Gagne JJ, Lii J, Liu J, Friedman S, Kim SC. Comparative risk of incident venous thromboembolism in patients with inflammatory bowel disease initiating tumour necrosis factor-α inhibitors or nonbiologic agents: a cohort study. CMAJ. (2017) 189:E1438–47. doi: 10.1503/cmaj.161485
41. van Ommen CH, Tabbers MM. Catheter-related thrombosis in children with intestinal failure and long-term parenteral nutrition: how to treat and to prevent? Thromb Res. (2010) 126:465–70. doi: 10.1016/j.thromres.2010.08.027
42. Billing H, Traunspurger A, Sturm E, Busch A. High incidence of proteinuria in children with chronic intestinal failure under long-term parenteral nutrition. J Pediatr Gastroenterol Nutr. (2018) 66:751-4.
43. Barbano B, Gigante A, Amoroso A, Cianci R. Thrombosis in nephrotic syndrome. Semin Thromb Hemost. (2013) 39:469–76. doi: 10.1097/MPG.0000000000001814
44. Geerts W. Central venous catheter-related thrombosis. Hematology Am Soc Hematol Educ Program. (2014) 2014:306–11. doi: 10.1182/asheducation-2014.1.306
45. Forauer AR, Theoharis CG, Dasika NL. Jugular vein catheter placement: histologic features and development of catheter-related (fibrin) sheaths in a swine model. Radiology. (2006) 240:427–34. doi: 10.1148/radiol.2402031129
46. Nifong TP, McDevitt TJ. The effect of catheter to vein ratio on blood flow rates in a simulated model of peripherally inserted central venous catheters. Chest. (2011) 140:48–53. doi: 10.1378/chest.10-2637
47. Esmon CT. The interactions between inflammation and coagulation. Br J Haematol. (2005) 131:417–30. doi: 10.1111/j.1365-2141.2005.05753.x
48. Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, et al. American Society of hematology 2018 guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. (2018) 2:3292–316. doi: 10.1182/bloodadvances.2018024786
49. Jaffray J, Young G. Direct oral anticoagulants for use in paediatrics. Lancet Child Adolesc Health. (2022) 6:207–14. doi: 10.1016/S2352-4642(21)00343-6
50. Holzhauer S, Male C, Monagle P, Bordbar M, van Ommen H, Raffini LJ. Validating direct oral anticoagulants (Doac) for use in children by the Throm-Ped Doac registry of the international pediatric thrombosis network. Blood. (2021) 138(Suppl.1):1063. doi: 10.1182/blood-2021-151005
51. Hakeam HA, Al-Sanea N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (Doacs). J Thromb Thrombolysis. (2017) 43:343–51. doi: 10.1007/s11239-016-1465-x
52. Naito T, Botwin GJ, Haritunians T, Li D, Yang S, Khrom M, et al. Prevalence and effect of genetic risk of thromboembolic disease in inflammatory bowel disease. Gastroenterology. (2021) 160:771–80 e4. doi: 10.1053/j.gastro.2020.10.019
53. Zitomersky NL, Levine AE, Atkinson BJ, Harney KM, Verhave M, Bousvaros A, et al. Risk factors, morbidity, and treatment of thrombosis in children and young adults with active inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2013) 57:343–7. doi: 10.1097/MPG.0b013e31829ce5cd
54. Chien KA, Hammad HT, Gerber L, Sheth S, Sockolow R, Kucine N. Pediatric gastroenterologists' approach to venous thromboembolism prophylaxis in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. (2018) 66:286–8. doi: 10.1097/MPG.0000000000001690
55. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, part 2: acute severe colitis-an evidence-based consensus guideline from the european crohn's and colitis organization and the european society of paediatric gastroenterology, hepatology and nutrition. J Pediatr Gastroenterol Nutr. (2018) 67:292–310. doi: 10.1097/MPG.0000000000002036
56. Sam JJ, Bernstein CN, Razik R, Thanabalan R, Nguyen GC. Physicians' perceptions of risks and practices in venous thromboembolism prophylaxis in inflammatory bowel disease. Dig Dis Sci. (2013) 58:46–52. doi: 10.1007/s10620-012-2435-6
57. Faye AS, Hung KW, Cheng K, Blackett JW, McKenney AS, Pont AR, et al. Minor hematochezia decreases use of venous thromboembolism prophylaxis in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2020) 26:1394–400. doi: 10.1093/ibd/izz269
58. Story E, Bijelic V, Penney C, Benchimol EI, Halton J, Mack DR. Safety of venous thromboprophylaxis with low-molecular-weight heparin in children with ulcerative colitis. J Pediatr Gastroenterol Nutr. (2021) 73:604–9. doi: 10.1097/MPG.0000000000003231
59. Klaassen ILM, Sol JJ, Suijker MH, Fijnvandraat K, van de Wetering MD, Heleen van Ommen C. Are low-molecular-weight heparins safe and effective in children? a systematic review. Blood Rev. (2019) 33:33–42. doi: 10.1016/j.blre.2018.06.003
60. Hansen R, Meade S, Beattie RM, Auth MK, Croft N, Davies P, et al. Adaptations to the current Ecco/Espghan guidelines on the management of paediatric acute severe colitis in the context of the covid-19 pandemic: a rand appropriateness panel. Gut. (2021) 70:1044–52. doi: 10.1136/gutjnl-2020-322449
61. Hansen R, Meade S, Torrente F, Kammermeier J, Croft NM, De Ridder L, et al. Thromboprophylaxis use in paediatric inflammatory bowel disease: an international rand appropriateness panel. J Crohn's Colitis. (2022) 16:i526–i7. doi: 10.1093/ecco-jcc/jjab232.716
62. Neelis E, de Koning B, van Winckel M, Tabbers M, Hill S, Hulst J, et al. Variation in organisation and clinical practice of paediatric intestinal failure teams: an international survey. Clin Nutr. (2018) 37:2271–9. doi: 10.1016/j.clnu.2017.11.008
63. Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, Parenteral Nutrition Guidelines Working G, et al. Guidelines on Paediatric Parenteral Nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (Espghan) and the European Society for Clinical Nutrition and Metabolism (Espen), Supported by the European Society of Paediatric Research (Espr). J Pediatr Gastroenterol Nutr. (2005) 41(Suppl.2):S1–87. doi: 10.1097/01.mpg.0000181841.07090.f4
64. Newall F, Barnes C, Savoia H, Campbell J, Monagle P. Warfarin therapy in children who require long-term total parenteral nutrition. Pediatrics. (2003) 112:e386. doi: 10.1542/peds.112.5.e386
65. Vegting IL, Tabbers MM, Benninga MA, Wilde JC, Serlie MJ, Tas TA, et al. Prophylactic anticoagulation decreases catheter-related thrombosis and occlusion in children with home parenteral nutrition. J Parenter Enteral Nutr. (2012) 36:456–62. doi: 10.1177/0148607111416482
66. Nagelkerke SCJ, Schoenmaker MHA, Tabbers MM, Benninga MA, van Ommen CH, Gouw SC. Prophylactic anticoagulation in children receiving home parenteral nutrition. J Parenter Enteral Nutr. (2021). doi: 10.1002/jpen.2298
67. Gonzalez-Hernandez J, Daoud Y, Styers J, Journeycake JM, Channabasappa N, Piper HG. Central venous thrombosis in children with intestinal failure on long-term parenteral nutrition. J Pediatr Surg. (2016) 51:790–3. doi: 10.1016/j.jpedsurg.2016.02.024
68. Kolacek S, Puntis JWL, Hojsak I, nutrition EEECwgopp. Espghan/Espen/Espr/Cspen guidelines on pediatric parenteral nutrition: venous access. Clin Nutr. (2018) 37:2379–91. doi: 10.1016/j.clnu.2018.06.952
69. Lambe C, Poisson C, Talbotec C, Goulet O. Strategies to reduce catheter-related bloodstream infections in pediatric patients receiving home parenteral nutrition: the efficacy of taurolidine-citrate prophylactic-locking. J Parenter Enteral Nutr. (2018) 42:1017–25. doi: 10.1002/jpen.1043
70. Chu HP, Brind J, Tomar R, Hill S. Significant reduction in central venous catheter-related bloodstream infections in children on Hpn after starting treatment with taurolidine line lock. J Pediatr Gastroenterol Nutr. (2012) 55:403–7. doi: 10.1097/MPG.0b013e31825bb0ae
71. Wendel D, Mezoff EA, Raghu VK, Kinberg S, Soden J, Avitzur Y, et al. Management of central venous access in children with intestinal failure: a position paper from the Naspghan intestinal rehabilitation special interest group. J Pediatr Gastroenterol Nutr. (2021) 72:474–86. doi: 10.1097/MPG.0000000000003036
Keywords: thrombosis, Crohn's disease, ulcerative colitis, short bowel syndrome, thromboprophylaxis, anticoagulation, children, gastroenterology
Citation: Klomberg RCW, Vlug LE, de Koning BAE and de Ridder L (2022) Venous Thromboembolic Complications in Pediatric Gastrointestinal Diseases: Inflammatory Bowel Disease and Intestinal Failure. Front. Pediatr. 10:885876. doi: 10.3389/fped.2022.885876
Received: 28 February 2022; Accepted: 08 April 2022;
Published: 28 April 2022.
Edited by:
Susanne Holzhauer, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Courtney Dawn Thornburg, University of California, San Diego, United StatesCopyright © 2022 Klomberg, Vlug, de Koning and de Ridder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lissy de Ridder, bC5kZXJpZGRlckBlcmFzbXVzbWMubmw=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.