
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr. , 24 June 2022
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.885308
This article is part of the Research Topic Hot Topics in Pediatrics View all 50 articles
 Gloria Lanzoni1,2
Gloria Lanzoni1,2 Camilla Sembenini1
Camilla Sembenini1 Stefano Gastaldo1
Stefano Gastaldo1 Letizia Leonardi1
Letizia Leonardi1 Vincenzo Pio Bentivoglio1
Vincenzo Pio Bentivoglio1 Giovanna Faggian2
Giovanna Faggian2 Luca Bosa2
Luca Bosa2 Paola Gaio2†
Paola Gaio2† Mara Cananzi1,2*†
Mara Cananzi1,2*†Pediatric esophageal dysphagia (PED) is an infrequent condition that can be determined by a large number of disorders. The etiologic diagnosis is challenging due to overlapping clinical phenotypes and to the absence of pediatric diagnostic guidelines. This review aims to summarize the most relevant causes of ED during childhood, highlight the clinical scenarios of PED presentation and discuss the indications of available diagnostic tools. Available information supports that PED should always be investigated as it can underlie life-threatening conditions (e.g., foreign body ingestion, mediastinal tumors), represent the complication of benign disorders (e.g., peptic stenosis) or constitute the manifestation of organic diseases (e.g., eosinophilic esophagitis, achalasia). Therefore, the diagnosis of functional PED should be made only after excluding mucosal, structural, or motility esophageal abnormalities. Several clinical features may contribute to the diagnosis of PED. Among the latter, we identified several clinical key elements, relevant complementary-symptoms and predisposing factors, and organized them in a multi-level, hierarchical, circle diagram able to guide the clinician through the diagnostic work-up of PED. The most appropriate investigational method(s) should be chosen based on the diagnostic hypothesis: esophagogastroduodenoscopy has highest diagnostic yield for mucosal disorders, barium swallow has greater sensitivity in detecting achalasia and structural abnormalities, chest CT/MR inform on the mediastinum, manometry is most sensitive in detecting motility disorders, while pH-MII measures gastroesophageal reflux. Further studies are needed to define the epidemiology of PED, determine the prevalence of individual underlying etiologies, and assess the diagnostic value of investigational methods as to develop a reliable diagnostic algorithm.
Dysphagia is defined as a disruption in the swallowing process that can involve any structure of the upper gastrointestinal (UGI) tract from the lips to the lower esophageal sphincter. The anatomical classification distinguishes oropharyngeal dysphagia (OD), in which the oral or pharyngeal phases of the swallowing process are involved, from esophageal dysphagia (ED) in which the underlying cause arises from the esophageal body or from the esophageal sphincters (1). Despite significant physiopathological differences, the distinction between OD and ED may be challenging, due to overlapping clinical presentation, especially in infants and young children (Figure 1). Regardless of the site involved, dysphagia can pose significant health issues and impair the child’s quality of life: the inability to eat properly can lead to dehydration and failure to thrive; the aspiration of food into the airways may cause recurrent respiratory infections and choking episodes; and, the difficulty in swallowing can elicit social isolation, anxiety, and depression (1–3).
Epidemiologic data regarding the prevalence of dysphagia in children are lacking. A nationwide US survey estimates that 0.9% of children, from 3 to 17 years of age, suffer from swallowing disorders with equal distribution between genders and mean age at onset of 8.2 years (4). While OD is prevalent in children with neurological impairment, the causes of dysphagia are more heterogeneous in children without developmental issues with ED being more relevant (5–7).
Pediatric ED (PED) can be the clinical manifestation of a wide variety of disorders. The majority of available studies focus on the specific etiologies of ED and, to the best of our knowledge, no guidelines or comprehensive recommendations are available for the diagnosis of PED. Due to the absence of a standardized diagnostic approach, the management of PED is extremely variable, especially as regards the employment of instrumental diagnostic techniques.
The aim of this review is to summarize the most relevant causes of PED, highlight the clinical key elements able to guide the diagnostic process, and discuss the available diagnostic tools with their specific indications. We also aim to propose a symptom-based diagnostic approach able to guide clinicians through the diagnostic work-up of PED.
We performed a narrative review of the literature as to satisfy the Scale for the Assessment of Narrative Review Articles (SANRA) (8). The review was structured around three research questions, all relevant to the aim of the study: (i) which are the most relevant causes of PED; (ii) what clinical key elements are able to guide the diagnostic process; (iii) what are the available diagnostic tools. A search of the literature published after January 2000 was performed employing the Scopus, Embase, PubMed and Cochrane Library databases. The research was focused to papers written in English and to studies performed in human subjects aged from 0 to 18 years old. Multiple combinations of keywords were employed, referring to dysphagia as a symptom (e.g., “dysphagia”), to the pediatric age (e.g., “children”), to specific etiologies (e.g., “eosinophilic esophagitis”), or to diagnostic techniques (e.g., “esophageal manometry”). Papers not relevant to the topic were excluded by screening of titles and abstracts. Paper eligibility was confirmed by direct assessment of full-text articles. When available, practice guidelines, systematic reviews, meta-analysis, and RCTs, were considered the preferred references. Findings from selected studies were combined and summarized to provide the best available evidence to address the above-mentioned research questions.
Based on the revision of the literature, we developed a symptom-based diagnostic diagram aiming to guide the clinician toward the identification of the etiology underlying PED. To graphically represent this process, we coded in HTML-Javascript a dynamic sunburst chart. The diagram represents in a circular graph hierarchical relational data distributed on different levels. Each level represents the detalization of the previous in a centrifugal way.
According to the underlying pathogenetic mechanism, esophageal disorders can be classified into mucosal, structural, and motility diseases as well as disorders caused by luminal esophageal obstruction (EO) or extrinsic compression (EC). The main causes of PED are reported in Table 1 and are synthetically described in Supplementary Table 1.
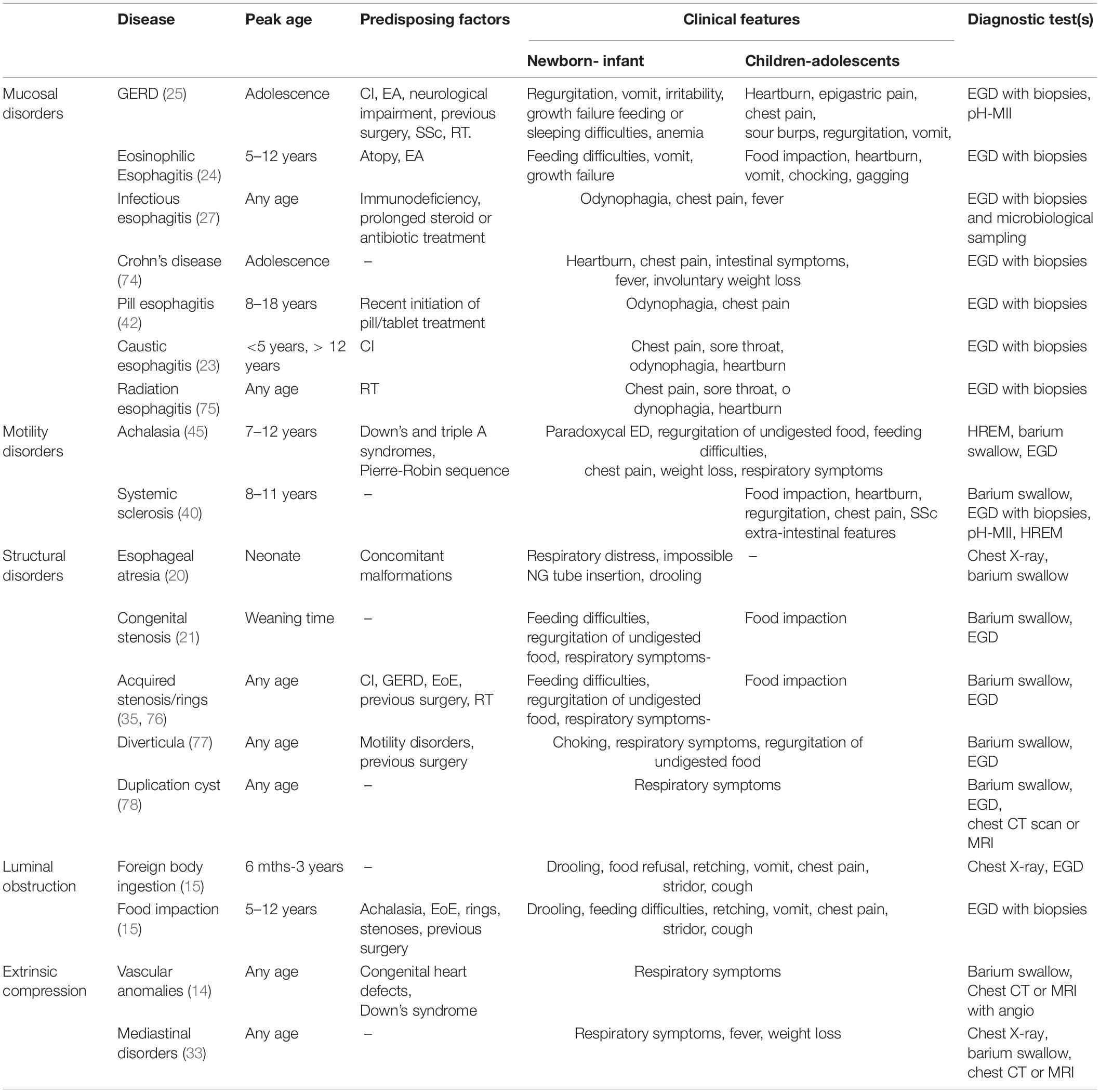
Table 1. Main causes of pediatric esophageal dysphagia along with representative disease features (peak age of incidence, predisposing factors, clinical features), and suggested diagnostic test(s).
From an epidemiological point of view, mucosal disorders constitute the most relevant cause of PED, with gastroesophageal reflux disease (GERD) and eosinophilic esophagitis (EoE) being the more frequent. The prevalence of GERD in children is 6% with an incidence that progressively increases with age (9). With a prevalence of 22.7 cases/100,000, EoE is far less frequent than GERD but is the leading cause of food impaction which constitutes the presenting symptom of disease in up to the 22% of children (6, 10). Primary esophageal motility disorders are a group of rare conditions subdivided, according to the Chicago Classification 4.0, into disorders of the esophagogastric junction outflow (i.e., Achalasia, Esophagogastric Junction Outflow Obstruction) and disorders of peristalsis [i.e., Distal Esophageal Spasm (DES), Hypercontractile Esophagus (nutcracker esophagus), Ineffective Esophageal Motility (IEM), and Absent Contractility] (11). Among the latter, achalasia is the one most commonly reported in the pediatric population but remains a rare disease with an incidence of 0.18 cases/100.000 children (12). No data are available regarding the epidemiology of the other primary esophageal motility disorders in children. Esophageal atresia (EA) has a prevalence of 0.7–3.2 cases/10,000 births and is the main representative of structural disorders causing PED (13). Dysphagia lusoria, due to an aberrant right subclavian artery, is the most frequent cause of esophageal EC (14). The esophagus is the most common site for an acute foreign body (FB) in the gastrointestinal tract. With a peak incidence between the ages of 6 months and 3 years, children make up the 80% of patients presenting to emergency departments with an esophageal FB (15).
When, after an appropriate evaluation, ED cannot be explained by another condition, functional dysphagia can be considered in the differential diagnosis. While it is the most prevalent esophageal disorder in adults, no data are available regarding its prevalence in the pediatric population (16). The diagnosis relies on the Rome IV criteria which define functional dysphagia as a sense of solid and/or liquid food sticking, lodging, or passing abnormally through the esophagus that arises in addition to all of the following criteria: (i) symptom onset at least 6 months before evaluation; (ii) symptom frequency of at least once a week; (iii) absence of evidence that esophageal mucosal or structural abnormality is the cause of the symptom; (iv) absence of evidence that gastroesophageal reflux or EoE is the cause of the symptom; (v) absence of major esophageal motor disorders (17).
Children with ED may present with a variety of complaints ranging from a sensation of food getting stuck in the throat or behind the sternum to a proper inability to swallow any kind of food or beverage with associated drooling (18). In the case of long-standing ED, compensatory behaviors, such as chewing for prolonged periods of time, using water or other lubricants to facilitate the passage of food, or avoiding problematic ingredients, can accompany and somewhat mask the presence of true dysphagia and therefore must always be investigated (19).
Infants usually present with non-specific complaints such as feeding difficulties and bolus regurgitation, thus making it more challenging to recognize ED in this age group.
Our review of the literature highlighted the following as relevant clinical elements able to guide the diagnostic work-up of PED.
While some disorders causing ED can develop at any age [e.g., infectious esophagitis (IE)], other conditions tend to arise at a typical age (Figure 2). Congenital disorders usually occur in neonates or infants: EA always presents within the first few hours of life (20), while congenital esophageal stenoses usually become evident at weaning or after the introduction of solid foods (21). FB ingestion (FBI) is typical of infants and preschool children (22). Caustic ingestion (CI) occurs in a bimodal fashion: accidental ingestion is most common under 5 years of age, while intentional ingestion, related to attempted suicide, mainly occurs in adolescents (23). EoE usually occurs in children between 5 and 12 years old (6, 24), and the incidence of GERD increases with age (9, 25).
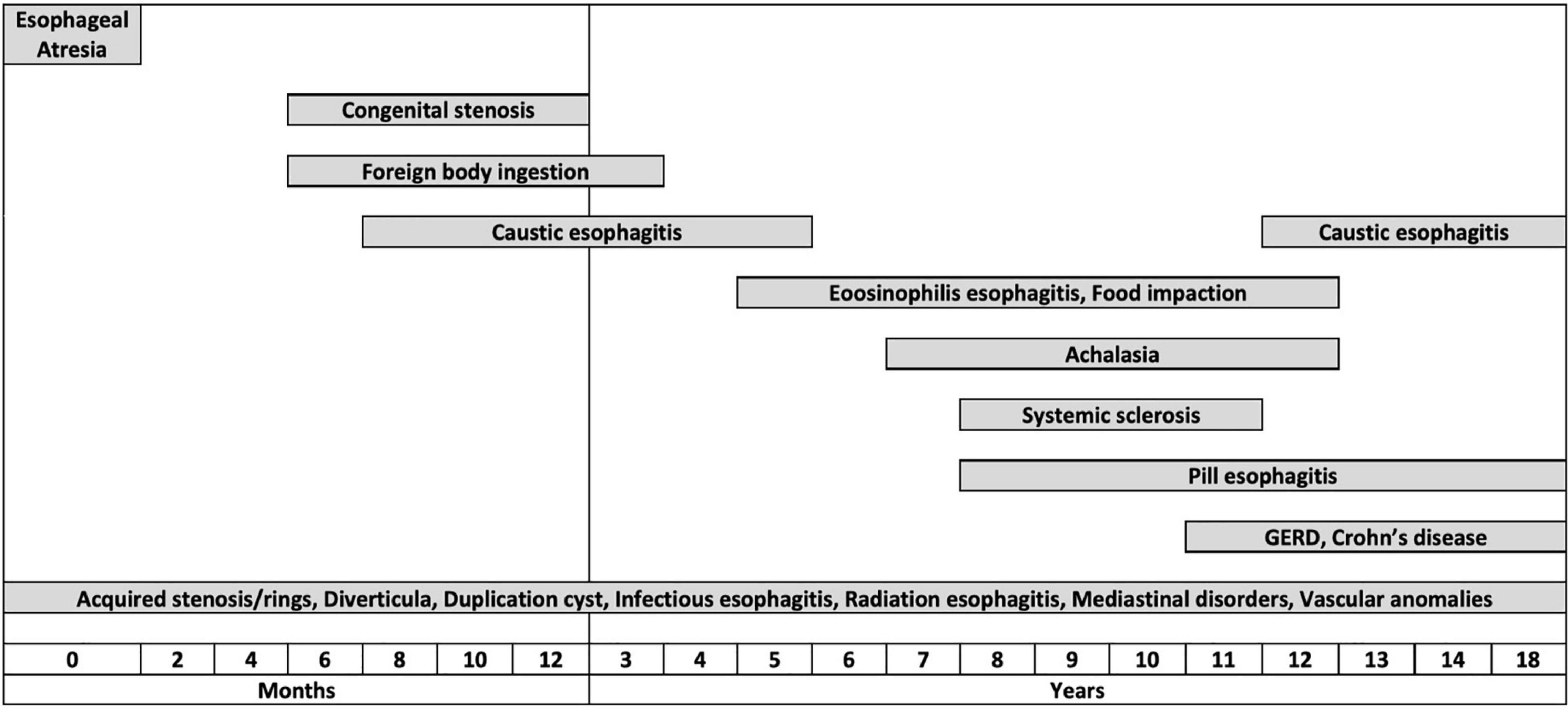
Figure 2. Graphical representation of the distribution of the different esophageal disorders by age groups.
The majority of esophageal disorders presenting with dysphagia cause a chronic or intermittent dysphagia with or without a progressive pattern.
ED can also present acutely with a sudden inability to swallow solids and/or liquids. Under these circumstances, EO should always be suspected, especially if sialorrhea is present. While FBI mostly concerns infants and toddlers with no underlying esophageal disfunction, food impaction is the most common cause of acute EO in older children and teenagers. The latter is usually the presenting symptom of an esophageal disorder, among which EoE is the most frequent (6, 26). Less frequent causes consist of peptic-, caustic- or radiation-induced strictures, Schatzki’s rings, esophageal diverticula, achalasia, and post-surgical complications (15). IE also presents acutely but, in this case, obstructive symptoms are usually absent, while fever and pain are predominant (27).
ED can be categorized based on the consistency of foods for which the swallowing process is impaired. Orthodox dysphagia is characterized by an impairment in managing solid foods which may later involve liquids, while paradoxical dysphagia presents with a difficulty in managing liquids from symptom onset. The first is a consequence of a mechanical obstruction of the esophagus (either intrinsic or extrinsic), while the latter is secondary to motility disorders (28, 29).
ED may be an isolated symptom or occur in association with other clinical findings.
UGI symptoms such as heartburn, epigastric pain, chest pain, and regurgitation are usually reported in GERD (7, 25). Food bolus impaction points toward the diagnosis of EoE (24). The combination of bolus regurgitation, chest pain, weight loss, and paradoxical dysphagia constitute the typical presentation of achalasia (30, 31).
Respiratory symptoms such as nocturnal cough and recurrent respiratory infections are also frequently reported in patients with esophageal achalasia due to recurrent aspiration or tracheal compression from the dilated esophagus (32). Sore throat, hoarseness, nocturnal cough, wheezing, and recurrent respiratory infections have been identified as atypical symptoms of GERD (25). Moreover, respiratory distress, stridor and wheezing can accompany dysphagia in the presence of any chest mass or vascular malformation causing a compression on mediastinal structures (14, 33).
Fever in association to odynophagia and chest pain suggest the diagnosis of IE or, in case of a compatible clinical history, may prompt the possibility of FBI complicated with esophageal perforation and acute mediastinitis (34). Mediastinal masses can be associated to the presence of paraneoplastic fever (33).
Several pre-existing conditions may predispose to the onset of dysphagia. Former esophageal surgery, radiation therapy and CI can lead to acquired stenosis and predispose to GERD (35). With a reported incidence as high as 70%, GERD is also common in children with neurological impairment (5). Achalasia has been linked to Down’s syndrome (36), Pierre-Robin sequence and other rare genetic disorders (37). Congenital heart defects and Down’s Syndrome are associated to vascular anomalies causing EC (14). Allergologic disorders and EA constitute well-established risk factors for the development of EoE (24, 38). Primary or acquired immunodeficiencies as well as prolonged antibiotic and steroid treatment favor the development of IE (34, 39). Immune-mediated disorders, such as systemic sclerosis (40) and Crohn’s disease can rarely involve the esophagus causing ED (41). Ongoing treatment with tablets/capsules should raise the possibility of pill-induced esophagitis especially when there is a close temporal relation (<10 days) with the use of NSAIDs, antibiotics (e.g., doxycycline, amoxicillin), L-arginine and iron, and the drug has been taken with little water at bedtime (42, 43).
The diagnostic work-up of PED can include a luminal, mucosal, anatomic, and functional assessment of the esophagus. Several diagnostic methodologies are available, the specific indications and diagnostic rates of which are discussed below. Moreover, pros and cons of the main diagnostic tools are summarized in Table 2.
The methods and the indications for performing EGD in children with ED have been reported by the ESPGHAN-ESGE guidelines (23).
In the context of GERD-related dysphagia, EGD is useful for: (i) defining the obstructive vs. non-obstructive nature of the symptom (stenosis vs. dysmotility); (ii) detect conditions that predispose to GERD (e.g., hiatal hernia); (iii) exclude conditions mimicking GERD such as EoE (7, 23). Indeed, the detection of > 15 eosinophils per HPF in at least 1 biopsy specimen of the esophageal mucosa is the gold standard for the diagnosis of EoE and the performance of 1–2 biopsies from the proximal, middle, and distal esophagus provides for a 97–100% sensitivity in the detection of esophageal eosinophilia (23, 24, 44). In IE, EGD reveals typical mucosal changes and provide for a microbiological diagnosis (39). EGD is the method of choice for detecting the characteristic features of pill-induced esophagitis which consist of single or multiple ulcers, surrounded by normal mucosa, usually located in the middle-third of the esophagus (42, 43).
EGD plays a diagnostic and therapeutic role in acute EO. Any symptomatic patient with suspected FBI should emergently undergo EGD as well as asymptomatic patients who swallowed sharp FB or button butteries. The endoscopic removal of asymptomatic blunt esophageal FB is also recommended within 24 h from the ingestion (23).
EGD can rise the suspicion of achalasia, and exclude pseudoachalasia, but is not diagnostic per se and may miss early disease. Abnormalities are present in the 90% of affected children with most common findings consisting of residual food in the esophagus (75%), closed stomach cardia (73%), esophageal enlargement (58%), and mucosal lesions (28%) (45). In patients with symptom relapse after surgery, EGD is useful to assess for reflux vs. recurrence of achalasia (30, 31).
EGD may also reveal a narrowing of the esophageal lumen caused by structural lesions or EC. In these circumstances, however, the definite diagnosis usually requires the employment of other diagnostic modalities including CT, MRI, and endoscopic ultrasound (46).
In children with a truly negative endoscopy (i.e., after an adequate biopsy sampling), radiologic studies and esophageal function tests should be warranted to investigate for subtle structural lesions (e.g., rings), EC and motility disorders (47).
Plain chest X-ray has a limited role in the assessment of PED. It can identify radiopaque FBs stuck into the esophagus, exclude the presence of pulmonary issues when fever or respiratory symptoms are present, and screen for pneumomediastinum or gross mediastinal enlargements (22).
Chest CT and MRI are needed to investigate the mediastinum when an esophageal EC is suspected. When coupled with angiography, these methodologies are the gold standard for the diagnosis of vascular anomalies causing dysphagia (14).
Barium swallow (BS) is useful in diagnosing esophageal webs, rings, stenoses, diverticula, tumors and EC (48, 49). Virtually all children with achalasia present at least one typical BS feature; the most common consist of slow contrast transit through the esophagus into the stomach (96.1%), esophageal dilation (94.1%), “bird’s beak” sign (82.4%), contrast retention in the esophagus (74%), and peristaltic anomalies (62%) (31, 45, 50). Besides providing excellent mucosal detail, BS is seldom used for the diagnosis of esophagitis and cannot establish or negate a diagnosis of GERD. However, it can detect conditions predisposing to GERD (e.g., hiatal hernia) and differentiate an obstructing vs. a slipped/loose fundoplication (7).
pH-metry or dual pH-multichannel intraluminal impedance (pH-MII) are not routinely recommended for the diagnosis of GERD (7). However, they can be helpful in the context of GERD-related dysphagia, as to: (i) correlate the symptom with acid/non-acid reflux events; (ii) clarify the role of acid/non-acid reflux in the etiology of esophagitis; (iii) evaluate for reflux in patients with EoE as these two conditions are not mutually exclusive; (iv) determine the efficacy of acid suppression in case of persisting dysphagia during treatment; (v) assess the onset of GERD after surgical and endoscopic treatments for achalasia (7, 24, 51). When available, pH-MII should be preferred to pH-monitoring and considered the gold standard diagnostic technique for the detection of GERD in the pediatric population (52). Indeed, since it allows the measurement of all reflux events irrespective of pH, pH-MII allows the detection of non-acid reflux episodes which have a high prevalence in the pediatric population, can be carried out on or off reflux therapy, and may be performed during continuous enteral feeding (49, 50, 52).
High-resolution esophageal manometry (HREM) represents the gold standard for the diagnosis of esophageal motility disorders, particularly achalasia (30, 31, 53). This technique uses a standardized swallow challenge to measure peristaltic pressures throughout the esophageal body (54). The Chicago classification algorithm classifies esophageal motility disorders according to manometric measurements of esophageal peristalsis (DCI, or Distal Contractile Integral, is the amplitude x duration x length [mmHg*s*cm] of the distal esophageal contraction above 20 mmHg, from the transition zone to the proximal margin of the lower esophageal sphincter; DL, or Distal Latency, is the interval between upper esophageal sphincter relaxation and the distal contraction) and esophago-gastric junction relaxation (IRP4s, or Integrated Relaxation Pressure, measured as the mean of the 4 s of lowest pressure across the lower esophageal sphincter in the 10 s window after swallow) (11). HREM has been recently combined with intraluminal impedance mapping (HRIM, High Resolution Impedance Manometry). This approach allows to directly evaluate the transit of the esophageal bolus using pressure-impedance metrics (IBP, or Intra-bolus Distension Pressure, indicating flow resistance; BFT, or Bolus Flow Time, indicating trans-esophagogastric junction emptying; and Impedance Ratio, indicating bolus transit failure), thus increasing the yield for detecting esophageal motility disorders (54). The use of the above-mentioned techniques has been validated in children (55), although some distinctions should be considered when interpreting the results. Patient characteristics and esophageal length and caliber have a significant impact on esophageal pressure topography metrics, thus, in pediatric patients reference ranges should be adjusted for size (55). Moreover, children under 7 years of age are often unable to tolerate the procedure and patient movement and crying can produce measurement artifacts that can impair study interpretation (54).
As said above (section “What Are the Clinical Key Diagnostic Elements for the Diagnosis of Pediatric Esophageal Dysphagia”, the clinical identification as well as the measurement of PED pose several challenges. To address this issue, questionnaires and clinical outcome metrics (COMs) have been developed to identify, quantify, and monitor the presence of a swallowing problem either independently from the underlying etiology or in specific pathological contexts (19).
The Eating Assessment Tool (EAT-10) (56) and its pediatric modified version (57) (PEDI-EAT-10) are symptom-based questionnaires that support the clinician in identifying the presence of a swallowing problem and in assessing the risk of penetration/aspiration (Supplementary Tables 2, 3).
The Reflux Symptom Index (RSI) (58) is a self-administered nine-item instrument to detect laryngopharyngeal reflux (Supplementary Table 4). It has been recently proven to be inaccurate in assessing the presence of gastroesophageal reflux in children and infants when compared to pH-MII. Indeed, although the number of acid refluxes directly correlates with the RSI, a normal RSI value does not exclude the presence of a pathological MII-pH (59).
Several scoring systems have been developed for the clinical evaluation of EoE. In adults, the Eosinophilic Esophagitis Activity Index was found to have the best validity and responsiveness in a systematic review: this tool focuses on dysphagia induced by food of different consistencies and on behavioral adaptation in daily life (60, 61). In children, the Pediatric Eosinophilic Esophagitis Symptom Score (PEESS) (62) has been validated to assess dysphagia in subjects from 2 to 18 years of age based on child and/or parent proxy scores (Supplementary Tables 5, 6). The aforementioned indexes are useful in assessing and monitoring EoE symptoms; nevertheless, they cannot be used to make assumptions on the biological activity of EoE, as they have a predictive unsatisfactory value (63).
The Eckardt score has been specifically developed for esophageal achalasia to establish clinical disease severity at diagnosis and to evaluate treatment efficacy during follow-up (30, 64, 65). The score is based on the three main symptoms associated with achalasia (dysphagia, regurgitation, and chest pain) in adjunct to weight loss included as a marker of the patient’s capacity to maintain nutrition (Supplementary Table 7). Unfortunately, the application of the Eckardt score in children is limited since the questionnaire is only validated for subjects older than 14 years of age (31, 65). A disease-specific quality of life measure has been developed and validated for children with achalasia (66). However, despite the availability of this questionnaire since 2010, it is seldom employed in pediatric clinical practice (31).
Our review of the literature shows that PED is caused by a heterogeneous group of disorders, among which EoE and GERD are the most frequent, summarizes the diverse clinical scenarios of PED presentation, and highlights the most appropriate investigational method(s) based on the diagnostic hypothesis. Despite available knowledge regarding individual etiologies, this revision also confirms the lack of pediatric epidemiological data and the absence of diagnostic guidelines regarding PED as a symptom.
In adults, ED is considered an alarm sign that may underlie the presence of esophageal carcinoma (67, 68). In children, ED is not clearly recognized as a red flag given the rarity of esophageal cancer in this age group. However, available information regarding the etiology of PED supports that this symptom should always be investigated as it can underlie life-threatening conditions (e.g., FBI, CI, mediastinal tumors), represent the complication of benign esophageal disorders (e.g., GERD-related stenosis) or constitute the clinical manifestation of an organic disease (e.g., EoE, achalasia). Therefore, in children as in adults, the diagnosis of functional dysphagia should only be made after demonstrating the absence of mucosal, structural, or esophageal motility abnormalities (17).
Several diagnostic protocols are available for the diagnosis of ED but none has been specifically made for children. No sufficient data are available to develop a diagnostic algorithm for PED or advocate for the superiority of a diagnostic test over another as first-line investigation.
We propose a diagnostic approach tailored on the main diagnostic suspect(s) deriving from the clinical evaluation of the child. To this end, we have identified six clinical key elements capable of recognizing patients who require an immediate medical evaluation (i.e., signs of acute EO and fever) or directing toward a diagnosis (i.e., history of food impaction, isolated dysphagia, paradoxical dysphagia, respiratory symptoms, UGI symptoms). As none of abovementioned clinical key elements can be univocally associated to a single etiology, we have also identified relevant complementary symptoms and predisposing factors that, combined to clinical key elements, can support the clinician throughout the diagnostic process. This symptom-based approach is graphically represented in a circular graph with hierarchical relational data distributed on different levels (Figure 3). Once one or more diagnostic hypotheses have been made, the most appropriate investigational method(s) should be chosen based on the specificities of individual tests. EGD has highest diagnostic yield for the evaluation of luminal and mucosal disorders. BS has a greater sensitivity in detecting achalasia and structural abnormalities, is non-invasive and can be used to confirm indication and define setting (diagnostic vs. operative) prior to perform EGD (28, 69, 70). Chest CT and MR provide detailed anatomical information on the mediastinum. Manometry is the most sensitive technique to detect motility disorders, while pH-MII evaluates the presence of acid/non-acid reflux (48, 49, 70).
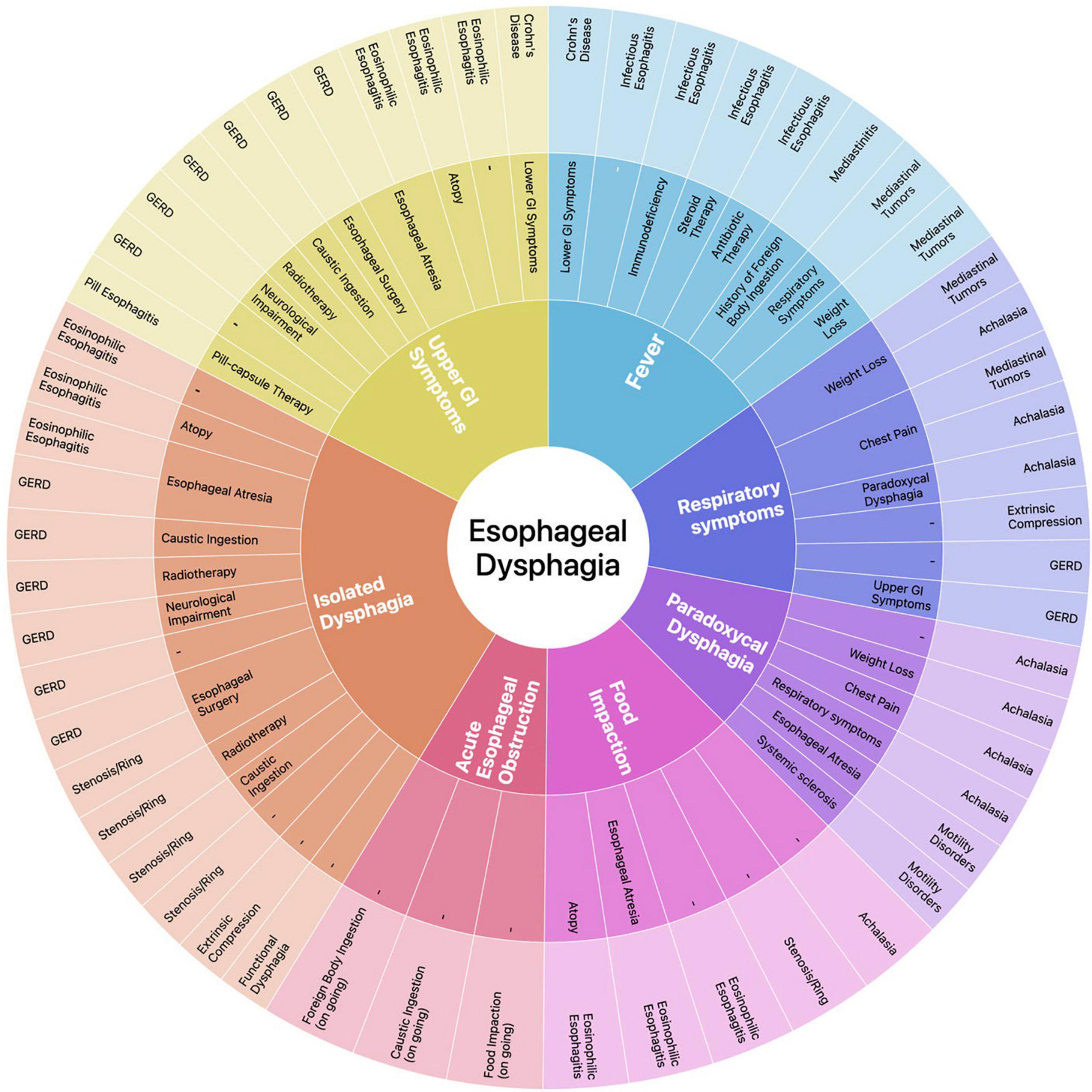
Figure 3. Sunburst diagram representing our proposed symptom-based diagnostic approach to ED. The diagram consists of four concentric circles (or levels) which, from the inside to the outside, represent in a hierarchical relation: (1) the clinical key elements that guide the diagnostic process; (2) relevant complementary symptoms and disease factors predisposing to a specific etiology that, in addition to the corresponding clinical key element, may address the differential diagnosis; (3) the most probable etiologic diagnosis/diagnoses of ED based on the clinical elements selected in the underlying levels. Each clinical key element is represented in a different color and this color is maintained throughout the corresponding section of the graph, albeit with different tones along the different levels (i.e., rings 1, 2, 3). Relevant complementary symptoms and predisposing factors in ring 2 make the etiologic diagnosis in ring 3 more likely; when no element is indicated it means that the final diagnosis may not be associated to any other clinical element. A dynamic version of the diagram is available in Supplementary Material.
To the best of our knowledge, this is the first comprehensive review regarding PED. Large scale studies are needed to define the epidemiology of PED, determine the prevalence of underlying etiologies, and assess the diagnostic value of available investigational methods as to establish the relative risk of disease and develop a reliable diagnostic algorithm. Prospective studies should be conducted, or a machine-learning approach implemented, to validate our symptom-based approach for the etiological definition of PED.
GL, PG, and MC wrote the manuscript, prepared tables and figures which all authors reviewed. GL, CS, SG, LL, VPB, and PG performed the literature search and summarized the results. GF and LB performed a critical review of the findings and participated in the preparation of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Pediatric Residency Program and the Department of Women’s and Children’s Health of the University Hospital of Padova for the support in producing and publishing this manuscript. We are grateful to Marco Tognin for his support in programming the Sunburst dynamic diagram.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.885308/full#supplementary-material
BS, Barium swallow; CI, caustic ingestion; CT, computed tomography; EA, esophageal atresia; EC, extrinsic compression; ED, Esophageal dysphagia; EGD, esophagogastroduodenoscopy; EoE, eosinophilic esophagitis; EO, esophageal obstruction; FB, foreign body; FBI, foreign body ingestion; GERD, gastroesophageal reflux disease; IE, infectious esophagitis; OD, oropharyngeal dysphagia; MRI, magnetic resonance; PED, pediatric esophageal dysphagia; UGI, upper gastrointestinal.
1. Dodrill P, Gosa MM. Pediatric dysphagia: physiology, assessment, and management. Ann Nutr Metab. (2015) 66(Suppl. 5):24–31. doi: 10.1159/000381372
2. van den Engel-Hoek L, de Groot IJ, de Swart BJ, Erasmus CE. Feeding and swallowing disorders in pediatric neuromuscular diseases: an overview. J Neuromuscul Dis. (2015) 2:357–69. doi: 10.3233/JND-150122
4. Bhattacharyya N. The prevalence of pediatric voice and swallowing problems in the United States. Laryngoscope. (2015) 125:746–50. doi: 10.1002/lary.24931
5. Romano C, van Wynckel M, Hulst J, Broekaert I, Bronsky J, Dall’Oglio L, et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. (2017) 65:242–64. doi: 10.1097/MPG.0000000000001646
6. Shaheen NJ, Mukkada V, Eichinger CS, Schofield H, Todorova L, Falk GW. Natural history of eosinophilic esophagitis: a systematic review of epidemiology and disease course. Dis Esophagus. (2018) 31:doy015. doi: 10.1093/dote/doy015
7. Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American society for pediatric gastroenterology, hepatology, and nutrition and the European society for pediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. (2018) 66:516–54.
8. Baethge C, Goldbeck-Wood S, Mertens S. SANRA-a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. (2019) 4:5. doi: 10.1186/s41073-019-0064-8
9. Martigne L, Delaage PH, Thomas-Delecourt F, Bonnelye G, Barthelemy P, Gottrand F. Prevalence and management of gastroesophageal reflux disease in children and adolescents: a nationwide cross-sectional observational study. Eur J Pediatr. (2012) 171:1767–73. doi: 10.1007/s00431-012-1807-4
10. Arias A, Perez-Martinez I, Tenias JM, Lucendo AJ. Systematic review with meta-analysis: the incidence and prevalence of eosinophilic oesophagitis in children and adults in population-based studies. Aliment Pharmacol Ther. (2016) 43:3–15.
11. Yadlapati R, Kahrilas PJ, Fox MR, Bredenoord AJ, Prakash Gyawali C, Roman S, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil. (2021) 33:e14058. doi: 10.1111/nmo.14058
12. Marlais M, Fishman JR, Fell JM, Haddad MJ, Rawat DJ. UK incidence of achalasia: an 11-year national epidemiological study. Arch Dis Child. (2011) 96:192–4. doi: 10.1136/adc.2009.171975
13. Nassar N, Leoncini E, Amar E, Arteaga-Vazquez J, Bakker MK, Bower C, et al. Prevalence of esophageal atresia among 18 international birth defects surveillance programs. Birth Defects Res A Clin Mol Teratol. (2012) 94:893–9. doi: 10.1002/bdra.23067
14. Levitt B, Richter JE. Dysphagia lusoria: a comprehensive review. Dis Esophagus. (2007) 20:455–60. doi: 10.1111/j.1442-2050.2007.00787.x
15. Fung BM, Sweetser S, Wong Kee Song LM, Tabibian JH. Foreign object ingestion and esophageal food impaction: an update and review on endoscopic management. World J Gastrointest Endosc. (2019) 11:174–92. doi: 10.4253/wjge.v11.i3.174
16. Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. (2021) 160:99.e–114.e. doi: 10.1053/j.gastro.2020.04.014
17. Aziz Q, Fass R, Gyawali CP, Miwa H, Pandolfino JE, Zerbib F. Functional esophageal disorders. Gastroenterology. (2016) 150:1368–79.
18. Sherman PM, Hassall E, Fagundes-Neto U, Gold BD, Kato S, Koletzko S, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. Am J Gastroenterol. (2009) 104:1278–95; quiz96.
19. Nguyen N, Furuta GT, Menard-Katcher C. Recognition and assessment of eosinophilic esophagitis: the development of new clinical outcome Metrics. Gastroenterol Hepatol (N Y). (2015) 11:670–4.
20. Pinheiro PF, Simoes e Silva AC, Pereira RM. Current knowledge on esophageal atresia. World J Gastroenterol. (2012) 18:3662–72.
21. Trappey AF III, Hirose S. Esophageal duplication and congenital esophageal stenosis. Semin Pediatr Surg. (2017) 26:78–86. doi: 10.1053/j.sempedsurg.2017.02.003
22. Kramer RE, Lerner DG, Lin T, Manfredi M, Shah M, Stephen TC, et al. Management of ingested foreign bodies in children: a clinical report of the NASPGHAN Endoscopy Committee. J Pediatr Gastroenterol Nutr. (2015) 60:562–74. doi: 10.1097/MPG.0000000000000729
23. Thomson M, Tringali A, Dumonceau JM, Tavares M, Tabbers MM, Furlano R, et al. Paediatric gastrointestinal endoscopy: European society for paediatric gastroenterology hepatology and nutrition and European Society of gastrointestinal endoscopy guidelines. J Pediatr Gastroenterol Nutr. (2017) 64:133–53. doi: 10.1097/MPG.0000000000001408
24. Papadopoulou A, Koletzko S, Heuschkel R, Dias JA, Allen KJ, Murch SH, et al. Management guidelines of eosinophilic esophagitis in childhood. J Pediatr Gastroenterol Nutr. (2014) 58:107–18. doi: 10.1097/MPG.0b013e3182a80be1
25. Poddar U. Gastroesophageal reflux disease (GERD) in children. Paediatr Int Child Health. (2019) 39:7–12.
26. De Matteis A, Pagliaro G, Corleto VD, Pacchiarotti C, Di Giulio E, Villa MP, et al. Eosinophilic esophagitis in children: clinical findings and diagnostic approach. Curr Pediatr Rev. (2020) 16:206–14. doi: 10.2174/1573396315666191004110549
27. Bordea MA, Pirvan A, Gheban D, Silaghi C, Lupan I, Samasca G, et al. Infectious esophagitis in romanian children: from etiology and risk factors to clinical characteristics and endoscopic features. J Clin Med. (2020) 9:939. doi: 10.3390/jcm9040939
28. Liu LWC, Andrews CN, Armstrong D, Diamant N, Jaffer N, Lazarescu A, et al. Clinical practice guidelines for the assessment of uninvestigated esophageal dysphagia. J Can Assoc Gastroenterol. (2018) 1:5–19. doi: 10.1093/jcag/gwx008
29. Kotilea K, Mahler T, Bontems P, Deviere J, Louis H. Management of esophageal motility disorders in children: a review. Acta Gastroenterol Belg. (2018) 81:295–304.
30. Vaezi MF, Pandolfino JE, Yadlapati RH, Greer KB, Kavitt RTACG. Clinical guidelines: diagnosis and management of achalasia. Am J Gastroenterol. (2020) 115:1393–411. doi: 10.14309/ajg.0000000000000731
31. van Lennep M, van Wijk MP, Omari TIM, Salvatore S, Benninga MA, Singendonk MMJ, et al. Clinical management of pediatric achalasia: a survey of current practice. J Pediatr Gastroenterol Nutr. (2019) 68:521–6. doi: 10.1097/MPG.0000000000002221
32. Tashiro J, Petrosyan M, Kane TD. Current management of pediatric achalasia. Transl Gastroenterol Hepatol. (2021) 6:33.
33. Ranganath SH, Lee EY, Restrepo R, Eisenberg RL. Mediastinal masses in children. AJR Am J Roentgenol. (2012) 198:W197–216.
34. Hoversten P, Kamboj AK, Katzka DA. Infections of the esophagus: an update on risk factors, diagnosis, and management. Dis Esophagus. (2018) 31:1–9. doi: 10.1093/dote/doy094
35. Ghiselli A, Bizzarri B, Ferrari D, Manzali E, Gaiani F, Fornaroli F, et al. Endoscopic dilation in pediatric esophageal strictures: a literature review. Acta Biomed. (2018) 89:27–32. doi: 10.23750/abm.v89i8-S.7862
36. Zarate N, Mearin F, Hidalgo A, Malagelada JR. Prospective evaluation of esophageal motor dysfunction in Down’s syndrome. Am J Gastroenterol. (2001) 96:1718–24. doi: 10.1111/j.1572-0241.2001.03864.x
38. Krishnan U. Eosinophilic esophagitis in children with esophageal atresia. Eur J Pediatr Surg. (2015) 25:336–44.
39. Rosolowski M, Kierzkiewicz M. Etiology, diagnosis and treatment of infectious esophagitis. Prz Gastroenterol. (2013) 8:333–7. doi: 10.5114/pg.2013.39914
40. Kadakuntla A, Juneja A, Sattler S, Agarwal A, Panse D, Zakhary N, et al. Dysphagia, reflux and related sequelae due to altered physiology in scleroderma. World J Gastroenterol. (2021) 27:5201–18. doi: 10.3748/wjg.v27.i31.5201
41. Ammoury RF, Pfefferkorn MD. Significance of esophageal Crohn disease in children. J Pediatr Gastroenterol Nutr. (2011) 52:291–4. doi: 10.1097/MPG.0b013e3181ec21b5
42. Bordea MA, Pirvan A, Sarban C, Margescu C, Leucuta D, Samasca G, et al. Pill -induced erosive esophagitis in children. Clujul Med. (2014) 87:15–8. doi: 10.15386/cjm.2014.8872.871.mab1
43. Hu SW, Chen AC, Wu SF. Drug-induced esophageal ulcer in adolescent population: experience at a single medical center in central Taiwan. Medicina (Kaunas). (2021) 57:1286. doi: 10.3390/medicina57121286
44. Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N, et al. Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology. (2018) 155:1022–33.e10. doi: 10.1053/j.gastro.2018.07.009
45. Jarzebicka D, Czubkowski P, Sieczkowska-Golub J, Kierkus J, Kowalski A, Stefanowicz M, et al. Achalasia in children-clinical presentation, diagnosis, long-term treatment outcomes, and quality of life. J Clin Med. (2021) 10:3917. doi: 10.3390/jcm10173917
46. Terui K, Saito T, Mitsunaga T, Nakata M, Yoshida H. Endoscopic management for congenital esophageal stenosis: a systematic review. World J Gastrointest Endosc. (2015) 7:183–91. doi: 10.4253/wjge.v7.i3.183
47. Triggs J, Pandolfino J. Recent advances in dysphagia management. F1000Res. (2019) 8:F1000FacultyRev–1527. doi: 10.12688/f1000research.18900.1
48. Ghazanfar H, Shehi E, Makker J, Patel H. The role of imaging modalities in diagnosing dysphagia: a clinical review. Cureus. (2021) 13:e16786. doi: 10.7759/cureus.16786
49. Debi U, Sharma M, Singh L, Sinha A. Barium esophagogram in various esophageal diseases: a pictorial essay. Indian J Radiol Imaging. (2019) 29:141–54. doi: 10.4103/ijri.IJRI_465_18
50. Rosenwald KD, Hayes K, Menard-Katcher C, Belkind-Gerson J. Implementation of a timed barium esophagram protocol for assessment of esophageal function in children. J Pediatr Gastroenterol Nutr. (2020) 71:470–5. doi: 10.1097/MPG.0000000000002829
51. Mousa HM, Rosen R, Woodley FW, Orsi M, Armas D, Faure C, et al. Esophageal impedance monitoring for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. (2011) 52:129–39. doi: 10.1097/MPG.0b013e3181ffde67
52. Quitadamo P, Tambucci R, Mancini V, Cristofori F, Baldassarre M, Pensabene L, et al. Esophageal pH-impedance monitoring in children: position paper on indications, methodology and interpretation by the SIGENP working group. Dig Liver Dis. (2019) 51:1522–36. doi: 10.1016/j.dld.2019.07.016
53. Yamasaki T, Tomita T, Mori S, Takimoto M, Tamura A, Hara K, et al. Esophagography in patients with esophageal achalasia diagnosed with high-resolution esophageal manometry. J Neurogastroenterol Motil. (2018) 24:403–9. doi: 10.5056/jnm17147
54. Omari TI, Krishnan U. What is the role of high-resolution oesophageal manometry in paediatrics? J Paediatr Child Health. (2020) 56:1754–9. doi: 10.1111/jpc.15057
55. Singendonk MMJ, Omari TI, Rommel N, van Wijk MP, Benninga MA, Rosen R, et al. Novel pressure-impedance parameters for evaluating esophageal function in pediatric achalasia. J Pediatr Gastroenterol Nutr. (2018) 66:37–42. doi: 10.1097/MPG.0000000000001647
56. Belafsky PC, Mouadeb DA, Rees CJ, Pryor JC, Postma GN, Allen J, et al. Validity and reliability of the eating assessment tool (EAT-10). Ann Otol Rhinol Laryngol. (2008) 117:919–24.
57. Soyer T, Yalcin S, Arslan SS, Demir N, Tanyel FC. Pediatric eating assessment tool-10 as an indicator to predict aspiration in children with esophageal atresia. J Pediatr Surg. (2017) 52:1576–9. doi: 10.1016/j.jpedsurg.2017.02.018
58. Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice. (2002) 16:274–7. doi: 10.1016/s0892-1997(02)00097-8
59. Mantegazza C, Mallardo S, Rossano M, Meneghin F, Ricci M, Rossi P, et al. Laryngeal signs and pH-multichannel intraluminal impedance in infants and children: the missing ring: LPR and MII-pH in children. Dig Liver Dis. (2020) 52:1011–6. doi: 10.1016/j.dld.2020.05.001
60. Schoepfer AM, Straumann A, Panczak R, Coslovsky M, Kuehni CE, Maurer E, et al. Development and validation of a symptom-based activity index for adults with eosinophilic esophagitis. Gastroenterology. (2014) 147:1255–66.e21. doi: 10.1053/j.gastro.2014.08.028
61. Warners MJ, Hindryckx P, Levesque BG, Parker CE, Shackelton LM, Khanna R, et al. Systematic review: disease activity indices in eosinophilic esophagitis. Am J Gastroenterol. (2017) 112:1658–69.
62. Franciosi JP, Hommel KA, DeBrosse CW, Greenberg AB, Greenler AJ, Abonia JP, et al. Development of a validated patient-reported symptom metric for pediatric eosinophilic esophagitis: qualitative methods. BMC Gastroenterol. (2011) 11:126. doi: 10.1186/1471-230X-11-126
63. Lucendo AJ, Molina-Infante J, Arias A, von Arnim U, Bredenoord AJ, Bussmann C, et al. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. (2017) 5:335–58. doi: 10.1177/2050640616689525
64. Taft TH, Carlson DA, Triggs J, Craft J, Starkey K, Yadlapati R, et al. Evaluating the reliability and construct validity of the Eckardt symptom score as a measure of achalasia severity. Neurogastroenterol Motil. (2018) 30:e13287. doi: 10.1111/nmo.13287
65. Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. (1992) 103:1732–8. doi: 10.1016/0016-5085(92)91428-7
66. Marlais M, Fishman JR, Rawat DJ, Haddad M. Development and validation of a disease-specific quality-of-life measure for children with achalasia. Eur J Pediatr Surg. (2010) 20:92–4. doi: 10.1055/s-0029-1243622
67. Stapley S, Peters TJ, Neal RD, Rose PW, Walter FM, Hamilton W. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. (2013) 108:25–31.
68. Jones R, Latinovic R, Charlton J, Gulliford MC. Alarm symptoms in early diagnosis of cancer in primary care: cohort study using General Practice Research Database. BMJ. (2007) 334:1040. doi: 10.1136/bmj.39171.637106.AE
70. Navaneethan U, Eubanks S. Approach to patients with esophageal Dysphagia. Surg Clin North Am. (2015) 95:483–9. doi: 10.1016/j.suc.2015.02.004
72. Rommel N, Hamdy S. Oropharyngeal dysphagia: manifestations and diagnosis. Nat Rev Gastroenterol Hepatol. (2016) 13:49–59.
73. Zerbib F, Omari T. Oesophageal dysphagia: manifestations and diagnosis. Nat Rev Gastroenterol Hepatol. (2015) 12:322–31. doi: 10.1038/nrgastro.2014.195
74. Ramaswamy K, Jacobson K, Jevon G, Israel D. Esophageal Crohn disease in children: a clinical spectrum. J Pediatr Gastroenterol Nutr. (2003) 36:454–8. doi: 10.1097/00005176-200304000-00006
75. Nesheiwat Z, Akbar H, Kahloon A, Mahajan K. Radiation Esophagitis. Treasure Island, FL: StatPearls (2022).
76. Towbin AJ, Diniz LO. Schatzki ring in pediatric and young adult patients. Pediatr Radiol. (2012) 42:1437–40. doi: 10.1007/s00247-012-2482-3
77. Lindholm EB, Hansbourgh F, Upp JR Jr., Cilloniz R, Lopoo J. Congenital esophageal diverticulum - a case report and review of literature. J Pediatr Surg. (2013) 48:665–8.
78. Sun CF, Chen CH, Ke PZ, Ho TL, Lin CH. Esophageal duplication cyst presenting with stridor in a child with congenital pulmonary airway malformation: a case report and literature review. Medicine (Baltimore). (2019) 98:e16364. doi: 10.1097/MD.0000000000016364
Antibiotic therapy, recent or ongoing prolonged antibiotic therapy; Atopy, former diagnosis of one or more atopic disorders; CI, history of caustic ingestion; Esophageal atresia, previous surgery for esophageal atresia; GERD, gastroesophageal reflux disease; Immunodeficiency, former diagnosis of congenital or acquired immunodeficiency; Lower GI symptoms, lower gastrointestinal symptoms (e.g., abdominal pain, chronic diarrhea, blood in stool); Neurologic impairment, child with neurological disability; Pill-capsule therapy, recent or ongoing treatment with pill or capsules; Radiotherapy, previous radiation therapy; Respiratory symptoms, associated respiratory symptoms (e.g., cough, recurrent infections); Steroid therapy, recent or ongoing prolonged steroid treatment; Esophageal surgery, previous surgery on the esophagus; Systemic sclerosis, former diagnosis of systemic sclerosis; Upper GI symptoms, upper gastrointestinal symptoms (e.g., heartburn, epigastric pain, sour burps, regurgitation, vomit).
Keywords: esophageal dysphagia, esophageal disorders, achalasia, eosinophilic esophagitis, peptic esophagitis, symptom based diagnosis, children, pediatric
Citation: Lanzoni G, Sembenini C, Gastaldo S, Leonardi L, Bentivoglio VP, Faggian G, Bosa L, Gaio P and Cananzi M (2022) Esophageal Dysphagia in Children: State of the Art and Proposal for a Symptom-Based Diagnostic Approach. Front. Pediatr. 10:885308. doi: 10.3389/fped.2022.885308
Received: 27 February 2022; Accepted: 06 June 2022;
Published: 24 June 2022.
Edited by:
Alfonso Galderisi, Hôpital Necker-Enfants Malades, FranceReviewed by:
Silvia Salvatore, University of Insubria, ItalyCopyright © 2022 Lanzoni, Sembenini, Gastaldo, Leonardi, Bentivoglio, Faggian, Bosa, Gaio and Cananzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mara Cananzi, bWFyYS5jYW5hbnppQGFvcGQudmVuZXRvLml0
†These authors have contributed equally to this work and share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.