
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 25 May 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.880786
Diastolic dysfunction often complicates myocardial ischemia with increased mortality rates. However, less is known about diastolic function after perinatal asphyxia in neonates with hypoxic-ischemic encephalopathy (HIE) during therapeutic hypothermia (TH) and rewarming.
Aim: The aim of this study was to assess diastolic function with tissue Doppler imaging (TDI) in neonates with moderate–severe HIE during TH and rewarming.
Method: Newborns at >36 weeks' gestation with moderate–severe HIE treated with TH were evaluated with targeted neonatal echocardiography (TNE), including TDI, within 24 h of TH initiation (T1), at 48–72 h of treatment (T2), and after rewarming (T3). These retrospective data were collected and compared with a control group of healthy babies at >36 weeks' gestation that was prospectively evaluated following the same protocol.
Results: A total of 21 patients with HIE + TH and 15 controls were included in the study. Myocardial relaxation before the onset of biventricular filling was prolonged in the HIE + TH group during TH with significantly longer isovolumic relaxation time (IVRT') in the left ventricle (LV), the septum, and the right ventricle (RV). This was associated with slower RV early diastolic velocity (e') and prolonged filling on T1. Total isovolumic time (t-IVT; isovolumic contraction time [IVCT'] + IVRT') and myocardial performance index (MPI') were globally increased in asphyxiated neonates. All these differences persisted after correction for heart rate (HR) and normalized after rewarming. TDI parameters assessing late diastole (a' velocity or e'/a' and E/e' ratios) did not differ between groups.
Conclusion: TDI evaluation in our study demonstrated a pattern of early diastolic dysfunction during TH that normalized after rewarming, whereas late diastole seemed to be preserved. Our data also suggest a possible involvement of impaired twist/untwist motion and dyssynchrony. More studies are needed to investigate the impact and therapeutic implication of diastolic dysfunction in these babies, as well as to clarify the role of TH in these findings.
Cardiovascular impairment is a frequent complication among neonates with hypoxic-ischemic encephalopathy (HIE) and has been associated with a worse neurodevelopmental outcome. Myocardial dysfunction in the context of altered cerebral blood flow autoregulation may delay brain reoxygenation after hypoxia-ischemia and consequently aggravate injury (1). Hemodynamic assessment has become a growing area of research in neonatal HIE on the grounds that a better understanding of the cardiovascular consequences of perinatal asphyxia with targeted treatment could potentially improve the outcomes. The identification of new therapeutic opportunities is of utmost importance because despite the clear benefits of therapeutic hypothermia (TH) on survival and neurological outcome, a substantial number of infants still die or remain severely affected in the long term.
In this regard, most studies have focused on the impact of perinatal hypoxia-ischemia on the systolic function of the left ventricle (LV). Only recently, the predominant vulnerability of the right ventricle (RV), and more importantly, the association of RV dysfunction with adverse neurologic outcomes, has been suggested (2, 3). Surprisingly, less attention has been paid to diastolic function in neonatal HIE, with only marginal references to it in some studies but, to the best of our knowledge, it has never been the focus of these investigations. This is striking since it is well known that myocardial ischemia in adults often results in diastolic dysfunction, even after minor myocardial damage or despite preserved LV ejection fraction (EF), and it is associated with increased mortality (4).
Diastole is a key component of heart function as adequate relaxation and ventricular filling are necessary for a normal systolic performance (5). Evaluation of diastolic function in neonates is challenging using conventional echocardiographic measurements (6). The opening and closure of the ventriculoarterial and atrioventricular valves define different mechanical events, namely, the isovolumic contraction and ejection phases during systole and the isovolumic relaxation, early, and late filling phases during diastole (7). Tissue Doppler imaging (TDI) is a relatively new technique that can provide direct measurements of myocardial velocities and timing of these myocardial events during the cardiac cycle (7) and appears to be less influenced by loading conditions and the geometry of the cardiac structures than conventional echocardiographic measurements (8). It has been demonstrated that diastolic TDI indices are strongly predictive of cardiac outcome and patient mortality, so assessment of diastolic ventricular function using TDI has been widely accepted in adult cardiology (6). Therefore, at present, TDI may be an interesting tool to assess biventricular diastolic function promptly and non-invasively (9). Although TDI is considered useful for the clinical management of some high-risk newborns, the information about its use in neonatal HIE is still scarce (10). Importantly, many studies, including TDI assessment in HIE, have been performed in babies not treated with TH (11). In fact, although TH is the standard of care for HIE newborns, the hemodynamic impact of TH and rewarming has not been fully explored yet (10, 12, 13).
The aim of this study was to assess diastolic function with TDI in newborns with moderate–severe HIE during TH and rewarming, with the hypothesis that diastolic dysfunction is another consequence of myocardial damage after HIE.
We conducted a retrospective study on newborns with moderate–severe HIE treated with TH at a tertiary neonatal intensive care unit (NICU).
All patients with HIE treated with TH (HIE + TH group) admitted to the NICU at Hospital Clínico San Carlos between October 2017 and October 2019, with echocardiographic evaluations, including TDI, were eligible for enrollment. Diagnosis of congenital heart disease, decision not to provide full life support on admission, or lack of TDI assessment were considered exclusion criteria (Figure 1).
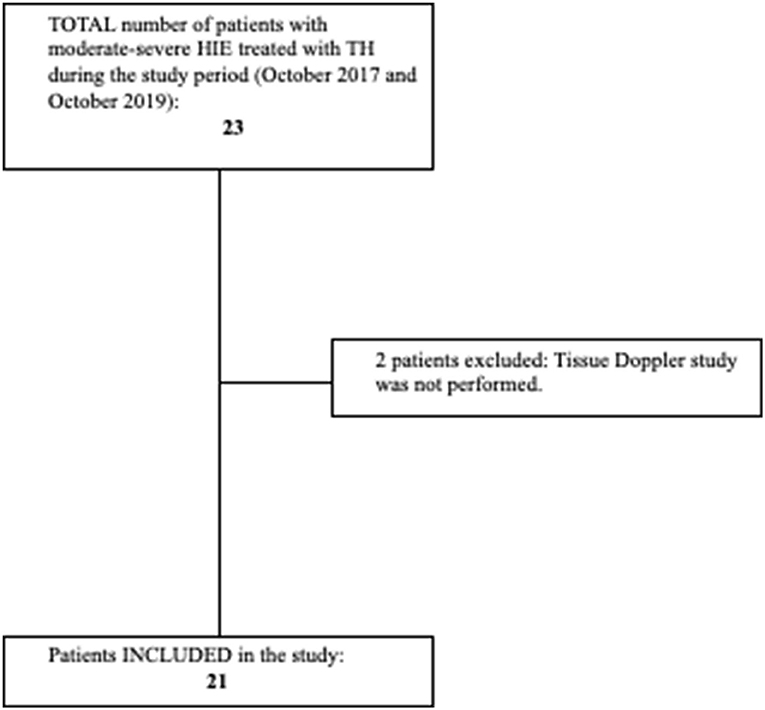
Figure 1. Flowchart showing patients with hypoxic-ischemic encephalopathy (HIE) included and excluded from the study. Criteria for initiation of TH: Age < 6 h, gestational age > 36 weeks, birthweight > 1,800 g. Signs of moderate–severe encephalopathy according to Sarnat score, and at least one of the following: 10-min Apgar score < 5, need for resuscitation beyond 10 min of life, cord pH or pH in the first h of life < 7.00, and base deficit ≥ 16 mmol/L in the first 60 min of life. Target rectal temperature of 33.5 ± 0.5°C was maintained for 72 h (Tecotherm Neo. Inspiration Healthcare, Leicester, UK). Rewarming was done at a rate of 0.5°C/h after cooling treatment was completed. Adapted and reprinted by permission from Springer Nature: European Journal of Pediatrics. Cerebral blood flow velocity and oxygenation correlate predominantly with right ventricular function in cooled neonates with moderate-severe hypoxic-ischemic encephalopathy (2). Copyright Springer Nature.
Their data were compared with a prospectively recruited control (CTL) group of healthy neonates (non-asphyxiated newborns with no signs of cardiovascular dysfunction and >36 weeks postconceptional age).
The study was approved by the local Ethics Committee. An exemption of formal consent was obtained for the inclusion of retrospective data. Parental informed consent was obtained before the enrollment of control babies.
We collected relevant demographic and clinical data of patients and controls. Gestational age, birth weight, gender, Apgar scores, cord pH, modified Sarnat score, and respiratory and cardiovascular support were recorded.
The neonatal echocardiography (TNE) evaluations were performed according to our NICU protocol within 24 h of TH initiation (T1), at 48–72 h of treatment (T2), and after rewarming (T3), and at equivalent time points in the CTL group, by three different operators (i.e., MJR, AC, and LA). These studies were performed as part of the routine care of these babies to guide treatment. The use of inotropes was indicated by the attending team based on ultrasound or on clinical parameters. Images were recorded and stored in digital format for offline analysis by a single investigator (MJR). Studies were performed using a portable ultrasound device (Mindray M7; Mindray Ltd., Hamburg, Germany) with a 12-MHz transducer probe. Standard transthoracic two-dimensional, M-mode, pulsed-wave (PW) Doppler, and PW TDI images were obtained for analysis.
Conventional echocardiographic measurements were performed according to published guidelines (refer to Supplemental Material) and averaged from three to five consecutive cardiac cycles (14).
The primary outcomes of the study were TDI myocardial diastolic velocities and time intervals. PW TDI was performed by adjusting the spectral pulsed Doppler signal filters to obtain a Nyquist limit of 15–20 cm/s and using the minimal optimal gain according to the published guidelines (7). TDI velocities were obtained from the apical four-chamber view by placing a pulsed wave Doppler sample (gate 2 mm) just below the lateral mitral annulus, the basal septal area, and the lateral tricuspid annulus (7, 15). The systolic velocity (s') and the early (e') and late (a') diastolic myocardial velocities were measured. On the PW Doppler tracings, five different time interval parameters were analyzed (Figure 2), namely, the isovolumic contraction time (IVCT', between the end of the a' wave to the beginning of the s' wave), the isovolumic relaxation time (IVRT', between the end of the s' wave to the beginning of the e' wave), the ejection time (ET', duration of the s' wave), the filling time (FT', measured from the beginning of the e' wave to the end of the a' wave), and the total isovolumic time (t-IVT'= IVCT'+ IVRT'). The e'/a', E/e' ratios, and the DTI-based myocardial performance index (MPI' = (IVCT'+IVRT')/ET') were also calculated.
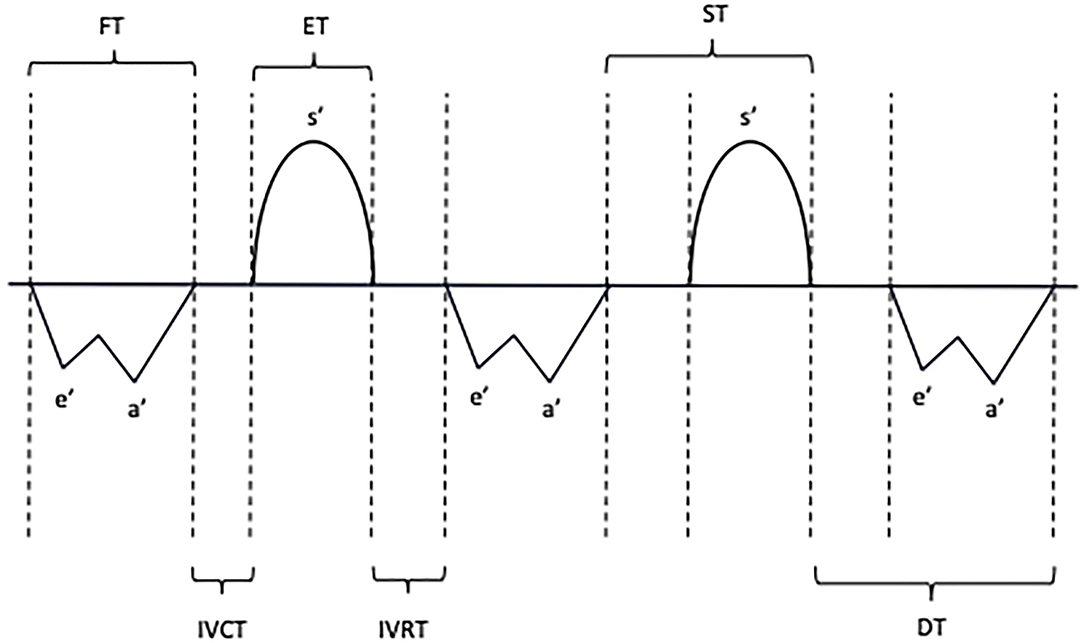
Figure 2. Schematic representation of systolic and diastolic myocardial velocities and time interval measurements with pulsed-wave tissue Doppler. S', Systolic velocity; E', Early diastolic velocity; A', Late diastolic velocity; IVCT, Isovolumic contraction time; IVRT, Isovolumic relaxation time; FT, Filling time; ET, Ejection time; ST, Systolic time; DT, Diastolic time.
Time intervals were corrected to the heart rate (HR) as calculated with the RR interval and reported as ET'/HR, IVCT'/HR, IVRT'/HR, FT'/HR, and t-IVT'/HR.
Considering the results of diastolic myocardial velocity measurements, for a statistic power of 90%, 1-α of 95% with d = −2, and S2 of 3.8, the calculated sample size was 16. The final number of babies in the control group was 15, because data from one of the babies had to be eliminated. All babies from the study group were included in the analysis. Comparison between HIE + TH and control groups was analyzed using generalized estimating equation (GEE) models with an exchangeable correlation structure. The cardiac variables were introduced in each model as dependent variables and the treatment group (TH vs. control), different time points, and the interaction term between group and time points as independent variables. The results are presented as the means and 95% confidence intervals obtained through the margins postestimation STATA command after fitting each model. The means of intragroup comparison between the treatment group (TH vs. control) in each time point and the intragroup differences, taking the time point one as reference, were made using the Bonferroni method for multiple comparisons. A value of p < 0.05 was accepted for statistical significance. The STATA version 15.0. software was used for the statistical analysis.
A total of 21 patients with HIE + TH (five girls and 16 boys) were consecutively included in the study as well as 15 controls (five girls and 10 boys) (Figure 1). Tables 1, 2 summarize the characteristics of these babies. Gestational age and birth weights were similar in both groups. Cord pH and 1- and 5-min Apgar scores were significantly lower in the HIE + TH group. Upon admission, the severity of the encephalopathy was classified as stage II in 42.9% of the asphyxiated babies, according to the modified Sarnat score, whereas 57.1% were classified as stage III. The majority of patients with HIE + TH were receiving inotropic support at the time of the echocardiographic evaluations, with 66.7, 85.7, and 61.9% of the babies treated during the first, second, and third assessment, respectively, with dobutamine as the first-line treatment. None of the patients received hydrocortisone. Notably, 20 patients with HIE were discharged home and one baby died after redirection of care.
The results of conventional echocardiographic measurements are described in the Electronic Supplementary Material.
TDI results are summarized in Tables 3–5.
Left ventricular (LV) myocardial systolic velocities were similar in both groups at all time points. In contrast, septal s' was decreased in patients with HIE + HT in T1 compared to controls and increased significantly after rewarming. RV s' did not differ from controls at any time point but it increased significantly in asphyxiated babies after rewarming.
The LV diastolic myocardial velocities were similar in both groups, without differences for early (e'LV) and late (a'LV) diastolic velocities, as well as E/e'LV and e'/a'LV ratios. Septal a' was significantly slower at T2 in asphyxiated neonates and increased significantly after rewarming, but e'SEPTAL and e'/a'SEPTAL did not differ between groups or time points. Regarding RV diastolic velocities, e' was significantly slower at the first assessment in the HIE + TH group and improved after rewarming. However, no differences were found for a'RV, E/e'RV, or e'/a'RV between groups and time points except for a significant increase in a'RV and e'/ a'RV after rewarming compared to baseline values in patients with HIE.
Isovolumic Contraction Time. LV, RV, and septal IVCT' were prolonged in the HIE + TH group in T1 compared to controls. This difference was significant at this time point even after correction for HR. IVCT' shortened significantly in T2 and T3 in asphyxiated neonates so that differences with the control group disappeared, except for the RV where it persisted significantly prolonged after rewarming. However, when corrected for HR, this difference in the RV was no longer significant.
Isovolumic Relaxation Time. Similarly, IVRT' was longer in the HIE + TH group at T1 and T2 in the LV, RV, and septum, and decreased significantly after rewarming. These differences remained significant after correction for HR.
Myocardial Performance Index. LV and RV MPI' were increased in asphyxiated babies in T1 and T2, compared to controls, and normalized after rewarming. Similarly, septal MPI' was increased at T1 in these groups but improved in subsequent assessments.
Ejection and Filling Times. Regarding the duration of ET', ET'LV was significantly shorter in T1 and T2, ET'SEPTAL was shorter after rewarming, and ET'RV was shorter in T2 and T3, in asphyxiated neonates as compared to controls, but none of these differences persisted after correction for HR. LV, septal, and RV FT' were significantly longer in infants with HIE at T1 compared to values at T2 and after rewarming, a difference that remained statistically significant after correction for HR. Moreover, when corrected for HR, FT'RV at the first assessment was also longer in the HIE + TH group compared to controls. In contrast, although TDI-measured FT' in the LV at T2, in the septum at T3, and in the RV at T2 were significantly shorter in the HIE + TH group compared to controls, these differences disappeared after controlling for HR. However, FT'RV was shorter in asphyxiated babies compared to controls after rewarming, with and without correction for HR.
Total Isovolumic Time. t-IVT (t'-IVT = IVCT' + IVRT') was significantly longer in LV, septum, and RV in babies with HIE during TH compared to controls, with a significant decrease in T2 and after rewarming.
In this retrospective study, we demonstrated the existence of a significant diastolic dysfunction in cooled neonates with moderate–severe HIE that improves progressively during treatment and normalizes after rewarming. TDI evaluation in our study showed a pattern of early diastolic dysfunction during TH, whereas late diastole seemed to be preserved.
This is one of the first studies specifically assessing biventricular diastolic function using TDI during TH and rewarming. We observed an increase in MPI' in both ventricles and the septum during TH that normalized after rewarming. Increased MPI is one of the most consistent echocardiographic findings in studies on HIE and reflects an alteration of combined systolic and diastolic function (16–19). In this context of global myocardial dysfunction, most investigations have focused on LV systolic function (11, 12, 20, 21), and less attention has been paid to RV function or diastolic performance. However, in high-risk newborns, diastolic heart failure may precede systolic failure and latent RV dysfunction may be present before LV failure appears (22). Newborns may be particularly vulnerable to diastolic dysfunction after HIE because, due to the relatively increased concentrations of collagen, the neonatal myocardium is less compliant, with shorter filling times and an increased dependency of diastolic function on atrial contraction (23).
Diastole can be divided into two different phases, namely, early and late diastole. The complex distribution and orientation of the different myocardial muscle layers are key anatomic factors that explain not only the highly efficient pumping of blood during systole but also the mechanisms of ventricular filling in early diastole. During IVRT, the clockwise relaxation of the transverse myocardial band and the cessation of contraction of the inner descending fibers, together with the contraction of the outer ascending layer, generate the powerful suction force that is critical for the rapid early filling of the LV after mitral valve opening (24). We found significantly increased IVRT' in both ventricles and the septum, even after correcting for HT-induced bradycardia, that improved progressively during treatment and normalized after rewarming. This finding suggests globally prolonged myocardial relaxation. IVRT' is a good non-invasive index of diastolic function that correlates well with invasive measurements of Tau, the time constant of ventricular relaxation, and its prolongation is associated with diastolic dysfunction (25, 26). Delayed relaxation has been shown to be an important contributor to heart failure with preserved EF (27). This can be explained by the fact that 50–60% of diastolic recoil occurs during IVRT and an intact myocardial untwisting is necessary for an adequate early diastolic function (28, 29). Moreover, 70% of ventricular filling takes place during early diastole. In agreement, prolonged IVRT' in our study was accompanied by a slower early myocardial diastolic velocity (e') in the RV, although not in the septum or the LV and longer RV FT'. TDI e' is a good indicator of ventricular relaxation that correlates well with invasive measurements (30, 31). Altered early filling affecting only the RV is in accordance with the observation of recent studies suggesting a particular vulnerability of the RV to perinatal hypoxia-ischemia (2, 3). A study on normothermic asphyxiated neonates describes biventricular prolongation of IVRT with slower e' only in the RV, but this was not interpreted as a representative of diastolic dysfunction (11).
Rapid early ventricular filling is followed by a transient decrease in flow (diastasis) after which atrial contraction collaborates to a further increase in ventricular filling during the late phase of diastole. Traditionally, relaxation parameters are used to characterize early diastole, whereas compliance and filling pressure parameters are used to characterize late diastole (8). Slow relaxation can limit filling and increase end-diastolic pressures when relaxation remains incomplete at the end of diastole (27). However, although early diastole was affected in our babies, late diastolic myocardial velocities seemed to be preserved during TH, except for a decreased septal a' in T2 that normalized after rewarming. Similarly, no differences were found for E'/A' ratio between babies with HIE and controls. In addition, ventricular compliance, as assessed with the E/e' ratio, remained unchanged, although this should be interpreted with caution. E/e' ratio correlates well with pulmonary capillary wedge pressure and provides a good estimation of ventricular filling pressure independent of relaxation or LV systolic function, and it has been evaluated in neonates in different contexts (3, 6, 9, 16, 30, 32–34). However, E/e' may be influenced by left-to-right shunts through the patent ductus arteriosus (PDA) or foramen ovale (FO), as probably happened in our study, so its role in neonates awaits further clarification (7). The influence of TH may explain the discrepancy between our findings regarding early and late diastole. As recently shown in experimental studies in adult pigs, time for complete ventricular relaxation (3.5 × Tau) may be within diastolic duration during TH despite the presence of diastolic dysfunction due to decreased spontaneous HRs (7, 27, 35, 36). This could explain why delayed relaxation and slowed early filling did not translate into increased filling pressures in our patients. However, in those animal models, if HR is increased with atrial pacing, the duration of diastole is shortened compromising filling (7). This situation may render the myocardium more vulnerable to moderate increases in HR as it happens with the use of inotropic drugs (up to 85.7% in our series). Interestingly, those experimental data as well as clinical studies in adults demonstrate that TH per se can induce diastolic dysfunction (37, 38). However, our results are consistent with those of previous studies in asphyxiated neonates not treated with TH, so hypoxia-ischemia seems to play a key role in the development of this condition in these babies (11, 20).
Prolongation of isovolumic relaxation can also be the result of alternative mechanisms, such as altered twist/untwist motion or intraventricular dyssynchrony, both of which impact early diastolic suction (39). In this regard, we found a prolongation of IVCT' during TH, even after correcting for HR, that normalized after rewarming. These results are in agreement with previous studies in non-cooled babies with HIE (11). IVCT' correlates with peak +dP/dt and is prolonged in systolic dysfunction (25). This fact may exacerbate diastolic dysfunction as impaired ventricular twisting may delay untwisting, reduce suction, and impair early diastolic filling (24). Moreover, coordinate systolic contraction itself is a major determinant of early diastolic ventricular filling, so dyssynchrony may have a negative impact on overall ventricular performance leading to prolonged contraction, as well as delayed and prolonged relaxation (40). t-IVT and MPI are sensitive indexes of ventricular dyssynchrony (40), and both parameters were globally prolonged in our study. Some studies have suggested that the increased post-ejection isovolumic period observed in patients with pulmonary hypertension can be explained by prolonged RV contraction beyond pulmonary valve closure due to increased afterload, and not by delayed isovolumic relaxation, and consequently, it does not reflect diastolic dysfunction in this context (41). This mechanism could partly explain the prolongation of IVRT' in our study, considering that PVR was significantly increased in the HIE + TH group (refer to Supplemental Material). However, the slower RV e' in our patients supports the presence of diastolic dysfunction. Besides, LV free wall IVRT' was also prolonged in our series.
Our study has some limitations. First, most patients were receiving dobutamine during the echocardiographic assessments and this could have influenced our results. In this regard, some studies have reported an improvement in diastolic function with this treatment (42). Thus, the frequent use of dobutamine in our series could have modified diastolic function and does not allow us to differentiate whether the progressive improvement observed in our patients was due to the use of inotropes or to spontaneous recovery. However, part of this improvement could also be attributed to the increased use of iNO in T2, as this drug could have improved myocardial function by reducing RV afterload. Dobutamine may also be responsible for the relatively high HR of some of our patients during TH. Furthermore, we could not discriminate whether the observed effects on diastolic function are the result of HIE, TH, or a combination of both factors. Finally, the retrospective design and the small number of patients evaluated may limit the extrapolation of our findings.
In conclusion, our study demonstrates the presence of diastolic dysfunction in neonatal HIE during TH characterized by globally prolonged IVRT' and decreased early diastolic RV myocardial velocity, regardless of HR, which normalized after rewarming, reflecting a prolonged and slowed myocardial relaxation. We also found data, suggesting an altered myocardial twist/untwist motion and ventricular dyssynchrony. Diastolic impairment may not be clinically evident in the setting of hypothermia-induced bradycardia, so echocardiographic assessment of myocardial function during TH should include the evaluation of diastolic function with appropriate techniques, such as TDI. Further studies are needed to investigate the impact of diastolic dysfunction on HIE as well as its potential therapeutic implications.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee in Clinical Research, Hospital Clinico San Carlos, Madrid, Spain. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
LA and MJR: study design. MJR, AC, and LA: acquisition of data. JM-O and IS: data analysis. MJR, JM-O, IS, and LA: interpretation of data. MJR, LA, and JM-O: manuscript drafting. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.880786/full#supplementary-material
1. Greisen G. Cerebral blood flow and oxygenation in infants after birth asphyxia. Clinically useful information? Early Hum Dev. (2014) 90:703–5. doi: 10.1016/j.earlhumdev.2014.06.007
2. Rodriguez MJ, Corredera A, Martinez-Orgado J, Arruza L. Cerebral blood flow velocity and oxygenation correlate predominantly with right ventricular function in cooled neonates with moderate-severe hypoxic-ischemic encephalopathy. Eur J Pediatr. (2020) 179:1609–18. doi: 10.1007/s00431-020-03657-w
3. Giesinger RE, El Shahed AI, Castaldo MP, Breatnach CR, Chau V, Whyte HE. el al. Impaired right ventricular performance is associated with adverse outcome after hypoxic ischemic encephalopathy. Am J Respir Crit Care Med. (2019) 200:1294–305. doi: 10.1164/rccm.201903-0583OC
4. Møller JE, Pellikka PA, Hillis GS, Oh JK. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. (2006) 114:438–44. doi: 10.1161/CIRCULATIONAHA.105.601005
5. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. (2007) 28:2539–50. doi: 10.1093/eurheartj/ehm037
6. Murase M. Assessing ventricular function in preterm infants using tissue Doppler imaging. Expert Rev Med Devices. (2016) 13:325–38. doi: 10.1586/17434440.2016.1153966
7. Nestaas E, Schubert U, de Boode WP, El-Khuffash A. European Special Interest Group ‘Neonatologist Performed Echocardiography' (NPE). Tissue Doppler velocity imaging and event timings in neonates: a guide to image acquisition, measurement, interpretation, and reference values. Pediatr Res. (2018) 84:18–29. doi: 10.1038/s41390-018-0079-8
8. Recher M, Botte A, Soquet J, Baudelet JB, Godart F, Leteurtre S. Assessment of left-ventricular diastolic function in pediatric intensive-care patients: a review of parameters and indications compared with those for adults. World J Pediatr. (2021) 17:21–30. doi: 10.1007/s12519-020-00369-x
9. Dokainish H. Tissue Doppler imaging in the evaluation of left ventricular diastolic function. Curr Opin Cardiol. (2004) 19:437–41. doi: 10.1097/01.hco.0000131538.55528.8f
10. Kluckow M. Functional echocardiography in assessment of the cardiovascular system in asphyxiated neonates. J Pediatr. (2011) 158:e13–8. doi: 10.1016/j.jpeds.2010.11.007
11. Matter M, Abdel-Hady H, Attia G, Hafez M, Seliem W, Al-Arman M. Myocardial performance in asphyxiated full-term infants assessed by Doppler tissue imaging. Pediatr Cardiol. (2010) 31:634–42. doi: 10.1007/s00246-010-9661-5
12. Nestaas E, Skranes JH, Støylen A, Brunvand L, Fugelseth D. The myocardial function during and after whole-body therapeutic hypothermia for hypoxic-ischemic encephalopathy, a cohort study. Early Hum Dev. (2014) 90:247–52. doi: 10.1016/j.earlhumdev.2014.01.014
13. Giesinger RE, Bailey LJ, Deshpande P, McNamara PJ. Hypoxic-ischemic encephalopathy and therapeutic hypothermia: the hemodynamic perspective. J Pediatr. (2017) 180:22–30. doi: 10.1016/j.jpeds.2016.09.009
14. Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr. (2011) 24:1057–78. doi: 10.1093/ejechocard/jer181
15. Jain A, El-Khuffash AF, Kuipers BCW, Mohamed A, Connelly KA, McNamara PJ, et al. Left ventricular function in healthy term neonates during the transitional period. J Pediatr. (2017) 182:197–203. doi: 10.1016/j.jpeds.2016.11.003
16. Aggarwal S, Natarajan G. Biventricular function on early echocardiograms in neonatal hypoxic-ischaemic encephalopathy. Acta Paediatr. (2017) 106:1085–90. doi: 10.1111/apa.13866
17. Hochwald O, Jabr M, Osiovich H, Miller SP, McNamara PJ, Lavoie PM. Preferential cephalic redistribution of left ventricular cardiac output during therapeutic hypothermia for perinatal hypoxic-ischemic encephalopathy. J Pediatr. (2014) 164:999–1004. doi: 10.1016/j.jpeds.2014.01.028
18. Karaarslan S, Alp H, Baysal T, Çimen D, Örs R, Oran B. Is myocardial performance index useful in differential diagnosis of moderate and severe hypoxic-ischaemic encephalopathy? A serial Doppler echocardiographic evaluation. Cardiol Young. (2012) 22:335–40. doi: 10.1017/S104795111200011X
19. Ichihashi K, Yada Y, Takahashi N, Honma Y, Momoi M. Utility of a Doppler-derived index combining systolic and diastolic performance (Tei index) for detecting hypoxic cardiac damage in newborns. J Perinat Med. (2005) 33:549–52. doi: 10.1515/JPM.2005.098
20. Wei Y, Xu J, Xu T, Fan J, Tao S. Left ventricular systolic function of newborns with asphyxia evaluated by tissue Doppler imaging. Pediatr Cardiiol. (2009) 30:741–6. doi: 10.1007/s00246-009-9421-6
21. Nestaas E, Støylen A, Brunvand L, Fugelseth D. Longitudinal strain and strain rate by tissue Doppler are more sensitive indices than fractional shortening for assessing the reduced myocardial function in asphyxiated neonates. Cardiol Young. (2011) 21:1–7. doi: 10.1017/S1047951109991314
22. Bussmann N, El-Khuffash A, Breatnach CR, McCallion N, Franklin O, Singh GK, et al. Left ventricular diastolic function influences right ventricular - Pulmonary vascular coupling in premature infants. Early Hum Dev. (2019) 128:35–40. doi: 10.1016/j.earlhumdev.2018.11.006
23. Marijianowski MM, van der Loos CM, Mohrschladt MF, Becker AE. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J Am Coll Cardiol. (1994) 23:1204–8. doi: 10.1016/0735-1097(94)90612-2
24. Buckberg GD, Nanda NC, Nguyen C, Kocica MJ. What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. J Cardiovasc Dev Dis. (2018) 5:33. doi: 10.3390/jcdd5020033
25. Cui W, Roberson DA, Chen Z, Madronero LF, Cuneo BF. Systolic and diastolic time intervals measured from Doppler tissue imaging: normal values and Z-score tables, and effects of age, heart rate, and body surface area. J Am Soc Echocardiogr. (2008) 21:361–70. doi: 10.1016/j.echo.2007.05.034
26. Thomas JD, Flachskampf FA, Chen C, Guerrero JL, Picard MH, Levine RA, et al. Isovolumic relaxation time varies predictably with its time constant and aortic and left atrial pressures: implications for the noninvasive evaluation of ventricular relaxation. Am Heart J. (1992) 124:1305–13. doi: 10.1016/0002-8703(92)90416-S
27. Schwarzl M, Alogna A, Zirngast B, Steendijk P, Verderber J, Zweiker D, et al. Mild hypothermia induces incomplete left ventricular relaxation despite spontaneous bradycardia in pigs. Acta Physiol. (2015) 213:653–63. doi: 10.1111/apha.12439
28. Rademakers FE, Buchalter MB, Rogers WJ, Zerhouni EA, Weisfeldt ML, Weiss JL, et al. Dissociation between left ventricular untwisting and filling. Accentuation by catecholamines. Circulation. (1992) 85:1572–81. doi: 10.1161/01.CIR.85.4.1572
29. Burns AT, La Gerche A, Prior DL, Macisaac AI. Left ventricular untwisting is an important determinant of early diastolic function. J Am Coll Cardiol Img. (2009) 2:709–16. doi: 10.1016/j.jcmg.2009.01.015
30. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. (2000) 102:1788–94. doi: 10.1161/01.CIR.102.15.1788
31. Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. (1997) 30:474–80. doi: 10.1016/S0735-1097(97)88335-0
32. Di Maria MV, Younoszai AK, Sontag MK, Miller JI, Poindexter BB, Ingram DA, et al. Maturational changes in diastolic longitudinal myocardial velocity in preterm infants. J Am Soc Echocardiogr. (2015) 28:1045–52. doi: 10.1016/j.echo.2015.04.016
33. Sehgal A, Malikiwi A, Paul E, Tan K, Menahem S. Right ventricular function in infants with bronchopulmonary dysplasia: association with respiratory sequelae. Neonatology. (2016) 109:289–96. doi: 10.1159/000442967
34. Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. (1997) 30:1527–33. doi: 10.1016/S0735-1097(97)00344-6
35. Nishimura Y, Naito Y, Nishioka T, Okamura Y. The effects of cardiac cooling under surface-induced hypothermia on the cardiac function in the in situ heart. Interact Cardiovasc Thorac Surg. (2005) 4:101–5. doi: 10.1510/icvts.2004.097188
36. Post H, Schmitto JD, Steendijk P, Christoph J, Holland R, Wachter R, et al. Cardiac function during mild hypothermia in pigs: increased inotropy at the expense of diastolic dysfunction. Acta Physiol. (2010) 199:43–52. doi: 10.1111/j.1748-1716.2010.02083.x
37. Kerans V, Espinoza A, Skulstad H, Halvorsen PS, Edvardsen T, Bugge JF. Systolic left ventricular function is preserved during therapeutic hypothermia, also during increases in heart rate with impaired diastolic filling. Intensive Care Med Exp. (2015) 3:41. doi: 10.1186/s40635-015-0041-6
38. Kuwagata Y, Oda J, Ninomiya N, Shiozaki T, Shimazu T, Sugimoto H. Changes in left ventricular performance in patients with severe head injury during and after mild hypothermia. J Trauma. (1999) 47:666–72. doi: 10.1097/00005373-199910000-00010
39. Smiseth OA, Thompson CR. Atrioventricular filling dynamics, diastolic function and dysfunction. Heart Fail Rev. (2000) 5:291–9.
40. Vancheri F, Vancheri S, Henein MY. Effect of age on left ventricular global dyssynchrony in asymptomatic individuals: a population study. Echocardiography. (2016) 33:977–83. doi: 10.1111/echo.13218
41. Mauritz GJ, Marcus JT, Westerhof N, Postmus PE, Vonk-Noordegraaf A. Prolonged right ventricular post-systolic isovolumic period in pulmonary arterial hypertension is not a reflection of diastolic dysfunction. Heart. (2011) 97:473–8. doi: 10.1136/hrt.2010.193375
Keywords: diastolic dysfunction, hypoxic-ischemic encephalopathy, therapeutic hypothermia, tissue Doppler, newborn, targeted neonatal echocardiography, cardiac function
Citation: Rodriguez MJ, Martinez-Orgado J, Corredera A, Serrano I and Arruza L (2022) Diastolic Dysfunction in Neonates With Hypoxic-Ischemic Encephalopathy During Therapeutic Hypothermia: A Tissue Doppler Study. Front. Pediatr. 10:880786. doi: 10.3389/fped.2022.880786
Received: 21 February 2022; Accepted: 21 April 2022;
Published: 25 May 2022.
Edited by:
Ana Alarcon, Hospital Sant Joan de Déu Barcelona, SpainReviewed by:
Javier Rodriguez Fanjul, Hospital Germans Trias i Pujol, SpainCopyright © 2022 Rodriguez, Martinez-Orgado, Corredera, Serrano and Arruza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luis Arruza, bHVpc2FycnV6YUB5YWhvby5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.