
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 12 April 2022
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.877759
Background: Human coronaviruses (HCoVs) are associated with upper respiratory tract infections. Although studies have analyzed the clinical and epidemiological characteristics of HCoV-associated infections, no multi-center studies have been conducted in Korean children. We aimed to describe the epidemiology and clinical characteristics of HCoV-associated infection in children.
Methods: We retrospectively reviewed medical records of children in whom HCoVs were detected using multiplex reverse transcriptase-polymerase chain reaction amplification in five centers from January 2015 to December 2019.
Results: Overall, 1,096 patients were enrolled. Among them, 654 (59.7%) patients were male. The median age was 1 year [interquartile range (IQR), 0–2 years]. HCoVs were identified mainly in winter (55.9%). HCoV-229E, HCoV-OC43, and HCoOV-NL63 were detected mainly in winter (70.9, 55.8, and 57.4%, respectively), but HCoV-HKU1 was mainly identified in spring (69.7%). HCoV-OC43 (66.0%) was detected most frequently, followed by HCoV-NL63 (33.3%), and HCoV-229E (7.7%). Two different types of HCoVs were co-detected in 18 samples, namely. Alphacoronavirus-betacoronavirus co-infection (n = 13) and, alphacoronavirus-alphacoronavirus co-infection (n = 5). No betacoronavirus-betacoronavirus co-infection was detected. Patients were diagnosed with upper respiratory tract infection (41.4%), pneumonia (16.6%), acute bronchiolitis (15.5%), non-specific febrile illness (13.1%), croup (7.3%), and acute gastroenteritis (5.1%). There were 832 (75.9%) hospitalized patients with a median duration of hospitalization of 4 days (IQR, 3–5 days); 108 (9.9%) patients needed supplemental oxygen with 37 (3.4%) needing high-flow nasal cannula or mechanical ventilation. There were no deaths.
Conclusion: HCoV-associated infections exhibit marked seasonality with peaks in winter. Patients with lower respiratory tract infection, a history of prematurity, or underlying chronic diseases may progress to a severe course and may need oxygen therapy.
Human coronaviruses (HCoVs) were identified as human respiratory pathogens in the 1960's (1). In addition to the recently discovered severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 2019, other HCoVs, such as severe acute respiratory syndrome coronavirus, Middle East respiratory syndrome coronavirus, SARS-CoV-2 and four common HCoVs (i.e., HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1) are known to cause respiratory infections in humans (2, 3).
Common HCoVs are found worldwide and cause diseases predominantly during winter and spring in temperate climates (4, 5). They are most frequently associated with upper respiratory tract infections (URIs) and are characterized by rhinorrhea, nasal congestion, sore throat, sneezing, and cough that may be associated with mild fever (4, 6–8), as well as acute otitis media or asthma exacerbations (4, 7–9). HCoVs are sometimes also associated with lower respiratory tract infections, including croup, bronchiolitis, and pneumonia, primarily in infants and immunocompromised patients (7, 10–16).
In Korea, while some studies analyzed clinical or epidemiological characteristics of HCoV-associated infection (14, 17–19), they were either single-center studies or focused on patients of all ages. Till date, there have been no multi-center studies focusing on children in Korea; hence, this study aimed to investigate the epidemiologic and clinical characteristics of HCoV-associated infections in children admitted to various hospitals in the Republic of Korea.
We conducted a retrospective chart review from January 2015 to December 2019 for children (0 to 18 years old) in whom HCoVs were detected using multiplex reverse transcriptase-polymerase chain reaction amplification (RT-PCR) at five hospitals in the southern part of Korea: Pusan National University Children's Hospital, Pusan National University Hospital, Kosin University Gospel Hospital, Kyungpook National University Children's Hospital, and Jeonbuk National University Children's Hospital. The following clinical characteristics of patients were analyzed: age, sex, date of admission, admission to intensive care unit, clinical diagnosis, history of prematurity and underlying chronic diseases, need for oxygen therapy, high-flow nasal cannula oxygen therapy or mechanical ventilation, presence of fever, presence of respiratory or gastrointestinal symptoms, presence of infiltrates on chest radiographs, length of hospital stay, and results of virologic study. We excluded patients in whom HCoVs were detected using RT-PCR 48 h or more after hospital admission. Patients who were diagnosed with Kawasaki disease, urinary tract infection, or other viral and bacterial infections were also excluded. To investigate the seasonal fluctuations of each HCoVs, the year was divided into spring (March-May), summer (June-August), autumn (September-November), and winter (December-February). The present study protocol was reviewed and approved by the Institutional Review Board of the Pusan National University Yangsan Hospital (approval No. 05-2020201). The requirement for informed consent was waived due to the retrospective study design.
Specimens of nasopharyngeal swabs for RT-PCR analyses were collected and two different RT-PCR tests were performed. The first test was a respiratory virus multiplex RT-PCR (Seoul Clinical Laboratories, Gyeonggi-do, Republic of Korea), and it was performed at all five hospitals. This test commenced in January 2015 at all the five hospitals. Testing was done for HCoV-229E, HCoV-OC43, HCoV-NL63, human adenovirus, human metapneumovirus, influenza A virus, influenza B virus, parainfluenza virus types 1-3, respiratory syncytial virus (RSV) A, RSV B, human rhinovirus A, human rhinovirus B, human rhinovirus C, and human bocavirus. The second test was the FILMARRAY Respiratory Panel (BioFiredx, Salt Lake City, USA) and the study was performed at two of the five hospitals. One hospital started in September 2018, and the other in January 2019. This test assessed for HCoV-229E, HCoV-OC43, HCoV-NL63, HCoV-HKU1, human adenovirus, human metapneumovirus, human rhinovirus, influenza A virus, influenza B virus, parainfluenza virus types 1- 4, RSV, Bordetella pertussis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae.
The Wilcoxon signed-rank test (non-parametric) and the χ2 test were used to compare non-normally distributed continuous variables and continuous variables, respectively. Statistical analyses were performed using SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). A value of P < 0.05 was considered significant.
Nasopharyngeal swab samples were obtained from 238,637 children and respiratory viruses were detected in 22,542 (9.4%) children. HCoVs were detected in 1,406 of 22,542 (6.2%) patients; 310 patients were excluded. In total, 1,096 patients were enrolled during the study period.
Among them, 664 (60.6%) patients were male, and the median age was 1 year [interquartile range (IQR), 0–2 years]. Co-infection with two different types of HCoVs was observed in 18 patients. The HCoVs were identified mainly in winter (55.9%) (see Figure 1). HCoV-OC43 (66.0%) was the most commonly detected HCoV, followed by HCoV-NL63 (33.3%) and HCoV-229E (7.7%). In the case of HCoV-HKU1, FILMARRAY Respiratory Panel (BioFiredx, Salt Lake City, USA) was performed in two institutions for about 1 year, and 18.1% of the samples that detected HCoVs using FILMARRAY Respiratory Panel (BioFiredx, Salt Lake City, USA) were confirmed to be positive for HCoV-HKU1.
HCoV-229E, HCoV-OC43, and HCoV-NL63 were mainly detected in winter (70.9, 55.8, and 57.4%, respectively), but HCoV-HKU1 was mainly detected in spring (69.7%). HCoV-229E and HCoV-OC43 are alphacoronavirus, and HCoV-NL63 and HCoV-HKU1 are betacoronavirus. Two different types of HCoVs were co-detected in 18 samples, which included. Alphacoronavirus-betacoronavirus co-infection (n = 13) and alphacoronavirus-alphacoronavirus co-infection (n = 5). No betacoronavirus-betacoronavirus co-infection was detected. Other viruses were co-detected with HCoVs in 44.5% of patients. Of these, two and three non-HCoV respiratory viruses were co-detected in 78 and 11 patients, respectively. Among them, RSV (28.9%) was the most detected virus, followed by human rhinovirus (27.7%) and human adenovirus (19.2%) (Table 1). The patients were diagnosed with URI (41.4%), pneumonia (16.6%), acute bronchiolitis (15.5%), non-specific febrile illness (13.1%), croup (7.3%), and acute gastroenteritis (5.1%). Of the total, 270 (24.6%) patients had a history of prematurity or underlying chronic diseases.
Of the 1,096 patients, 832 (75.9%) were hospitalized, and the median duration of hospitalization was 4 days (IQR, 3–5 days). Oxygen supply was needed in 108 (9.9 %) patients, and 37 (3.4%) needed high-flow nasal cannula oxygen therapy or mechanical ventilation. These 37 patients were diagnosed with pneumonia (51.4%), acute bronchiolitis (16.2%), croup (16.2%), URI (13.5%), and asthma attack (2.7%). Out of them, 23 (62.2%) had a history of prematurity or underlying chronic diseases. Among the hospitalized patients, respiratory virus co-detection was found in 18 (48.6%) patients. Human rhinovirus (36.5%) was the most commonly detected virus, followed by RSV (27.3%), influenza viruses (13.6%), human metapneumovirus (13.6%), and parainfluenza virus (4.5%). When comparing patients who needed high flow-nasal cannula oxygen therapy or mechanical ventilation with those who did not, no significant differences in patient's age and sex were found (P = 0.349, P = 0.620) (Table 2). Patients in need of high-flow nasal cannula oxygen therapy or mechanical ventilation had significantly more history of prematurity or underlying chronic diseases (P < 0.001), and the ratio of pneumonia was high (P < 0.001).
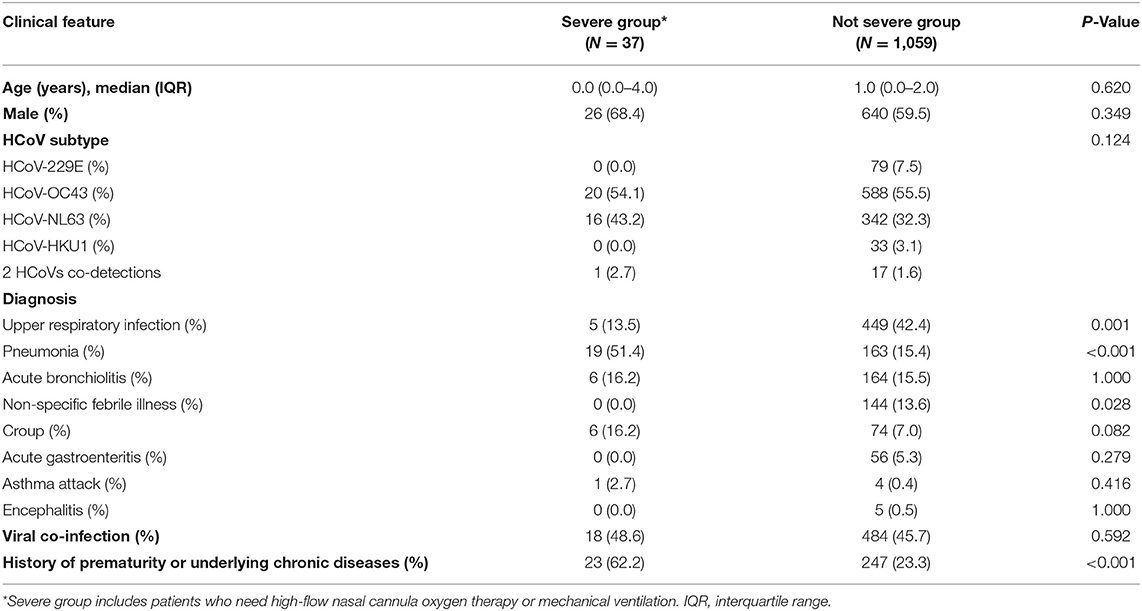
Table 2. Clinical characteristics associated with HCoV infection: comparison of patients who needed high-flow nasal cannula oxygen therapy or mechanical ventilation with those who did not.
According to the type of HCoV, there were no significant differences in patient's sex (P = 0.173) and patients who were detected with HCoV-229E were older than those detected with HCoV-OC43 (P = 0.001) (Table 3). The most common clinical diagnosis associated with all four types of HCoVs was URI. While, HCoV-229E and HCoV-HKU1 were more frequently associated with non-specific febrile illness (P = 0.027), HCoV-OC43 and HCoV-NL63 were more frequently associated with pneumonia (P = 0.001) and croup (P < 0.001), respectively. There were significant differences in the viral co-infection in HCoV-229E- and HCoV-NL63-infected patients (P < 0.001). There were no significant differences in the history of prematurity or underlying chronic diseases (P = 0.539).
Comparing single infections and viral co-infections, there were no significant differences in patient's age and sex (P = 0.148, P = 0.931) (Table 4). The clinical diagnosis was also significantly different between both groups, as pneumonia and acute bronchiolitis were more frequent in viral co-infections (P < 0.001, P < 0.001), whereas non-specific febrile illness was more prevalent in HCoV single infections (P < 0.001). There was no significant difference in the requirement for hospital admission (P = 0.116); however, the duration of hospital admission was longer in the viral co-infection group than in the single-infection group (P < 0.001). There were no significant differences in the history of prematurity or underlying chronic diseases and clinical severity.
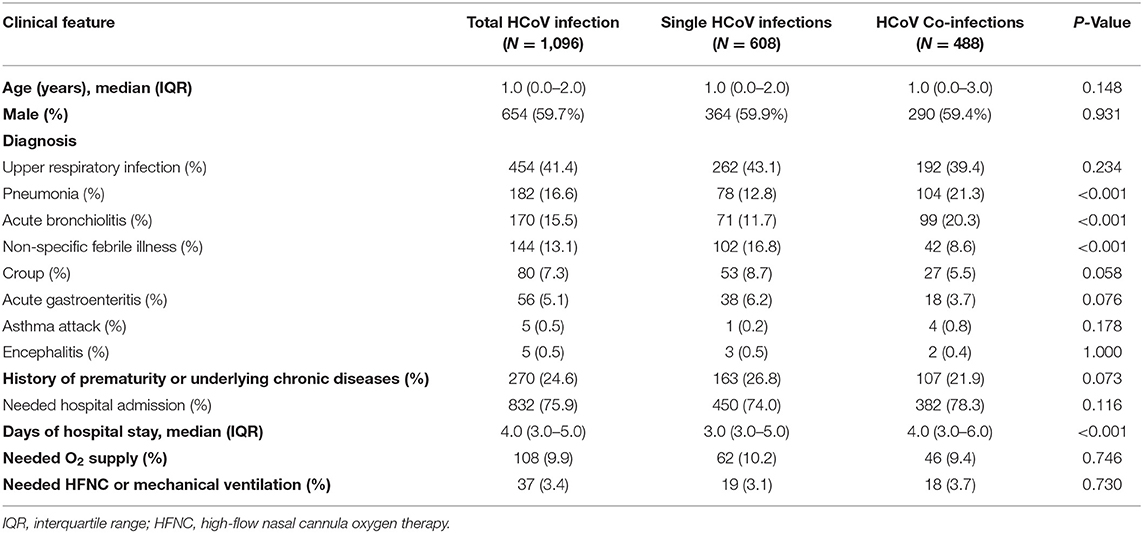
Table 4. Clinical characteristics associated with HCoV infection: comparison between single infection and viral co-infection.
We investigated the prevalence pattern of HCoVs for 5 consecutive years, from 2015 to 2019. During the study period, HCoV-229E, HCoV-OC43, and HCoV-NL63 showed marked seasonality with peaks in winter and were rarely detected in summer. Meanwhile, HCoV-HKU1 showed a marked seasonality with peaks in spring. HCoV-OC43 was the most commonly detected HCoV. These findings are consistent with those of earlier studies (4, 14, 16, 18, 20). However, our finding that HCoV-HKU1 showed a marked seasonality with peaks in spring is not consistent with that of earlier studies, which have shown that HCoV-HKU1 infections peak in winter and spring (20, 21). We could only investigate HCoV-HKU1 in two centers and for only a short duration. Therefore, further studies are required to better understand the patterns of activity of HCoV-HKU1.
In our study, two different types of HCoVs were co-detected in 18 samples. They were alphacoronavirus-betacoronavirus co-infection (n = 13) and alphacoronavirus-alphacoronavirus co-infection (n = 5). Betacoronavirus-betacoronavirus co-infection was not detected in any samples. The lack of significant sequence identity between the alphacoronavirus spike proteins and betacoronavirus cousins (<25%) suggests that they would be unlikely to have significant cross-reactivity. SARS-CoV-2 is also a betacoronavirus, and interest in cross-reactive antibodies against seasonal betacoronaviruses and SARS-CoV-2 have increased since the latter occurred in 2019. There were some studies about cross-reactive antibodies against seasonal betacoronaviruses and SARS-CoV-2 (22–26). Although it was reported that seasonal betacoronavirus antibodies led to a rise in the level of cross-reactive antibodies against SARS-CoV-2, its association with protection is not yet clear (26). Further studies would be needed to determine this interaction, though due to the high level of sequence and structural homology of betacoronaviruses spike proteins, such a cross-reactivity.
In our study, there were no cases of death despite some patients having had a history of prematurity or underlying chronic diseases. Most patients (75.9%) were hospitalized, and 9.9% of patients needed oxygen supply or high-flow nasal cannula oxygen therapy; however, all patients recovered and were discharged. There are a few studies about the clinical features of HCoV-associated infection in adults (14–16). One study retrospectively investigated HCoV-associated infections in patients of all ages from January 2018 to March 2020 (14). In that study, there were no cases of deaths in children, and the mortality rate was high in older individuals. The authors suggested that the reasons for the high mortality rate in older patients with infection can be the presence of comorbid diseases, the poor immunity, or the high probability of pneumonia. In our study, 24.6% of patients had a history of prematurity or underlying chronic diseases, and 16.6% of patients were diagnosed with pneumonia, but there were no deaths. Our study showed that unlike adults, HCoV-associated infection generally has a mild to moderate evolution with a favorable clinical outcome in children.
HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 are known to be associated with URI in children (4, 6, 8). Our study also showed that URI was the most common clinical diagnosis associated with all four types of HCoVs. There are a few studies showing that HCoV-OC43, and HCoV-NL63 are associated with lower respiratory tract infections (17, 27, 28). Our study showed HCoV-OC43 was more frequently associated with pneumonia. Compared with other HCoVs, HCoV-NL63 is more frequently associated with croup, and it is the second most common cause after parainfluenza virus type 1 (29, 30). Our study also showed that HCoV-NL63 was more frequently associated with croup than the other HCoVs. In our study, five patients were diagnosed with encephalitis. Among them, three patients were detected with HCoV-OC43, and the other two patients were detected with HCoV-229E and HCoV-NL63. Coronavirus can cause neurological diseases in animals; however, the exact association with neurological diseases in humans is not yet known. There are few studies about the association between HCoVs and neurological diseases. A Finnish study provided evidence of HCoV-OC43 infection in six patients with acute neurologic episodes (31). Another case study reported the presence of acute demyelinating encephalitis accompanying HCoV-OC43 respiratory tract infection, wherein HCoV-OC43 RNA was detected in the spinal fluid using PCR (32). However, further investigations will be needed to establish whether HCoVs are related causally to any neurologic disease in humans.
In our study, the 3.4% of patients who needed high flow nasal cannula oxygen therapy or mechanical ventilation were detected with HCoV-NL63 or HCoV-OC43. Among them, HCoV-NL63 and HCoV-OC43 were detected in 44.7 and 55.3% of patients, respectively. In a Belgian study, among children aged ≤ 5 years admitted to the hospital, the highest burden was associated with HCoV-OC43, followed by HCoV-NL63 (16). In adults, a study showed that patients detected with HCoV-OC43 were twice as likely to require oxygen or intubation than those with other strains (15); another study showed that mortality was higher in patients infected with HCoV-OC43 than those infected with other HCoVs (14). Comparing single infections and viral co-infections in our study, there were no significant differences in the requirement for hospital admission and clinical severity, as characterized by the need for oxygen supply, high-flow nasal cannula oxygen therapy, or mechanical ventilation. Considering these points, it is thought that viral co-infection is not related to the severity of the disease. Our study showed HCoV-OC43 was more frequently associated with pneumonia and that HCoV-NL63 was more frequently associated with croup. Because pneumonia and croup are diseases that can cause difficulty in breathing, we surmise that patients with HCoV-NL63 and HCoV-OC43 needed more high-flow nasal cannula oxygen therapy or mechanical ventilation. The reason for the virulent prognosis of HCoVs is still unclear, and further investigation is needed to better understand this topic.
Our study has some limitations that should be noted. First, the study was retrospective in nature, which has inherent bias. Second, respiratory multiplex RT-PCR testing was not performed on all patients who visited the hospitals. If the symptoms were mild or typical of URI, it was highly likely that the test was not performed; and if hospitalization was required, the test would have, in all likelihood, been performed. The proportion of patients who were diagnosed with non-specific febrile illness and URI may have been higher than that reported in our study. Third, we only investigated HCoV-HKU1 in two centers and for a short duration. Further studies with longer duration are needed to better understand the epidemiology and clinical features of HCoV-HKU1.
HCoV-associated infection has marked seasonality with peaks in winter, and it generally has a mild-to-moderate evolution with a favorable clinical outcome. Patients with lower respiratory tract infection, a history of prematurity, or underlying chronic diseases may need oxygen and may progress to a severe course, necessitating careful observation.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
KJ helped to write the initial draft and collect the data. S-HC helped to collect the data and performed statistical analysis. CO helped to collect the data contributed on revisions of the initial draft. HK, BC, and DJ helped to collect the data. SP conceived the study and contributed on revisions of the initial draft. All authors approved the final version of the report.
This work was supported by a 2-year Research Grant from Pusan National University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.877759/full#supplementary-material
1. Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. (1965) 1:1467–70. doi: 10.1136/bmj.1.5448.1467
2. Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. (2016) 24:490–502. doi: 10.1016/j.tim.2016.03.003
3. Da Costa VG, Moreli ML, Saivish MV. The emergence of SARS, MERS and novel SARS-2 coronaviruses in the 21st century. Arch Virol. (2020) 165:1517–26. doi: 10.1007/s00705-020-04628-0
4. Talbot HK, Shepherd BE, Crowe JE, Griffin MR, Edwards KM, Podsiad AB, et al. The pediatric burden of human coronaviruses evaluated for twenty years. Pediatr Infect Dis J. (2009) 28:682–7. doi: 10.1097/INF.0b013e31819d0d27
5. Vabret A, Dina J, Gouarin S, Petitjean J, Tripey V, Brouard J, et al. Human (non-severe acute respiratory syndrome) coronavirus infections in hospitalised children in France. J Paediatr Child Health. (2008) 44:176–81. doi: 10.1111/j.1440-1754.2007.01246.x
6. Fouchier RAM, Hartwig NG, Bestebroer TM, Niemeyer B, De Jong JC, Simon JH, et al. A previously undescribed coronavirus associated with respiratory disease in humans. Proc Natl Acad Sci USA. (2004) 101:6212–6. doi: 10.1073/pnas.0400762101
7. Bastien N, Robinson JL, Tse A, Lee BE, Hart L, Li Y. Human coronavirus NL-63 infections in children: a 1-year study. J Clin Microbiol. (2005) 43:4567–73. doi: 10.1128/JCM.43.9.4567-4573.2005
8. Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. (2006) 44:2063–71. doi: 10.1128/JCM.02614-05
9. Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. (1995) 310:1225–9. doi: 10.1136/bmj.310.6989.1225
10. McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. (1974) 130:502–7. doi: 10.1093/infdis/130.5.502
11. Woo PC, Lau SK, Chu CM, Chan KH, Tsoi HW, Huang Y, et al. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J Virol. (2005) 79:884–95. doi: 10.1128/JVI.79.2.884-895.2005
12. Pene F, Merlat A, Vabret A, Rozenberg F, Buzyn A, Dreyfus F, et al. Coronavirus 229E-related pneumonia in immunocompromised patients. Clin Infect Dis. (2003) 37:929–32. doi: 10.1086/377612
13. Falsey AR, Walsh EE, Hayden FG. Rhinovirus and coronavirus infection–associated hospitalizations among older adults. J Infect Dis. (2002) 185:1338–41. doi: 10.1086/339881
14. Kim T, Choi H, Shin TR, Ko Y, Park YB, Kim HI, et al. Epidemiology and clinical features of common community human coronavirus disease. J Thorac Dis. (2021) 13:2288–99. doi: 10.21037/jtd-20-3190
15. Kozak R, Prost K, Yip L, Williams V, Leis JA, Mubareka S. Severity of coronavirus respiratory tract infections in adults admitted to acute care in Toronto, Ontario. J Clin Virol. (2020) 126:104338. doi: 10.1016/j.jcv.2020.104338
16. Fischer N, Dauby N, Bossuyt N, Reynders M, Gérard M, Lacor P, et al. Monitoring of human coronaviruses in Belgian primary care and hospitals, 2015–20: a surveillance study. Lancet Microbe. (2021) 2:e105–e14. doi: 10.1016/S2666-5247(20)30221-4
17. Chung JY, Han TH, Kim SW, Koo JW, Hwang ES. Human coronavirus infection in hospitalized children with community-acquired pneumonia. Korean J Pediatr Infect Dis. (2007) 13:69–74. doi: 10.14776/kjpid.2007.14.1.69
18. Choi YY, Kim YK, Choi EH. Clinical and epidemiological characteristics of common human coronaviruses in children: a single center study, 2015–2019. Pediatr Infect Vaccine. (2021) 28:101–9. doi: 10.14776/piv.2021.28.e10
19. Kim KY, Han SY, Kim HS, Cheong HM, Kim SS, Kim DS. Human coronavirus in the 2014 winter season as a cause of lower respiratory tract infection. Yonsei Med J. (2017) 58:174–9. doi: 10.3349/ymj.2017.58.1.174
20. Killerby ME, Biggs HM, Haynes A, Dahl RM, Mustaquim D, Gerber SI, et al. Human coronavirus circulation in the United States 2014-2017. J Clin Virol. (2018) 101:52–6. doi: 10.1016/j.jcv.2018.01.019
21. Lee WJ, Chung YS, Yoon HS, Kang C, Kim K. Prevalence and molecular epidemiology of human coronavirus HKU1 in patients with acute respiratory illness. J Med Virol. (2013) 85:309–14. doi: 10.1002/jmv.23465
22. Hicks J, Klumpp-Thomas C, Kalish H, Shunmugavel A, Mehalko J, Denson JP, et al. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal betacoronaviruses. J Clin Immunol. (2021) 41:906–13. doi: 10.1007/s10875-021-00997-6
23. Wang C, van Haperen R, Gutierrez-Alvarez J, Li W, Okba NMA, Albulescu I, et al. A conserved immunogenic and vulnerable site on the coronavirus spike protein delineated by cross-reactive monoclonal antibodies. Nat Commun. (2021) 12:1715. doi: 10.1038/s41467-021-21968-w
24. Guo L, Wang Y, Kang L, Hu Y, Wang L, Zhong J, et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect. (2021) 10:664–76. doi: 10.1080/22221751.2021.1905488
25. Sagar M, Reifler K, Rossi M, Miller NS, Sinha P, White LF, et al. Recent endemic coronavirus infection is associated with less-severe COVID-19. J Clin Invest. (2021) 131:e143380. doi: 10.1172/JCI143380
26. Anderson EM, Goodwin EC, Verma A, Arevalo CP, Bolton MJ, Weirick ME, et al. Seasonal human coronavirus antibodies are boosted upon SARS-CoV-2 infection but not associated with protection. Cell. (2021) 184:1858–64. doi: 10.1016/j.cell.2021.02.010
27. Gaunt ER, Hardie A, Claas EC, Simmonds P, Templeton KE. Epidemiology and clinical presentations of the four human coronaviruses 229E, HKU1, NL63, and OC43 detected over 3 years using a novel multiplex real-time PCR method. J Clin Microbiol. (2010) 48:2940–7. doi: 10.1128/JCM.00636-10
28. Chiu SS, Chan KH, Chu KW, Kwan SW, Guan Y, Poon LL, et al. Human coronavirus NL63 infection and other coronavirus infections in children hospitalized with acute respiratory disease in Hong Kong, China. Clin Infect Dis. (2005) 40:1721–9. doi: 10.1086/430301
29. Van Der Hoek L, Sure K, Ihorst G, Stang A, Pyrc K, Jebbink MF, et al. Croup is associated with the novel coronavirus NL63. PLoS Med. (2005) 2:e240. doi: 10.1371/journal.pmed.0020240
30. Sung JY, Lee HJ, Eun BW, Kim SH, Lee SY, Lee JY, et al. Role of human coronavirus NL63 in hospitalized children with croup. Pediatr Infect Dis J. (2010) 29:822–6. doi: 10.1097/INF.0b013e3181de9c2e
31. Riski H, Hovi T. Coronavirus infections of man associated with diseases other than the common cold. J Med Virol. (1980) 6:259–65. doi: 10.1002/jmv.1890060309
Keywords: human coronavirus, multiplex polymerase chain reaction, children, multi-center study, prognosis
Citation: Jo KJ, Choi S-H, Oh CE, Kim HA, Choi BS, Jo DS and Park SE (2022) Epidemiology and Clinical Characteristics of Human Coronaviruses-Associated Infections in Children: A Multi-Center Study. Front. Pediatr. 10:877759. doi: 10.3389/fped.2022.877759
Received: 17 February 2022; Accepted: 21 March 2022;
Published: 12 April 2022.
Edited by:
Tauqeer Hussain Mallhi, Al Jouf University, Saudi ArabiaReviewed by:
Alessandra Pierangeli, Sapienza University of Rome, ItalyCopyright © 2022 Jo, Choi, Oh, Kim, Choi, Jo and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Su Eun Park, cHNlcHNlQG5hdmVyLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.