
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 27 April 2022
Sec. Pediatric Pulmonology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.876882
This article is part of the Research Topic Translational Research in Pediatric Respiratory Diseases - From Bench to Bedside View all 6 articles
Introduction: The identification of gene-environment interactions allows the recognition of groups with higher risk of morbidity. This study evaluated the interaction between the presence of TLR4 gene polymorphisms and Ascaris infection with severe bronchiolitis in a tropical Colombian region.
Methods: We included all infants younger than 24 months hospitalized due to bronchiolitis in Hospital centers in the county of Rionegro, Colombia. To identify interaction between severe bronchiolitis and presence of TLR4 polymorphisms and Ascaris infection, we used log-binomial regression.
Results: Four hundred and seventeen infants were hospitalized due to bronchiolitis, of which 115 (27%) had severe bronchiolitis. In infants with respiratory syncytial virus (RSV) acute infection and positive anti-Ascaris IgE, TLR4 Asp299Gly was associated to low risk of severe bronchiolitis (OR 0.09, CI 95% 0.01–0.48). Conversely, in infants RSV negative with negative anti-Ascaris IgE, TLR4 Asp299Gly was associated with an increased risk of severe bronchiolitis (OR 14.5, CI 95% 2.2–96).
Conclusion: In our population there is an interaction between the presence of severe bronchiolitis, TLR4 Asp299Gly and Ile399Thr polymorphisms, anti-Ascaris IgE levels and RSV. This association should be evaluated in other populations to elucidate its role in the pathogenesis of severe bronchiolitis.
Respiratory syncytial virus (RSV) bronchiolitis is the most important cause of lower respiratory tract infection in children worldwide (1, 2). The disease places a substantial clinical and economic burden, not only on healthcare systems, but also on families and society, mainly in low-to- middle-income countries (LMICs) where more than 90% of the deaths occur (3–6). The treatment consists only in respiratory and hemodynamical support since there is no a specific intervention or vaccination yet (7). Identifying patients at higher risk of morbidity and mortality is essential to plan preemptive strategies in the future. Different determinants of bronchiolitis severity have been described, among them RSV infection, smoking, pre-existing diseases, absence of breastfeeding (8).
A gene-environment interaction as documented between toll like receptor 4 (TLR4) D299G single nucleotide polymorphism and environmental endotoxin exposure reveals a possible T helper 2 (Th2) polarizations in patients with severe RSV bronchiolitis, which may become useful for early identification of these patients at risk of severe RSV disease (9). However, in tropical countries, in addition to exposure to endotoxin, the presence of Ascaris lumbricoides infection has been associated with a Th2 profile in asthmatic or recurrent wheezing children (10). Although, both environmental stimuli may share similar immunological pathways, the gene-environment interaction between TLR4 polymorphisms and infection with A. lumbricoides has not been evaluated. Previously, we made a first report analyzing clinical and sociodemographic risk factors associated with severe bronchiolitis in a retrospective cohort of children with bronchiolitis <2 years of age in Rionegro, Colombia (8). In this study and, using the same population, we study aimed to evaluate the association between the presence of RSV bronchiolitis with TRL4 polymorphisms and Ascaris infection in infants under 2 years old.
The methodology and characteristics of this population were reported previously (8). We included all children with bronchiolitis <2 years old in two hospital centers in the municipality of Rionegro Colombia, between January 2019 to December 2019. All infants diagnosed with bronchiolitis, according to the national clinical guideline of bronchiolitis, were included (8). We excluded infants who did not present lower respiratory infection or positive bacterial cultures on admission or confirmed whooping cough. The Institutional Review Board of the University of Antioquia approved the study protocol (No. 18/2015).
We collected sociodemographic, medical, and clinical variables from medical records in the hospital centers. All variables that were not fully documented in the clinical history were obtained by directly interviewing the parents or caregivers in person or by telephone, previous informed consent to the responsible for the children. Severe bronchiolitis was identified in patients who had an increased respiratory rate, retractions, and oxygen saturation of 90% or less (11). Nasopharyngeal aspirate (NPA) was taken during the first 48 h in the emergency unit using a standardized technique. RSV was confirmed using direct immunofluorescence. There was no data available for other viruses. ImmunoCAP (Thermo Fisher Scientific) was used to measure the levels of specific IgE antibodies against Ascaris spp. where it was considered positive when the specific IgE levels were equal to or greater than 0.35 kUA/L. DNA was obtained from whole blood samples using the Gentra Puregene kit (Gentra Systems) and DNA was quantified using a Beckman Coulter DU640 spectrophotometer. The REPLI-g kit (QIAGEN) was used for the amplification of the DNA samples. The TLR4 Asp299Gly and Ile399Thr polymorphisms were evaluated by allelic discrimination using Applied Biosystems assays that had probes with specific fluorophores for each of the polymorphisms. The thermal profile for PCR consisted of an initial denaturation at 95°C for 10 min, followed by 35 cycles at 92°C for 15 s and 60°C for 1 min. An ABI 7000 (Applied Biosystems) was used for PCR amplification and subsequent characterization of the genotypes for each polymorphism. Controls were used for the correct identification of genotypes in patient samples.
We analyzed the differences of continuous variables using the unpaired t-test or Wilcoxon's signed-rank test, whichever was appropriate, categorical variables were analyzed using the chi-square test or Fisher's exact test. We included only in the final model variables associated with severe bronchiolitis with values of P < 0.2 or that changed the effect estimate by over 10% after their inclusion, to identify factors independently associated with severe bronchiolitis. We also included in the model variables widely known and related to severe bronchiolitis such as age, smoking at home and exclusive breastfeeding (8, 12). A log-binomial regression model was performed with a backward elimination method, used with a P-value of 0.05 as the limit value for the model entry (7). Hosmer–Lemeshow test was used to evaluate he goodness of fit of the model. Two-tailed, and the significance level of P < 0.05 were used in all statistical tests were. All data were analyzed with Statistical Package Stata 15.0 (Stata Corporation, College Station, TX).
During the study period, 417 infants with bronchiolitis were included. Table 1 shows the clinical characteristics of the infant population evaluated. Sixty-six percentage of the patients were <6 months, most of them males (60%), RSV was detected in 200 patients. TLR4 Asp299Gly (AG or AA) was present in 59 patients (14%), while TLR4 Ile399Thr (CC or CT) was present in 286 patients (68%). All the alleles of the TLR4 Asp299Gly and Ile399Thr polymorphisms were in Hardy-Weinberg equilibrium. Positive anti-Ascaris IgE levels (>0.35 kU/L) were observed in 74 patients (18%).
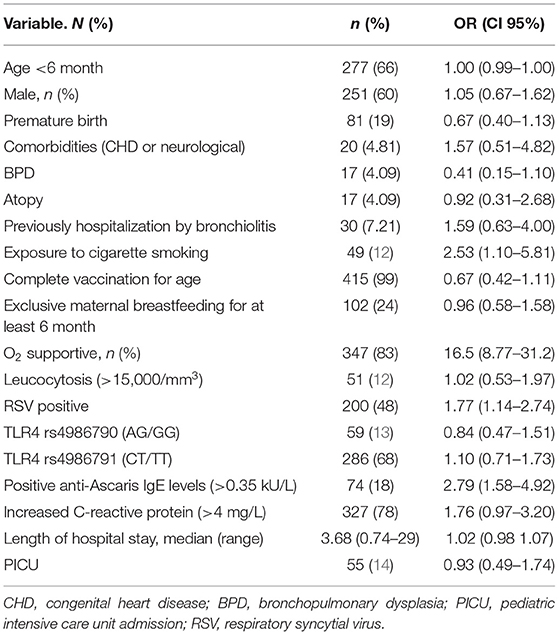
Table 1. Univariate analysis of demographic features and clinical information associated with severe bronchiolitis.
Among all 417 infants, 115 (27%) had severe bronchiolitis. Exposure to smoking in home (OR 2.53, CI 95%, 1.10–5.81), positive anti-Ascaris IgE levels (OR 2.79, CI 95% 1.58–4.92), RSV (OR 1.77, CI 95% 1.14–2.74), crepitation 3.06 (OR, CI 95% 1.19–5.23), and pneumonia (OR 2.54, CI 95% 1.31–4.89) were associated with severe bronchiolitis, Table 1.
After modeling, we detected a statistically significant interaction between severe bronchiolitis and TRL4-IgE Ascaris in RSV-positive (P = 0.000) and non-RSV-positive children (P = 0.000). In infants with positive anti-Ascaris IgE levels, TLR4 Asp299Gly was associated with low risk of severe bronchiolitis independent of the presence or absence of RSV infection. Conversely, in infants with negative anti-Ascaris IgE levels, TLR4 Asp299Gly was associated with higher risk of severe bronchiolitis independent of the presence or absence of RSV infection (see Table 2).
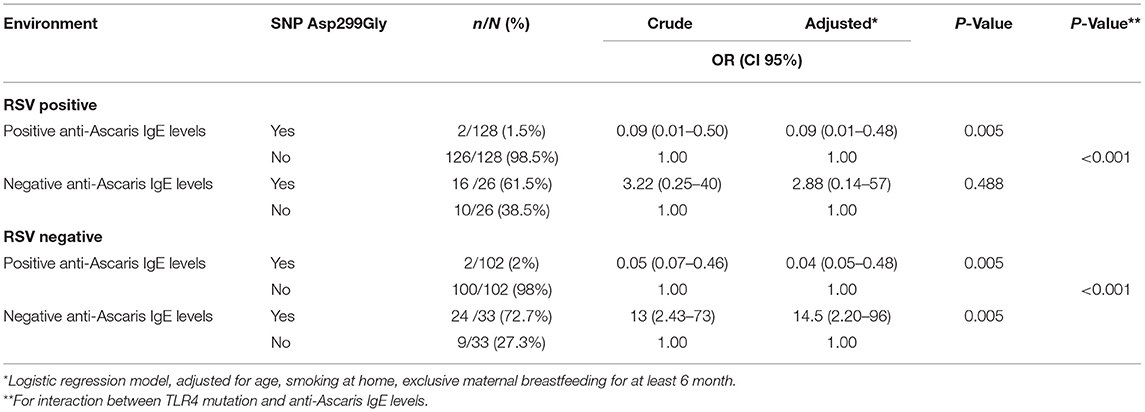
Table 2. OR for severe bronchiolitis: association with TLR4 mutation according to anti-Ascaris IgE Levels and RSV.
Our study identifies an interaction between severe bronchiolitis, TRL4, anti-Ascaris IgE levels and RSV. This interaction has been previously reported with bacterial endotoxin exposure (14). Caballero et al. (9), reported a significant association between TLR4 Asp299Gly and the environment with different levels of endotoxins even after adjusting for risk factors that have an effect on the severity of RSV bronchiolitis. In this study patients with high endotoxin levels, TLR4 Asp299Gly mutation was associated with less risk of severe bronchiolitis (OR 0.20 CI 95% 0.06–0.64), while in infants with low endotoxin levels there was a tendency of higher risk of severe bronchiolitis (OR 8.96 CI 95% 0.98–81.6). In our study we observed a similar result with anti-Ascaris IgE levels. Previously evidence has demonstrated that A. lumbricoides induces an enhanced Th2-biased immune response that cause symptoms in susceptible individuals mediated by cross-reactivity among components of both sources intensifies the Th2 response associated to allergens such as tropomyosins (13).
Ascaris lumbricoides induce the adaptive immune response through its interaction with the extracellular domain of the TLR4 receptor(cite). Our study shows that this effect is regulated by the presence of variants in the TLR4 gene, such as TLR4 Asp299Gly which have been associated with a loss of function of this receptor in the plasma membrane according to previous studies (15). Caballero revealed that children with severe RSV exhibited a high GATA3/T-bet levels, which manifested as a high IL-4/IFN-γ in respiratory secretions. The IL-4/IFN-γ present in children with severe RSV is indicative of Th2 polarization. Murine models of RSV infection showed that endotoxin exposure, the Tlr4 genotype, and Th2 cell polarization influence disease phenotypes (9). Due to the similarity of the findings, it is possible that the mechanisms by which the bacterial endotoxin generates this antagonistic interaction are the same as those occurring in Ascaris infection, a hypothesis to be studied further.
Our study has some limitations. We were unable to include other variables such as environmental pollution and other genetic factors considered important for susceptibility to the disease, and residual confounding cannot be excluded. Second, the study was conducted in a reference hospital center, so the patients included represent the high spectrum of severity. Despite the above, the similarity of our population regarding clinical characteristics, risk factors and seasonality of bronchiolitis in our country coincides with the characteristics of other populations also evaluated for this disease, which suggests strength and consistency in our results (16). Given the retrospective nature would be possible bias due to missing data. However, in all patients the data was collected in electronic records or directly to the patient in outpatient consultations that these patients have in the hospital after their hospitalization. We concluded that in our population there is an interaction between the presence of severe bronchiolitis, TLR4 Asp299Gly and Ile399Thr polymorphisms, anti-Ascaris IgE levels and RSV. This association should be evaluated in other populations to elucidate its role in the pathogenesis of severe bronchiolitis.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Review Board of the University of Antioquia approved the study protocol (No. 18/2015). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CHD, congenital heart disease; BPD, bronchopulmonary dysplasia; PICU, pediatric intensive care unit admission; RSV, respiratory syncytial virus; NPA, nasopharyngeal aspirate.
1. Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. (2010) 375:1545–55. doi: 10.1016/S0140-6736(10)60206-1
2. Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. (2013) 132:e341–8. doi: 10.1542/peds.2013-0303
3. Buendia JA, Patino DG. Disability-adjusted life years for acute bronchiolitis in infants in Colombia. Pan Afr Med J. (2021) 39:236. doi: 10.11604/pamj.2021.39.236.25761
4. Berman S. Epidemiology of acute respiratory infections in children of developing countries. Rev Infect Dis. (1991) 13(Suppl 6):S454–62. doi: 10.1093/clinids/13.Supplement_6.S454
5. Buendia JA, Patino DG. Costs of respiratory syncytial virus hospitalizations in Colombia. Pharmacoecon Open. (2021) 5:71–6. doi: 10.1007/s41669-020-00218-7
6. Villamil JPS, Polack FP, Buendía JA. Disability-adjusted life years for respiratory syncytial virus in children under 2 years. BMC Public Health. (2020) 20:1679. doi: 10.1186/s12889-020-09796-x
7. Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. (2003) 143(5 Suppl):S112–7. doi: 10.1067/S0022-3476(03)00508-0
8. Buendía JA, Patino DG. Risk factors for severe bronchiolitis in Colombia. Trop Doct. (2021) 51:434–7. doi: 10.1177/00494755211002032
9. Caballero MT, Serra ME, Acosta PL, Marzec J, Gibbons L, Salim M, et al. TLR4 genotype and environmental LPS mediate RSV bronchiolitis through Th2 polarization. J Clin Invest. (2015) 125:571–82. doi: 10.1172/JCI75183
10. Zakzuk J, Casadiego S, Mercado A, Alvis-Guzman N, Caraballo L. Ascaris lumbricoides infection induces both, reduction and increase of asthma symptoms in a rural community. Acta Trop. (2018) 187:1–4. doi: 10.1016/j.actatropica.2018.07.016
11. Ralston SL, Lieberthal AS, Meissner HC, Alverson BK, Baley JE, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. (2014) 134:e1474–502. doi: 10.1542/peds.2014-2742
12. Legg JP, Hussain IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. (2003) 168:633–9. doi: 10.1164/rccm.200210-1148OC
13. Ahumada V, Garcia E, Dennis R, Rojas MX, Rondon MA, Perez A, et al. IgE responses to Ascaris and mite tropomyosins are risk factors for asthma. Clin Exp Allergy. (2015) 45:1189–200. doi: 10.1111/cea.12513
14. Richard K, Piepenbrink KH, Shirey KA, Gopalakrishnan A, Nallar S, Prantner DJ, et al. A mouse model of human TLR4 D299G/T399I SNPs reveals mechanisms of altered LPS and pathogen responses. J Exp Med. (2021) 218: e20200675. doi: 10.1084/jem.20200675
15. Fonceca AM, Zosky GR, Bozanich EM, Sutanto EN, Kicic A, McNamara PS, et al. Accumulation mode particles and LPS exposure induce TLR-4 dependent and independent inflammatory responses in the lung. Respir Res. (2018) 19:15. doi: 10.1186/s12931-017-0701-z
Keywords: severe bronchiolitis, polymorphism, respiratory syncytial virus, Ascaris. suum, Colombia
Citation: Buendía JA, Lindarte EF and Polack FP (2022) TLR4 Gene Polymorphisms Interaction With Ascaris Infection in Severe RSV Bronchiolitis. Front. Pediatr. 10:876882. doi: 10.3389/fped.2022.876882
Received: 16 February 2022; Accepted: 29 March 2022;
Published: 27 April 2022.
Edited by:
Diego Raul Hijano, St. Jude Children's Research Hospital, United StatesReviewed by:
Fernando Ferrero, Hospital Pedro de Elizalde, ArgentinaCopyright © 2022 Buendía, Lindarte and Polack. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jefferson Antonio Buendía, amVmZmVyc29uLmJ1ZW5kaWFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.